Abstract
Rationale:
Impulsivity and individual differences in subjective response to alcohol are risk factors for alcohol problems and possibly endophenotypes for alcohol dependence. Few prior studies have addressed relationships between the two constructs.
Objectives:
To predict subjective responses to ethanol, we tested self-reported impulsiveness, ethanol dose condition (high dose, low dose or placebo) and time (7 timepoints) along with interactions among these variables.
Methods:
The present study is a secondary analysis of data from a within subject, placebo-controlled, dose-ranging ethanol administration study using IV infusion with a clamping technique to maintain steady-state breath alcohol concentration. The sample consisted of healthy, non-alcohol dependent social alcohol drinkers between the ages of 21-30 (N=105). Participants at varying levels of impulsivity were compared with regard to stimulant and subjective responses to three ethanol dose conditions over time.
Results:
Individuals with higher impulsivity reported stronger stimulant and weaker sedative response to alcohol, particularly at the higher dose. Higher impulsivity was associated with a steeper increase in stimulant effects during the first half of clamped ethanol infusion with the higher dose.
Conclusions:
These results suggest that impulsive individuals may experience enhanced reinforcing, stimulant effects and relatively muted aversive, sedative effects from alcohol. These subjective responses may relate to enhanced risk of alcohol problems among more impulsive individuals.
Keywords: Alcohol, stimulation, sedation, reinforcement, disinhibition, family history, dose ranging, social drinker, young adult, gender
INTRODUCTION
Alcohol use disorders are prevalent (Hasin et al. 2007; Wittchen and Jacobi 2005) and excessive alcohol use is associated with substantial cost to the public (Rehm et al. 2009). Identification of risk factors for alcohol problems could help to target prevention and intervention efforts. Two risk factors and potential endophenotypes (i.e., genetically-related intermediate phenotypes) for alcohol dependence are subjective response to alcohol (Crabbe et al. 2010) and impulsivity (Dick et al. 2010). Several models have been proposed to understand relationships between each of these constructs and risk for alcohol dependence.
Subjective response may reflect individual differences in sensitivity to alcohol’s pharmacologic effects (Morean and Corbin 2010). Schuckit and colleagues have reported results supporting a low response model (e.g., Schuckit and Gold 1988; Schuckit et al. 1984), in which muted subjective responses to alcohol in the laboratory predict greater risk of alcohol dependence longitudinally (Schuckit and Smith 2000). Low subjective response is thought to be problematic due to diminished experience of sedative effects that provide a signal to slow down or stop consuming alcohol. In lieu of this signal, individuals may drink frequently to hazardous levels (Morean and Corbin 2010; Schuckit 1994). A strong relationship is believed to exist between family history of alcohol problems and low subjective response (Pollock 1992; Quinn and Fromme 2011) though not all studies have confirmed this relationship (de Wit and McCracken 1990; Vogel-Sprott and Chipperfield 1987). A recent meta-analysis found support for the low response model among studies comparing family history positive and negative individuals using oral alcohol administration paradigms (Quinn and Fromme 2011).
An alternative to the low response model, called the differentiator model (Newlin and Thomson 1990), has also been proposed. The differentiator model suggests that those at greatest risk of alcohol-related problems experience enhanced reinforcing, stimulant effects on the ascending limb of the blood alcohol curve and muted adverse, sedative effects on the descending limb. The aforementioned meta-analysis offered support for the differentiator model with regard to heavy compared to light drinking, but not with respect to family history status (Quinn and Fromme 2011). This result highlights the unique contribution of level of alcohol use and familial vulnerability to alcohol-related outcomes. Based on their findings, King et al. (2011) have proposed a modified version of the differentiator model, which posits elevated stimulant and dampened sedative effects among higher risk drinkers across limbs of the blood alcohol curve.
Impulsivity has also been recognized as a risk factor for problem drinking and a possible alcohol dependence endophenotype (Dick et al. 2010). In multiple studies, impulsivity has been related to alcohol use (Sher and Trull 1994; Verdejo-Garcia et al. 2008), alcohol-related problems (Littlefield et al. 2010) and dependence (Bjork et al. 2004). Genetic studies have concluded that impulsivity and related constructs (e.g., conduct disorder) share a common genetic liability with alcohol and other substance use disorders (Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000). Thus, while they are distinct constructs, impulsivity and subjective response to alcohol may each be manifestations of genetic risk for alcohol dependence.
To date, most studies on the relationship between impulsivity and subjective response to alcohol have yielded non-significant or equivocal findings. These include non-significant results from studies involving self-report (Corbin et al. 2008—unpublished findings; Magrys et al. 2013; Rose and Grunsell 2008) and behavioral task measures of impulsivity (Schuckit et al. 2012). Other studies have reported difficult to interpret associations that were limited to particular measures, subgroups and/or timepoints/limbs of the blood alcohol curve. Among male and female non-dependent drinkers (mean age = 30), Nagoshi et al. (1991) reported a significant association between self-reported impulsivity and enhanced sedative response, but only among males on the descending limb of the blood alcohol curve. Among male and female social drinkers (mean age = 26) Shannon et al. (2011) reported significant, inverse relationships between impulsive performance on a go/stop behavioral task and arousal reported at a timepoint on the ascending limb and stimulation reported at a timepoint on the descending limb. No significant relationships were found involving self-reported impulsivity. In addition, Yip et al. (2012) reported diminished subjective intoxication among young males with histories of hypomania, which is often associated with impulsive behavior (Moeller et al. 2001). However, the relationship between self-reported impulsivity and subjective response was not significant. These equivocal findings were all from studies involving single, oral alcohol doses.
Intravenous (IV) administration using a clamping procedure to maintain steady state blood alcohol levels is a potentially advantageous approach to investigating relationships between subjective response and impulsivity. While oral and fixed IV administration are valuable approaches, both have limitations including variable absorption, side effects (e.g., nausea) and variable blood alcohol levels (BALs) (O'Connor et al. 1998; Ramchandani et al. 1999). Ethanol administration via IV infusion titrated to a breathalyzer reading and clamped at a steady state (Gilman et al. 2012; Ramchandani et al. 1999; Roh et al. 2011) enables direct comparisons of the effects of a specific dose of ethanol between groups without these confounds. This clamping procedure has been used safely in healthy participants with targeted BALs from 50-150 mg/dl (Morzorati et al. 2002; Ramchandani et al. 1999; Subramanian et al. 2002).
Given the lack of clear prior findings, continued research on the relationship between the constructs of impulsivity and subjective effects of alcohol is of high clinical importance. The present report is a secondary analysis of data from a study designed to compare the effects of two ethanol doses and placebo on subjective response to ethanol and cognitive function in non-alcohol-dependent, family history positive versus negative young adults (Kerfoot et al. 2013). In the parent study, participants responded to IV ethanol administration with clamping technique in a dose-related, time-dependent manner on measures of subjective effects and showed impaired coordination and impaired performance on cognitive measures. However, no significant differences were found between family history positive and negative individuals. To our knowledge, among studies examining relationships between impulsivity and subjective response, ours is the first to utilize data from IV administration and the first involving multiple alcohol doses. Use of multiple doses is important as dosage is a key factor in subjective response (Morean and Corbin 2010; Schuckit et al. 1984). The use of lower risk social drinkers allows for investigation of relationships between impulsivity and subjective response to alcohol with minimal confounding by heavy alcohol exposure, which has been found to affect both subjective response to alcohol (Morean and Corbin 2008) and impulsive behavior (see Leeman et al. 2009).
METHODS
Sample and Preliminary Procedures
Healthy individuals were recruited by advertisement and compensated for participating. One-hundred eighty participants completed at least one test day. Participants who completed a self-report measure of impulsiveness (n = 105) that was added after the beginning of the parent study comprise the sample for this report. Inclusion criteria were: (i) age 21-30 and (ii) medically and neurologically healthy based on history, physical examination, electrocardiogram, and screening laboratories. Exclusion criteria were: (i) for women: positive pregnancy test or intention to engage in sex without use of birth control; (ii) alcohol naïve; (iii) lifetime DSM-IV diagnosis of any psychiatric disorder including substance use disorders except non-treatment-seeking individuals with alcohol abuse; (iv) any history of counseling or psychotherapy except family therapy focused on relatives; (v) unwillingness to be alcohol free for 48 hours before each test day; (vi) positive urine drug toxicology on test days; (vii) adoptees with no contact with family members; and (viii) a history of maternal alcoholism.
Randomized participants were classified as family history positive (FHP) or negative (FHN) defined as follows: (1) FHP had a biological father and another first or second-degree biological relative with history of alcohol dependence, while (2) FNH had no history of alcohol dependence in any first or second-degree relative. Individuals had to be able to report on first and second-degree relatives in order to participate. The institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine approved this study.
After signing informed consent, subjects began baseline screening. Eligible participants were scheduled for three separate test days a minimum of three days apart under double-blind conditions in randomized order. Participants were told they would receive ethanol on two of the three test days. Test days included high concentration ethanol (targeted breath alcohol concentration [BrAC] = 100mg%), low concentration ethanol (target BrAC= 40mg%) or placebo, within-subjects. Participants fasted overnight before each session. They reported to the Biological Studies Unit at VA Connecticut Healthcare System, West Haven campus, at 9:00am each test day. Before testing, participants underwent urine drug and breathalyzer screening. Participants were tested for cannabis, barbiturates, benzodiazepines, cocaine, opiates and methadone. After all tests returned negative, an IV line was placed. Participants were then given a light breakfast. See Kerfoot et al. (2013) for further detail regarding procedures.
Ethanol Infusion
Ethanol administration procedures were in accordance with established guidelines (NIAAA 2005). Infused ethanol was a solution of ethanol 6% (v/v) in 0.9% saline solution via a computerized pump (Braun Horizon NXT) to obtain a predetermined steady state (“clamped”) BrAc. Loading phase rate was determined using a MATLAB (1987) calculation, including participant sex, age, weight and height to generate linear ascension to target BrAc in approximately 20 min. After target BrAC was reached, the infusion pump rate was adjusted so that participants were maintained within ±5 mg% of target BrAc for 60 min. BrAc was measured every 2 min during the ascending phase and every 2-8 min during steady-state by Alcotest 7410-plus device (Dräger Safety AG & Co. KGaA, Lübeck, Germany). Self-reports of subjective response to ethanol were collected at +10, +30 and +60 min. after target BrAC was reached. For placebo infusion, IV bottles marked the same as those used for ethanol infusion were utilized. BrAC testing and pump alterations mirrored procedures used during ethanol test days. After the 60 min. period during which steady state BrAC was maintained using the clamping procedure, ethanol infusion ended. BrAC readings and subjective response continued to be collected after the end of ethanol infusion at +110, +140, +170 and +230 minutes after target BrAC was achieved (see Figures 1 and 2).
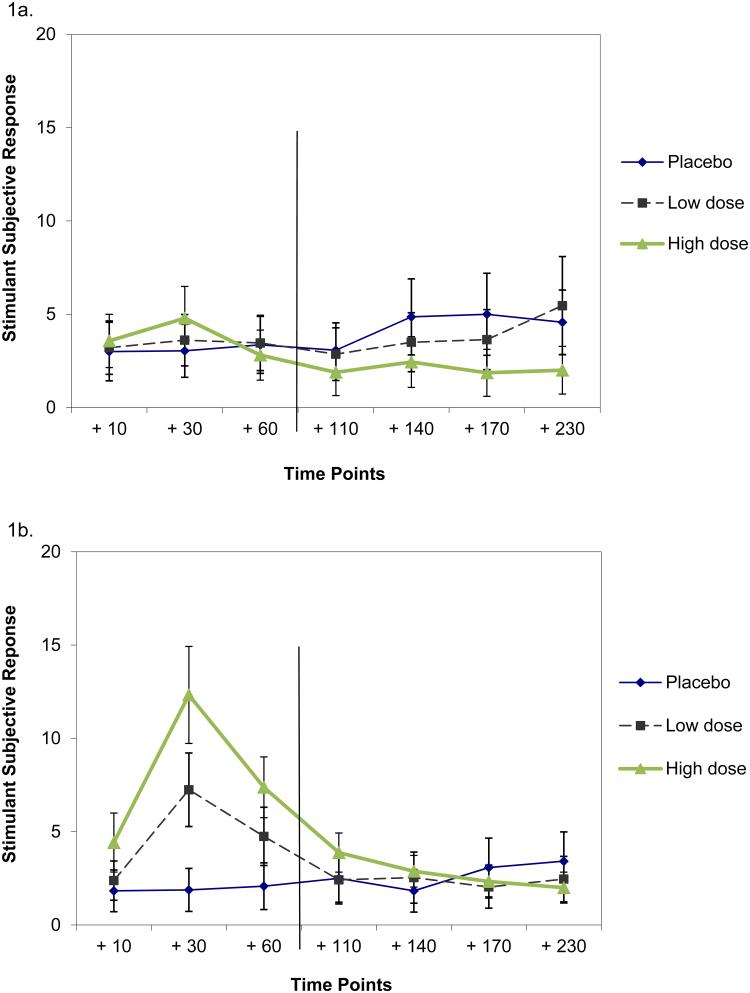
Figure 1.
Stimulant subjective response to IV ethanol by dose condition over time among participants at the lowest (1a) and highest (1b) quartile for self-reported impulsivity. Timepoints are with respect to the time at which steady state BrAC was first reached using a clamping procedure, which required approximately 20 minutes. Ethanol infusion occurred during the +10, +30 timepoints and +60 timepoints and ended after the +60 timepoint, which is indicated with a vertical line after the +60 timepoint. Equal spacing of timepoints was for parsimony and thus is not to scale.
Measures
Assessments administered at the screening appointment included demographic items and the following self-reports:
Impulsivity
Barratt Impulsiveness Scale, Version 11 (BIS-11; Patton et al. 1995) score was the main predictor variable. The BIS-11 is a 30-item self-report questionnaire with items rated on a four-point scale. Recent evidence called into question the psychometric quality of the subscales of the BIS-11 (Steinberg et al. in press), thus only the total score was utilized (α=.70).
Past 30-day alcohol use
The Timeline Follow-back (TLFB) (Sobell and Sobell 2003) is a structured interview that utilizes a calendar including memory prompts (e.g., holidays) to assist with recall of daily alcohol consumption. TLFB data have been shown to be valid and reliable out to 12 months (Sobell and Sobell 2003).
Family history of alcohol dependence
The alcohol portion of the Family History Assessment Module (FHAM; Rice et al. 1995) was administered at screening. The FHAM is a structured interview that contains diagnostic items related to first- and second-order relatives.
Assessments collected during study sessions included the following:
Subjective response to ethanol
The Biphasic Alcohol Effects Scale (BAES) (Martin et al. 1993) was administered at regular intervals during and after ethanol infusion (see Figures 1-2). Ratings were analyzed beginning at the first post-infusion time point (+10 minutes after target BrAC was reached). The BAES is a 14-item self-report measure with sub-scales to assess subjective experiences of alcohol stimulation (i.e., “elated,” “energized,” “excited,” “stimulated,” “talkative,” “up,” “vigorous”; α=.95) and sedation (“down,” “heavy head,” “inactive,” “sedated,” “slow thoughts,” “sluggish,” “difficulty concentrating”; α=.91). Participants rated their experience of each effect due to alcohol on 11-point scales anchored by “not at all” (0) and “extremely” (10).
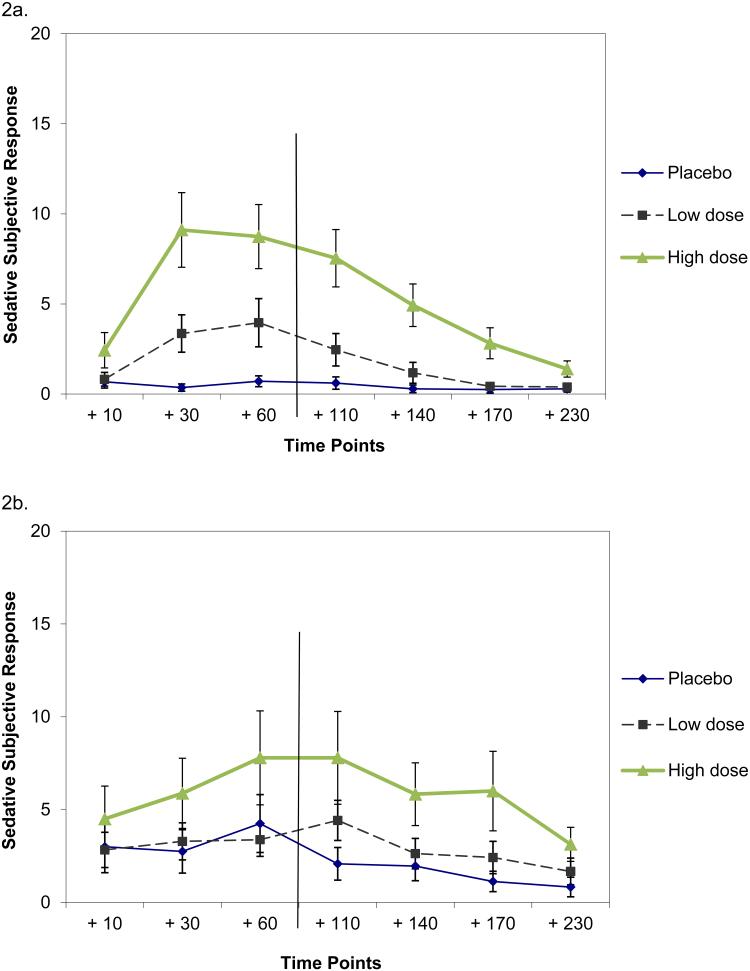
Figure 2.
Sedative subjective response to IV ethanol by dose condition over time among participants at the lowest (2a) and highest (2b) quartile for self-reported impulsivity. Timepoints are with respect to the time at which steady state BrAC was first reached using a clamping procedure, which required approximately 20 minutes. Ethanol infusion occurred during the +10 and +30 timepoints and +60 timepoints and ended after the +60 timepoint, which is indicated with a vertical line after the +60 timepoint. Equal spacing of timepoints was for parsimony and thus is not to scale.
Physiologic response and BAL
Blood pressure and pulse readings were taken while participants were seated at multiple timepoints throughout the test sessions. Blood alcohol levels were also taken approximately 10 minutes before the end of ethanol infusion.
Statistical Analyses
We checked normality for continuous variables. All subjective response variables were skewed, particularly in the placebo condition due to infrequent nonzero response, and efforts to log transform these variables were not successful as the variables remained heavily skewed following these transformations. As a result, all outcomes were analyzed using a nonparametric approach for repeated measures (Brunner et al. 2002) in which data were first rank-ordered, then fitted using a mixed effects model with unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS). Primary outcome variables were self-reported stimulation and sedation on the BAES. We utilized separate models to predict each. All models included three predictor variables and their interactions: 1. self-reported impulsivity; 2. ethanol dose condition (high dose, low dose or placebo); and 3. time (7 timepoints: +10, +30, +60, +110, +140, +170 and +230 min.). Dose and time were within-subject variables. Timepoints were anchored to the time when target BrAC was first achieved. For instance, the +10 timepoint occurred 10 minutes after target BrAC was reached, approximately 30 minutes after IV ethanol infusion began. Given that this nonparametric approach is not amenable to continuous variables, we divided impulsivity scores into quartiles and created a 4-level ordinal variable. We added additional variables (i.e., test day [1, 2 or 3], gender and family history) and three-way interactions with time and dose condition involving these variables to models subsequently. Additional effects that were statistically significant at p<.05 and improved model fit significantly based on likelihood ratio testing were retained in the final models.
Nonparametric analyses were the most appropriate option given the non-normal subjective response data. However, due to concern about ambiguity in the interpretation of rank order scores and loss of information from converting impulsivity score into an ordinal variable, we confirmed results of the final nonparametric models using linear mixed effects (LME) models with random subject effects and other predictors treated as fixed effects. Results from the LME models were very similar to the ATS results and thus are not reported here (results available from the first author).
In the event of significant relationships involving impulsivity, we took two steps to interpret these results. First, we plotted subjective response scores by self-reported impulsivity quartile. Second, when we found impulsivity by time interactions, we fitted additional models to examine changes between the individual timepoints during ethanol infusion (between the +10 and +30 timepoints and between the +30 and +60 timepoints) and across the post-infusion timepoints (from the +110 through the +230 timepoint) to determine when significant associations with impulsivity were most relevant. Alpha was Bonferroni-corrected in these additional models.
We used LME models to test relationships between impulsivity and physiologic response (i.e., blood pressure and pulse) by dose condition and timepoint. To confirm the validity of the ethanol infusion and clamping procedure, which was based on BrAC, we analyzed blood alcohol results taken approximately 10 minutes before the end of ethanol infusion.
RESULTS
Participants
Descriptive data (N = 105) overall and by impulsivity quartile are reported in Table 1. Ninety-nine participants (94.3%) completed all three test days. Among partial-completers, 3 received placebo only, 1 received 40mg% only and the other 2 received both ethanol doses but no placebo. Data from all 105 participants were included. The orders in which ethanol dose conditions occurred were compared across impulsivity quartiles and found to be highly similar, X2 (df = 14) = 7.02, p = .957. The sample was 48.6% female, mainly Caucasian (80%) and highly educated with a mean of 16.4 years of schooling (SD = 2). Sample means were approximately 6 drinking days in the past 30 days with an average of just over 2.5 drinks per drinking day. Mean total score (59.33[SD=8.83]) on the BIS-11 approximated a normative adult score (Stanford et al. 2009). More impulsive participants were significantly younger, less educated, reported more drinking days and overall drinks at baseline (Table 1).
Table 1.
Sample descriptives overall (N=105) and by impulsivity quartile (level 1: least impulsive, level 4: most impulsive)
| Categorical variables | Percentage | ||||
|---|---|---|---|---|---|
|
| |||||
| Impulsivity quartile | |||||
|
| |||||
| Level 1 (n=28) |
Level 2 (n=25) |
Level 3 (n=28) |
Level 4 (n=24) |
Overall (N=105) |
|
| Female | 57.1 | 36 | 60.7 | 37.5 | 48.6 |
| Race/ethnicity | |||||
| White | 71.4 | 80 | 78.6 | 91.7 | 80 |
| African American | 10.7 | 0 | 17.9 | 8.3 | 9.5 |
| Asian | 7.1 | 16 | 3.6 | 0 | 6.7 |
| Other | 10.7 | 4 | 0 | 0 | 3.8 |
| Family history positive | 42.9 | 28 | 42.9 | 41.7 | 39 |
| Current cigarette smoking | 3.6 | 4 | 7.7 | 12.5 | 6.9 |
|
| |||||
| Continuous variables | Mean (SD) | ||||
|
| |||||
| Level 1 (n=28) |
Level 2 (n=25) |
Level 3 (n=28) |
Level 4 (n=24) |
Overall (N=105) |
|
|
| |||||
| Age* | 24.9(2.8) | 25.5(2.8) | 23.5(2.4) | 24(2.4) | 24.5(2.7) |
| Years of Education* | 16.3(1.7) | 17.2(2.2) | 16.4(1.4) | 15.6(2.2) | 16.4(2) |
| Barratt Impulsiveness total score (BIS-11)*** |
49.2(3) | 56.2(1.8) | 61.8(1.8) | 71.6(5.9) | 59.3(8.8) |
| Past 30-day baseline alcohol use | |||||
| Drinking days** | 3.8(3.3) | 5.4(4.4) | 7.3(3.5) | 7.4(5.4) | 5.9(4.4) |
| Total drinks* | 9.9(9.1) | 14.4(15.1) | 23.5(35.6) | 25.6(22.8) | 18.2(23.7) |
| Drinks per drinking day | 2.3(1.6) | 2.3(1.5) | 2.7(2.4) | 3.5(2.6) | 2.7(2.1) |
Significant difference across impulsivity quartiles at
p <.001,
p <.01,
p <.05.
There were no significant differences across impulsivity quartiles for any of the categorical variables in the top half of the table.
Analyses to confirm BAL
BAL results taken about 10 minutes before IV infusion ended confirmed significant differences across dose conditions. No participants registered a positive BAL in the placebo condition. Mean BALs were 47.6mg/dl (SD=.053) in the low dose (target = 40mg/dl) and 115mg/dl (SD=.098) in the high dose condition (target = 100mg/dl). In addition, Kerfoot et al. (2013) reported that low and high dose ethanol produced expected, significant effects on multiple subjective response variables in a dose-related manner in this study.
Prediction of stimulant response
A significant three-way interaction indicated that higher impulsivity was associated with stronger stimulant response (Table 2). These results were found while holding other variables constant: family history, gender and test day (Table 2). To illustrate this effect, plots of stimulant response by dose and timepoint among the most and least impulsive quartiles are given in Figure 1. After reviewing plots of subjective response by dose over time among the four impulsivity quartiles, our next step in interpreting the three-way interaction was to fit additional nonparametric models to assess changes between the timepoints when clamped ethanol infusion occurred (between the +10 and +30 timepoints and between the +30 and +60 timepoints) and across the timepoints following the end of clamped ethanol infusion (from the +110 through the +230 timepoint). In a model to compare the +10 with the +30 timepoint, the three-way interaction was again significant (num df = 1.93, ATS = 7.37, p = 0.001). While differences were not observable by impulsivity level or dose condition at the +10 timepoint, the increase in stimulation between the +10 and +30 timepoints was significantly greater at higher impulsivity levels. Further, this change was most pronounced during high-dose ethanol administration (Figure 1). In a model to assess change between the +30 and +60 timepoints, the dose by impulsivity interaction was significant (num df = 1.92, ATS = 8.05, p < 0.001), but the three-way interaction was not (num df = 1.92, ATS = 0.30, p = 0.73). Again, the more impulsive participants were, the stronger their stimulant response, particularly in the high ethanol condition. However, this relationship applied the same way across the +30 and +60 timepoints, thus the lack of a significant three-way interaction.
Table 2.
Final nonparametric mixed effects models involving rank order versions of the stimulant and sedative response variables
| Stimulant response final model | |||
|---|---|---|---|
|
| |||
| Effect | Num df | ATS value | p value |
| Ordinal impulsivity score | 1 | 0.01 | 0.938 |
| Dose condition (placebo, low or high dose) | 1.61 | 0.44 | 0.602 |
| Timepoint (7 timepoints: +10 through +230) | 2.83 | 0.91 | 0.432 |
| Dose × timepoint | 7.63 | 1.72 | 0.091 |
| Impulsivity score × dose | 1.61 | 7.16 | 0.002 |
| Impulsivity score × timepoint | 2.83 | 2.96 | 0.034 |
| Impulsivity score × dose × timepoint | 7.63 | 2.37 | 0.017 |
| Test day | 2 | 15.06 | <0.001 |
| Gender | 1 | 3.86 | 0.05 |
|
| |||
| Sedative response final model | |||
|
| |||
| Effect | Num df | ATS value | p value |
|
| |||
| Ordinal impulsivity score | 1 | 0.93 | 0.336 |
| Dose condition | 1.92 | 29.49 | <0.001 |
| Timepoint | 4.02 | 17.43 | <0.001 |
| Dose × timepoint | 7.88 | 6.94 | <0.001 |
| Impulsivity × dose | 1.92 | 3.49 | 0.033 |
| Impulsivity × timepoint | 4.02 | 2.05 | 0.084 |
| Impulsivity × dose × timepoint | 7.88 | 3.57 | 0.001 |
| Test day | 2 | 4.62 | 0.001 |
| Family history | 1 | 8.96 | 0.003 |
Num df: numerator degrees of freedom, ATS: ANOVA-type statistic. Impulsivity was measured with the Barratt Impulsiveness Scale, version 11 (BIS-11; Patton et al. 1993). For these analyses, BIS-11 scores were divided into quartiles and a 4-level ordinal version of the variable was created. Models were tested including main effects of gender, test day and family history and three-way interactions by time and dose condition involving these variables for each outcome, however only effects that were significant a p < .05 and improved model fit were retained in the final models reported here.
In addition, the main effect of test day was significant due to a tendency for ratings to decline between the first and second day. The main effect of gender was significant due to males giving higher ratings. There were no significant interactions by dose and timepoint for either test day or gender. Neither family history nor an interaction involving family history met criteria for inclusion in the final model (Table 2).
In a model to assess stimulant response across timepoints following the end of clamped ethanol infusion (+110 through +230), the dose by impulsivity interaction was significant (num df = 1.55, ATS = 6.39, p = 0.004), but the three-way interaction was not (num df = 4.14, ATS = 1.02, p = 0.396). The significant two-way interaction was due to low impulsive participants reporting somewhat stronger stimulation than high impulsive participants with the placebo and low dose while there were no significant differences by impulsivity level with the high dose during these later time points.
Prediction of sedative response
A significant three-way interaction indicated that higher impulsivity was associated with weaker sedative response (Table 2). We based this conclusion on examination of plots of sedative response among the most and least impulsive participants (Figure 2). These results were found while holding other variables constant: family history, gender and test day (Table 2). After examination of the plots, we again fit additional nonparametric models to assess changes between timepoints during ethanol infusion. These findings paralleled results for stimulant response but in the opposing direction. In a model examining change between the +10 and +30 timepoints, the three-way interaction was significant (num df = 1.82, ATS = 4.48, p = 0.014). While differences were not observable at the +10 timepoint, the increase in sedation between the +10 and +30 timepoints was greater at lower impulsivity levels. As with stimulant response, this change was most pronounced during high-dose ethanol administration (Figure 2). In a model to assess change between the +30 and +60 timepoints, also paralleling the simulant results, the dose by impulsivity interaction was significant (num df = 1.99, ATS = 7.25, p = 0.001), but the three-way interaction was not (num df = 1.99, ATS = 0.13, p = 0.874). This meant that the less impulsive participants were, the stronger their sedative response, particularly in the high dose condition, but that this relationship applied in the same way across the +30 and +60 timepoints, thus the lack of a significant three-way interaction.
There was a significant main effect of test day where sedation ratings on the third day were lower than on the other two days. A significant main effect of family history was due to lower ratings among family history positive participants. However, there were no significant interactions by dose and timepoint for test day or family history. Neither a gender main effect nor an interaction involving gender met criteria for inclusion in the final model (Table 2).
In a model to assess sedative response across timepoints following ethanol infusion (the +110 through +230 timepoints), the three-way dose by impulsivity interaction was significant (num df = 4.66, ATS = 4.65, p < 0.001). As can be observed in Figure 2, among less impulsive participants, sedative response continued to be stronger in the high dose than in the low dose condition. Sedative response also declined steadily following the end of ethanol infusion among less impulsive participants. However, the decline in sedative response was less consistent among more impulsive participants after the end of infusion.
Alternate analyses
Due to significant differences across impulsivity quartiles (Table 1), alternate models were tested including age, years of education and baseline alcohol use as covariates. None of these variables significantly predicted subjective response and their inclusion in models did not change the study results, thus findings from these alternate models were not reported here.
Analyses of physiologic effects
LME models were conducted to predict physiologic measures for comparison with results involving the subjective response variables. While there were significant effects of dose condition and timepoint, impulsivity was not a significant predictor of blood pressure or pulse.
DISCUSSION
The main finding is that participants with higher impulsivity reported stronger stimulant and weaker sedative effects during IV ethanol infusion. Notably, higher impulsivity was associated with a steeper increase in stimulant effects during the first half of clamped ethanol infusion (between the +10 and +30 timepoints). In contrast, lower impulsivity was associated with a steeper increase in sedative response in the first half of clamped ethanol infusion. These findings were most pronounced following the high dose targeting a BrAC of 100mg%. These results were found regardless of family history and gender effects. The findings shifted following the end of clamped ethanol infusion, such that differences by impulsivity level dissipated and were no longer as clear. It is not surprising that we observed changes in subjective response after the end of ethanol infusion, given that BALs were no longer uniform and had begun to decline. Impulsivity did not influence physiologic responses to alcohol.
An advantage of the clamping procedure used in this study is the ability to observe changes in subjective response over time that are not due to rate of BAL change or rate of alcohol elimination. An interesting additional finding was that after the rise in stimulant response during the first half of clamped ethanol infusion, stimulant response began to decline between the +30 and +60 timepoints among the most impulsive participants while steady state levels of ethanol were maintained. This pattern was most readily observable in the high dose condition and to a lesser extent in the low dose condition. These results are indicative of acute tolerance (O’Connor et al. 1998) to stimulant effects among the most impulsive individuals, even in the absence of declining BALs. Acute tolerance could represent an additional risk factor for impulsive individuals that could lead to heavy drinking in order to obtain higher BALs in the hopes of maintaining their initial stimulant response.
Regarding other predictors, we found significant family history and gender main effects. Consistent with the report from the parent study (Kerfoot et al., 2013), which utilized the full sample of 180 participants, we did not find a family history by dose condition by timepoint interaction. In the present study, irrespective of timepoint, family history positive individuals reported significantly less sedation than family history negative individuals; and males reported more stimulation than females. Given the lack of significant interactions, it is difficult to know whether these results are attributable to family history status or gender differences in subjective response, alcohol-related expectancies or some other response tendency. Thus, these results regarding family history and gender should be interpreted with caution.
Our primary findings of enhanced stimulation and weaker sedative effects among more impulsive drinkers pertain more closely to the differentiator model (Newlin and Thomson 1990) than to the low response model (Schuckit and Smith 2000; Schuckit and Gold 1988; Schuckit et al. 1984). These theoretical models posit particular patterns of subjective response among higher-risk drinkers (e.g., those with a family history of alcohol problems or heavy drinkers). Here, we are extrapolating higher risk to include more impulsive individuals given established relationships between impulsivity and various negative outcomes (Verdejo-Garcia et al. 2008). The differentiator model, and not the low response model, would predict the enhanced stimulant effects reported by the more impulsive participants in this study. However, our findings relate most closely to the modified differentiator model proposed by King and colleagues (King et al. 2011). In the present study, weaker sedative response among more impulsive individuals was observable during the first half of clamped IV ethanol infusion. The modified differentiator model, which states that stronger stimulant and weaker sedative response among higher-risk drinkers are observable across limbs of the blood alcohol curve, would predict this finding. In contrast, the original differentiator model posits weaker sedation among higher-risk drinkers only during the descending limb of the blood alcohol curve (Newlin and Thomson, 1990).
A relationship between impulsivity and stronger stimulant and weaker sedative effects has implications for our understanding of risk factors for alcohol problems. Based on the present findings, the risk of alcohol problems linked to impulsivity (Verdejo-Garcia et al. 2008) may be due at least in part to enhanced stimulant and/or dampened sedative responses. This finding is in contrast to prior studies that have offered null and equivocal results relating impulsivity to subjective response (Corbin et al. 2008—unpublished findings; Magrys et al. 2013; Nagoshi et al. 1991; Rose and Grunsell 2008; Schuckit et al. 2012; Shannon et al. 2011; Yip et al. 2012). To our knowledge, among studies addressing relationships between impulsivity and subjective response, ours is the first to utilize data from IV administration. Use of IV ethanol infusion and clamping procedures may have facilitated observation of relationships between impulsivity and subjective response given the absence of potential confounds associated with oral administration (e.g., variable alcohol absorption and metabolism).
Enhanced stimulation and weaker sedation among more impulsive individuals may be genetically mediated. Impulsivity and related constructs (e.g., conduct disorder) share common genetic liability with alcohol and other substance disorders (Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000) and individual differences in subjective response to alcohol have also been tied to genetic factors (Heath et al. 1999; Roh et al. 2011; Schuckit 1999). However, relationships among subjective response to alcohol, impulsivity and alcohol dependence risk are likely to be complex. Family history and impulsivity were not related significantly in the present study and similar null results have been reported earlier (Schuckit and Smith 2000).
The present study had limitations. IV ethanol administration with clamping technique offers design advantages but introduces departures from external validity given that alcohol is typically consumed orally. Differences between IV administration and typical oral alcohol consumption may explain the magnitude of subjective response reported in this study, which was noticeably lower than in most oral administration studies involving comparable alcohol doses (e.g., Addicott et al. 2007; King et al. 2011). Young adults, who were primarily Caucasian and highly educated, comprised the sample, which limits generalizability of the results to other populations. In addition, the parent study was designed to make comparisons of subjective response to ethanol between family history positive and negative individuals. In order to draw clearer conclusions about differences by family history status with minimal confounding by effects of substantial alcohol exposure, the study recruited only non-alcohol-dependent social drinkers. Similarly, in the present study, differences across impulsivity levels are more likely to be attributable to implications of impulsivity rather than to effects of substantial alcohol exposure. While we believe this was a sound approach, it leaves unanswered the question of whether the patterns of subjective response by impulsivity level reported here would also apply to heavy drinkers from the community or within a clinical sample of patients with alcohol use disorder. This is an important potential future research direction. Both patterns of subjective response (King et al., 2011) and impulsivity (Littlefield et al., 2010) have been found to predict alcohol-related outcomes prospectively. Thus, it would be important to determine whether drinkers at high risk with respect to both subjective response and impulsivity are at particularly high risk for continued heavy drinking and negative consequences. A paradigm in which subjective response to IV ethanol with clamping procedure is examined by impulsivity level could also benefit future medication development (i.e., testing medications to decrease stimulation and/or increase sedation among highly impulsive, heavy drinkers).
To our knowledge, among studies examining relationships between subjective response and impulsivity, ours is the first to utilize data from IV administration and the first involving multiple alcohol doses. Our results suggest that impulsive individuals may experience enhanced reinforcing, stimulant effects and muted aversive, sedative effects. These subjective responses may relate to enhanced risk of alcohol problems among more impulsive people. This pattern of subjective response needs to be replicated and long-term implications of enhanced stimulant and weaker sedative response in impulsive individuals should be addressed in future studies.
ACKNOWLEDGMENTS
This research was supported by grants P50 AA012870, K01 AA 019694, K05 AA014715, the VA VISN1 MIRECC, the VA Alcohol Research Center, ABMRF/the Foundation for Alcohol Research and the Connecticut Department of Mental Health and Addiction Services. The authors acknowledge the important contributions of Angelina Genovese, R.N.C., M.B.A., Elizabeth O’Donnell, R.N., Michelle Lynn SanPedro, R.N. and Willie Ford of the Neurobiological Studies Unit of the VA Connecticut Healthcare System, West Haven Campus, West Haven, CT. The authors would also like to thank Elisa Gagliardi and Christine Nogueira, B.A. of the Department of Psychiatry, Yale School of Medicine for editorial assistance.
Footnotes
FINANCIAL DISCLOSURES
The authors report that they have no financial conflicts of interest with respect to the content of this research. Dr. O’Malley is a member ACNP/ASCP workgroup, the Alcohol Clinical Trial Initiative, sponsored by Abbott Laboratories, Alkermes, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, Pfizer and Schering Plough; past partner, Applied Behavioral Research; contract, Eli Lily & Company, Nabi Biopharmaceuticals; medication donations, Pfizer, Inc.; Advisory Board, Lundbeck; consultant, Alkermes, GlaxoSmithKline; Scientific Panel of Advisors, Hazelden Foundation; received an honorarium from the University of Florida to provide a grand rounds lecture. Dr. Petrakis has served as an advisor to Alkermes. All other authors have no financial disclosures.
Clinicatrials.gov registration # NCT00612352
REFERENCES
- Addicott MA, Marsh-Richard DM, Mathias CW, Dougherty DM. The biphasic effects of alcohol: comparisons of subjective and objective measures of stimulation, sedation, and physical activity. Alcohol Clin Exp Res. 2007;31:1883–1890. doi: 10.1111/j.1530-0277.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to contol subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons; New York, NY: 2002. [Google Scholar]
- Corbin WR, Gearhardt A, Fromme K. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology. 2008;197:327–337. doi: 10.1007/s00213-007-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith GT, Olausson P, Mitchell S, Leeman RF, O'Malley SS, Sher KJ. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacol. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, Correlates, Disability, and Comorbidity of DSM-IV Alcohol Abuse and Dependence in the United States. Arch Gen Psychiat. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K. A review of expectancy theory and alcohol consumption. Addiction. 2001;96:57–72. doi: 10.1046/j.1360-0443.2001.961575.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott C, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiat. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL. Effects of Family History of Alcohol Dependence on the Subjective Response to Alcohol using the Intravenous Alcohol Clamp. Alcohol Clin Exp Res. 2013;37:2011–2018. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiat. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Leeman RF, Grant JE, Potenza MN. Behavioral and neurological foundations for the moral and legal implications of intoxification, addictive behaviors and disinhibition. Behav Sci Law. 2009;27:237–259. doi: 10.1002/bsl.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK. Do changes in drinking motives mediate the relation betwen personality change and "maturing out" of problem drinking? J Abnorm Psychol. 2010;119:93–105. doi: 10.1037/a0017512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrys SA, Olmstead MC, Wynne-Edwards KE, Balodis IM. neuroendocrinological responses to alcohol intoxication in healthy males: relationship with impulsivity, drinking behavior, and subjective effects. Psychophysiology. 2013;50:204–9. doi: 10.1111/psyp.12007. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Pyschiat. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: The relative influence of early response and acquired tolerance. Addict Behav. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective Response to Alcohol: A Critical Review of the Literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O'Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR, Rodriguez LA. Impulsivity, Sensation Seeking, and Behavioral and Emotional Responses to Alcohol. Alcohol Clin Exp Res. 1991;15:661–667. doi: 10.1111/j.1530-0277.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- NIAAA National Advisory Council on Alcohol Abuse and Alcoholism . Recommended council guidelines on ethyl alcohol administration in human experimentation- Revised. Bethesda; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping Breath Alcohol Concentration Reduces Experimental Variance: Application to the Study of Acute Tolerance to Alcohol and Alcohol Elimination Rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiat. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective Response to Alcohol Challenge: A Quantitative Review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Li T-K. A Preliminary Study of Acute Responses to Clamped Alcohol Concentration and Family History of Alcoholism. Alcohol Clin Res Exp. 1999;23:1320–1330. [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol- use disorders. Alcohol and Global Health 1. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roh S, Sachio M, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li T-K, Higuchi S. Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alcohol Clin Exp Res. 2011;35:400–407. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Grunsell L. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcohol Clin Exp Res. 2008;32:1096–104. doi: 10.1111/j.1530-0277.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiat. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. New findings in the genetics of alcoholism. J Amer Med Assoc. 1999;281:1875–1876. doi: 10.1001/jama.281.20.1875. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiat. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Morrison CR, Gold EO. A pragmatic alcoholism treatment outcome scale. Am J Drug Alcohol Ab. 1984;10:125–131. doi: 10.3109/00952998409002660. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The Relationships of a Family History of Alcohol Dependence, a Low Level of Response to Alcohol and Six Domains of Life Functioning to the Development of Alcohol Use Disorders. J Stud Alcohol. 2000:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Trim RS, Cesario E, Saunders G, Sanchez C, Campbell N. Comparison Across Two Generations of Prospective Models of How the Low Level of Response to Alcohol Affects Alcohol Outcomes. J Stud Alcohol Drugs. 2012;73:195–204. doi: 10.15288/jsad.2012.73.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon EE, Staniforth ER, McNamara J, Bernosky-Smith KA, Liguori A. Response inhibition impairments predict alcohol-induced sedation. Alcohol Alcoholism. 2011;46:33–8. doi: 10.1093/alcalc/agq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. National Institute on Alcohol Abuse & Alcoholism; Bethesda, MD: 2003. [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Indiv Differ. 2009;47:385–395. [Google Scholar]
- Steinberg L, Sharp C, Stanford MS, Tharp AT. New Tricks for an Old Measure: The Development of the Barratt Impulsiveness Scale- Brief (BIS-Brief) Psychol Assessment. doi: 10.1037/a0030550. in press. [DOI] [PubMed] [Google Scholar]
- Subramanian M, Heil S, Kruger M, Collins K, Buck P, Zawacki T, Abbey A, Sokol R, Diamond M. A three-stage alcohol clamp procedure in human subjects. Alcohol Clin Exp Res. 2002;26:1479–1483. doi: 10.1097/01.ALC.0000034038.41972.36. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav R. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, Chipperfield B. Family History of Problem Drinking among Young Male Social Drinkers: Behavioral Effects of Alcohol. J Stud Alcohol Drugs. 1987;48:430–436. doi: 10.15288/jsa.1987.48.430. [DOI] [PubMed] [Google Scholar]
- Wall A-M, Thrussell C, Lalonde RN. Do alcohol expectancies become intoxicated outcomes? A test of social-learning theory in a naturalistic bar setting. Addict Behav. 2003;28:1271–1283. doi: 10.1016/s0306-4603(02)00253-8. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe--a critical review and appraisal of 27 studies. Eur Neuropsychopharm. 2005;15:357–76. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Yip SW, Doherty J, Wakeley J, Saunders K, Tzagarakis C, de Wit H, Goodwin GM, Rogers RD. Reduced subjective response to acute ethanol administration among young men with a broad bipolar phenotype. Neuropsychopharmacol. 2012;37:1808–15. doi: 10.1038/npp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]