Abstract
Computerized treadmill gait analysis in models of toxicant exposure and neurodegenerative disorders holds much potential for detection and therapeutic intervention in these models, and researchers must validate the technology that assists in that data collection and analysis. The present authors used a commercially available computerized gait analysis system that used (a) a motorized treadmill on retired breeder male C57BL/6J mice, (b) the toxicant-induced (1-methyl-1-, 2-, 3-, 6-tetrahydropyridine) MPTP mouse model of Parkinson’s disease (PD), and (c) the superoxide dismutase 1 (SOD1) G93A transgenic mouse model of amyotrophic lateral sclerosis (ALS). The authors compared the detection of deficits by computerized treadmill gait analysis in MPTP-treated mice with inked-paw stride length and correlated these measures to dopamine (DA) loss. The authors found that the computerized treadmill gait analysis system did not distinguish MPTP-treated mice from vehicle controls, despite a nearly 90% deficit of striatal DA. In contrast, decreases in inked-paw stride length correlated strongly with DA losses in these same animals. Computerized treadmill gait analysis could neither reliably distinguish SOD1 G93A mutant mice from controls from 6 to 12 weeks of age nor detect any consistent early motor deficits in these mice. On the basis of the authors’ findings, they inferred that computerized gait analysis on a motorized treadmill is not suited to measuring motor deficits in either the MPTP mouse model of PD or the SOD1 G93A mouse model of ALS.
Keywords: amyotrophic lateral sclerosis, behavioral assessment, gait analysis, motor impairment, MPTP, Parkinson’s disease, SOD
Animal models of toxicant exposure and neurodegenerative disease are important tools for assessing toxic outcomes and for the screening and testing of potential therapeutic interventions at the preclinical stages. Early recognition of behavioral deficits and the quantitative measurement of these deficits are a requirement for the evaluation of the progression of pathology and response to therapies. In Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS), clinical deficits are characterized by motor problems such as bradykinesia, tremor, and rigidity in PD (Marsden, 1984; Morris, Huxham, McGinley, & Iansek, 2001; Olanow & Tatton, 1999; Phillips, Bradshaw, Iansek, & Chiu, 1993); and muscle weakness, fasciculation, and impaired speech in ALS (Francis, Bach, & DeLisa, 1999; Jackson & Bryan, 1998; Walling, 1999). Deviations from normal motor activity or ability are quantified in experimental animals by common testing paradigms such as open field locomotor activity, balance beam walking, ability to remain on a rotating cylinder (rotarod), and measurement of stride length, stance width, and paw angle using inked paws. These testing methods have been used to assess motor deficits with varying levels of success in the 1-methyl 4-phenyl 1-, 2-, 3-, 6-tetrahydropyridine (MPTP) mouse model of PD and the superoxide dismutase 1 (SOD1) G93A mutant mouse model of ALS (Schallert, Whishaw, Ramirez, & Teitelbaum, 1978; Barneoud, Lolivier, Sanger, Scatton, & Moser, 1997; Sedelis, Schwarting, & Huston, 2001; Meredith & Kang, 2006).
Our laboratories have been interested in developing and refining methods for behavioral detection of impairments in animals with chemically and genetically induced movement disorders. One such model is the MPTP mouse model of PD (Mokry, 1995; Tillerson, Caudle, Reveron, & Miller, 2002; Tillerson, Caudle, Reveron, & Miller, 2003; Tillerson & Miller, 2003; Tillerson et al., 2006; Caudle, Tillerson, Reveron, & Miller, 2007). We have shown that inked-paw stride length and parameters of the inverted grid test are dependent on dopamine (DA) and are compromised upon MPTP administration (Tillerson, Caudle, et al., 2002; Tillerson & Miller, 2003). Furthermore, we have shown that these behavioral deficits caused by MPTP can be rescued by the administration of L-dihydroxyphenylalanine (L-DOPA) or behavioral manipulations (Tillerson, Caudle, et al., 2002; Tillerson, Cohen, et al., 2002; Tillerson et al., 2003; Tillerson & Miller, 2003).
In SOD1 G93A mutant mice, motor abnormalities can be reliably detected at different ages by using rotarod, incline plane, stride length, and balance beam (Gurney et al., 1994; Barneoud, Lolivier, Sanger, Scatton, & Moser, 1997; Fischer et al., 2004; Fischer et al., 2005). We have extensively tested these mice for motor pathology as well as behavioral impairments and have observed a deficit in the ability to complete the rotarod task starting at approximately 12 weeks old. This is coincident with major denervation of the neuromuscular junction (NMJ), actively degenerating ventral root axons, and a significant loss of alpha motor neurons (Fischer et al., 2004; Fischer et al., 2005). Furthermore, other studies have shown a selective and progressive degeneration of fast firing neuromuscular synapses and motor unit numbers beginning as early as 6 weeks old (Kennel, Finiels, Revah, & Mallet, 1996; Frey et al., 2000; Fischer et al., 2004). Transgenic mice studies have suggested that the denervation of the NMJ is the most critical determinant of motor deficits in SOD1 G93A mice (Gould et al., 2006). In addition, our investigations show there is already a 30% loss of innervated NMJs at 7 weeks old (Fischer et al., 2004).
Gait analysis systems with computerized data acquisition and analysis have been suggested as a better way to monitor gait dysfunction and disease progression in models of genetic and toxicant-induced neurological disorders (Kale, Amende, Meyer, Crabbe, & Hampton, 2004; Amende et al., 2005; Wooley et al., 2005). As compared with the methods that do not use computerized acquisition and analysis, the computerized gait analysis systems are marketed as a more efficient, sensitive, and reliable way to measure motor deficits. Commercially available treadmill gait analysis systems were envisioned to replace the laborious and limited scope of tests such as inked-paw stride length and rotarod by using a digital paw print readout, complete with analysis parameters derived from observing the paw contact area over time.
We utilized a commercially available computerized treadmill gait analysis system for two reasons: (a) to increase the throughput (ease of increasing the number of mice tested per group) and (b) to increase the sensitivity (detecting deficits in animals with lesser amounts of neuronal damage) of our mouse experiments. We compared the system directly with the inked-paw stride length test in the MPTP mouse model of PD and correlated these measures to DA deficits. Also, we tested whether the system could reveal differences in mutant mice versus control mice, as well as disease progression in the SOD1 G93A model of ALS. These readily testable movement disorder models could demonstrate the potential of a treadmill gait analysis system with computerized data acquisition and analysis to detect early deficits and test therapeutics at the early stages. Our results showed that the computerized treadmill gait analysis system did not detect any changes in the motorized treadmill-running gait parameters of the MPTP-treated mice and found two early differences in SOD1 G93A mice that did not correlate to disease progression. Our data enable us to conclude that the inability of the treadmill gait analysis system to find significant and consistent changes in the aforementioned disease models reflects a limitation of using a motorized treadmill to assess these gait dysfunction disorders.
Method
Gait Analysis
We used a Mouse Specifics (Cambridge, MA) treadmill and high-speed camera setup as advised by Mouse Specifics, Inc., and as described (Kale et al., 2004; Amende et al., 2005). Thus, we acclimated mice to the testing chamber for 3 min before the transparent treadmill belt was started. Fluorescent and fiber-optic halogen light was used to illuminate the chamber from above and below, respectively, to create the necessary contrast between paws and body for accurate data collection. A high-speed camera (Basler Vision Technologies, Allentown, PA) mounted below the belt captured 80 frames/ s, and these frames were recorded using the Mouse Specifics DigiVideo software. Data was analyzed by DigiGait software by Mouse Specifics. The treadmill belt was set to run at 20 cm/s, which the manufacturer noted as an appropriate average running speed for mice. Video was collected for 10 s (∼800 frames), and gait parameters were calculated from approximately 15 DigiGait-scorable strides/paw. Strides in which DigiGait failed to properly detect paw placement or misidentified another body part (i.e., nose or tail) as a paw were not scored; this error occurred rarely and is easily corrected within the software. Right and left forepaw (FP) data were averaged, and this average was considered the FP score for that parameter; the same was done with hind-paw (HP) parameters. All retired breeder mice were pretested on the treadmill, and those that could run at 20 cm/s were divided into four groups for DA depletion studies, which commenced 1 week later. The vast majority (90%) of retired breeder mice runs well at this speed; however, some of them could not run at pretesting and were removed from the study. All SOD1 G93A mice ran at 20 cm/s with no obvious problems.
Mice
Male retired breeder C57BL/6J mice, 8–10 months old, were used for the MPTP model studies. Male and female adolescent SOD1 G93A transgenic mice with C57BL/6J age-matched controls were used for ALS model studies (all mice from Jackson Labs, Bar Harbor, ME). Mice were kept on a 12:12 light/dark cycle (lights on at 7:00 a.m.) and had free access to tap water and LabDiet 5001 rodent chow from PMI Laboratories International (Brentwood, MO). All animal treatments were conducted according to standards set forth by Emory University and in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Grossblatt, 1996).
Dopamine Depletion
MPTP was administered in two subcutaneous doses of MPTP (5, 10, or 15 mg/kg, free base) from Sigma (St. Louis, MO) in 0.9% saline, given 12 hr apart (doses indicated as 2 × 5, 2 × 10, or 2 × 15). Mice were tested 1 week after the last dose and sacrificed for neurochemistry the following day. This paradigm has consistently shown substantial loss of striatal DA at this time point. Retired breeder mice are particularly suited to MPTP-induced DA depletion studies because they show no recovery of DA or stride length up to 4 weeks after intoxication (Tillerson, Caudle, et al., 2002; Tillerson et al., 2003; Tillerson & Miller, 2003; Caudle, Tillerson, Reveron, & Miller, 2007).
Verification of Neurotransmitter Loss by HPLC-ECD
HPLC-ECD was used to verify MPTP-induced DA loss as described (Richardson & Miller, 2004). Briefly, striatal tissue was sonicated in 0.1 M of perchloric acid for 30 s. Each sample was centrifuged at 14,000 × g for 10 min. The resulting supernatant was subjected to a 0.22-µm filter by centrifugation, and 20 µl of the filtrate was analyzed. The potential applied to the first electrode was set at −70 mV and increased in increments of 50 mV to a final potential of 350 mV. The mobile phase consists of 4-mM citrate, 8-mM ammonium acetate, 54-µM EDTA, 230-µM 1-octanesulfonate, and 5% methanol, pH 2.5. Separation was achieved using an ESA MD-150 micropore column (150 × 3.2–mm i.d.; 3-µm particle size) with a flow rate of 0.6 ml/min. DA levels are expressed as ng per mg striatal tissue wet weight.
Inked-Paw Stride Length
Inked-paw stride length was performed as described (Tillerson, Caudle, et al., 2002). Briefly, mice were trained to walk a narrow path between two inverted cages over paper (approximately 30 cm) toward their individual home cages. This test is performed under fluorescent lighting similar to what mice experience inside the treadmill testing chamber. The front paws were inked, and the mice were allowed to walk toward their home cage across the paper without stimulation. Toe-to-toe measures were taken and averaged (approximately 4 strides/paw). Mice were retested or strides were not scored when there was a complete halt of forward motion or running.
Statistics
Measures of single parameter retired breeder FP versus HP were subjected to Student’s t test, and differences were considered significant at p < .05. Single parameter MPTP dose response data were analyzed as a one-way analysis of variance (ANOVA) using Student Newman-Keuls post hoc test, and groups were considered significantly different at p < .05. Single parameter SOD1 G93A data were analyzed by two-way ANOVA using genotype and age as factors with Student Newman-Keuls post hoc test, and these were considered significant at p < .05. Note that all gait parameters derived from the analysis software were extracted from the change in paw contact area over time. Gait data were analyzed as raw values and converted to percentage control for presentation.
Results
Treadmill Gait Parameters in Retired Breeder C57BL/6J Mice
An illustration showing the derivation of many gait parameters (from paw contact area over time) can be seen in Figure 1A, and the derivation of others appears from the digital paw print in Figure 1B. Baseline measures of C57BL/6J retired breeder male mice (8–10 months old) running at 20 cm/s were compiled, and gait parameter outputs from the analysis program are represented in Table 1. These are intended as reference values for mice of this breed and age and to show that there are consistent data produced by the analysis software. When FP versus HP gait parameters were compared, we found that several of the measures exhibited significant differences. Stride time, swing time, stride length, and stride frequency were not different. Stance time of the HPs was greater than that of the FPs because of longer length of the limbs. This situation led to a significant difference in parameters dependent on stance time for calculation, because the HPs also have more propel time and less brake time than do FPs. The significant differences found are most likely real differences in gait parameters and were evident because the test group was very large (n = 60).
FIGURE 1.
(A) Derivations of gait parameters from paw contact area plotted over time and (B) stride length and stance width measurements on digital paw prints, as generated by DigiGait software.
TABLE 1.
Gait Parameters of C57BL/6J Retired Breeder Male Mice Running at 20 cm/s
| Variable | M | SE M |
|---|---|---|
| FP swing, ms | 116.61 | 1.92 |
| HP swing, ms | 112.21 | 2.13 |
| FP brake, ms | 54.77 | 1.73 |
| HP brake, ms | 43.07 | 1.40 |
| FP propel, ms | 149.34 | 2.22 |
| HP propel, ms | 168.61 | 2.11 |
| FP stance, ms | 203.89 | 1.92 |
| HP stance, ms | 211.48 | 1.52 |
| FP stride, ms | 320.12 | 3.39 |
| HP stride, ms | 323.49 | 3.12 |
| FP stride length, cm | 6.40 | 0.07 |
| HP stride length, cm | 6.47 | 0.06 |
| FP stride length, variability cm | 0.94 | 0.04 |
| HP stride length, variability cm | 0.81 | 0.04 |
| FP stride frequency, Hz | 3.24 | 0.04 |
| HP stride frequency, Hz | 3.22 | 0.04 |
| FP stance width, cm*** | 1.42 | 0.02 |
| HP stance width, cm | 2.37 | 0.02 |
| FP stance width, variability cm*** | 0.37 | 0.02 |
| HP stance width, variability cm*** | 0.22 | 0.01 |
| FP paw angle, deg*** | 4.47 | 0.30 |
| HP paw angle, deg | 16.65 | 0.41 |
| FP step angle, deg*** | 67.60 | 0.96 |
| HP step angle, deg | 58.21 | 1.06 |
| FP step angle, variability deg | 13.47 | 0.81 |
| HP step angle, variability deg | 12.91 | 0.63 |
Note. FP = forepaw; HP = hind-paw.
p < .001.
Gait in MPTP-Treated Mice
Mice injected with 0.9% saline or MPTP (two injections of 5, 10, or 15 mg/kg, 12 hr apart, n = 12–15 per dose) were run at 20 cm/s, and their gait parameters were compared in Figure 2. The loss of DA did not produce any significant changes in treadmill gait parameters at any dose of MPTP. FP stride length variability and FP step angle variability were nonsignificantly increased as one may expect with a loss of limb control, but two other variability measures that one may expect to increase—HP stance width variability and HP step angle variability—inexplicably decreased. MPTP-treated mice were also administered L-DOPA (15 mg/kg, i.p.) 20 min after benserazide (12.5 mg/kg, i.p.) and retested on the treadmill. No significant differences were found between gait parameters tested after L-DOPA was given (data not shown).
FIGURE 2.
Changes in gait parameters 1 week after two injections of 0, 5, 10, or 15 mg/kg 1-methyl phenyl 1, 2, 3, 6-tetrahydro-pyridine (MPTP). Analysis of variance (ANOVA) p values appear above analyzed groups. There were no significant differences; error bars represent SE M.
Mice injected with MPTP exhibited a range of striatal DA loss as assessed by HPLC-ECD for DA (see Figure 3). There was a significant loss of DA at each dose of MPTP (each dose p < .001 compared with saline control and p < .05 compared with other MPTP doses). Mice receiving two doses of 5 mg/kg MPTP lost 43 ± 6.5%, two doses of 10 mg/kg lost 78 ± 1.2%, and two doses of 15 mg/kg lost 89 ± 1.2%. Mice treated with saline had 20.36 ± 0.28 ng/mg striatal tissue DA. Figure 4 shows the dose-dependent decrease in inked-paw stride length after MPTP-induced loss of DA. Generally, mice that lost approximately 80% and more of striatal DA decreased inked-paw stride length. Figure 5A – B depicts two representative digital paw print readouts from the analysis program, for a saline-treated mouse and a 2 × 15 mg/kg MPTP-treated mouse that had nearly the same stride length when running on a treadmill despite almost 90% DA loss in the MPTP-treated mouse. Figure 5C – D shows that mice from these dose groups can be readily distinguished using the inked-paw stride length test.
FIGURE 3.
Dopamine (DA) levels by HPLC-ED. 2 × 5 1-methyl phenyl 1, 2, 3, 6-tetrahydropyridine (MPTP) group had 43% loss of DA (p < .001), 2 × 10 MPTP group had 78% loss of DA (p < .001), and 2 × 15 MPTP group had 89% loss of DA (p < .001) compared to saline control group. Groups marked with different letters indicate significant difference of at least p < .05. Error bars represent SE M.
FIGURE 4.
Stride length by inked paw measurement. 2 × 5 1-methyl phenyl 1, 2, 3, 6-tetrahydropyridine (MPTP) group suffered no loss of stride length, 2 × 10 MPTP group displayed shorter stride length (p < .05), and 2 × 15 MPTP group exhibited severely reduced stride length (p < .001) compared to saline control group. Groups marked with different letters indicate significant difference of at least p < .05. Error bars represent SE M.
FIGURE 5.
(A) Representative digital paw prints using DigiGait from saline control at 6.4-cm forepaw (FP) stride length; (B) 2 × 15 MPTP at 6.2-cm FP stride length; (C) representative inked FP prints from saline control at 7.5-cm FP stride length; and (D) 2 × 15 MPTP at 4.8-cm FP stride length. Scale bar represents 2 cm.
Gait in SOD1 G93A Mice
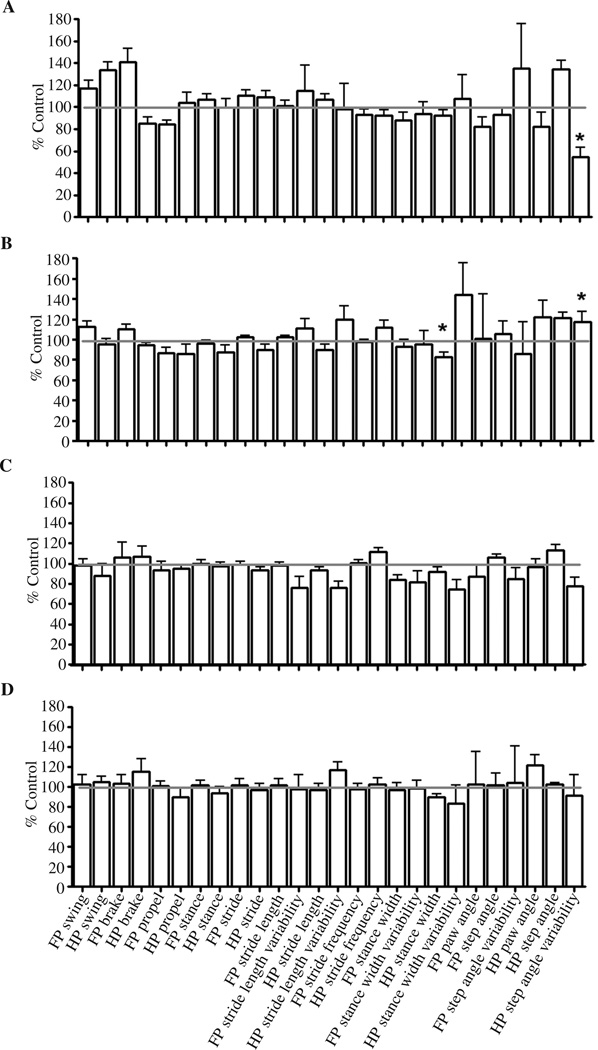
Gait analysis of SOD1 G93A mice (n = 6; 3 male mice, 3 female mice) was performed at 20 cm/s, because this speed is intermediate among the speeds that researchers have found to show significant differences in SOD1 G93A mice in a previous study (Wooley et al., 2005). The results revealed one significant difference from controls at 6 weeks old: HP step angle (p < .05). This difference in HP step angle was also present at 8 weeks old (p < .05) but not at 10 or 12 weeks old. Also at 8 weeks old, HP stance width had decreased (p < .05), but this is not seen at any other age. Apart from these differences, there were no significant deficits related to genotype or age in gait behavior.
Discussion
We undertook the present study to test whether a computerized method for assessment of gait in experimental mice is a viable way to determine toxicant-induced or genetically induced alterations in motor behavior. In addition, we investigated whether the computerized method is superior to the traditional, low-tech methods available in most laboratories for testing deficits after MPTP intoxication. These questions are important for determining the most efficient and sensitive way for testing progression of disease and response to therapy in experimental animals. In addition, technology-based systems are quite expensive, and the justification for such an investment requires just this sort of analysis.
Table 1 shows baseline measures of gait parameters in C57BLJ retired breeder mice running at 20 cm/s. These data demonstrate that the DigiGait system is capable of collecting raw paw contact area over time and using software algorithms to produce 26 measures of gait. Because of the different sizes of girdles and bone lengths of the limbs attached to the FPs and HPs, one would expect some differences in gait parameters among them (see Figure 1 for explanation of gait parameter calculation). Some parameters showed statistical differences among FP and HP measures, whereas others, such as stride length, stride frequency, and stride time were not significantly different, in agreement with previous studies (Clarke & Still, 1999; Clarke & Still, 2001; Stanford, Vorontsova, Surgener, Gerhardt, & Fowler, 2002). There was a significant stance time difference between FPs and HPs (p < .05) and, because stance time comprises brake time and propel time, these measures were also different among FPs and HPs. Paw angle, step angle, and stance width were also significantly different among FPs and HPs, in agreement with Amende et al. (2005).
Retired breeder C57BL/6J mice given 2 × 10 mg/kg and 2 × 15 mg/kg showed an average of 78% and 89% loss of striatal DA, respectively, and these mice did not display a reduction in stride length or a significant change in any other measured gait parameter using the Mouse Specifics Digi-Gait system. Amende et al. (2005) reported that adolescent C57BL/6J mice that had been given a 3 × 30 mg/kg MPTP at 24 hr apart and tested at 24 hr after the last dose exhibited a decrease in stride length, an increase in stride frequency, and a decrease in stride duration. Amende et al. suggested that the loss of DA after MPTP is greater than 50% (although this was not determined in their study), and they concluded that DA deficit may or may not be the reason for reduction in stride length. The present study, however, shows that despite a demonstrated loss of DA leading to a reduction in inked-paw stride length, gait analysis on the treadmill cannot distinguish between MPTP and saline treatments. Two notable differences between Amende et al.’s study and the present study are (a) time between MPTP administration and testing and (b) belt speed. The adolescent mice were tested 24 hr after the last MPTP injection to minimize the recovery that researchers have often observed in mice of this age, whereas the retired breeders have shown no recovery of inked-paw stride length even up to 4 weeks after MPTP administration using our protocol (Tillerson, Caudle, et al., 2002). Also, Amende et al.’s treadmill was set at 34 cm/s, whereas our treadmill was set at 20 cm/s. The speed in our study was chosen on the basis of our own experiments that determined that most (90%) of the retired breeder mice could run at this speed. We attempted to test mice at 14 cm/s, the speed at which they run across paper in the test of inked-paw stride length (14.1 ± 0.3 cm/s), but the speed control mechanism on our system would not allow such a low speed.
The measures of variability in stride length, stance width, and step angle held the most potential for extracting a viable marker of DA loss because of the alterations of these parameters in humans with PD (Vieregge, Stolze, Klein, & Heberlein, 1997). For measures of coordination, we expected that FP stride variability and FP step angle variability would have increased after treatment with MPTP. Similarly, we expected that HP stance width variability and HP step angle variability would decrease with MPTP intoxication and DA loss. Neither of these changes in gait variability were observed. Inked-paw stride length, however, revealed significant FP stride length deficits in 2 × 10 and 2 × 15 MPTP dose groups. We have shown that the test of inked-paw stride length is better suited to this model because it more accurately reflects a Parkinsonian behavior: It forces the mice to initiate each movement and therefore can show the hesitation that shortens stride length. The inked-paw stride length is low-tech, and we only measured one output, but the inked-paw stride length correlated well with striatal DA levels (R2 = .61, p < .0001). It is interesting that these differences were in mice that moved more slowly (14.1 ± 0.3 cm/s) than the treadmill speed of 20 cm/s, whereas Amende et al. (2005) found a difference in stride length between control and MPTP mice at 34 cm/s. Considering that the test of inked-paw stride length finds differences at a speed lower than that of tests on the treadmill and that Amende et al. (2005) found differences at a higher speed, we hypothesized that the treadmill speed of 20 cm/s would have shown significant differences in gait after MPTP intoxication. Because of the lack of potential exhibited by the computerized treadmill gait analysis system at this speed, and considering the personnel expense and animals required for an extensive characterization, we did not pursue belt speed-response experiments.
A study by researchers at the Jackson labs reported that stance time can be used to differentiate SOD1 G93A mice from controls at 8 and 10 weeks old (Wooley et al., 2005). We were unable to confirm this in our system. In fact, only HP step angle and HP stance width distinguished SOD1 G93A mice from control mice. This difference was statistically different at 6 and 8 weeks old, respectively, but disappeared by 10 weeks old. Neither of these measures showed deficits that were exacerbated with time, and we suspect that the measured differences that we saw may be artifacts of analysis rather than reflections of motor deficits in these mice. One possible explanation for the inability to separate control mice from mutant mice lies with the chosen belt speed. We ran our mice at 20 cm/s, but the largest difference among genotypes that Wooley et al. reported was at 17 cm/s. However, Wooley et al. also reported a slight difference at 23 cm/s. Another confound may be due to the analysis software that researchers have used. Although we used one brand of image capture and analysis program, Wooley et al. utilized competing commercial software for their analyses. For example, the TreadScan program (CleverSys, Inc., Reston, VA) uses different algorithms for paw recognition and gait parameter calculation, and that difference may have affected the final results.
We found that the computerized treadmill gait analysis system was not useful for identifying motor abnormalities in the MPTP mouse model of PD. In these same mice, however, we could detect dose-dependent motor deficits by inked-paw stride length. A major difference between these two measures is that the inked-paw measures require the mice to walk under their own power and initiative, whereas the computerized treadmill gait analysis system forces the mouse to walk at a specific speed. Research on patients with peripheral neuropathy has shown that treadmill walking normalizes locomotor patterns and reduces the effectiveness of correlating interstride variability with gait abnormalities (Dingwell et al., 1999; Dingwell & Cusumano, 2000; Dingwell, Cusumano, Cavanagh, & Sternad, 2001). Additionally, PD patients also display the same stride length as healthy control participants when walking at identical speed on a treadmill (Zijlstra, Rutgers, & Van Weerden, 1998). However, when moving over ground under their own power, they display reduced stride length (Knutsson, 1972; Chien et al., 2006). Thus, the backward-moving force of the treadmill may allow the mice to bypass areas of motor control that lead to hesitation when damaged.
We also found that the computerized treadmill gait analysis system was not able to consistently identify motor problems in SOD1 G93A mouse model of ALS (see Figure 6). Many laboratories, including our own, have documented motor abnormalities in this model by 12 weeks old using rotarod, and others have shown this using inclined plane and balance beam testing (Barneoud et al., 1997; Fischer et al., 2004; Fischer et al., 2005). We did not detect early deficits in gait parameters or any deficits that progressed to a more robust motor dysfunction with time using the computerized treadmill gait analysis system despite having shown these mice have pathological changes in motor end plates at these and older ages (Fischer et al., 2004). It is possible that the rotarod distinguishes the SOD1 G93A mice because the loss of hind limb muscle strength affects this assay more than treadmill running. Because of previous rotarod studies from our lab and others, we believe the lack of finding consistent changes in the gait of SOD1 G93A mice in the chosen ages reflects a limitation of testing this disease model on a moving treadmill and not necessarily an error in behavioral methodology, faulty equipment, or software malfunctions. We do not believe that our findings can be explained by inexperience or user error because our laboratories have demonstrated proficiency in many types of behavioral assays, including those of motor function (Manning-Bog et al., 2007; Tillerson, Caudle, et al., 2002; Tillerson et al., 2003; Tillerson & Miller, 2003; Fischer et al., 2004; Fischer et al., 2005; Tillerson et al., 2006; Caudle et al., 2007).
FIGURE 6.
Changes in gait parameters of SOD1 G93A mice over time relative to age-matched control C57BL mice. (A) At 6 weeks old, hind-paw (HP) step angle is increased (p < .05). (B) At 8 weeks old, HP step angle is increased (p < .05), and HP stance width is decreased (p < .05). (C) No differences in gait were found at 10 weeks old. (D) No differences in gait were found at 12 weeks old. Error bars represent SE M. FP = forepaw.
We were surprised to find that the low-tech method of ink-paw analysis was more sensitive (identified DA loss-correlated motor deficits), more efficient (data acquisition and analysis were faster because only one parameter was measured), and more economical (paper and ink costs were much less than those of a computerized system) than computer-driven image analysis in assessing behavioral dysfunction in the MPTP mouse model of PD. The data indicate that using a computerized gait analysis on a motorized treadmill is not an adequate method for measuring motor deficits in the MPTP mouse model of PD or the SOD1 G93A mutant mouse model of ALS. Our data suggest that the treadmill homogenizes the gait of mice, perhaps bypassing the normal visual and proprioceptive feedback of walking (Herbin, Hackert, Gase, & Renous, 2007). In fact, mice can recover normal hind-limb locomotion on a treadmill only 2 weeks after spinalization, although they cannot walk (Leblond, L’Esperance, Orsal, & Rossignol, 2003). A computerized system that accurately records and analyzes over-ground walking gait could be a viable replacement for the inked-paw and other behavioral tests. Additionally, treadmill-based systems may be useful for other toxicant or disease models, but our data recommend against their incorporation into studies of the MPTP mouse model of PD and the SOD1 G93A model of ALS.
REFERENCES
- Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson’s disease and Huntington’s disease. Journal of Neuroengineering Rehabilitation. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barneoud P, Lolivier J, Sanger DJ, Scatton B, Moser P. Quantitative motor assessment in FALS mice: A longitudinal study. Neuroreport. 1997;8:2861–2865. doi: 10.1097/00001756-199709080-00012. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Tillerson JL, Reveron ME, Miller GW. Use-dependent behavioral and neurochemical asymmetry in MPTP mice. Neuroscience Letters. 2007;418:213–216. doi: 10.1016/j.neulet.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Chien SL, Lin SZ, Liang CC, Soong YS, Lin SH, Hsin YL, et al. The efficacy of quantitative gait analysis by the GAITRite system in evaluation of parkinsonian bradykinesia. Parkinsonism Related Disorders. 2006;12:438–442. doi: 10.1016/j.parkreldis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiology and behavior. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Development and consistency of gait in the mouse. Physiology and behavior. 2001;73:159–164. doi: 10.1016/s0031-9384(01)00444-9. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10:848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. Journal of Biomechanical Engineering. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Ulbrecht JS, Boch J, Becker MB, O’Gorman JT, Cavanagh PR. Neuropathic gait shows only trends towards increased variability of sagittal plane kinematics during treadmill locomotion. Gait and Posture. 1999;10:21–29. doi: 10.1016/s0966-6362(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Davis AA, Tennant P, Wang M, Coleman M, et al. The WldS gene modestly prolongs survival in the SOD1G93A fALS mouse. Neurobiology of Disease. 2005;19:293–300. doi: 10.1016/j.nbd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Experimental Neurology. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Francis K, Bach JR, DeLisa JA. Evaluation and rehabilitation of patients with adult motor neuron disease. Archives of Physical Medicine and Rehabilitation. 1999;80:951–963. doi: 10.1016/s0003-9993(99)90089-8. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. Journal of Neuroscience. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. Journal of Neuroscience. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossblatt N. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994 Jun;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Herbin M, Hackert R, Gasc JP, Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behavioural Brain Research. 2007;181:173–179. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Bryan WW. Amyotrophic lateral sclerosis. Seminars in Neurology. 1998;18:27–39. doi: 10.1055/s-2008-1040859. [DOI] [PubMed] [Google Scholar]
- Kale A, Amende I, Meyer GP, Crabbe JC, Hampton TG. Ethanol’s effects on gait dynamics in mice investigated by ventral plane videography. Alcoholism, Clinical and Experimental Research. 2004;28:1839–1848. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: An electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- Knutsson E. An analysis of Parkinsonian gait. Brain. 1972;95:475–486. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- Leblond H, L’Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. Journal of Neuroscience. 2003;23:1141–1149. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, Caudle WM, Perez XA, Reaney SH, Paletzki R, Isla MZ, et al. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiology of Disease. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The pathophysiology of movement disorders. Neurologic Clinics. 1984;2:435–459. [PubMed] [Google Scholar]
- Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Movement Disorders. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- Mokry J. Experimental models and behavioural tests used in the study of Parkinson’s disease. Physiological Research. 1995;44:143–150. [PubMed] [Google Scholar]
- Morris ME, Huxham FE, McGinley J, Iansek R. Gait disorders and gait rehabilitation in Parkinson’s disease. Advances in Neurology. 2001;87:347–361. [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annual Review of Neuroscience. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Phillips JG, Bradshaw JL, Iansek R, Chiu E. Motor functions of the basal ganglia. Psychological Research. 1993;55:175–181. doi: 10.1007/BF00419650. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicology Letters. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. Compulsive, abnormal walking caused by anticholinergics in akinetic, 6-hydroxydopamine-treated rats. Science. 1978 Mar;199:1461–1463. doi: 10.1126/science.564552. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behavioural Brain Research. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: Exacerbation by nomifensine. Neuroscience Letters. 2002;333:195–198. doi: 10.1016/s0304-3940(02)01105-9. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behavioural Brain Research. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Experimental Neurology. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. Journal of Neuroscience. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J Neuroscience Methods. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Vieregge P, Stolze H, Klein C, Heberlein I. Gait quantitation in Parkinson’s disease: Locomotor disability and correlation to clinical rating scales. Journal of Neural Transmission. 1997;104:237–248. doi: 10.1007/BF01273184. [DOI] [PubMed] [Google Scholar]
- Walling AD. Amyotrophic lateral sclerosis: Lou Gehrig’s disease. American Family Physician. 1999;59:1489–1496. [PubMed] [Google Scholar]
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle and Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra W, Rutgers AW, Van Weerden TW. Voluntary and involuntary adaptation of gait in Parkinson’s disease. Gait Posture. 1998;7:53–63. doi: 10.1016/s0966-6362(97)00037-4. [DOI] [PubMed] [Google Scholar]