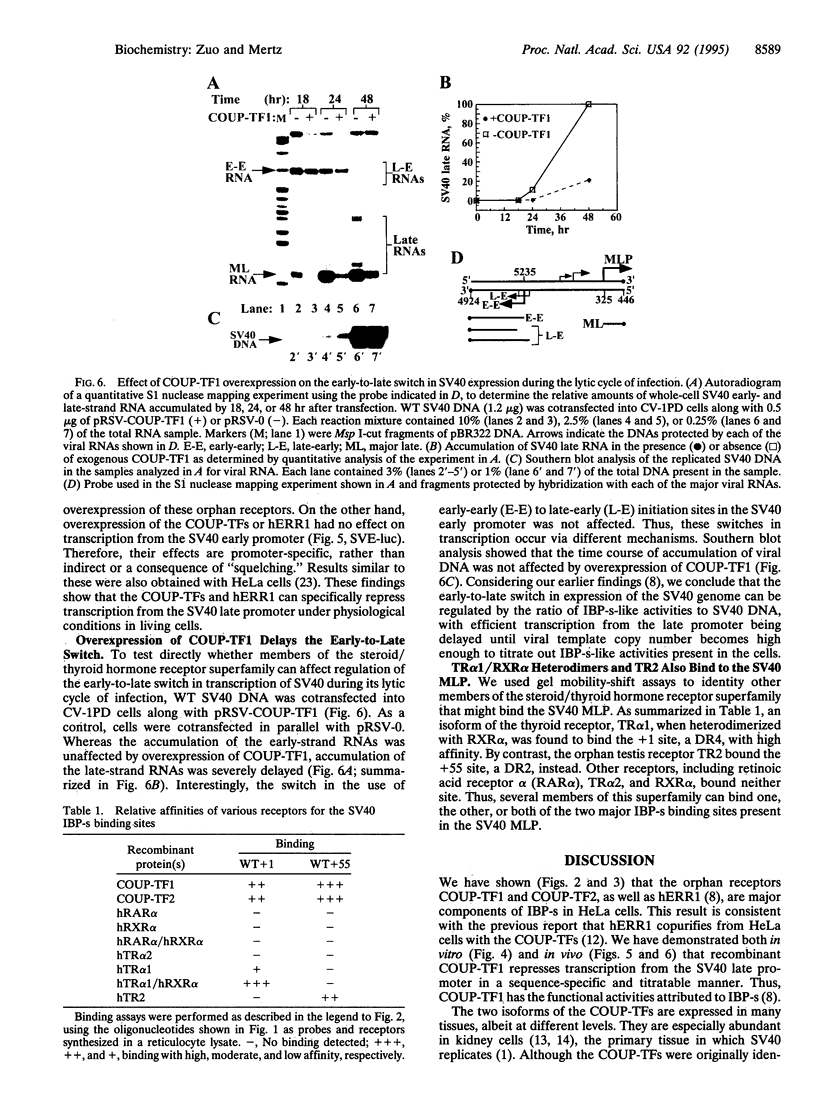
Abstract
Transcription of the late genes of simian virus 40 (SV40) is repressed during the early phase of the lytic cycle of infection of binding of cellular factors, called IBP-s, to the SV40 late promoter; repression is relieved after the onset of viral DNA replication by titration of these repressors. Preliminary data indicated that one of the major components of IBP-s was human estrogen-related receptor 1 (hERR1). We show here that several members of the steroid/thyroid hormone receptor superfamily, including testis receptor 2, thyroid receptor alpha 1 in combination with retinoid X receptor alpha, chicken ovalbumin upstream promoter transcription factors 1 and 2 (COUP-TF1 and COUP-TF2), as well as hERR1, possess the properties of IBP-s. These receptors bind specifically to hormone receptor binding sites present in the SV40 major late promoter. Recombinant COUP-TF1 specifically represses transcription from the SV40 major late promoter in a cell-free transcription system. Expression of COUP-TF1, COUP-TF2, or hERR1 in monkey cells results in repression of the SV40 late promoter, but not the early promoter, in the absence of the virally encoded large tumor antigen. Overexpression of COUP-TF1 leads to a delay in the early-to-late switch in SV40 gene expression during the lytic cycle of infection. Thus, members of this superfamily can play major direct roles in regulating expression of SV40. Possibly, natural or synthetic ligands to these receptors can serve as antiviral drugs. Our findings also provide the basis for the development of assays to screen for the ligands to testis receptor 2 and hERR1.
Full text
PDF
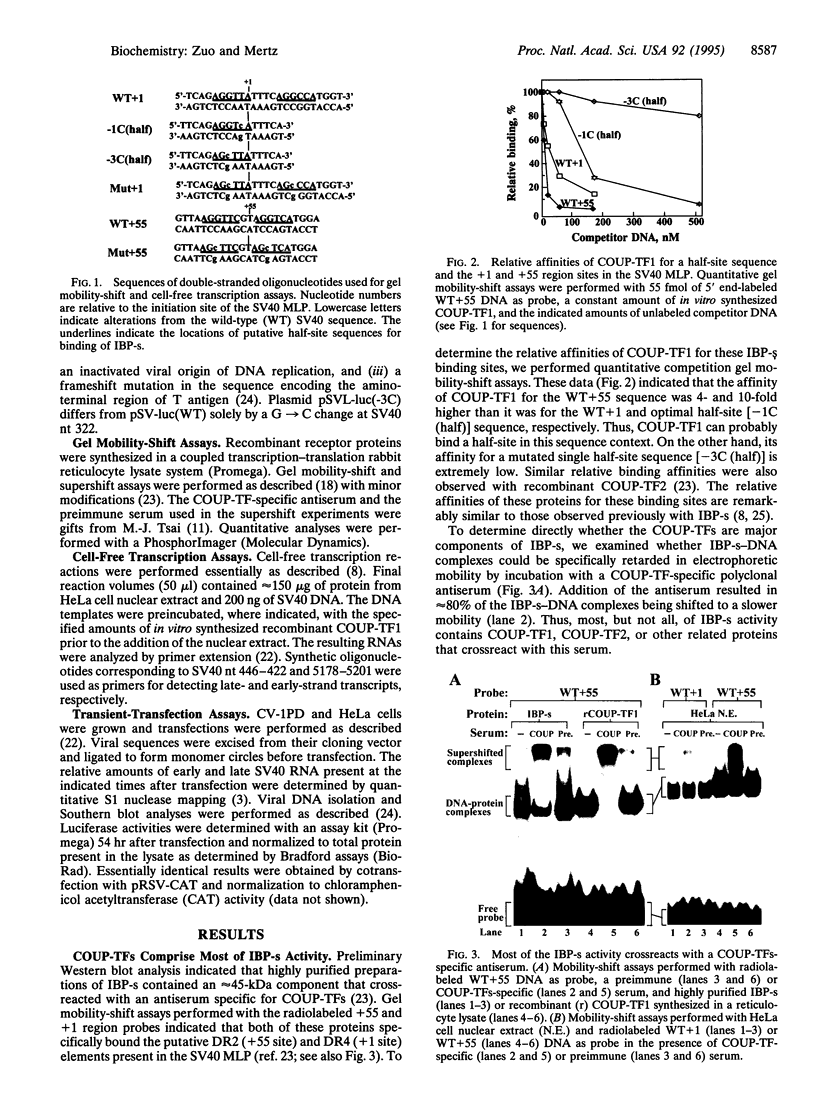
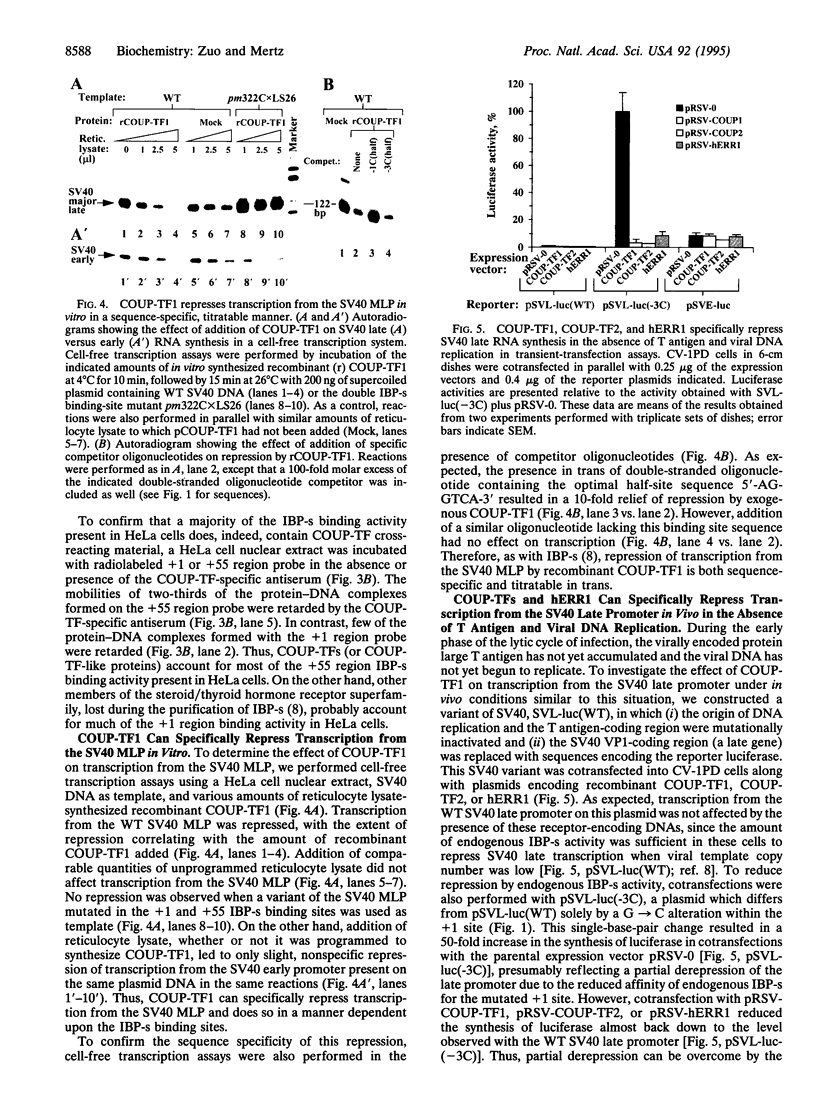

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. A downstream-element-binding factor facilitates assembly of a functional preinitiation complex at the simian virus 40 major late promoter. Mol Cell Biol. 1990 Jul;10(7):3635–3645. doi: 10.1128/mcb.10.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Kokontis J., Acakpo-Satchivi L., Liao S., Takeda H., Chang Y. Molecular cloning of new human TR2 receptors: a class of steroid receptor with multiple ligand-binding domains. Biochem Biophys Res Commun. 1989 Dec 15;165(2):735–741. doi: 10.1016/s0006-291x(89)80028-2. [DOI] [PubMed] [Google Scholar]
- Chang C., Kokontis J. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem Biophys Res Commun. 1988 Sep 15;155(2):971–977. doi: 10.1016/s0006-291x(88)80591-6. [DOI] [PubMed] [Google Scholar]
- Cooney A. J., Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993 Feb 25;268(6):4152–4160. [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991 Jun;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Garcia A. D., Ostapchuk P., Hearing P. Functional interaction of nuclear factors EF-C, HNF-4, and RXR alpha with hepatitis B virus enhancer I. J Virol. 1993 Jul;67(7):3940–3950. doi: 10.1128/jvi.67.7.3940-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V., Yang N., Segui P., Evans R. M. Identification of a new class of steroid hormone receptors. Nature. 1988 Jan 7;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Good P. J., Welch R. C., Ryu W. S., Mertz J. E. The late spliced 19S and 16S RNAs of simian virus 40 can be synthesized from a common pool of transcripts. J Virol. 1988 Feb;62(2):563–571. doi: 10.1128/jvi.62.2.563-571.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz G. Z., Mertz J. E. Bidirectional promoter elements of simian virus 40 are required for efficient replication of the viral DNA. Mol Cell Biol. 1986 Oct;6(10):3513–3522. doi: 10.1128/mcb.6.10.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz G. Z., Mertz J. E. The enhancer elements and GGGCGG boxes of SV40 provide similar functions in bidirectionally promoting transcription. Virology. 1988 Apr;163(2):579–590. doi: 10.1016/0042-6822(88)90299-1. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lee M. O., Hobbs P. D., Zhang X. K., Dawson M. I., Pfahl M. A synthetic retinoid antagonist inhibits the human immunodeficiency virus type 1 promoter. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5632–5636. doi: 10.1073/pnas.91.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Kadowaki Y., Fukushige S., Shimizu S., Semba K., Yamanashi Y., Matsubara K., Toyoshima K., Yamamoto T. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988 Dec 9;16(23):11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A., Sakurai A., Bell G. I., DeGroot L. J. Characterization of a third human thyroid hormone receptor coexpressed with other thyroid hormone receptors in several tissues. Mol Endocrinol. 1988 Nov;2(11):1087–1092. doi: 10.1210/mend-2-11-1087. [DOI] [PubMed] [Google Scholar]
- Park H. Y., Davidson D., Raaka B. M., Samuels H. H. The herpes simplex virus thymidine kinase gene promoter contains a novel thyroid hormone response element. Mol Endocrinol. 1993 Mar;7(3):319–330. doi: 10.1210/mend.7.3.8387156. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Cooney A. J., Kuratani S., DeMayo F. J., Tsai S. Y., Tsai M. J. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4451–4455. doi: 10.1073/pnas.91.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Mertz J. E. Functional binding of the "TATA" box binding component of transcription factor TFIID to the -30 region of TATA-less promoters. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5814–5818. doi: 10.1073/pnas.89.13.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Zuo F., Murray E. E., Loritz K., Mertz J. E. SV40 early-to-late switch involves titration of cellular transcriptional repressors. Genes Dev. 1993 Nov;7(11):2206–2219. doi: 10.1101/gad.7.11.2206. [DOI] [PubMed] [Google Scholar]