Abstract
Green wild plants (dirctly before flowering) and seeds of Hyoscyamus aureus were collected from natural habitat at Al Qalamon region in Syria. Seeds were surface sterilized and cultured in vitro, after 21 days from germination stem-derived callus was induced on two different nutrient media. Tropane alkaloids were extracted from wild plants and 30 days old in vitro plants and callus, and then analyzed using GC-MS. Genetic variation was also studied between the wild and in vitro plants and the callus culture lines using twenty ISSR markers. The results showed that there were significant variations in tropane alkaloids contents between the wild plants, the in vitro plants and the callus culture lines. The highest content of hyoscyamine was in callus on line A medium, but the highest content of scopolamine was in the wild plants. However, the lowest content of tropane alkaloids was in callus on line B medium. Also the ISSR analyses showed that there was genetic variation between the wild and in vitro plants and the callus culture lines.
Keywords: Hyoscyamus aureus, Callus culture, Tropane alkaloids
Introduction
The genus Hyoscyamus, belongs to Solanaceae family, is well known for the production of tropane alkaloids, which constiute one of the largest groups of pharmaceutically and economically important plant secondary metabolites. The tropane alkaloids are widely used for their antispasmodic, anti-cholinergic, analgesic and sedative properties (Zhang et al. 2004; Oksman-Caldentey 2007; Tytgat and Guido 2007; Frank and Rene 2008). Hyoscyamine and scopolamine, commercially important anesthetic and anti-spasmodiac drugs, are the two most important Solanaceae alkaloids. They produced in roots and then translocated to the aerial parts of the plant (Hashimoto et al. 1993). Owing to the cost and time factors involved, the chemical synthesis of these alkaloids has been proved to be difficult and commercially infeasible. They are still isolated exclusively from plants, several species belonging to Solanaceae such as Atropa, Datura, Scopolia, Douboisia and Hyoscyamus, to cater to the needs of pharmaceutical industry (Kang et al. 2004; Cardillo et al. 2010).
Tissue culture techniques have an important role in the development of methods that allow synthesis of a high yield of active substances (Berlin and Sasse 1985). During the past few years, considerable efforts have been made to develop an economically feasible method of in vitro production of tropane alkaloids (Zolala and Farsi1 M, Gordan HR, Mahmoodnia M, 2007). Much research has, therefore, been conducted to discover a suitable alternative method for producing tropane alkaloids through in vitro procedures, including callus and suspension cultures, protoplast cultures, somatic hybridization and root cultures (Oksman-caldentey and Strauss 1986; Koul et al. 1986; Oksman-Caldentey 1986; Oksman-Caldentey 1987; Oksman-Caldentey et al. 1987a; 1987b). Promising results were obtained from wild type root cultures of Hyoscyamus species. Tropane alkaloids were produced at relatively high levels in cultured roots of seven Hyoscyamus species, where hyoscyamine content varied from 0.04% to 1.1% of dry weight, while scopolamine content varied from 0.06% to 0.3% of dry weight, depending on the plant species (Hashimoto et al. 1986). The cell culture, with a careful selection for cells that produce a high levels of active substances and the optimization of culture conditions to increase the accumulation of active substances, could be an effective method of active substances production with the possibility of economical exploitation (Buite and Tramper 1992; Ravishankar and Venkataraman 1993; Dixon 1999; Rao 2000; Mulabagal and Tsay 2004). Moreover, callus culture created a new source of secondary metabolites; it is more efficient than extract these secondary metabolites directly from the plant. However, few studies compared between the production of secondary metabolites directly from the plant and the production from the tissue resulted from callus culture Zenk et al. 1977; Mulabagal and Tsay 2004).
Somaclonal variation describes genetic changes in plants that become apparent either during or after in vitro culture of plant cells, callus or organs (Larkin and Scowcroft 1981). This variation may be caused through pre-existing genetic variation occurred in the explant and the variation induced by the in vitro conditions (Skirvin et al. 1994). As somaclonal variation may imply an advantage as a source of variability for new lines, it is important to achieve a rapid and easy method to assess the genetic stability of the propagated plants at the earliest stage of plant growth. ISSR markers have been successfully used for the detection of somaclonal variation in various micropropagated plants (Carvalho et al. 2004; Martins et al. 2004; Ramage et al. 2004; Modgil et al. 2005). ISSR is highly discriminative, reliable and cost-effective (Pradeep et al. 2002; Mehrotra et al. 2012.
The extraction of tropane alkaloids from wild plants and the chemical synthesis of these alkaloids are difficult and commercially infeasible, so alternative production methods are developed for their production, such as in vitro plants and tissue cultures, metabolic engineering manipulation and recombinant plants. Therefore, this study aimed to compare the tropane alkaloids production capacity between the wild and in vitro plants and two callus culture lines of H. aureus, and to determine the optimal conditions for increasing the content of tropane alkaloids in callus culture. And since the modifications of the callus culture and in vitro grown plants from the wild plants could be essential, it was necessary to use ISSR molecular markers to find differences at the gene level.
Materials and methods
Plant material
Wild and in vitro plants
Green wild plants (dirctly before flowering) and seeds of Hyoscyamus aureus were collected from natural habitat at Al Qalamon region in Syria. For in vitro plants, seeds were surface sterilized with ethanol 70% for 1 min, 1.5% sodium hypochlorite for 20 min and washed three times with sterile distilled water. Then, they were cultivated on Murashige and Skooge (MS) medium (Murashige and Skoog 1962), and incubated for growth under long-day conditions (16 h light/8 h dark) at a constant temperature of 24 ± 2 °C.
Callus induction
Shoots (1 cm long) of twenty one days old in vitro plants were used as a source of explants for establishing the callus cultures. Shoots were planted in sterile glass tubes containing two nutrient media for callus induction (line A medium and line B medium) which are described in Table 1 (Vollosovich et al. 1979). Callus were subcultured on the same induction media every 30 days.
Table 1.
Components of used media in callus induction
| Line A medium | Line B medium | ||
|---|---|---|---|
| Component | concentration | Component | concentration |
| KNO3 | 1.1 g/l | KNO3 | 1.9 g/L |
| NH4NO3 | 0.5 g/l | ||
| MgSO4 | 0.5 g/l | NH4NO3 | Lg/ |
| KCL | 0.07 g/l | ||
| (NH4) 2SO4 | 0.3 g/l | MgSO4.7H2O | g/L 0.37 |
| Ca (NO3) 2.4H2O | 0.9 g/l | ||
| (NH4) 2H2PO4 | 0.1 g/l | CaCL2. H2O | 0.44 g/L |
| NH4H2PO4 | 0.6 g/l | KH2PO4 | 0.17 g/L |
| Thiamine-HCL (B1) | 1mg/ml | Thiamine – HCL (B1) | 1mg/ml |
| Pyridoxine (B6) | 0.5 mg/ml | ||
| Nicotine acid (P.P.) | 0.5 mg/ml | 2.4-D | 0.1 mg/L |
| Kinetin) K) | 1 mg/ml | ||
| Naphthalene aceticacid (NAA) | 2mg/ml | ||
| Glycine | 2 mg/ml | BAP | 0.2 mg/L |
| Myo-Inositol | 80 mg/L | ||
| Casein | 500 mg/L | ||
| FeSO4 .7 H2O | 27.85 mg/L | FeSO4 .7 H2O | 27.85 mg/L |
| Na2 . EDTA | 37.25 mg/L | Na2 . EDTA | 37.25 mg/L |
| MnSo4.5H2O | 24.1,mg/L | MnSo4.5H2O | 24.1,mg/L |
| ZnSO4.7H2O | 10.62 mg/L | ZnSO4.7H2O | 10.62 mg/L |
| H3PO3 | 6.2 mg/L | H3PO3 | 6.2 mg/L |
| KI | 0.83 mg/L | KI | 0.83 mg/L |
| Na2MoO4.2H20 | 0.25 mg/L | Na2MoO4.2H20 | 0.25 mg/L |
| CuSO4.2H20 | 0.025 mg/L | CuSO4.2H20 | 0.025 mg/L |
| CoCl2.6H2O | 0.025 mg/L | CoCl2.6H2O | 0.025 mg/L |
| Sucrose | g 50 | Sucrose | g 30 |
| Agar | 7 g | Agar | 7 g |
Callus formation
Percentage of callus formation was inscrolled at the end of incubation period according to this equation (Namdeo et al. 2006):
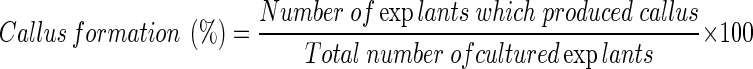 |
Callus fresh and dry weight
Fresh weight of callus was recorded at 30 days old, pieces of fresh callus were extracted and put on sterile aluminum foil and the remains of the medium were removed using scalpels. Fresh weight was measured using a sensitive balance. After that callus was separately dried at 45 °C in an air drying oven until having constant weight, then dry weight was recorded.
Callus characterization
Some indicators were studied to evaluate the callus, such as appearance, color, texture and growth strength of callus. These qualities were measured in 3 replicates per treatment and at rate of 7 tubes per replicate.
Determination of tropane alkaloids
Tropane alkaloids were extracted from wild plants and 30 days old in vitro plants and callus. The wild and in vitro plants and callus were separately dried at 45 °C in an air drying oven until having constant weight and manually grinded to powder. And then, 5 g of powdered were soaked in 50 ml H2SO4 (0.4 N) for 2 h, filtrate under pressure, samples then were made alkaline (pH = 9 by NH4OH, 25%). Alkaloids were extracted twice with 30 ml Cl2CH2. After centrifugation at 4000 rpm for 10 min, the pellucid phase and filtrate were collected. Then 4 g of sodium sulphate anhydrate were added to each sample and that was evaporated under reduced pressure to dryness. The alkaloid extracts were dissolved in 3 ml methanol and 1 μl injected into GC-MS (Doerk et al. 1991).
GC-MS was carried out on a gas chromatograph (Agilent 7890 A). A mass spectrometer (model MS 5975C) with an ion trap detector in full scan (80-325 amu) under electron impact ionization (70 eV) was used. The chromatographic column for the analysis was HP-5-MS capillary column (30 m X0.25 mm, film thickness 0.25 μm). The carrier gas used was helium at a flow rate of 1 mL/min. 1 μL crude alkaloid fractions were injected and analyzed. Column initial temperature was 70 °C for 1 min and then increased to 250 °C with a 9 °C/min heating ramp and subsequently kept at 250 °C for 13 min. The injection was performed in splitless mode at 280 °C. All the calculations concerning the quantitative analysis were performed with external standardization by measurement of the peak areas.
Standard calibration curves for hyoscyamine and scopolamine were constructed by injecting different contents of each. Three replicates were performed for each injection and the mean value of the area under the peaks was plotted against the corresponding content.
DNA isolation and ISSR analysis
After 30 days of culture, fresh leaves of wild and in vitro plants and callus of H. aureus were harvested and directly frozen in liquid nitrogen. Total DNA was isolated using CTAB method with slight modifications (Tewary and Suryanarayana 2007). DNA pellet was dissolved in 100 μl sterile distilled water and then 8 μl of DNA solution with 2 μl of loading dye were loaded in each slot at the 1 % agarose gel containing SYBR green. DNA was detected and photographed under UV-light. DNA concentration was calculated by the absorbance at 260 nm, while the purity of DNA was checked by the absorbance ratio: 260/280.
ISSR-PCR reaction was performed in 25 μl reaction volume containing 50 ng genomic DNA, 0.3 μlTaq DNA polymerase, 1x-10x PCR buffer, 2 mM MgCl2, 100 mMdNTPs and 5 pmol ISSR primers (Table 2). Amplification was carried as follows: Initial denaturation (one cycle) at 95 °C for 5 min. followed by 40 cycles: as follows: denaturation at 94 °C for 1 min, annealing at 40 °C for 1 min, elongation at 72°C for 2 min; final extension at 72 °C for 10 min.
Table 2.
ISSR primers codes and Sequences
| No. | Primer code | Sequence |
|---|---|---|
| 1 | ISSRHA1 | (CCA) 5 |
| 2 | ISSRHA2 | GAG (CAA) 5 |
| 3 | ISSRHA3 | (CT) 8TG |
| 4 | ISSRHA4 | (CT) 8AC |
| 5 | ISSRHA5 | (CT) 8GC |
| 6 | ISSRHA6 | (CA) 6GG |
| 7 | ISSRHA7 | CA) 6AG |
| 8 | SSRHA8 | (AG) 8 |
| 9 | ISSRHA9 | (GAG) 3GC |
| 10 | ISSRHA10 | (CA) 6GT |
| 11 | ISSRHA11 | (CAC) 5 |
| 12 | ISSRHA12 | (CTC) 3GC |
| 13 | ISSRHA13 | (GA) 6GG |
| 14 | ISSRHA14 | (AG) 8TG |
| 15 | ISSRHA15 | (ATG) 5 |
| 16 | ISSRHA16 | (GCC) 5 |
| 17 | ISSRHA17 | (CA) 6AC |
| 18 | SSRHA18 | (CA) 6AC |
| 19 | ISSRHA19 | (AC) 8 |
| 20 | ISSRHA20 | (CAC) 3GC |
Amplification mixture was mixed with 2 μl of the gel loading dye {Sucrose 40 % and Bromo phenol Blue 0.25 % (w/v)} and electrophoresed on 2 % agarose gel stained with sybr green along with 50 ng of 100 bp ladder for 1h at constant 100 V. The gels were pictured in Gel-Doc system.
Molecular weights for ISSR-PCR products, in base pairs, were estimated using TotalLab software (Ultra · Lum Inc., Claremont, Calif.). The data was exported to binary format (presence = “1” and absence = “0”). Then 0/1 matrix was used to calculate genetic distance using Jaccard coefficient, and the resultant dissimilarity matrix was employed to construct dendrogram using Unweighted Pair Group Method of Arithmetic Means (UPGMA) as implemented in POWER MARKER with the tree viewed using TREEVIEW software.
Statistical analysis
Morphological and biochemical results were analyzed using GENSTAT.7 software to determine the less significant differences (LSD) at a confidence level of 95 %.
Results and discussion
Many studies have indicated the easy and obvious ability to callus formation from different parts of Hyoscyamus plants Basue and Chand 1998; Ibrahim et al. 2009). In this study, Hyoscyamu aureus plants have easily induced callus on both used media. The highest percentage of callus formation was on line A medium at 100 %, while it was 90 % on line B medium (Table 3). Also, fresh and dry weights of stem-derived callus were higher on line A medium (103 and 9.39 g, respectively) than that on line B medium (72 and 6.28 g, respectively) (Table 3). The highest formation percentage and fresh and dry weight on line A medium could be explained by the highest content of nutrient elements and sucrose in line A medium (50 g/L in comparison with 30 g/L in line B medium), which support the cells division ratio and increase the bio-mass of induced callus (Grewal et al. 1979; Ramage et al. 2004). In addition to the role of nutrient elements, the growth regulators and vitamins added to the medium play an essential role in callus formation and growth (Acquaach 2004). The line B medium contained the auxin 2,4-Dichlorophenoxyacetic acid (2,4-D), which known by its strong influence in callus formation, and cytokinin 6-Benzylaminopurine (6-BAP), which has a strong influence in cell division and prevent roots formation, in balanced proportions (Rossignol et al. 1990). Moreover, medium B was devoid from kinetin which decreases cells division and consequently fresh and dry weight of callus (Ibrahim et al. 2009). The results show also that callus on both used media had a white creamy color, a coherent texture and a granular desirable appearance on used media.
Table 3.
Callus formation (%) and fresh and dry weigh (g) in used media. Values are the mean (±S.E.) of five replicates, and different letters within columns indicate significant differences (P <0.05)
| Dry weigh | Fresh weigh | Callus formation | Medium |
|---|---|---|---|
| 9.39 ± 0.14 a | 103 ± 1.32 a | 100 ± 2.00 a | Line A |
| 6.28 ± 0.17 b | 72 ± 1.20 b | 90 ± 1.73 b | Line B |
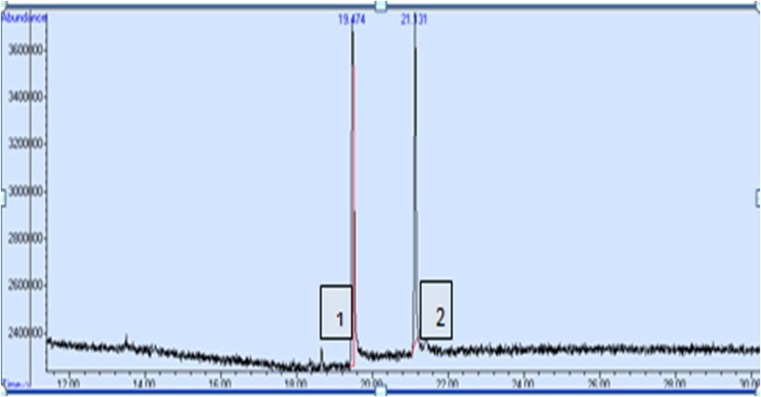
The results in Table 4 show that there were significant differences between the induced callus and the wild and in vitro plants in the content of tropane alkaloids. The highest content of hyoscyamine was in callus on line A medium (0.19 % of dry weight) without significant difference with the in vitro plants (0.17 %). However, the content of scopolamine was higher in the wild plants (0.242 % of dry weight) than in the in vitro plants (0.063 %) and in the callus on line A medium (0.020 %). The lowest contents of tropane alkaloids were in callus on line B medium (0.06 % of hyoscyamine and no trace of scopolamine). Difference in contents of alkaloids between callus cultures and wild and in vitro plants could be explained by the influence of overlapping growth regulators depending on the type and concentration of auxin and cytokinin used. Auxin and cytokinin could increase or decrease the amount of accumulated alkaloids, due to the fact that growth regulators increase the absorption of minerals and accumulation of carbohydrates (Richter 1993). Also growth regulators stimulate the production of amino acids, such as ornithine which forms the basis of building tropane alkaloids (Mann 1987). In addition, these hormones may have an indirect effect on the putrescine N-methyl transferase (PMT) and the hyoscyamine 6β-hydroxylase (H6H) enzymes which are responsible for the production of hyoscyamine and converting hyoscyamine to scopolamine respectively (Rocha et al. 2002). Higher concentration of hyoscyamine than of scopolamine in line A callus and in vitro plants could be explained that Hyoscyamus plants produce hyoscyamine as the main alkaloid and then convert it to scopolamine by H6H enzyme (Elisabit et al. 2003; Ute et al. 2009). Our results are in accordance with these reported by Nabil et al. (2009), Kadi and Yahia (2007) and Kim et al. (2002). It was observed also that hyoscyamine always shows a retention time less than scopolamine (Fig. 1), this indicates that scopolamine is more familiar with the material of the gaseous chromatographic column, and more volatile with the used inert gas. These results accord with that of Eava et al. (1998)) on H. muticus.
Table 4.
Contents of tropane alkaloids (hyoscyamine and scopolamine, % of dry weight) in wild and in vitro plants and callus cultures of H. aureus. Values are the mean (±S.E.) of five replicates, and different letters within columns indicate significant differences (P <0.05)
| Hyoscyamine | Scopolamine | |
|---|---|---|
| Wild plants | 0.10 ± 0.001 b | 0.242 ± 0.002 a |
| In vitro plant | 0.17 ± 0.006 a | 0.063 ± 0.001 b |
| Line A | 0.19 ± 0.006 a | 0.020 ± 0.001 c |
| Line B | 0.06 ± 0.012 c | 0.000 ± 0.000 d |
Fig. 1.
Retention time of hyoscyamine (1) and scopolamine (2)
To estimate the probable somaclonal variation derived from in vitro and callus culture, we subjected the wild plants, the in vitro plants and the callus on line A and B media to ISSR analysis. Out of the 20 ISSR primers initially tested, 19 gave distinct and reproducible band patterns. A total of 254 bands were generated. The number of bands varied from 3 (ISSRHA12) to 25 (ISSRHA2 and ISSRHA16), with an average of 15.86 per primer (Table 5). Sixteen primers were polymorphic, and the highest percentage of polymorphic was detected with ISSRHA20 (93.75%), while the other primers gave 26.67 – 86.96% polymorphism with an average of 53%.
Table 5.
The number of amplified and polymorphic bands and percentage of polymorphism in callus cultures and in vitro plants of H. aureus using ISSR markers
| Primer | Number of amplified bands | Number of polymorphic bands | Percentage of polymorphism |
|---|---|---|---|
| ISSRHA1 | 23 | 20 | 86.96 |
| ISSRHA2 | 25 | 12 | 48.00 |
| ISSRHA3 | 0 | - | - |
| ISSRHA4 | 10 | 7 | 70.00 |
| ISSRHA5 | 15 | 12 | 80.00 |
| ISSRHA6 | 12 | 4 | 33.33 |
| ISSRHA7 | 22 | 16 | 72.73 |
| ISSRHA8 | 17 | 11 | 64.71 |
| ISSRHA9 | 15 | 9 | 60.00 |
| ISSRHA10 | 4 | 2 | 50.00 |
| ISSRHA11 | 15 | 0 | 0.00 |
| ISSRHA12 | 3 | 2 | 66.67 |
| ISSRHA13 | 19 | 9 | 47.37 |
| ISSRHA14 | 12 | 0 | 0.00 |
| ISSRHA15 | 13 | 7 | 53.85 |
| ISSRHA16 | 25 | 17 | 68.00 |
| ISSRHA17 | 20 | 17 | 85.00 |
| ISSRHA18 | 15 | 4 | 26.67 |
| ISSRHA19 | 8 | 0 | 0.00 |
| ISSRHA20 | 16 | 15 | 93.75 |
| Total | 254 | 164 | - |
| Mean | 15.86 | 8.63 | 53 |
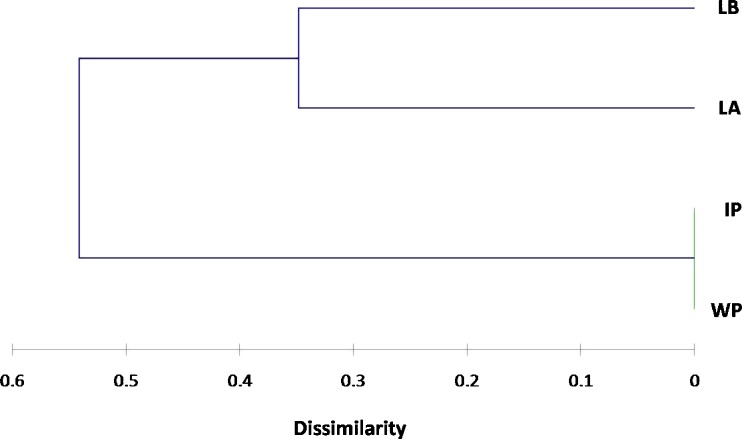
The Jaccard's dissimilarity coefficients calculated based on the ISSR data ranged from 0.000 (between wild and in vitro plants) to 0.605 (between wild plants and callus on line B medium) (Table 6). Cluster analysis was used on the basis of the dissimilarity coefficients. Wild plants, in vitro plants and callus cultures were divided into two broad groups (I and II) (Fig. 2). Group I contained callus on line A medium and callus on line B medium and their dissimilarity coefficient was 0.348. Group II included the wild and in vitro plants, which separated from group I by dissimilarity coefficient of 0.54. This analysis clarified the genetic variation induced by the callus culture conditions, and the high genetic stability of in vitro plants. It has been assumed that certain growth regulators are mutagenic, and the high somaclonal variation that was observed in callus cultures in this study could be attributed to the effect of the used growth regulators.
Table 6.
The Jaccard's dissimilarity coefficients calculated based on the ISSR data
| Wild plants | In vitro plants | Line A | Line B | |
|---|---|---|---|---|
| Wild plants | 0.000 | |||
| In vitro plants | 0.000 | 0.000 | ||
| Line A | 0.477 | 0.477 | 0.000 | |
| Line B | 0.605 | 0.605 | 0.348 | 0.000 |
Fig. 2.
UPGMA dendrogram derived from Jaccard's dissimilarity coefficients calculated between wild plants (WP), in vitro plants (IP), callus culture on line A medium (LA) and callus culture on line B medium (LB) based on amplification profiles generated by ISSR markers
In conclusion, the in vitro plants and the callus cultures, especially on line A medium, could be alternative methods of tropane alkaloids production. Also, ISSR markers have provided a useful assessing technique of genetic stability in the in vitro and callus cultures of Hyoscyamus aureus.
References
- Basue P, Chand S. Embryogenesis and plant regeneration from callus cultures derived from unpollinated ovaries of H. muticus L. J. Plant Biochem. Biotech. 1998;7:302–305. doi: 10.1007/s002990050397. [DOI] [PubMed] [Google Scholar]
- Berlin J, Sasse F. Selection and screening techniques for plant cell cultures. Advanced Biochemistry and Engeneering Volume. 1985;31:99–132. [Google Scholar]
- Buite L, Tramper J. Strategies to improve the production of secondary metabolites with plant cell cultures a literature review. J Biotechnol. 1992;23:111–143. doi: 10.1016/0168-1656(92)90087-P. [DOI] [Google Scholar]
- Cardillo AB, Otálvaro AAM, Busto VD, Talou JR, Velásquez LME,Giulietti AM (2010) Scopolamine, anisodamine and hyoscyamine production by Brugmansia candida hairy root cultures in bioreactors. Process Biochemistry45 (9): 1577-1581
- Carvalho LC, Goulao L, Oliveira C, Goncalves JC, Amancio S. RAPD assessement for identification of clonal identity and genetic stabitlity of in vitro propagated Chestnut hybrids. Plant Cell Tiss Org Cult. 2004;77:23–27. doi: 10.1023/B:TICU.0000016482.54896.54. [DOI] [Google Scholar]
- Dixon RA. Plant natural products: the molecular genetic basis of biosynthetic diversity. Curr Opin Biotechnol. 1999;10:192–197. doi: 10.1016/S0958-1669(99)80034-2. [DOI] [PubMed] [Google Scholar]
- Doerk K, Witte L, Wilham A. Identification of tropane alkaloids in hairy root culture of Hyoscyamus albus. Z Naturforsch. 1991;46:519–521. [Google Scholar]
- Eava M, Salo JP, Oksman-Caldentey KJ. Determination of the main tropane alkaloids from transformed Hyoscyamusmuticus plants by capillary zone electrophoresis. Journal of Pharmaceutical and Biomedical Analaysis. 1998;16:717–722. doi: 10.1016/S0731-7085(97)00121-0. [DOI] [PubMed] [Google Scholar]
- Elisabit M, Jouhikainen K, Tammela P, Palazon J, Rosa M, Cusido RM, Pinol MT, Teeri TH, Oksman-Caldentey KM. Effect of PMT gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J Exp Botany. 2003;54:203–211. doi: 10.1093/jxb/erg014. [DOI] [PubMed] [Google Scholar]
- Frank P, Rene A (2008) Natural compounds as drugs. Volumel Bikhauser 350
- Grewal S, Koul S, Ahuja A, Atal CK. Hormonal control of growth organogenesis and alkaloid production in vitro cultures of Hyoscyamus muticus Linn. Indian J Exp Biol. 1979;17:558–561. [Google Scholar]
- Hashimoto T, Yukimune Y, Yamada Y. Tropane Alkaloid Production In Hyoscyamus Root Cultures. J Plant Physiol. 1986;124(1–2):61–75. doi: 10.1016/S0176-1617(86)80178-X. [DOI] [Google Scholar]
- Hashimoto T, Yun DJ, Yamada Y. Purification of tropane alkaloid in genetically engineered root cultures. Phytochemistry. 1993;32(3):713–718. doi: 10.1016/S0031-9422(00)95159-8. [DOI] [Google Scholar]
- Ibrahim AI, Abd El Kawi M, Nower A, Abdel Motaal A, Abd El Aal A. Alkaloid Production and Organogenesis from Callus of Hyoscyamusmuticus L. In vitro Journal of Applied Sciences Research. 2009;5(1):82–92. [Google Scholar]
- Kadi K, Yahia A. Effect of phytohormones 2,4-D and Kinitin, Application on alkaloids accumulation in Hyoscyamusalbus L. Science and Technologie. 2007;25:13–17. [Google Scholar]
- Kang YM, Chen JY, Ouyang W, Qiao JT, Reyes VC. Serotonin modulates hypothalamic neuronal activity. Int J Neurosci. 2004;114:293–319. doi: 10.1080/00207450490264115. [DOI] [PubMed] [Google Scholar]
- Kim Y, Wysluzie EB, Weathers JP. Secondary metabolism of hairy root cultures bioreactors in vitrocell. dev. Boil-plant. 2002;38:1–10. [Google Scholar]
- Koul S, Auja A, Grewal S. Growth and Alkaloid Production in Suspension Cultures of Hyoscyamusmuticus as Influenced by Various Cultural Parameters. Planta Med. 1986;47:11–16. doi: 10.1055/s-2007-969939. [DOI] [PubMed] [Google Scholar]
- Larkin PJ, Scowcroft WR. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theoretical Appl. Genetics. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Mann J (1987) Secondary metabolism. Clarendon press, pp 353-366
- Martins M, Sarmento D, Oleveira MM. Genetic stability of micropropagted almond plantlets as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004;23:492–496. doi: 10.1007/s00299-004-0870-3. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Khwaja O, Kukreja AK, Rahman L (2012) ISSR and RAPD based evaluationof genetic stability of encapsulated microshoots of Glycyrrhizaglabra following 6 months of storage. Mol. Biotechnoldoi 10.1007/s12033-011-9491-6 [DOI] [PubMed]
- Modgil M, Mahajan K, Chakrabarti SK, Sharma DR, Sobti RC. Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Sci Hort. 2005;104:151–160. doi: 10.1016/j.scienta.2004.07.009. [DOI] [Google Scholar]
- Mulabagal V, Tsay HS. Plant Cell Culture an alternative and efficient source for the production of biologically important secondary metabolites. Internat J Appl Sci Eng. 2004;2:29–48. [Google Scholar]
- Murashige T, Skoog F. Arevised Medium for Rapid growth and bioassays with Tobacco tissue culture. Plant physic. 1962;15(1):473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nabil JV, Celedonio G, Angel GR, Rafael Z. Cloning characterization and analysis of expression prophils of a cDNA encoding a hyoscyamine 6B-hydroxylase (H6H) from AtropabaeticaWillk. Plant Physiol Biochem. 2009;47:20–25. doi: 10.1016/j.plaphy.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Namdeo AG, Mahadik RR, Kadam SS. Cost effective medium for callus initiation from Catharanthusroseus leaves. Pharmacogn Mag. 2006;2(8):227–231. [Google Scholar]
- Oksman-Caldentey KM, Strauss A, Hiltunen R. Optimization of Some Parameters in Cultivating Scopolamine producing Cells of Hyoscyamusmuticus. Acta Pharm Fenn. 1987;96(2):91–99. doi: 10.1055/s-2007-969287. [DOI] [PubMed] [Google Scholar]
- Oksman-Caldentey KM, Vuorela H, Strauss A, Hiltunen R. Variation in the Tropane Alkaloid Content of Hyoscyamusmuticus Plants and Cell Culture Clones. Planta Med. 1987;53:349–354. doi: 10.1055/s-2006-962736. [DOI] [PubMed] [Google Scholar]
- Oksman-Caldentey KM. Production of Hyoscyamine and Scopolamine in Some Solanaceous Plants and Cell Cultures. Acta Pharm Fenn. 1986;96(2):49–58. [Google Scholar]
- Oksman-Caldentey KM (1987) Scopolamine and Hyoscyamine Production by Plants and Cell Cultures of Hyoscymusmuticus. PhD thesis, University of Helsinki, Helsinki, Finland
- Oksman-Caldentey KM. Tropane and nicotine alkaloid biosynthesis-novel approaches towards biotechnological production of plant-derived. Pharmaceuticals. Curr Pharm. Biotechnol. 2007;8:203–210. doi: 10.2174/138920107781387401. [DOI] [PubMed] [Google Scholar]
- Oksman-caldentey KM, Strauss A. Somaclonal Variation of Scopolamine Content in Protoplast-derived Cell Culture Clones of Hyoscyamusmuticus. Planta Med. 1986;52(1):6–12. doi: 10.1055/s-2007-969053. [DOI] [Google Scholar]
- Pradeep RM, Sarla N, Siddiq EA. Intersimple sequence repeat (ISSR (polymorphism and its application in plantbreeding. Euphytica. 2002;128:9–17. doi: 10.1023/A:1020691618797. [DOI] [Google Scholar]
- Ramage CM, Borda AM, Hamill SD, Smith MK (2004) A simplified PCR test for early detection of dwarf off-types in micropropagated Cavendish banana (Musaspp. AAA). Sci. Hort. 103: 145-151
- Rao RS. Biotechnological production of phyto-pharmaceuticals. Bio-chemistry Molecular Biology Biophysies. 2000;4:73–102. [Google Scholar]
- Ravishankar GA, Venkataraman LV (1993) Pole of plant cell cultures in food biotechnology: commercial prospectus and problems. Oxford IBH Press, New Delhi, pp 255-274
- Richter G. Metabolism de vegetaux, physiologieetbiochimie. Lausanne: Presses polytechniques et universitaires Romandes; 1993. pp. 431–454. [Google Scholar]
- Rocha P, Stemzel O, Parr A, Walton N, Christon P, Drager B, Leech MJ. Functional expression of tropinonereductase I (tr1) and Hyoscyamine 6-B-hydroxylase H6H from H. niger in Nicotianatabacum. Plant Sci. 2002;162:905–913. doi: 10.1016/S0168-9452(02)00033-X. [DOI] [Google Scholar]
- Rossignol M, Santoni V, Zponanski S, Wand Vansuyt G (1990) Differential sensitivity to auxin at the plasma membrane level. Nij Kamp, pp 498-503
- Skirvin RM, Mcpheeters KD, Norton M. Sources and frequency of somaclonal variation. Hort Sci. 1994;29:1232–1237. [Google Scholar]
- Tewary PK, Suryanarayana N (2007) DNAisolation and RAPD analysis of Tasarfoodplants (Terminalia Spp.).Ad. Plant. Sci. 20 (II): 319-321
- Tytgat A, Guido N. Hyoscinebutylbromide: A review of its use in the treatment of abdominal cramping and pain. Drugs. 2007;67(9):1343–1357. doi: 10.2165/00003495-200767090-00007. [DOI] [PubMed] [Google Scholar]
- Ute R, Grit R, Qune-Katrin F, Bettina R, Birgit D. Overexpression of tropinone reductase alters alkaloid composition in Atropa belladonna root culture. Exp. Botany Oxford Journals. 2009;56:645–652. doi: 10.1093/jxb/eri067. [DOI] [PubMed] [Google Scholar]
- Vollosovich AG, Puchinina TN, Nikolaeva LA. Optimization of macroelemts for tissue culture of Rauwolfia serpentina Benth. Journal of Russian Grow Resources. 1979;15(4):516–526. [Google Scholar]
- Zenk MH, El–Shagi H, Arens H, Stockigt J, Well EW, Deus B (1977) Formation of the Indole Alkaloids Serpentine and ajmalicine in cell suspenstion culture of CatharanthusRoseus. New York, pp 27–43
- Zhang L, Ding R; Chai Y, Bonfill M, Moyano E, Oksman K, Caldentey M, Xu T, Pi Y, Wang Z, Zhang H, Kai G, Liao Z, Sun X, Tang K (2004) Engineering tropane biosynthetic pathway in Hyoscyamusniger hairy root cultures. Proc. Natl. Acad. Sci. U.S.A. 101 6786e6791 [DOI] [PMC free article] [PubMed]
- Zolala J, Farsil M, Gordan HR, Mahmoodnia M. Producing a High Scopolamine Hairy Root Clone in Hyoscyamus muticus through Transformation by Agrobacterium rhizogenes. Agric Sci Technol. 2007;9:327–339. [Google Scholar]