Abstract
Nepeta pogonosperma is an important medicinal plant with anti-inflammatory effects. An efficient and reliable transformation system for this plant was developed through optimization of several factors which affected the rate of Agrobacterium rhizogenes mediated transformation. Five bacterial strains, A4, ATCC15834, LBA9402, MSU440 and A13, two explant types, leaves and stems, and several co-cultivation media were examined. The maximum rate of hairy root induction was obtained from stem explants using MSU440 and ATCC15834 bacterial strains. A drastic increase in the frequency of transformation (91 %) was observed when MS medium lacking NH4NO3, KH2PO4, KNO3 and CaCl2. Hairy root lines were confirmed by polymerase chain reaction (PCR) using primers of the rolB gene. According to Southern blot analysis, one T-DNA copy was inserted into each of the hairy root lines. In the present study, transgenic hairy roots have been obtained trough genetic transformation by A. rhizogenes harbouring two plasmids, the Ri plasmid and pBI121 binary vector harbouring gus reporter gene. Expression of the gus gene in transgenic hairy root was confirmed by histochemical GUS assay.
Keywords: Agrobacterium rhizogenes, Hairy root, Nepeta pogonosperma
Introduction
Medicinal plants are the most important source of drugs for people all around the world (Tripathi and Tripathi 2005). The global demand for herbal medicine is growing (Khan et al. 2009). Nepeta genus has 67 species growing in different regions of Iran; most of them are used in traditional medicine for their anticonvulsant, anti-cough and asthma, antiseptic and diuretic effects. Nepeta pogonosperma Jamzad et Assadi was identified as a new species in 1984 (Jamzad and Assadi 1984). Sefidkon and Akbarinia (2003) has demonstrated that 4aα-7α-7aβ-nepetalactone and 1, 8-cineole are the main compounds in N. pogonosperma essential oil. Anti-inflammatory effects of these compounds have also been investigated in previous studies (Silva et al. 2003; Miceli et al. 2005).
Biotechnological tools such as in vitro regeneration and genetic transformation are important for multiplication and metabolic engineering of medicinal plants. Agrobacterium rhizogenes is a gram-negative soil bacterium responsible for hairy root induction at the site of infection by transferring T-DNA of Ri plasmid. Hairy root grows much faster and it maintains genetic and biochemical stability. Hairy root can be cultivated in a simple medium without phytohormones (Tao and Li 2006; Pratapchandran and Potty 2011). Moreover, hairy root culture are becoming attractive for expressing recombinant proteins (Ghiasi et al. 2012; Ono and Tian 2011).
Several factors affect the rate of A. rhizogenes mediated transformation. Henzi et al. (2000) indicated that the use of 200 mM acetocyringone in LB medium for bacterial growth, 50 mM acetocyringon and 1 mM arginine in the co-cultivation medium showed significant improvement in A. rhizogenes transformation of Brassica oleracea L. In another study, Sharafi et al. (2013d) modified the medium of co-cultivation (lacking KH2PO4, NH4NO3, KNO3 and CaCl2) for reaching to high efficient transformation of Papaver bracteatum.
In this study, we describe an efficient protocol for the development of hairy root culture as a biotechnological tool for secondary metabolite production from N. pogonosperma. The improved transformation was achieved by modifying macroelement compounds in co-cultivation medium. Different strains of A. rhizogenes and N. pogonosperma explants were evaluated in this study. Also transgenic hairy roots have been obtained via transformation by A. rhizogenes harbouring pBI121 binary vector harbouring gus reporter gene. To the best of our knowledge, it is the first study on N. pogonosperma transformation and hairy root production.
Materials and methods
Seed sterilization and germination
Seeds of N. pogonosperma were procured from Alamout Medicinal Plant Research Center (Qazvin province, Iran). The seeds were surface sterilized. They were cultured in half strength MS medium (Murashige and Skoog 1962), solidified with 0.7 % (w/v) agar. The medium was adjusted to pH 5.8 before adding agar. The cultures were maintained under a 16 h photoperiod regime with fluorescent light at 25º C in a culture room.
Preparation of A. rhizogenes strains
Five strains of A. rhizogenes (ATCC 15,834, LBA9402, A4, A13, and MSU440) (All of them were provided by the bank of microbes at the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran) were used in this study. A single bacterial colony from each strain was inoculated in 10 ml of LB medium. The culture was placed on rotary shaker (160 rpm) at 28 °C for 24 h. The bacterial suspensions were centrifuged at 4,000 rpm for 10 min at 4 °C. The pellets were re-suspended in 20 ml MS liquid medium supplemented with 100 μM acetocyringone.
Co-cultivation and establishment of hairy root culture
Explants (leaf and stem) were pre-cultured for two days on MS medium supplemented with 2 mg/l 6-benzyl-aminopurine (BA). The explants were immersed in bacterial suspension (optical density at 600 nm, OD = 0.5-0.8) for 10 min and then blotted on sterile filter paper and incubated in a co-cultivation medium consisted of MS salts and vitamins along with 50 mg/l sucrose and 100 μM acetocyringone (designated as Medium 1). This medium was subsequently adjusted by removing the following compounds: KH2PO4 (Medium 2); KH2PO4, CaCl2 (Medium 3); KH2PO4, NH4NO3, KNO3 (Medium 4); KH2PO4, NH4NO3, KNO3, CaCl2 (Medium 5) based on our previous studies (Sharafi et al. 2013a,b,c). Control explants were used with similar treatments without inoculation with A. rhizogenes. After two days, they were transferred to MS medium containing 400 mg/l cefotaxime to eliminate A. rhizogenes. They were sub-cultured every week by reducing the concentration of cefotaxime.
Transgenic hairy roots induction using pBI121 binary vector
The best bacterial strain and explant (MSU440 and stem, respectively) obtained from the previous experiment were selected for this part of study. The pBI121 vector was mobilized by freeze–thaw method into A. rhizogenes strain MSU440. Bacteria were grown in liquid LB medium supplemented with 50 mg/l kanamycin to mid-growth phase (OD600 =0.6). The cells were collected by centrifugation and resuspended at a density of OD600 = 0.8 in liquid inoculation medium (MS salts-NH4NO3, KNO3, KH2PO4, and vitamins containing 50 g sucrose/l and 100 μM acetocyringone).
Polymerase chain reaction analysis and Southern hybridization
Genomic DNA was extracted from 8 unrelated samples of hairy root and a normal root (control) of N. pogonosperma using a modified CTAB protocol for DNA extraction from medicinal plant species (Ibrahim, 2011). PCR analysis was done using the rolB specific primers for samples. Primer sequences used are as follows: 5′-GCTCTTGCAGTGCTAGATTT-3′ and 5′-GAAGGTGCAAGCTACCTCTC-3′. Amplification products were separated by electrophoresis on 0.8 % agarose gel in 0.5× TBE buffer, stained with ethidium bromide and visualized under UV trans-illuminator.
Genomic DNA (15 μg) of hairy roots and control (normal root) of N. pogonosperma were digested using XbaI (Fermentas Co., Germany) separated on 0.8 % agarose gel and subsequently transferred to a nylon membrane. Probe was designed on the basis of rolB sequence, using the DIG DNA labeling Kit (Roche Co., Germany). Prehybridization, hybridization, washing and detection were performed according to the instruction manual of the DIG Labeling and Detection System (Roche Co., Germany). Also, PCR was performed using genomic DNA of transgenic hairy roots as a target and the oligonucleotide primers for gus gene as follow: Forward primer: 5′-GGTGGTCAGTCCCTTATGTTACG-3′ and reverse primer 5′- CCGGCATAGTTAAAGAAATCATG -3′.
Histochemical GUS assay
The hairy roots and roots from a normal plant were examined for histochemical GUS expression by soaking in X-Gluc solution (Jefferson 1987).
Shoot regeneration from hairy roots
Hairy roots and tumors induced by A. rhizogenes were transferred to regeneration media. MS solid media supplemented 0.5 mg/l BA in companion with 0.1 mg/l NAA, pH 5.8 was used as regeneration medium. After one month, regenerated shoots were excised and cultivated on root induction medium (MS medium supplemented with 0.5 mg/l IBA).
Statistical analysis
The experiments were laid on a completely randomized design (CRD) with 3 replications and 9 explants cultured in each Petri dish. The data collected were subjected to analysis of variance test. The means were compared using Duncan’s multiple range tests.
Results
Effect of A. rhizogenes strains, types of explant and different co-cultivation medium
Five different Agrobacterium rhizogenes strains: ATCC15834, A4, LBA9402, A13, MSU440 were examined for transformation ability. Hairy roots of Nepeta pogonosperma were initiated from stem and leaf explants after one week. They were transferred to 1/2 MS liquid medium for more growth after three weeks (Fig. 1). N. pogonosperma stems and leaves were susceptible to infection by all A. rhizogenes strains, as shown by the percentage of each explant from which hairy roots emerged. The highest rate of hairy root induction was found using strain MSU440 followed by strains A13 and ATCC15834, respectively. All of the A. rhizogenes strains led to hairy root induction and also caused the formation of tumorigenic calli using the stem explants. Stem explants were the best explants for A. rhizogenes mediated transformation in N. pogonosperma. Leaf explants showed necrosis with a low rate of hairy root induction (data not shown).
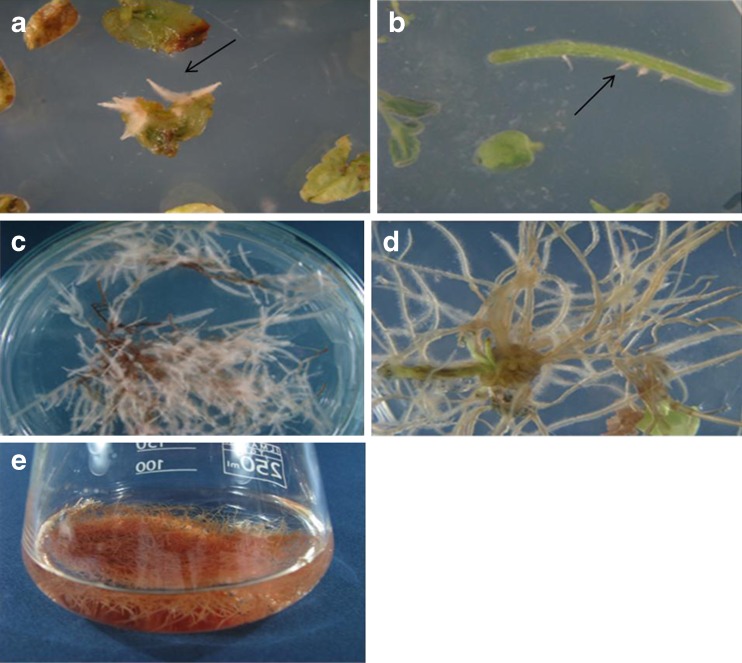
Fig. 1.
A. rhizogenes mediated transformation in N. pogonesperma; a hairy root induction in leaf explants after 3 weeks of inoculation; b induction of hairy root on wound site of stem, after 4 weeks of inoculation using strain MSU440; d Hairy root growth after 4 weeks of inoculation using ATCC15834 strain; c Hairy root growth after 4 weeks of inoculation using strain MSU440; e Hairy root culture in liquid MS medium; f, g, h induction of adventitious shoot from hairy roots and calli; i growth of regenerated shoots after one month
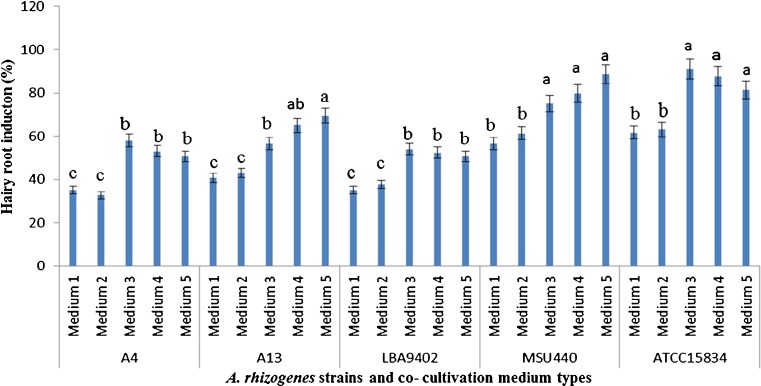
Stem explants as a suitable explant were evaluated for testing the effect of elimination of macro element salts in co-cultivation media with five A. rhizogenes strains. The maximum rate of transformation in full strength MS medium (medium 1), was observed at 61 % in the ATCC15834 strain. On the other hand, using medium 3 or 4 showed 91 and 87.6 % hairy root induction by the same strain.
Transformation frequency in medium 5, was significantly increased in comparison with the full strength MS medium (medium 1) in all strains and resulted in 50.6, 50.6, 69.3, 88.6 and 81.3 % hairy root induction frequency in A4, LBA9402, A13, MSU440, and ATCC15834 strains, respectively (Fig. 2).
Fig. 2.
Effect of different A. rhizogenes strains and co-cultivation media on percentage of hairy root induction in N. pogonesperma. The data were obtained as a mean of four replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s multiple range test. Vertical lines represent standard errors
PCR and southern bolt analysis
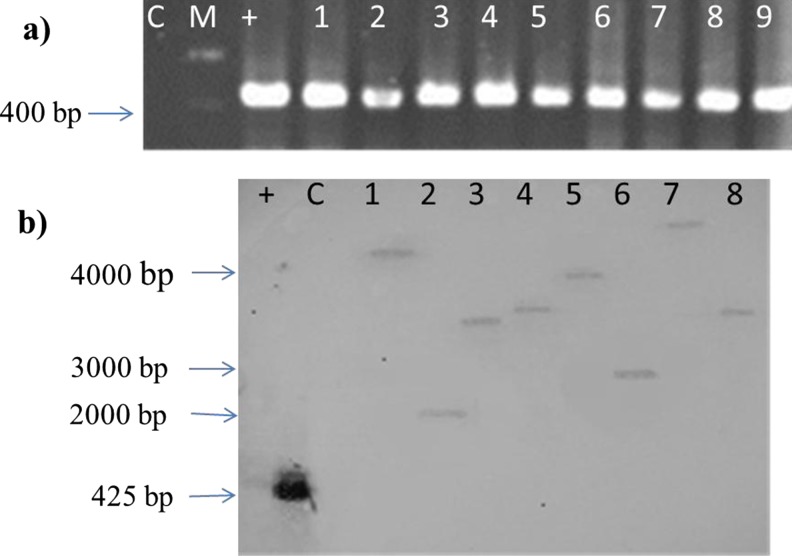
PCR was performed to investigate presence of the rolB gene transferred from Ri plasmid. PCR led to amplification of rolB gene in all transgenic hairy root lines. T-DNA copy number in transgenic hairy root lines was determined by Southern blot analysis on nylon membranes and the results showed that one T-DNA copy number was inserted into all hairy root lines, while no hybridization signal was observed in the untransformed root (Fig. 3).
Fig. 3.
Molecular analysis of hairy roots. a) PCR analysis for detection of the rolB gene in hairy roots of N. pogonesperma M: Molecular size marker (1 kb ladder Fermentase); 1–9: hairy roots, C: negative control (non-transformed root); +: positive control (Ri plasmid). b) Southern blot analysis of hairy roots (1–8) and an untransformed root (C). DNA samples were digested with XbaI and hybridized to rolB probe. Molecular markers are indicated on the left
Transgenic hairy root production
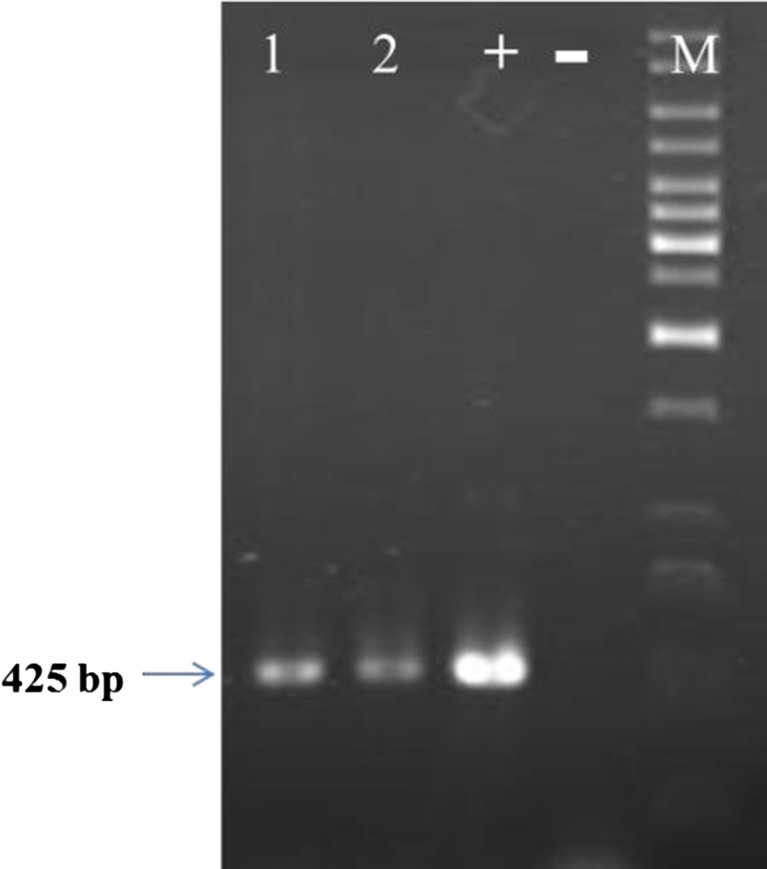
Twenty nine independent hairy root lines were separated from 22 explants (total explants were 24) and culture in MS liquid media 3 weaks after inoculation. Approximately, 35 % (10 lines) of the hairy root lines were transgenic as analysed by PCR and histochemical GUS assay (Fig. 4 and Fig. 5). Transformation efficiency (the frequency of co-cultivated leaf explants which produced independent hairy-root transgenic lines) of 41.7 % was obtained.
Fig. 4.
PCR analysis of hairy roots (1,2) and normal root (−); M: 1 kb size marker; positive control (+) is plasmid DNA (pBI121)
Fig. 5.
Histochemical GUS assay; a normal root; b hairy root
Shoot regeneration from hairy root cultures
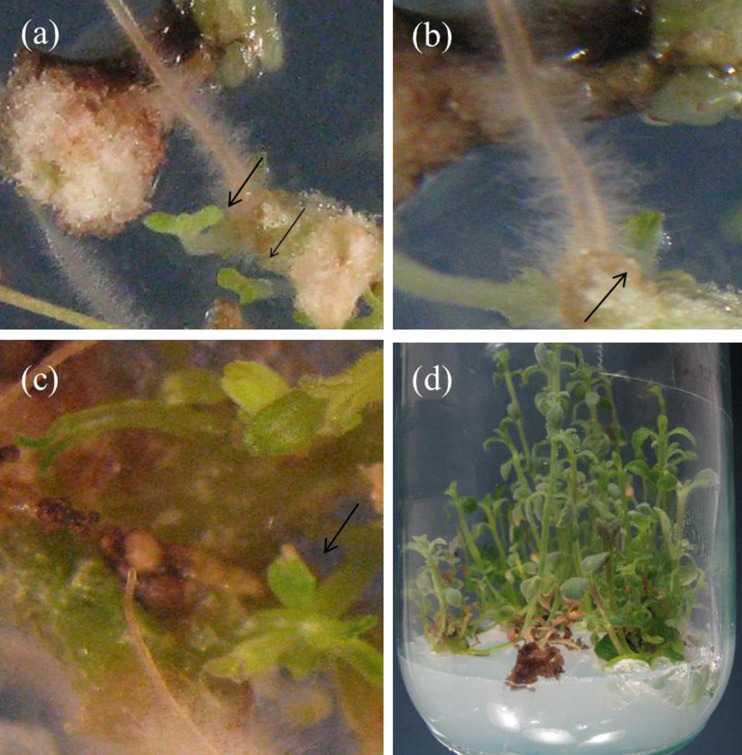
After 2 weeks most hairy root segments induced callus in regeneration medium. Some small shoot points were formed on the surface of calli from hairy roots after 3 to 4 weeks (Fig. 6 a, b, c). the induced shoots were cut and culture on MS medium for more growth (Fig. 6 d). then they were cultivated on root induction medium for rooting.
Fig. 6.
Adventitious shoot regenerated from hairy roots of N. pogonesperma; a, b induction of adventitious shoot from hairy root after 3 weeks c growth of regenerated shoots after 4 weeks from hairy root; d shoots originated from hairy roots after 6 weeks
Discussion
Previous studies have shown that several factors such as genotype, explant, physical and chemical factors, strain of A. rhizogenes can affect genetic transformation by A. rhizogenes (Kumar et al. 1991; Sharafi et al. 2013b). Selection of explant type and effective A. rhizogenes strain is very important factor affecting frequency of transformation. In present study it was observed that stem explants were much susceptible than leaf to infection by all A. rhizogenes strains which were tested. The highest frequency of hairy root induction was achieved in case of MSU440 and ATCC15834 strains using stem explants. Strain A4 produced low hairy root induction in comparison to other strains. This could be due to presence of different plasmids in this strain (Nguyen et al. 1992).
Co-cultivation is very important in transformation processes, since bacterial attachment, T-DNA transfer and integration to plant genome is made at this stage. Transformation can increase according to changes in co-culture media; our results suggest that macro-elements in co-cultivation media have inhibitory effects on the transformation of N. pogonosperma via A. rhizogenes. Compared to the MS full strength medium, cell proliferation of A. rhizogenes around the explants during co-cultivation in media four and five was highly increased. The bacterial overgrowth in these media proved the inhibitory effect of macro-elements on the proliferation of A. rhizogenes during co-cultivation time (data not shown). Previous studies showed that low level of PO4 can activate the expression of virG and could be a positive signal to induce the infection of plants (Winans 1990; Dupre et al. 2000) indicating that a medium with lacking mineral components was the best co-cultivation medium to achieve the highest rate of transformation in Ginkgo biloba. Similar result was reported in the case hairy root induction of Papaver bracteatum (Sharafi et al. 2013c).
The main outcome of the present study is introduction of a reliable and well defined protocol to induce high frequency of hairy root induction and increase the growth rate in N. pogonosperma. The A. rhizogenes strains MSU440 and ATCC15834, stem explant and medium lacking KH2PO4, NH4NO3, KNO3, CaCl2 as best strains, explant and co-cultivation medium respectively are proposed as most suitable for N. pogonosperma transformation. Transformation efficiency can be over 40 % using binary vector (co-transformation of rol gene and target gene) by this protocol. The transgenic hairy roots can be used as explants for plantlet regeneration to obtain stable transgenic plants (Sharafi et al. 2013a).
Acknowledgments
This research was a part of MS thesis of the first author and supported by National Institute of Genetic Engineering and Biotechnology (NIGEB) of Iran and Novin Giti Gene Biotech. Company (NG ene Biotech Co.) located in biotechnology incubator center of NIGEB.
Refences
- Dupre P, Lacoux Y, Neutelings G, Mattar-Lavrain D, Fliniaux MA, David A, Jacquin-Dubreuil A. Genetic transformation of Ginkgo biloba by Agrobacterium tumefaciens. Physiol Plant. 2000;108:413–419. doi: 10.1034/j.1399-3054.2000.t01-1-100411.x. [DOI] [Google Scholar]
- Ghiasi SM, Salmanian AH, Sharafi A, Kazemi R, Jafari M, Chinikar S, Zakeri S. Molecular farming, an effective system for the production of immunogenic Crimean-Congo hemorrhagic fever virus glycoprotein (CCHF) Prog Biol Sci. 2012;2:12–19. [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- Henzi MX, Christey MC, McNeil DL. Factors influence Agrobacterium rhizogenes mediated transformation of broccoli (Brassica oleracea L. var.italica) Plant Cell Rep. 2000;19:994–999. doi: 10.1007/s002990000221. [DOI] [PubMed] [Google Scholar]
- Ibrahim RIH. A modified CTAB protocol for DNA extraction from young flower petals of some medicinal plant species. Gene Conserve. 2011;40:165–182. [Google Scholar]
- Jamzad Z, Assadi M. New species of the genera Nepeta and Ajuga (Labiatae) from Iran. Iranian J Bot. 1984;2:95–102. [Google Scholar]
- Khan MY, Aliabbas S, Kumar V, Rajkumar S. Recent advances in medicinal plant biotechnology. Indian J Biotechnol. 2009;8:9–22. [Google Scholar]
- Kumar V, Jones B, Davey MR. Transformation by Agrobacterium rhizogenes and regeneration of transgenic shoots of the wild soybean Glycine argyrea. Plant Cell Rep. 1991;10:135–138. doi: 10.1007/BF00232044. [DOI] [PubMed] [Google Scholar]
- Miceli N, Taviano MF, Giuffrida D, Trovato A, Tzakou O, Galati EM. Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham. J Ethnopharmacol. 2005;97:261–266. doi: 10.1016/j.jep.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nguyen C, Bourgaud F, Forlot P, Guckert A. Establishment of hairy root cultures of Psoralea species. Plant Cell Rep. 1992;11:424–427. doi: 10.1007/BF00234375. [DOI] [PubMed] [Google Scholar]
- Ono NN, Tian L. The multiplicity of hairy root cultures: prolific possibilities. Plant Sci. 2011;180:439–446. doi: 10.1016/j.plantsci.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Pratapchandran R, Potty VP. Initiation of hairy roots from Canavalia sp. using Agrobacterium rhizogenes 15834 for the co-cultivation of arbuscular mycorrhizal fungi, Glomus microcarpum. J Agric Technol. 2011;7:235–245. [Google Scholar]
- Sefidkon F, Akbarinia A. Essential oil composition of Nepeta pogonosperma jamzad et assadi from Iran. J of Essential Oil Res. 2003;15:327–328. doi: 10.1080/10412905.2003.9698601. [DOI] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Azadi P, SharafiA A. Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants. 2013 doi: 10.1007/s12298-013-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Dehsara B, Hosseini Khalifani B. Increasing morphinanalkaloid production by over-expressing salutaridinol 7- o – acetyltransferase in Iranian poppy hairy roots. Plant Cell Tissue Organ Cult. 2013;29:2125–2131. [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Hosseini Khalifani B, Razavi K. Metabolic engineering of morphinan alkaloids by over expression of code none reductase in transgenic hairy root of Papaver bracteatum. Biotechnol Lett. 2013;35:445–453. doi: 10.1007/s10529-012-1080-7. [DOI] [PubMed] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Razavi K, Ntui VO. A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum. Plant Cell Tiss Organ Cult. 2013;113:1–9. doi: 10.1007/s11240-012-0246-2. [DOI] [Google Scholar]
- Silva J, Abebe W, Sousa SM, Duarte VG, Machado MIL, Matos FJA. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol. 2003;89:277–283. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Tao J, Li L. Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. South African J Bot. 2006;72:211–216. doi: 10.1016/j.sajb.2005.07.010. [DOI] [Google Scholar]
- Tripathi L, Tripathi JN. Role of biotechnology in medicinal plants. Trop J Pharm Res. 2005;2:243–253. doi: 10.4314/tjpr.v2i2.14607. [DOI] [Google Scholar]
- Winans SC. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]