Abstract
Purpose
To compare the outcomes of nephron-sparing options (e.g., partial nephrectomy [PN]) and low-surgical-morbidity options (e.g., radical nephrectomy [RN]) in elderly patients with limited life expectancy.
Materials and Methods
We retrospectively reviewed 135 patients aged 70 years or older who underwent RN (n=82) or PN (n=53) for clinical T1 stage renal masses between January 2000 and December 2012. Clinicopathologic data were thoroughly analyzed and compared between the RN and PN groups. The modification of diet in renal disease equation was used to estimate glomerular filtration. Overall survival and cardiac events were assessed by using Kaplan-Meier survival analysis and Cox proportional-hazards regression modeling.
Results
Over a median follow-up period of 59.72 months, 17 patients (20.7%) in the RN group and 3 patients (5.7%) in the PN group died. Chronic kidney disease (<60 mL/min/1.73 m2) developed more frequently in RN patients than in PN patients (75.6% vs. 41.5%, p<0.001). The 5-year overall survival rate did not differ significantly between the RN and PN groups (90.7% vs. 93.8%; p=0.158). According to the multivariate analysis, the Charlson comorbidity index score was an independent predictor of overall survival (hazard ratio [HR], 2.679, p=0.037). Type of nephrectomy was not significantly associated with overall survival (HR, 2.447; p=0.167) or cardiac events (HR, 1.147; p=0.718).
Conclusions
Although chronic kidney disease was lower after PN, overall survival and cardiac events were similar regardless of type of nephrectomy.
Keywords: Aged, Cardiovascular diseases, Kidney, Mortality, Nephrectomy
INTRODUCTION
The greatest increase in the incidence of renal cell carcinoma (RCC) occurs in the later years of life, with the highest incidence reported in patients between 75 and 84 years of age (approximately 56 cases per 100,000 persons) [1,2]. Most cases of kidney cancer are found incidentally owing to the widespread use of cross-sectional imaging. This increase in incidence has results in stage migration. Today, most newly detected cancers are small renal masses at the time of diagnosis [3,4]. An observational study reported that active surveillance is associated with an increased risk of kidney cancer death among Medicare beneficiaries between 75 and 79 years of age and an attenuated difference for patients aged 80 years or older in the United States [5]. Therefore, older patients with small renal masses require surgical management. Extirpative surgery is the gold standard for treatment, and partial nephrectomy (PN) is favored over radical nephrectomy (RN) because of equivalent oncologic control and the potential benefits of maximum renal function and reduced cardiovascular sequelae [6,7]. However, surgically induced chronic kidney disease (CKD) is associated with a relatively low risk of progressive renal function decline, and survival does not appear to be substantially affected on intermediate-term follow-up examinations [8]. Hence, it is difficult to determine if nephron-sparing options (such as PN) or easier surgical options (such as RN) benefit elderly patients with limited life expectancy. The goal of this study was to compare overall survival and cardiac events in older patients treated by RN or PN for clinical T1 stage renal masses.
MATERIALS AND METHODS
After Institutional Review Board approval was obtained from Asan Medical Center, data on patients who underwent nephrectomy for a clinical T1 stage renal mass between January 2000 and December 2012 were retrospectively analyzed. A total of 204 patients aged ≥70 years were treated at our institution. Patients who received <1 year of follow-up examinations after our review (n=57) and patients who were initially diagnosed with metastatic lesions (n=6) were excluded. Another 6 patients were excluded because of decreased renal function before nephrectomy. The study population thus consisted of 135 patients with a suspected clinical T1 stage renal mass at the initial evaluation. Patients in this cohort were treated by using either RN (n=82, 60.7%) or PN (n=53, 39.3%). Among patients who received PN, open (n=33, 62.2%), laparoscopic (n=10, 18.9%), or robot-assisted laparoscopic surgical modalities (n=10, 18.9%) were used as the initial treatments. In the RN group, open (n=28, 34.1%) or laparoscopic modalities (n=54, 65.9%) were used.
1. Management and follow-up
Initial management was selected by the patient and attending physician after considering tumor size, radiographic appearance, overall patient health, life expectancy, and available treatment options. Comorbidity was evaluated by using the Charlson comorbidity index. Cancer recurrence was determined according to the clinical and radiological findings. Oncological outcomes-including overall and cancer-specific survival-were obtained from the patient, medical, and electronic medical records and radiographic reports. In some instances, direct telephone calls to patients or their families were needed to obtain the required information. Recurrence was defined as any new soft-tissue masses >10 mm that were previously undetected by computed tomography (CT); biopsy was not routinely performed to confirm the diagnosis. Local recurrence was defined as recurrent disease at the surgical site, whereas distant recurrence was defined as any evidence of disease outside the renal fossa, including visceral, bone, and lung metastases.
2. Evaluation of kidney function
Serum creatinine was measured by using standard laboratory methods 1 to 7 days before surgery and perioperatively. To calculate the estimated glomerular filtration rate (eGFR), postoperative serum creatinine levels were measured <1 year after surgery. Patient age and sex were used to calculate the GFR by using the modification of diet in renal disease equation [9]. In accordance with the National Kidney Foundation guidelines for CKD, CKD was defined as an eGFR <60 mL/min/1.73 m2 (≥stage 3) [10,11].
3. End points
The primary end points of the analysis included cardiac events, cardiac deaths, and all-cause mortality after nephrectomy. Cardiac events included myocardial infarction, ischemic stroke, transient ischemic attack, percutaneous coronary intervention, coronary artery bypass graft surgery, cerebrovascular disease, and hospitalization for the diagnosis of acute angina, congestive heart failure, coronary artery disease, or peripheral vascular disease [12]. Cancer-specific mortality was attributed to patients with evidence of renal cell cancer progression before death, and other cancer-specific mortality was attributed to patients with evidence of cancer progression before death. Cardiovascular-related deaths included deaths attributed to ischemic heart disease, congestive heart disease, ischemic stroke, and peripheral vascular disease, whereas patient deaths by other causes were classified as other-cause mortality. Follow-up and all-cause mortality data were available through December 2013. Survival duration was calculated as the interval between the date of the surgical procedure and the date of mortality or last follow-up examination.
4. Statistical analyses
Parametric data are shown as the mean±standard deviation. The Student t-test was used to compare continuous parametric data, and the chi-square and Fisher exact tests were used to compare the indicated data according to treatment type (RN vs. PN) and covariates. Overall survival and cardiac events were estimated by using the Kaplan-Meier method. Univariate and multivariate analyses were used to evaluate all-cause mortality and cardiac events. Adjustments for prespecified clinical characteristics (e.g., age, sex, American Society of Anesthesiologists score) were performed by using Cox proportional-hazards modeling. Associations are provided as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two-tailed, with p-values <0.05 considered statistically significant. All analyses were performed by using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
The characteristics of our study patients who underwent RN or PN for suspected localized T1 renal masses are listed in Table 1. Significant differences between most pretreatment variables indicated that treatment type was not evenly distributed across these patients. For example, patients who underwent RN were older (74.8±3.4 years vs. 73.4±2.0 years, p=0.008) and were diagnosed with larger tumors (4.44±1.3 cm vs. 2.42±0.89 cm, p<0.001) than were PN-treated patients. There was no significant preoperative between-group difference in eGFR (67.77±22.9 mL/min/1.73 m2 vs. 69.42±22.1 mL/min/1.73 m2, p=0.682), but postsurgical eGFR was reduced to a significantly greater extent in the RN group than in the PN group (47.54±14.7 mL/min/1.73 m2 vs. 61.98±20.3 mL/min/1.73 m2, respectively, p<0.001). A total of 116 conventional, 10 papillary, 3 unclassified, and 6 benign tumors (5 oncocytoma and 1 angiomyolipoma) were diagnosed. According to the Fuhrman nuclear grading system, 77 patients had grade 1 to 2 tumors (42 RN patients [51.2%] vs. 35 PN patients [66.0%]) and 58 patients had grade 3 to 4 tumors (40 RN patients [48.8%] vs. 18 PN patients [34.0%]). In terms of previous medical history, hypertension had been diagnosed in 48 RN patients (58.5%) and 40 PN patients (75.5%), but no statistically significant difference was determined between the groups. There was also no significant difference between the groups in terms of the incidence of preoperative cardiovascular disease (7 RN patients [8.5%] vs. 8 PN patients [15.1%]). The median follow-up duration was 56.20 months (mean, 59.72 months; range, 15.60-159.00 months).
TABLE 1.
Characteristics of the study patients of 70 years and older with renal masses
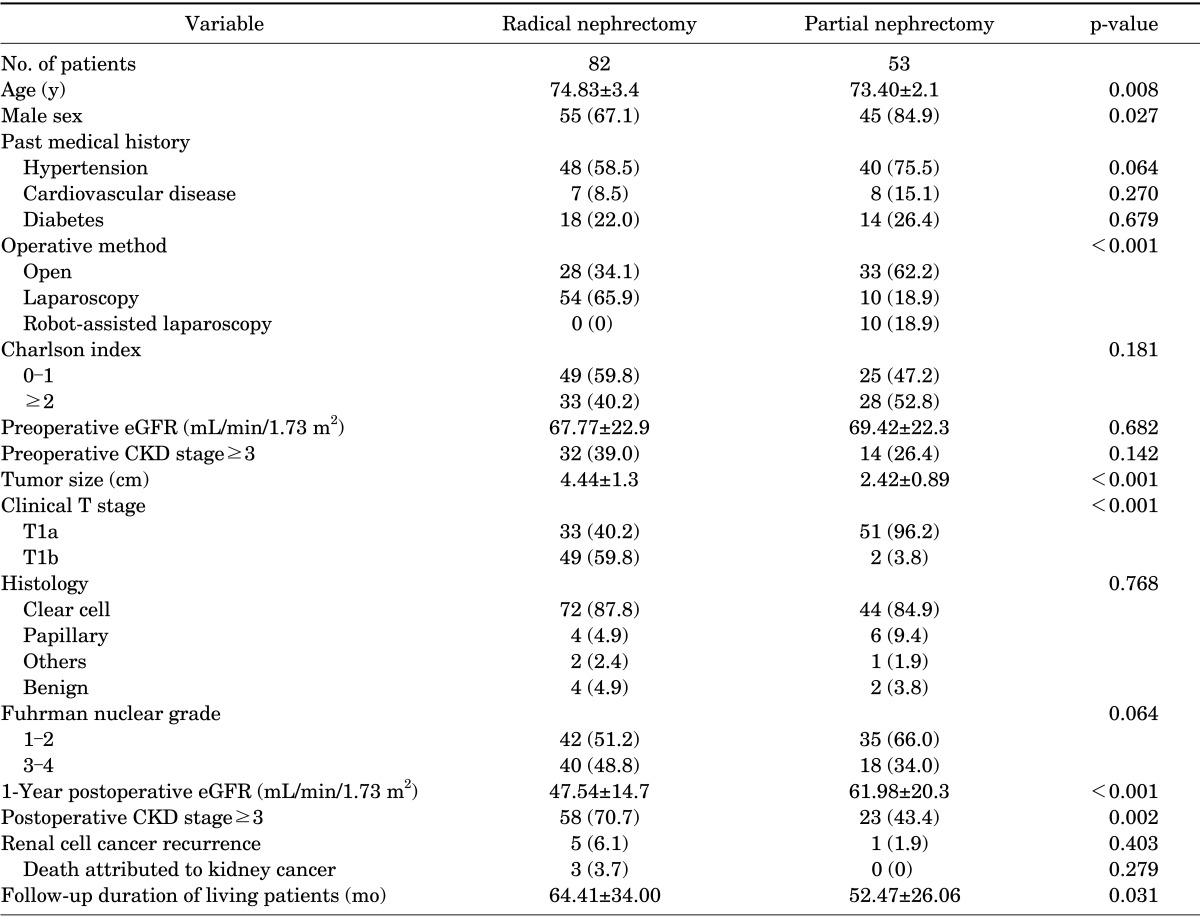
Values are presented as mean±standard deviation or number (%).
eGFR, estimate glomerular filteration rate; CKD, chronic kidney disease.
Overall, 20 patients (14.8%) died during the study period. Three deaths were attributed to cancer-specific death. In seven patients, the deaths were attributed to other cancer deaths (1 prostate cancer, 1 bladder cancer, 3 liver cancer, 1 lung cancer, and 1 lymphoma). Four deaths were attributed to cardiovascular death. Three deaths were attributed to renal failure-induced death, and three deaths were attributed to other causes. Cancer recurrence was detected in 6 patients (4.4%), including 3 patients with deaths that were attributed to RCC and 3 patients who survived with distant metastases.
CKD was diagnosed in 34.1% of patients before surgery and 60.0% of patients after surgery. A greater median reduction in the eGFR occurred after RN (28.3%) relative to PN (9.9%), resulting in a much larger proportion of patients with newly diagnosed CKD after RN (44.3%) than after PN (18.4%). After surgery, 9 patients were newly diagnosed with cardiovascular disease (6 RN patients vs. 3 PN patients) and 2 patients developed recurrent cardiovascular disease (1 RN patient vs. 1 PN patient).
The Kaplan-Meier curves between the RN and PN groups are shown in Fig. 1. Estimates of overall survival are shown in Fig. 1A, and the Kaplan-Meier curves show the overall survival difference according to type of surgery. Kaplan-Meier estimates of 5-year overall survival for patients with a clinical T1 stage renal mass who received RN or PN were 90.7% (95% CI, 77-91) and 93.8% (95% CI, 80-98), respectively. Fig. 1B shows cardiac-event-free survival stratified by type of surgery. In the PN group, the 5-year probability of freedom from cardiac events was 66.3%; in the RN group, the probability was 62.9%.
FIG. 1.
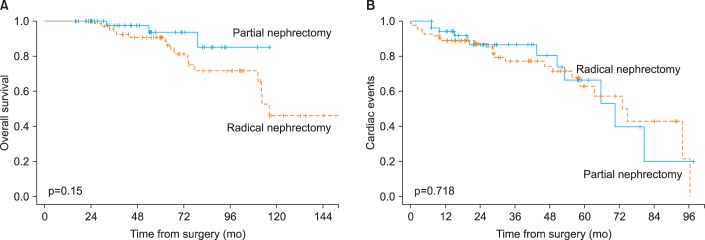
Kaplan-Meier survival curves comparing radical nephrectomy vs. partial nephrectomy. (A) Overall survival, (B) cardiac events.
To evaluate the effects of management type on overall survival while still controlling for potential confounding covariates within each treatment group, we performed multivariate Cox proportional-hazards analysis (Table 2). The Charlson comorbidity index was a significant predictor of mortality, demonstrating an HR value of 2.697 (95% CI, 1.063-6.841) for preoperative status (p=0.037). Surgical method (p=0.089), tumor size (p=0.797), preoperative CKD (p=0.474), and postoperative eGFR (p=0.178) were not associated with overall survival according to the univariate and multivariate Cox proportional-hazards analyses.
TABLE 2.
Univariate and multivariate Cox regression models for predicting overall survival
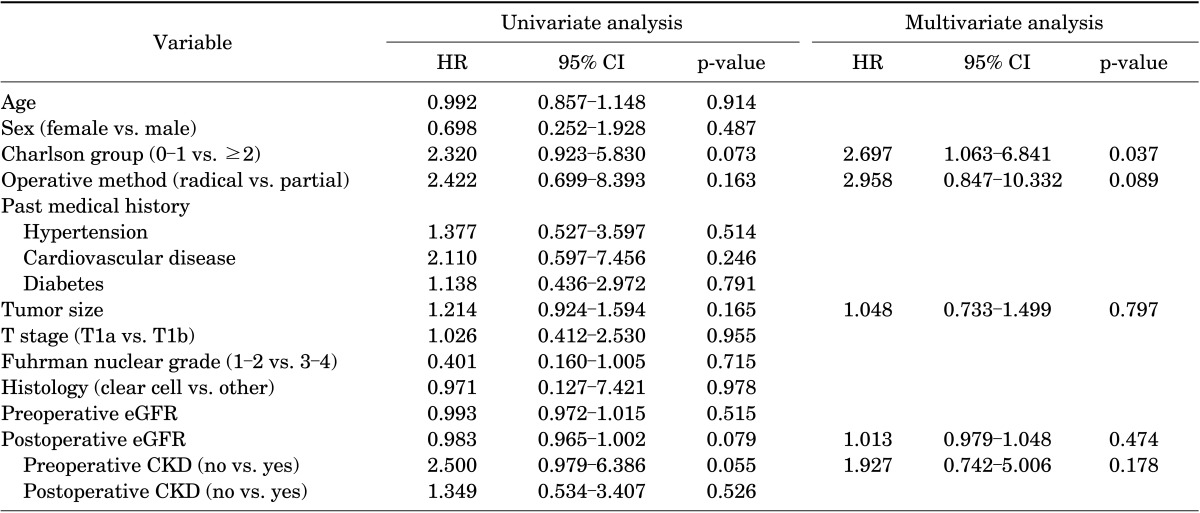
HR, hazard ratio; CI, confidence interval; eGFR, estimate glomerular filteration rate; CKD, chronic kidney disease.
In total, 36 patients experienced at least 1 cardiac event after nephrectomy, including 24 patients (29.3%) in the RN group and 12 patients (22.6%) in the PN group. According to the multivariate analysis, preoperative CKD and history of hypertension were the most significant factors associated with cardiac events (HR, 2.611; 95% CI, 1.293-5.273; p=0.007; and HR, 2.350; 95% CI, 1.017-5.430; p=0.046, respectively) (Table 3). The risk of experiencing a cardiac event was greater in the RN group than in the PN group, but this finding was not statistically significant (HR, 1.137; p=0.718).
TABLE 3.
Univariate and multivariate Cox regression models for predicting cardiac events
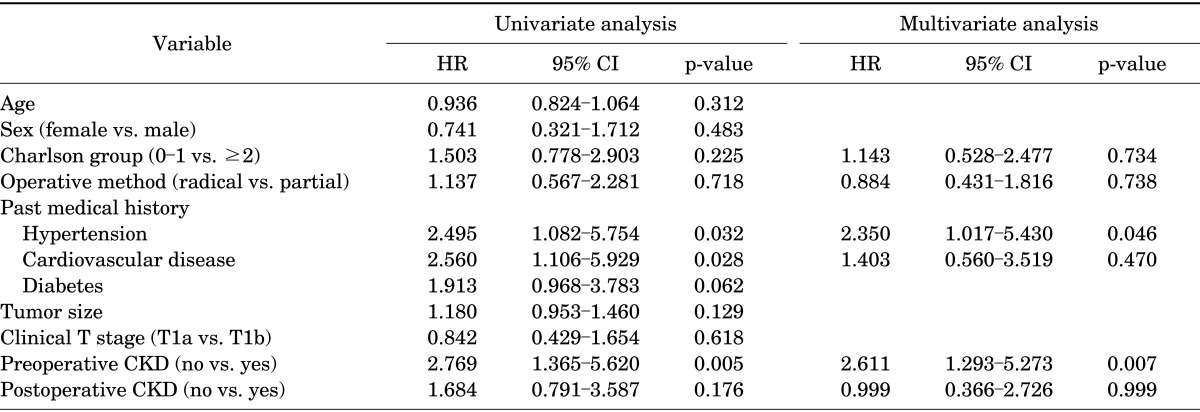
HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease.
DISCUSSION
Despite the potential benefits of PN, population-based data suggest that adoption of PN has been slow [13]. PN represented <25% of all kidney cancer surgeries in 2008 [13], perhaps because of the technical challenges associated with performing this surgery on complex tumors and the fear of complications in physically frail patients [14].
PN was initially reserved for treating renal masses in patients at high risk of developing renal failure after kidney surgery. In a randomized prospective phase III trial, Van Poppel et al. [15] reported equivalent oncological outcomes after PN and RN and suggested that PN be considered an acceptable approach for treating small asymptomatic RCC. Most especially, because patients are living longer, factors that place a patient at risk of CKD-associated morbidity and mortality must be considered. Decreased renal function is associated with increased risk of cardiovascular death [12], which is the leading cause of mortality in elderly patients. In a population-based competing-risk analysis, Hollingsworth et al reported that competing causes of mortality account for 28% of elderly patient deaths (age≥70 years) and concluded that active surveillance might be a reasonable strategy for treating some tumors [16]. Most recently, using data from the Surveillance, Epidemiology, and End Results cancer registry, which includes Medicare claims, Huang et al. [17] reported that RN is associated with an increased risk of death and cardiovascular events after surgery. Surgical candidates with preexisting CKD are at the highest risk for end-stage renal disease and stand to benefit the most from PN because RN could result in more extensive nephron loss and worse predicted outcomes. According to studies performed on a heterogeneous population of patients aged ≥75 years, interventions for clinical T1 stage RCC are not associated with improvement in overall survival because advanced age and comorbidity burden are associated with death from any cause, and the most common cause of death was cardiovascular [18].
In a previous study on CKD after surgery, surgically induced CKD was associated with a relatively low risk of progressive renal functional decline, and the impact on survival did not appear to be substantial [8]. Therefore, preoperative renal function is an important factor to consider along with other patient and tumor characteristics when choosing the type of nephrectomy to perform (i.e., partial or radical) [19]. Our current data confirm that postoperative renal function was poorer in the RN group than in the PN group, but there was no statistically significant difference between the RN and PN groups in terms of overall survival. We found no significant differences between groups in terms of medical history; most notably, there were no differences in cardiovascular disease. Before loss to follow-up, we noted that 4 patients postoperatively developed cardiovascular disease (3 RN patients [2.6%] vs. 1 PN patient [2%], p=0.821), and only 2 patients died of cardiovascular disease (1 in each group).
Few studies evaluate eGFR or the risk of outcomes in the general population in terms of kidney function. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study (1992, 18-year follow-up) did not determine a significant association between eGFR of 30-60 mL/min/1.73 m2, risk of death from any cause, or death from cardiovascular causes [20]. The authors of the National Health and Nutrition Examination Survey I study explained that the association between moderate renal insufficiency and cardiovascular disease, as reported in other epidemiologic studies, appears to result from the co-occurrence of renal insufficiency and other traditional cardiovascular disease risk factors.
In the last decade, PN has been more widely adopted and surgical techniques have been modified to reduce the risk of complications, ease convalescence, and better preserve the functions of the remnant kidney [21,22,23]. Others have noted the underutilization of PN in older patients and have reported age as an independent predictor of radical surgery over PN in large populations, despite control for comorbidities [17]. This indicates that absolute age remains an inappropriately important factor in the decision-making process. In some cases, a high Eastern Cooperative Oncology Group score and high Charlson comorbidity index, rather than age, were associated with increased complications [24]. In comparison with the outcomes of laparoscopic, open RN, and open PN, our complication rates were similar to those reported in younger patients [25,26]. Some general population evaluations also report a higher rate of procedure-related complications after PN than after RN [27,28]. Interpreting published complication rates after these procedures is made even more difficult by the presence of strong selection biases. The true morbidity associated with RN and PN in elderly patients and in patients with significant comorbidities may be greater than that suggested in the literature, which tends to report results from highly specialized tertiary referral centers [29]. Therefore, RN is a possible treatment for clinical stage T1 renal masses in patients >70 years of age. There were several limitations to our present study.
First, this was a retrospective study, which leads to an inherent selection bias that cannot be overcome. Another limitation of the present study was the between-group differences in follow-up duration and the short duration of the follow-up period. Last, we could not fully evaluate the cause of death in some patients, which could have biased our evaluations of mortality and cardiovascular-induced death.
CONCLUSIONS
Although the rate of CKD is lower in patients who receive PN than in those who receive RN, these two approaches demonstrate similar overall survival outcomes when used to treat RCC in older patients. For clinical stage T1 masses, RN might be an effective surgical treatment for older patients regardless of their CKD status before surgery. Evaluations of any underlying morbidities and provision of individualized consultations of the risks and benefits of each surgical modality are required before selecting the surgical method and treating RCC in older patients.
Footnotes
The authors have nothing to disclose.
References
- 1.Roos FC, Hampel C, Thuroff JW. Renal cancer surgery in the elderly. Curr Opin Urol. 2009;19:459–464. doi: 10.1097/MOU.0b013e32832f0c7d. [DOI] [PubMed] [Google Scholar]
- 2.Katz DL, Zheng T, Holford TR, Flannery J. Time trends in the incidence of renal carcinoma: analysis of Connecticut Tumor Registry data, 1935-1989. Int J Cancer. 1994;58:57–63. doi: 10.1002/ijc.2910580111. [DOI] [PubMed] [Google Scholar]
- 3.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 5.Patel HD, Kates M, Pierorazio PM, Hyams ES, Gorin MA, Ball MW, et al. Survival after diagnosis of localized T1a kidney cancer: current population-based practice of surgery and nonsurgical management. Urology. 2014;83:126–132. doi: 10.1016/j.urology.2013.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettus JA, Jang TL, Thompson RH, Yossepowitch O, Kagiwada M, Russo P. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc. 2008;83:1101–1106. doi: 10.4065/83.10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Lane BR, Campbell SC, Demirjian S, Fergany AF. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649–1655. doi: 10.1016/j.juro.2012.11.121. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 10.Goolsby MJ. National Kidney Foundation Guidelines for chronic kidney disease: evaluation, classification, and stratification. J Am Acad Nurse Pract. 2002;14:238–242. doi: 10.1111/j.1745-7599.2002.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Patel SG, Penson DF, Pabla B, Clark PE, Cookson MS, Chang SS, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 14.Sivarajan G, Huang WC. Current practice patterns in the surgical management of renal cancer in the United States. Urol Clin North Am. 2012;39:149–160. doi: 10.1016/j.ucl.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007;51:1606–1615. doi: 10.1016/j.eururo.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 17.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors: is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane BR, Abouassaly R, Gao T, Weight CJ, Hernandez AV, Larson BT, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer. 2010;116:3119–3126. doi: 10.1002/cncr.25184. [DOI] [PubMed] [Google Scholar]
- 19.Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363–2368. doi: 10.1016/j.juro.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 21.Dulabon LM, Lowrance WT, Russo P, Huang WC. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116:2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol. 2011;186:394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 23.Lane BR, Russo P, Uzzo RG, Hernandez AV, Boorjian SA, Thompson RH, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol. 2011;185:421–427. doi: 10.1016/j.juro.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 24.O'Malley RL, Hayn MH, Hellenthal NJ, Kim HL, Underwood W, 3rd, Schwaab T. Safety and outcomes of surgical treatment of renal cell carcinoma in the elderly. Can J Urol. 2012;19:6111–6117. [PubMed] [Google Scholar]
- 25.Staehler M, Haseke N, Stadler T, Bader M, Karl A, Becker A, et al. Renal surgery in the elderly: morbidity in patients aged >75 years in a contemporary series. BJU Int. 2008;102:684–687. doi: 10.1111/j.1464-410X.2008.07794.x. [DOI] [PubMed] [Google Scholar]
- 26.Varkarakis I, Neururer R, Harabayashi T, Bartsch G, Peschel R. Laparoscopic radical nephrectomy in the elderly. BJU Int. 2004;94:517–520. doi: 10.1111/j.1464-410X.2004.04994.x. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130–134. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 28.Corman JM, Penson DF, Hur K, Khuri SF, Daley J, Henderson W, et al. Comparison of complications after radical and partial nephrectomy: results from the National Veterans Administration Surgical Quality Improvement Program. BJU Int. 2000;86:782–789. doi: 10.1046/j.1464-410x.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 29.Abouassaly R, Alibhai SM, Tomlinson GA, Urbach DR, Finelli A. The effect of age on the morbidity of kidney surgery. J Urol. 2011;186:811–816. doi: 10.1016/j.juro.2011.04.077. [DOI] [PubMed] [Google Scholar]


