Abstract
A rapid and efficient plant propagation system through shoot tip explants was established in Vitex trifolia L., a medicinally important plant belonging to the family Verbenaceae. Multiple shoots were induced directly on Murashige and Skoog (MS) medium consisting of different cytokinins, 6-benzyladenine (BA), kinetin (Kin) and 2-isopentenyl adenine (2-iP), BA at an optimal concentration of 5.0 μM was most effective in inducing multiple shoots where 90 % explants responded with an average shoot number (4.4±0.1) and shoot length (2.0±0.1 cm) after 6 weeks of culture. Inclusion of NAA in the culture medium along with the optimum concentration of BA promoted a higher rate of shoot multiplication and length of the shoot, where 19.2±0.3 well-grown healthy shoots with an average shoot length of 4.4±0.1 cm were obtained on completion of 12 weeks culture period. Ex vitro rooting was achieved best directly in soilrite when basal portion of the shoots were treated with 500 μM indole-3-butyric acid for 15 min which was the most effective in inducing roots, as 95 % of the microshoots produced roots. Plantlets went through a hardening phase in a controlled plant growth chamber, prior to ex-vitro transfer. Micropropagated plants grew well, attained maturity and flowered with 92 % survival rate. The results of this study provide the first report on in vitro plant regeneration of Vitex trifolia L. using shoot tip explants.
Keywords: Keywords, Shoot tip explant, Multiple shoot induction, Ex vitro rooting
Introduction
Medicinal and aromatic plants are an important source of medicines and play a significant role in world health care system. Vitex trifolia L. is a stout, aromatic shrub or shrubby tree that grow up to 6 m found wild in several parts of India, which is traditionally used by the tribes and native medical practioners for the treatment of various ailments including liver disorders, tumours, rheumatic pains, inflammation, sprains fever and used in the treatment of tuberculosis (Anonymous 2003). The leaves are used for improving memory, useful in pain, remove bad taste in mouth and cure fever (Kirtikar and Basu 1991), treating hair loss (Varier 2003) and excessive bleeding during menstruation (Islam et al. 2011). The aerial parts of this plant are useful in the treatment of diabetes (Pullaiah and Naidu 2003). Besides, the plant also possesses larvicidal, wound healing, anti HIV, anticancer, trypanocidal, antibacterial and antipyretic activities (Li et al. 2005; Woradulayapinij et al. 2005; Kannathasan et al. 2007; Manjunatha et al. 2007).
The plant can be propagated through seeds or root suckers but these methods are not very efficient in producing sufficient number of planting stocks as the germination frequency of the seeds is poor and is slow growing, age and season dependent. Natural stand of the plant are fast disappearing in India because of indiscriminate and overexploitation (Hiregoudar et al. 2006). Recently, the plant is Red listed by IUCN with Low-Risk status (Nagaveni and Rajanna, 2013). Thus, conventional propagation through seeds and vegetative cuttings are not adequate solution to meet the demand for this medicinal plant. Alternative propagation methods would be beneficial in accelerating large scale multiplication, improvement and conservation of the plant. Tissue culture techniques paved the way for mass production of plants in a short time span to meet the increasing demand and offer a viable tool for mass propagation and multiplication in a variety of medicinal plant species (Faisal et al. 2005; Ahmad and Anis 2007; Anis et al. 2012). To our knowledge, there is no published report on multiplication of Vitex trifolia from shoot tips. The plant regeneration from meristems is considered to be one of the most promising ways for multiplying a selected true-to-type plant. Such individuals are genetically similar showing the same agronomic characteristics. Therefore, the current study was designed to establish a rapid in vitro regeneration system through shoot tip explants.
Materials and methods
Initiation and establishment of aseptic cultures
Shoot tip explants (0.3-0.5 cm), collected from a three years old mature tree were used as explants for establishing a protocol for mass production of V. trifolia plantlets. Shoots containing shoot tip were collected and any larger leaves were removed and washed under running tap water for 20 min, treated with 5 % (v/v) of a laboratory detergent, Labolene (Qualigens Fine Chemicals, Mumbai, India) for 5 min. This was followed by 3-4 washes with distilled water (DW). Surface sterilization was carried out using 0.1% (w/v) HgCl2 for 3-5 min followed by repeated washes with autoclaved sterile distilled water. The sterilized single excised shoot tip was implanted on sterile shoot induction medium.
Culture media and conditions
The nutrient medium used in all the experiments consisted of MS (Murashige and Skoog 1962) salts and vitamins with 3% (w/v) sucrose (Qualigens Fine Chemicals, Mumbai, India) and 0.8% (w/v) bacteriological grade agar. All the salts used were of analytical grade. The medium was adjusted to 5.8 pH using 1 N NaOH or HCl. All the culture vials were incubated in culture room at 25 ± 20 C under 16/8 h (light/dark) cycle with a light intensity of 50 μmolm-2 s-1 supplied by 40 W cool-white fluorescent tubes (Philips Electronics India Ltd.) and the relative humidity was maintained between 50-60%.
Multiple Shoot induction and proliferation
For multiple shoot induction, shoot tips segment was placed on MS (Murashige & Skoog 1962) medium supplemented with various cytokinins (BA, Kin and 2iP) at different concentrations (0.0, 0.5, 1.0, 2.5, 5.0, 7.5, and 10.0 μM) either singly or in combination with auxins (IBA, NAA, IAA) as listed in Table 2 and 3. All the cultures were transferred into fresh medium after every 3 weeks.
Table 2.
Effect of different concentrations of cytokinins and auxins on multiple shoot regeneration from shoot tip segments of V. trifolia in MS medium after 12 weeks of culture
| BA | Kin | 2iP | NAA | IBA | IAA | % Response | Mean no. of shoots per explant | Mean shoot length (cm) |
|---|---|---|---|---|---|---|---|---|
| 5.0 | 0.1 | _ | _ | 90 | 18.2 ± 0.6b | 3.6 ± 0.0ij | ||
| 5.0 | 0.5 | _ | _ | 94 | 19.2 ± 0.3a | 3.9 ± 0.0fghi | ||
| 5.0 | 1.0 | _ | _ | 91 | 18.4 ± 0.4b | 3.4 ± 0.1j | ||
| 5.0 | _ | 0.1 | _ | 90 | 17.0 ± 0.1d | 3.7 ± 0.0ghij | ||
| 5.0 | _ | 0.5 | _ | 92 | 18.1 ± 0.1bc | 4.0 ± 0.0defgh | ||
| 5.0 | _ | 1.0 | _ | 91 | 17.4 ± 0.6cd | 3.6 ± 0.1ij | ||
| 5.0 | _ | _ | 0.1 | 90 | 16.9 ± 0.3d | 3.7 ± 0.1hij | ||
| 5.0 | _ | 0.5 | 93 | 17.4 ± 0.1cd | 3.9 ± 0.0efghi | |||
| 5.0 | _ | _ | _ | _ | 1.0 | 91 | 16.8 ± 0.3d | 3.4 ± 0.0j |
| _ | 5.0 | _ | 0.1 | _ | _ | 90 | 6.4 ± 0.0efgh | 4.1 ± 0.0bcdef |
| _ | 5.0 | _ | 0.5 | _ | _ | 92 | 7.2 ± 0.1e | 4.2 ± 0.1bcdef |
| _ | 5.0 | _ | 1.0 | _ | _ | 91 | 6.1 ± 0.0gh | 4.0 ± 0.1efgh |
| _ | 5.0 | _ | _ | 0.1 | _ | 90 | 6.0 ± 0.0gh | 4.2 ± 0.1bcdef |
| _ | 5.0 | _ | _ | 0.5 | _ | 92 | 7.0 ± 0.0ef | 4.4 ± 0.1ab |
| _ | 5.0 | _ | _ | 1.0 | _ | 91 | 6.1 ± 0.0gh | 4.1 ± 0.0bcdef |
| _ | 5.0 | _ | _ | _ | 0.1 | 90 | 6.0 ± 0.1gh | 4.0 ± 0.0cdefg |
| _ | 5.0 | _ | _ | _ | 0.5 | 91 | 6.4 ± 0.1efgh | 4.2 ± 0.1bcdef |
| _ | 5.0 | _ | _ | _ | 1.0 | 89 | 5.9 ± 0.0h | 4.0 ± 0.0cdefg |
| _ | _ | 5.0 | 0.1 | _ | _ | 90 | 6.3 ± 0.0fgh | 4.1 ± 0.0bcdef |
| _ | _ | 5.0 | 0.5 | _ | _ | 92 | 7.0 ± 0.0ef | 4.4 ± 0.1abcd |
| _ | _ | 5.0 | 1.0 | _ | _ | 91 | 6.1 ± 0.0gh | 4.2 ± 0.1abcdef |
| _ | _ | 5.0 | _ | 0.1 | _ | 90 | 5.9 ± 0.0h | 4.3 ± 0.1abcde |
| _ | _ | 5.0 | _ | 0.5 | _ | 91 | 6.8 ± 0.1efg | 4.6 ± 0.1a |
| 5.0 | 1.0 | 91 | 6.0 ± 0.0gh | 4.2 ± 0.1bcdef | ||||
| 5.0 | 0.1 | 90 | 6.0 ± 0.1gh | 4.3 ± 0.0abcdef | ||||
| 5.0 | 0.5 | 91 | 6.1 ± 0.0gh | 4.4 ± 0.2abc | ||||
| 5.0 | 1.0 | 90 | 5.9 ± s0.0h | 4.1 ± 0.0bcdef | ||||
Values represent means ± standard error of 5 randomly selected readings of 20 replicates per treatment in three repeated experiments. Means sharing the same letter are not significantly different (P = 0.05) using Duncan’s multiple range test
Table 3.
Effect of different concentrations of IBA on ex vitro rooting of in vitro raised shoots of Vitex trifolia L., after 4 weeks of transfer
| IBA(μM) | % Response | Mean no. of roots/shoots | Mean root length (cm) |
|---|---|---|---|
| 0 | 0 | 0.0 ± 0.0e | 0.0 ± 0.0e |
| 100 | 80 | 3.1 ± 0.3d | 1.7 ± 0.0d |
| 200 | 82 | 3.4 ± 0.2d | 1.9 ± 0.0cd |
| 300 | 90 | 5.5 ± 0.1b | 2.0 ± 0.1bcd |
| 400 | 94 | 5.0 ± 0.1bc | 2.5 ± 0.3b |
| 500 | 95 | 7.0 ± 0.1a | 3.1 ± 0.1a |
| 1000 | 92 | 4.6 ± 0.1c | 2.3 ± 0.1bc |
Values represent means ± standard error of 20 replicates per treatment in three repeated experiments. Means followed by the same letter are not significantly different (P = 0.05) using Duncan’s multiple range test
* Significant at P = 0.05
Ex vitro rooting
For root induction, individual in vitro microshoot (4–5 cm long) were excised from shoot clusters and their basal cut ends were dipped in double distilled water containing various concentrations of IBA (100, 200, 300, 500 and 1000 μM) for 15 min, washed with distilled water and planted in thermocol cups (8 cm diameter) filled with autoclaved soilrite. These were covered with clear polyethylene bags to ensure high humidity and maintained at 25 ± 2o C under 16/8 h light/dark cycle in growth chamber.
Acclimatization
Four weeks following planting, percentage of rooting response, root number and root length were measured. The polybags were opened gradually over a period of four weeks in order to acclimatize the plantlets in greenhouse condition. After the formation of proper root system, the plantlets were transferred into thermocol cups with soilrite, garden soil, vermiculite and vermiculite-garden soil mixture (1:1) and placed in green house. After one month, surviving plants were transferred to field.
Recording of Data and Statistical analysis
The multiplication potential of the explants was estimated by recording the data on number of shoots per explants and shoot length after six and 12 weeks of culture. Each treatment consisted of 20 replicates and all the experiments were repeated thrice. Data on percentage of rooting, mean number of roots and root length per shoot were recorded after four weeks of transfer to rooting media. All the experiments were repeated thrice with 20 explants for each treatment. The data obtained was analyzed using statistical software, SPSS Version 17 (SPSS Inc. Chicago, USA) and means were compared using Duncan’s multiple range test (DMRT) at 5 % level of significance. All the results were expressed in Mean ± Standard error.
Result and Discussion
Effect of Cytokinin
The plant regeneration from shoot tip segments is considered to be one of the most promising ways for multiplying a selected variety true to its type showing the same agronomic characteristics. The explants cultured on MS basal medium without any growth regulator did not show any morphogenetic response and failed to produce shoots, even after 4 weeks of culture. Generally, a cytokinin is required for shoot induction and proliferation. However its effective type and optimal concentration varies with the system (Park et al. 2008). A differential response with regard to shoot bud induction was observed when shoot tip explants were cultured on a medium supplemented with different concentrations of BA, Kin and 2-iP (0.0-10 μM) as listed in (Table 1). Swelling of the dormant axillary bud took place within 7 days followed by differentiation of multiple shoot induction after 6 weeks of culture (Fig. 1a). BA was found more effective than Kin and 2iP as seen in the number of shoot bud formation after 6 weeks of culture. The reason for effectiveness of the BA may be because of its ability to stimulate the plant tissues to metabolize the natural endogenous hormones or alternatively could induce the production of natural hormone system for the induction of shoot organogenesis. Among the various concentrations of cytokinins tested, BA (5.0 μM) showed the highest shoot regeneration frequency (90 %) and number of shoots (4.4±0.1) with maximum shoot length (2.0±0.1) cm after 6 weeks of culture (Table 1, Fig. 1b). This concentration was considered as the optimal growth regulator for shoot regeneration in V. trifolia among the treatments tested. The potential of BA on multiple shoot bud differentiation has been demonstrated in a number of plant species using a variety of explants (Usha et al. 2007; Jain and Bashir 2010; Kanchanapoonm et al. 2010; Ahmad et al. 2013). The regeneration frequencies and number of shoots decline with an increasing concentration of cytokinin beyond the optimal level. Similar results have been reported in other Vitex species like Vitex agnus-castus (Balaraju et al. 2008), Vitex negundo (Ahmad et al. 2013). When Kin was used as the sole source of cytokinin, a slight basal callusing was observed with a low regeneration frequency (Table 2). A maximum of (3.7±0.0) shoots with the highest percentage (89%) of responding explants was produced at 5.0 μM Kin after 6 weeks of incubation. These results clearly showed that presence of BA on shoot multiplication was more prolific with BA over Kin and in contrast better shoot elongation was observed with kin over BA in this species which is in consistence with earlier reports on many woody plant species (Anis et al. 2010; Naz et al. 2011; Ahmad and Anis 2011; Ahmad et al. 2013). The use of 2iP too resulted in the induction of few shoot buds with a low regeneration frequency, the highest being 3.8±0.0 shoots with 86% response at 5.0 μM concentration after 6 weeks of culture, but the elongation of regenerated shoots was maximum compared to BA or Kin (Table 1).
Table 1.
Effect of different concentrations of cytokinins on multiple shoot regeneration from shoot tip segments of V. trifolia in MS medium after 6 and 12 weeks of culture
| PGRs(μM) | % Response | 6 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|---|
| BA | Kn | 2iP | Mean no. of shoots per explant |
Mean shoot length (cm) | Mean no. of shoots per explant |
Mean shoot length (cm) | |
| 0.0 | 0.0 | 0.0 | 0 | 0.0 ± 0.0 k | 0.0 ± 0.0i | 0.0 ± 0.0 g | 0.0 ± 0.0g |
| 0.5 | 75 | 2.4 ± 0.1gh | 1.4 ± 0.1h | 4.3 ± 0.5de | 2.1 ± 0.2f | ||
| 1.0 | 78 | 3.3 ± 0.1cde | 1.7 ± 0.0fgh | 4.6 ± 0.1d | 2.3 ± 0.1ef | ||
| 2.5 _ |
83 | 3.9 ± 0.0b | 1.8 ± 0.2efgh | 6.0 ± 0.4c | 2.8 ± 0.2bcde | ||
| 5.0 | 90 | 4.4 ± 0.1a | 2.0 ± 0.1cdef | 10.3 ± 0.2a | 3.2 ± 0.2b | ||
| 7.5 | 86 | 3.5 ± 0.1bcd | 1.7 ± 0.1fgh | 7.6 ± 0.3b | 2.4 ± 0.2def | ||
| 10.0 | 80 | 2.8 ± 0.1hi | 1.5 ± 0.1gh | 6.8 ± 0.3bc | 2.1 ± 0.1f | ||
| 0.5 | 78 | 1.7 ± 0.1j | 1.9 ± 0.1defg | 2.9 ± 0.3f | 2.4 ± 0.1ef | ||
| 1.0 | 80 | 2.4 ± 0.1ghi | 2.2 ± 0.1bcde | 3.3 ± 0.4ef | 2.6 ± 0.1cdef | ||
| 2.5 | 85 | 2.9 ± 0.0ef | 2.3 ± 0.0abcd | 4.4 ± 0.3de | 3.0 ± 0.2abcd | ||
| 5.0 | 89 | 3.7 ± 0.0bc | 2.8 ± 0.1a | 5.9 ± 0.1c | 3.4 ± 0.0a | ||
| 7.5 | 88 | 3.1 ± 0.1def | 2.6 ± 0.1ab | 4.5 ± 0.5d | 2.6 ± 0.2cdef | ||
| 10.0 | 78 | 2.9 ± 0.2ef | 2.2 ± 0.1bcde | 3.3 ± 0.1ef | 2.2 ± 0.0f | ||
| 0.5 | 77 | 2.2 ± 0.2gh | 2.2 ± 0.1bcde | 2.6 ± 0.1f | 2.8 ± 0.2bcde | ||
| 1.0 | 80 | 2.6 ± 0.2fgh | 2.4 ± 0.1abc | 3.0 ± 0.1f | 3.1 ± 0.1abc | ||
| 2.5 | 84 | 3.0 ± 0.1def | 2.6 ± 0.1ab | 3.5 ± 0.1def | 3.3 ± 0.1b | ||
| 5.0 | 86 | 3.8 ± 0.0bc | 2.7 ± 0.1ab | 5.8 ± 0.5c | 3.5 ± 0.1a | ||
| 7.5 | 87 | 3.0 ± 0.1def | 2.5 ± 0 .1ab | 4.4 ± 0.2de | 2.8 ± 0.0bcde | ||
| 10.0 | 78 | 2.0 ± 0.1ij | 2.4 ± 0.2abc | 4.2 ± 0.1de | 2.4 ± 0.2ef | ||
Values represent means ± standard error of 5 randomly selected readings of 20 replicates per treatment in three repeated experiments. Means sharing the same letter are not significantly different (P = 0.05) using Duncan’s multiple range test
Multiplication and proliferation of shoots
The combined effects of cytokinins along with an auxin was also studied for their ability to affect the shoot multiplication rate by taking the optimized concentration (5.0 μM) of different cytokinins in combination with different concentrations (0.1, 0.5, 1.0 μM) of auxins (NAA, IAA, IBA). Inclusion of any of the auxins together with cytokinins enhanced both shoot regeneration frequency with shoot number and shoot length per explants as summarized in Table 1. The highest shoot regeneration frequency (94 %) and maximum mean number (19.2 ± 0.3) and length (4.4 ± 0.1) of shoots per explant was found with a combination of 5.0 μM BA and 0.5 μM NAA (Table 3; Fig. 1c, d). Such stimulating effects of combinations of these two hormones are well supported by earlier studies on Adenium obesum (Kanchanapoom et al. 2010) and Vitex negundo (Usha et al. 2007, Ahmad et al. 2013). This positive effect of BA + NAA on differential morphogenetic response may be due to apical dominance. Apical dominance is controlled by the ratio of auxin and cytokinin and widely recognized to be caused by the action of basipetally transported auxin from apex and its consequent inhibition of axillary bud growth (Samir 2004). In Dianthus chinensis, high efficiency shoot bud formation and plant regeneration were achieved when the culture medium contained both BA and NAA (Kantia and Kotharis 2002). Inclusion of higher concentration of auxins suppressed the regeneration pathway as it induced basal callusing, which is not desirable for direct shoot regeneration. Our results substantiate with earlier findings in Adenium obesum (Kanchanapoom et al. 2010), Lippia nodiflora (Priya and Ravindhran, 2011) and Vitex negundo (Usha et al. 2007; Ahmad et al. 2013). Thus, it can be inferred from the above results that the interactive effect of BA and NAA could ensure better in vitro regeneration and their synergism in proper concentration was extremely favourable for multiplication.
Effect of Kin with different auxins at the same concentrations was also evaluated, and results have been summarized in Table 2. The presence of NAA along with Kin in medium further improved the shoot induction as well as the shoots length per explant. Among the various concentrations of NAA tested with Kin (5.0 μM), the concentration of 0.5 μM NAA proved to be the optimum with the highest response of 92 % cultures with a mean number (7.2 ± 0.1) of shoots per explant (Table 3). Similar culture response on a medium containing Kin and NAA has been observed in Chlorophytum arundinaceum (Lattoo et al. 2006). Similarly, the promoting effect of Kin with IBA and IAA too enhanced better shoot multiplication and elongation which is in accordance with earlier findings of Teucrium stocksianum (Bouchoucke and Ksiksi 2007).
Interactive effect of combinations of auxins along with 2iP (5.0 μM) also yielded favorable results (Table 2). Thus the results of combination of auxins and cytokinins in the present investigated reported here clearly support the findings that the interaction of auxins (NAA, IBA and IAA) and cytokinins (BA, Kn and 2iP) is necessary for in vitro organogenesis.
Effect of explants collection season
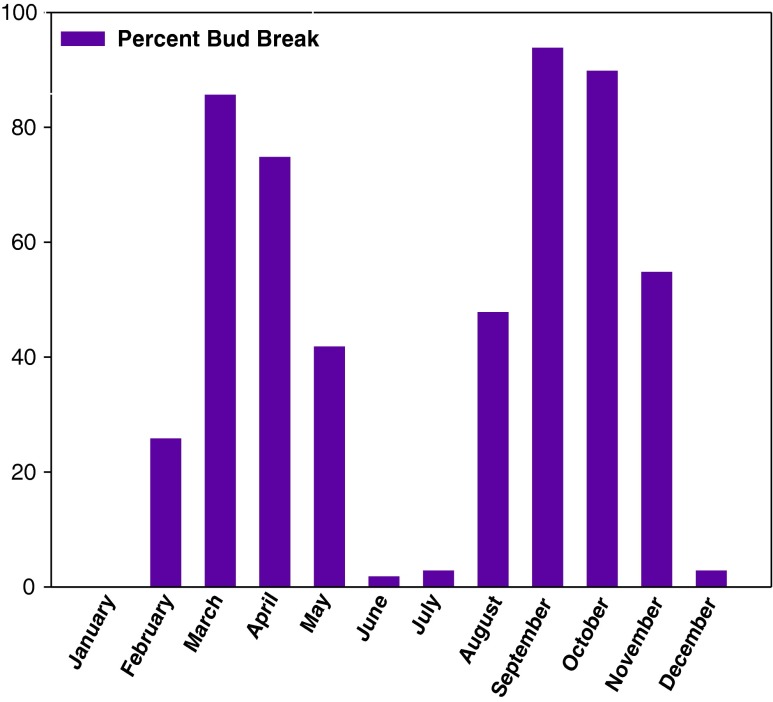
The success of in vitro response of the explants does not only depend on the genotype but is strongly affected by the quality of the donor plants. The latter is influenced by environmental conditions like temperature, photoperiod, light intensity and light quality, etc. Seasonal changes greatly influence explants establishment and have a marked effect on the concentration of various plant growth regulators in the cambial zone (Siril and Dhar 1997). Shoot tip collection season proved to play an important role in inducing bud break in Vitex trifolia since it is a redundant tree and availability of fresh sprout is season dependent. Thus, the availability of explants for the clonal propagation becomes a limitation (Ahmad and Anis 2011). Highest frequency of bud break (94 %) coupled with maximum number of shoots formed (19 shoots per explants) was obtained from shoot tip explants collected during the month of mid-September to November. Other explanting periods were comparatively less suitable (Fig. 2). The actively growing season was known to be more responsive for bud break than others. This may be due to the presence of different concentration of various plant growth regulators in the cambial zone. High levels of growth promoting substances and low growth inhibitors in actively growing shoots during the September-November and February-March may be responsible for high explants establishment during these months. Seasonally dependent responses of tissue cultures were reported sporadically in the literature (Carman et al. 1987).
Figure 1.
a. Initiation of shoots on BA (5.0 μM) + NAA (0.5 μM) after 4 weeks of culture-Inset—an excised shoot tip segment after one week of culture, b. Shoot multiplication on BA (5.0 μM) + NAA (0.5 μM) after 6 weeks of culture, c. Shoot elongation and proliferation on BA (5.0 μM) + NAA (0.5 μM) after 12 weeks of culture, d. Mass multiplication on BA (5.0μM) + NAA (0.5μM) after 12 weeks of culture, e. Ex vitro rooting of in vitro raised shoots on IBA (500 μM) after 4 weeks of culture.f. An acclimatized plant in soilrite. Scale bar indicates 1 cm
Figure 2.
Effect of explant collection season on culture establishment on MS supplemented with BA (5.0 μM) and NAA (0.5 μM) in Vitex trifolia
Effect of subculturing
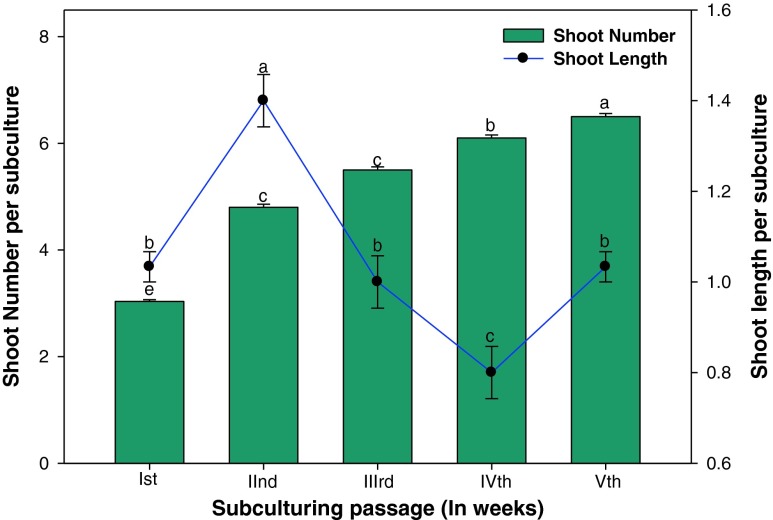
In many plant species, micropropagation requires two media– propagation medium and shoot elongation medium– making the micropropagation procedures cumbersome and uneconomical (Debergh and Maene 1981). Some researchers have added gibberellic acid (GA3) to the shoot multiplication medium to achieve simultaneous shoot multiplication and elongation; but sometimes it drastically affected the shoot multiplication rate (Sahoo and Chand 1998). In our work, shoot multiplication and subsequent elongation was achieved on the same medium. Shoot multiplication rate was found to be highest upto the fourth subculturing passages after which the shoot number decreased. Similar effect of subculturing was observed in Bacopa monniera where shoot induction and multiplication increased up to the third subculture passage, after which the frequency and number of shoots decreased (Tiwari et al. 2001). An average of 18-25 shoots (Fig. 3) were obtained from shoot tip after fourth subculture passage on BA (5.0 μM) and NAA (0.5 μM). The results obtained confirm the positive effect of relatively high doses of BA (5.0 μM) on the production of shoots and axillary-buds during successive subculturing passages (Fig. 3). In Cassia angustifolia, shoot production increased during subculturing and stabilized in the fifth passage (Siddique and Anis 2007). The increase shoot number, due to repeated transfer of the mother explants, may be owing to suppression of apical dominance during subculture that induced basal dominant meristematic cells to form new shoots (Shekhawat and Shekhawat 2011). The well-developed shoots were regularly removed and transferred to rooting medium. The enhanced multiplication of shoots during subsequent subculturing substantiates the earlier report on Albizia chinensis (Sinha et al. 2000), Vitex negundo (Usha et al. 2007) and Holarrhena antidysenterica (Raha and Roy 2001). Thus, the regenerating medium (MS) containing BA (5.0 μM) and NAA (0.5 μM) may be recommended as the best suitable medium for long-term maintenance of regenerative potential of shoot tip explants of V. trifolia.
Figure 3.
Effect of subculture passages on shoot tip culture obtained on BA (5.0 μM) + NAA (0.5 μM) in V. trifolia L
Ex vitro rooting
The induction of roots is an important procedure to form the complete plantlets. In vitro regenerated shoots generally lack roots, therefore ex-vitro rooting experiment was carried out. Individual shootlets were excised from shoot clusters and dipped in different concentrations of IBA for 15 min following transfer to pots containing soilrite and acclimatized according to procedure explained in material and methods. The number of root and root length per shootlet increased significantly with an increase in the concentrations of IBA and 500 μM was proved to be the best for highest root formation frequency (95 %) with maximum (7.0 ± 0.1) roots and root length (3.1 ± 0.1) per shoot (Table 3) (Fig. 1e). Ex vitro rooting of microcuttings was also reported for Vitex negundo (Ahmad and Anis 2007) and Rotula aquatica (Martin 2003). They noticed a better survival rate of plantlets through ex vitro rooting. Given the reduced time for establishment, auxin-pulse treated ex vitro rooting is the more favorable method than in vitro rooting. Rooting in microshoots of Vitex trifolia with this technique eliminates the additional in vitro rooting step reported earlier (Hiregoudar et al. 2006). Shootlets with well developed root system were transferred to pots for hardening and acclimatization.
Acclimatization
The period of transition during the process of hardening after transfer from the in vitro to the ex vitro environment is considered to be the most important step in tissue culture. One important factor during acclimatization is the type of potting material used. Of the four different types of planting substrates used (soilrite, garden soil, vermiculite and (1:1) vermiculite-garden soil mixture), the highest survival rate (92 %) was achieved in soilrite (Table 4, Fig. 1f). Plantlets with well-developed root and shoot system were successfully hardened off inside the growth room with different planting substrate. Soilrite being more porous substrate holds more water than vermiculite, vermiculite-garden soil mixture (1:1) and garden soil, and thus promoted better growth of tender roots of tissue culture raised plants during hardening. Similar observations using different planting substrates were observed in Tecomella undulate (Varshney and Anis 2012). After one month, surviving plants were transferred to field.
Table 4.
Hardening of in vitro grown plants on different planting substrates for their acclimatization and establishment in natural conditions
| Planting substrates | No. of plants transferred | No. of acclimatized plants | Acclimatization percentage |
|---|---|---|---|
| Soilrite | 25 | 21.0 | 84 |
| Vermiculite | 25 | 18.7 | 75 |
| Vermiculite-garden soil mixture(1:1) | 25 | 23.0 | 92 |
| Garden soil | 25 | 16.2 | 65 |
Conclusion
Micropropagation plays a key role in the successful multiplication and conservation of many valuable medicinal plants. The present study describes a procedure for improving in vitro micropropagation and a successful acclimatization of V. trifolia. The results clearly show that tissue culture techniques can play an important role in large scale multiplication as well as germplasm conservation of elite genotypes of V. trifolia, rich in secondary metabolites of high pharmaceutical properties.
Acknowledgments
Md. Rafique Ahmed is thankful to the University Grants Commission (UGC), Goverment of India, New Delhi for the award of a Junior Research Fellowship (F1-17.1/2011/MANF-MUS-MAN-581 (SA-III/manfugc). Research support provided by the Department of Science and Technology and University Grants Commission under DST-FIST (2011-16) and UGC-SAP, DRS-I (2009-2014) Programme respectively is duly acknowledged.
References
- Ahmad N, Anis M. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agro Syst. 2007;71:195–200. doi: 10.1007/s10457-007-9078-1. [DOI] [Google Scholar]
- Ahmad N, Anis M. An efficient in vitro process for recurrent production of cloned plants of Vitex negundo L. Eur. J. For. Res. 2011;130:135–144. doi: 10.1007/s10342-010-0415-y. [DOI] [Google Scholar]
- Ahmad N, Khan MI, Ahmed S, Javed SB, Faisal M, Anis M, Rehman S, Umair SM. Change in total phenolic content and antibacterial activity in regenerants of Vitex negundo L. Acta Physiol Plant. 2013;35:791–800. doi: 10.1007/s11738-012-1120-x. [DOI] [Google Scholar]
- Anis M, Varshney A, Siddiqui I. In vitro clonal propagation of Balanites Egyptiaca (L.) Del. Agro Syst. 2010;2:151–158. doi: 10.1007/s10457-009-9238-6. [DOI] [Google Scholar]
- Anis M, Siddique I, Naz R, Ahmed MR, Aref IM (2012) Advances in Micropropagation of a Highly Important Cassia species- A Rev New Persp in Plant Protect 192-206.
- Anonymous (2003) The wealth of India – Raw Materials,Vol. X, Council for Scientific and Industrial Research, New Delhi, p.525.
- Balaraju K, Agastian P, Preetamraj JP, Asokiyaraj S, Ignacimuthu S. Micropropagation of Vitex agnus-castus (Verbenaceae)- a valuable medicinal plant. In Vitro Cell Dev Biol Plant. 2008;44:436–441. doi: 10.1007/s11627-008-9155-9. [DOI] [Google Scholar]
- Bouchoucke N, Ksiksi T. An efficient in vitro plant regeneration system for the medicinal plant Teucrium stocksianum Boiss. Plant Biotechnol. J. 2007;1:179–184. doi: 10.1007/s11816-007-0033-4. [DOI] [Google Scholar]
- Carman JG, Jefferson NE, Cambell WF. Induction of embryogenic Triticum aestivum calli. II. Quantification of organic addenda and other culture variable effects. Plant Cell Tiss. Org Cult. 1987;10:115–128. doi: 10.1007/BF00035909. [DOI] [Google Scholar]
- Debergh PC, Maene LJ. A scheme for commercial propagation of ornamental plants by tissue culture. - Sci. Progress. Hortic. 1981;14:335–345. doi: 10.1016/0304-4238(81)90047-9. [DOI] [Google Scholar]
- Faisal M, Ahmad N, Anis M. Shoot multiplication in Rauvolfia tetraphylla using thidiazuron. Plant Cell Tiss Org Cult. 2005;80:187–190. doi: 10.1007/s11240-004-0567-x. [DOI] [Google Scholar]
- Hiregoudar LV, Murthy HN, Bhat JG, Nayeem A, Hema BP, Hahn EJ, Paek KY. Rapid clonal propagation of Vitex trifolia. Biol Plant. 2006;50(2):291–294. doi: 10.1007/s10535-006-0023-3. [DOI] [Google Scholar]
- Islam F, Jahan IF, Seraj S, Malek I, Sadat AFMN, Bhuiyan MSA, Swarna A, Sanam S, Rahmatullah M. Variations in Disease and Medicinal Plant Selection among Folk Medicinal Practitioners: a Case Study in Jessore District, Bangladesh. American Eurasian Journal of Sustainable Agriculture. 2011;5(2):282–291. [Google Scholar]
- Jain AK, Bashir M (2010) Efficient Micropropagtion Protocol for Portulaca grandiflora. Hook. using shoot tip explants, New York Science Journal, 3 (10).
- Kanchanapoom K, Sunheem S, Kanchanapoom K. In vitro Propagation of Adenium obesum (Forssk.) Roem. and Schult. Not. Bot. Hort. Agrobot. Cluj. 2010;38(3):209–213. [Google Scholar]
- Kannathasan K, Senthilkumar A, Chandrasekaran M, Venkatesalu V. Differential larvicidal efficacy of four species of Vitex against Culex quiquefasciatus larvae. Parasitology ResParasatilogy Res. 2007;101:1721–1723. doi: 10.1007/s00436-007-0714-5. [DOI] [PubMed] [Google Scholar]
- Kantia A, Kothari SL. High efficiency adventitious shoot bud formation and plant regeneration from leaf explants of Dianthus chinensis L. Sci Horti. 2002;96:205–212. doi: 10.1016/S0304-4238(02)00081-X. [DOI] [Google Scholar]
- Kirtikar KR, Basu BD (1991) Indian medicinal plants, Lalit Mohan Basu, Allahabad. India 1935–1944
- Lattoo SK, Bamotra S, Dhar RS, Khan S, Dhar AK. Rapid plant regeneration and analysis of genetic fidelity of in vitro derived plants of Chlorophytum arundinaceum Baker-an endangered medicinal herb. Plant Cell Rep. 2006;25:499–50. doi: 10.1007/s00299-005-0103-4. [DOI] [PubMed] [Google Scholar]
- Li WX, Cui CB, Cai B, Wang HY, Yao XS. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J Asian Nat Prod ResJ Asian. 2005;7:615–626. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- Manjunatha BK, Vidya SM, Krishna V, Mankani KL, Singh SD, Manohara YN. Comparative evaluation of wound healing potency of Vitex trifolia L. and Vitex altissima L. Phytother Res. 2007;21:457–461. doi: 10.1002/ptr.2094. [DOI] [PubMed] [Google Scholar]
- Martin KP. Rapid in vitro multiplication and ex vitro rooting of Rotula aquatica Lour., a rare rhoeophytic woody medicinal plant. Plant Cell Rep. 2003;21:415–420. doi: 10.1007/s00299-002-0547-8. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. - Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nagaveni C, Rajanna L. In-vitro Flowering in Vitex trifolia L. Botany Researh InternationalS. 2013;6(1):13–16. [Google Scholar]
- Naz R, Anis M, Aref IM. High frequency shoot regeneration through cotyledonary node explants of Bauhinia tomentosa L., a woody leguminous tree. J of Horti Sci and Biotech. Hortic. Sci. Biotechnol. 2011;86:37–42. [Google Scholar]
- Park SY, Kim YW, Moon HK, Murthy HN, Choi YH, Cho HM. Micropropagation of Salix pseudolasiogne from nodal explants. Plant Cell TIss Org Cult. 2008;93:341–346. doi: 10.1007/s11240-008-9362-4. [DOI] [Google Scholar]
- Pullaiah T, Naidu KC (2003) Antidiabetic plants in India and Herbal bases antibiotic research. Recency, New Delhi Recency New Delhi 314–315
- Raha S, Roy CS. In vitro plant regeneration in Holarrhena antidysenterica wall through high frequency axillary shootproliferation. In Vitro Cell Dev Biol Plant. 2001;37:232–236. doi: 10.1007/s11627-001-0041-y. [DOI] [Google Scholar]
- Sahoo Y, Chand PK. Micropropagation of Vitex negundo L., a woody aromatic medicinal shrub, through high frequency axillary shoot proliferation. - Plant Cell Rep. 1998;18:301–307. doi: 10.1007/s002990050576. [DOI] [PubMed] [Google Scholar]
- Samir CD. Clonal propagation of dwarf raspberry (Rubus pubescens Raf.) through in vitro axillary shoot proliferation. Plant Growth Regul. 2004;43:179–186. doi: 10.1023/B:GROW.0000040110.53216.6a. [DOI] [Google Scholar]
- Shekhawat MS, Shekhawat NS. Micropropagation of Arnebia hispidissima (Lehm). DC. and production of alkannin from callus and cell suspension culture. Acta Physiol Plant. 2011;33:1445–1450. doi: 10.1007/s11738-010-0680-x. [DOI] [Google Scholar]
- Siddique I, Anis M (2007) In vitro shoot multiplication and plantlet regeneration from nodal explants of Cassia angustifolia (Vahl.): a medicinal plant Acta Physiol Plant 29: 233-238.
- Sinha RK, Majumdar K, Sinha S. In vitro differentiation and plant regeneration of Albizia chinensis. (OBS.) MERR In Vitro Cell Dev Biol Plant. 2000;36:370–373. doi: 10.1007/s11627-000-0066-7. [DOI] [Google Scholar]
- Siril EA, Dhar U. Micropropagation of mature Chinese tallow tree (Sapium sebiferum Roxb.) Plant Cell Rep. 1997;16:637–640. doi: 10.1007/BF01275506. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Tiwari KN, Singh BD. Comparative studies of cytokinins on in vitro propagation of Bacopa monniera. Plant Cell Tiss Org. 2001;66:9–16. [Google Scholar]
- Usha PK, Benjamin S, Mohanan KV, Raghu AV. 2007 An efficient micropropagation system for Vitex negundo L., an important woody aromatic medicinal plant, through shoot tip culture. Research J. of Botany 2: 102-107.
- Varier PS (2003) Indian medicinal plants. Orient Longman Hyderabad India 387–395
- Woradulayapinij W, Soonthornchareonnon N, Wiwat C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J. Ethnopharmacol. 2005;101(1–3):84–89. doi: 10.1016/j.jep.2005.03.030. [DOI] [PubMed] [Google Scholar]