Abstract
Withania ashwagandha, belonging to the family Solanaceae, is an important medicinal herb of India with restricted geographic distribution. It is a rich source of withaferin A (WA) and other bioactive withanolides. In the present study a rapid in vitro mass propagation protocol of W. ashwagandha was developed from nodal explants. Nodal explants were cultured on MS medium supplemented with various concentrations and combinations of plant growth regulators (PGRs). The highest number of regenerated shoots per ex-plant (33 ± 2.7) and highest WA (13.4 ± 1.15 mg/g of DW) production was obtained on MS medium supplemented with 5.0 μM 6-benzyladenine (BA) and 1.0 μM Kinetin (Kn). In vitro raised shoots were further rooted on half-strength MS medium containing 2.0 μM Indole-3-butyric acid (IBA) and analyzed for WA production. The rooted plantlets when transferred to poly bags in the greenhouse showed 90 % survival frequency. Levels of WA were higher in the in vitro and ex vitro derived shoot and root tissues as compared to field grown mother plants. In an attempt to further maximize WA production, shoot cultures were further grown in liquid MS medium supplemented with 5.0 μM 6-benzyladenine (BA) and 1.0 μM Kinetin (Kn). Root cultures were grown on half strength MS liquid medium fortified with 2.0 μM of IBA. WA production in the liquid cultures was significantly higher compared to the static composition of the same media. This protocol, first of its kind in this plant, can be successfully employed for conservation, proliferation and large-scale production of WA. The regenerated plants can also be used in traditional medicine as an alternative to naturally collected plants.
Keywords: High performance liquid chromatography, In vitro conservation, Medicinal plant, Plant growth regulators, W. ashwagandha, Withaferin A
Introduction
Withania ashwagandha belonging to the family Solanaceae is a plant of immense medicinal value largely due the presence of array of bioactive withanolides. It is an annual self-fertilizing herb with restricted geographic distributed growing exclusively as small-cultivated populations only in drier regions of India (Mir et al. 2013). Recently, it has been delineated form wild germplasm of W. somnifera using multidisplinary genome diagnostic approaches (Mir et al. 2010; Kumar et al. 2011). Withania is an extensively used medicinal herb in the Indian traditional systems of medicine. The roots of the plant are used in constipation, rheumatism, nervous exhaustion, loss of memory, loss of muscular energy, syphilis and gastrointestinal disorders (Tuli et al. 2009; Mir et al. 2012). The plant is a rich source of pharmacologically active withanolides including withaferin A (WA, anticancer molecule) that are prospective high-value drug candidates. The leaves contain two times more WA than the common species, W. somnifera which has been extensively investigated both in terms of pharmacology and phytochemistry. WA is an effective anticancer molecule widely used for the treatment of wide range of cancers (Kaileh et al. 2007; Mayola et al. 2011; Hahm et al. 2013). The other therapeutic value ascribed to bioactive withanolides include anti-oxidative, immune-modulatory, anti-stress, cardio-protective, anti-inflammatory, aphrodisiac, anti-stress, cardio-protective and neuroprotective (Ahmad et al. 2005; Tuli et al. 2009).
Owing to the ever-increasing demand of bioactive withanolides, the species is being indiscriminately exploited, thereby threatening its stock in the wild. Pharmaceutical industry generally uses field-grown plants by randomly uprooting the whole plants to meet the current demand of bioactive withanolides (Mir et al. 2014a, b). Long gestation period coupled with slow growth rate and low concentration of active compounds are the other impediments with the extraction of withanolide from plant source. The complex accumulation patterns and the structural complexity of WA also preclude its large scale extraction and chemical synthesis.
Poor seed viability and germination restricts its propagation through sexual means, and propagation via seeds causes a wide range of variations among the plants. The quality and quantity of withanolide constituents are highly affected by genotype and environmental conditions. In this scenario, plant cell and organ culture of an elite genotype is an attractive alternative for large-scale propagation and for the production of bioactive withanolide (Mir et al. 2014a). It is the most suited approach for the conservation and commercial propagation of rare and endangered medicinal species to reduce the dependence on the natural habitat for the supply of raw drugs (Baskaran et al. 2013; Mir et al. 2014a). Plant tissue culture could also provide a means of disease free healthy clones for extraction of pure drugs molecules. It also offer an elegant alternative for homogeneous, controlled production of metabolites, throughout the year, especially when we take commercial demand into account (Gawde and Paratkar, 2012; Mir et al. 2014a). Further in vitro propagation through shoot tip and nodal explants is an easy and economic way for obtaining large number of consistently uniform and true-to-type plants.
The present study was therefore, aimed to develop a high-frequency rapid in vitro regeneration system for mass propagation, conservation and for the production of WA in an elite genotype (WA02) of W. ashwagandha. Additionally, the communication documents the capacity of regenerated plantlets and liquid cultures to accumulate greater quantity of WA in comparison with naturally-grown plants. To our knowledge this is the first study to develop the in vitro regeneration protocol and withanolides accumulation insights in W. ashwagandha.
Materials and methods
Plant material
Plant material was selected for the present study from Ashwagandha Gene Bank stocks at the Indian Institute of Integrative Medicine (IIIM), Jammu, India. A withaferin A rich accession of W. ashwagandha (WA02) characterized previously was used as a source of explants. The voucher specimen of the plant vide specimen voucher number RRL 50412 was deposited in the Herbarium of IIIM.
Culture media and conditions
Culture media consisted of Murashige and Skoog (1962) basal medium, liquid (root and shoot culture) or solidified with 0.8 % (w/v) agar (shoot proliferation and rooting), supplemented with 3 % (w/v) sucrose and plant growth regulators (PGRs) of various concentrations (Table 1 and 2). All PGRs were purchased from Sigma–Aldrich (St. Louis; MO; USA). The pH of the medium was adjusted to 5.8-5.9 prior to the addition of 0.8 % agar (w/v). The medium was sterilized by autoclaving at 15 psi and 121 ºC for 20 min and the cultures were incubated in a growth chamber under a 16:8 h photoperiod, illuminated with cool white fluorescent (F 40 T 12/CW/EG) lamp at a photon flux density of 100 μ mol m−2 s−1 at 25 ± 2 ºC and 65–70% relative humidity. The root cultures were maintained in darkness.
Table 1.
Effects of PGRs (BA, Kn and IAA) on multiple shoot proliferation, average shoot length, percentage of ex-plant response and WA content of W. ashwagandha cultured on MS static medium after five weeks
| PGR Concentration (μM) | No. of shoots/explants | Average shoot length (cm) | Percentage of explants response | WA (mg/g DW) | ||
|---|---|---|---|---|---|---|
| BA | Kn | IAA | ||||
| 0.5 | - | - | 16 ± 2.25a | 3.9 ± 0.30a | 90 | 9.60 ± 1.25 |
| 1.0 | - | - | 25 ± 3.35b | 3.9 ± 0.35a | 90 | 9.68 ± 1.20 |
| 2.0 | - | - | 26 ± 3.25b | 4.8 ± 0.50b | 90 | 12.13 ± 0.80 |
| 3.0 | - | - | 26 ± 2.75b | 4.8 ± 0.50b | 90 | 12.50 ± 0.85 |
| 5.0 | - | - | 29 ± 3.25c | 5.0 ± 0.25b | 100 | 13.2 ± 1.25 |
| 7.0 | - | - | 26 ± 3.25b | 5.3 ± 0.55c | 100 | 13.05 ± 1.20 |
| 10.0 | - | - | 25 ± 2.50b | 5.3 ± 0.30c | 100 | 12.77 ± 1.35 |
| - | 1.0 | - | 12 ± 2.25d | 3.0 ± 0.23d | 80 | 8.85 ± 1.15 |
| - | 2.0 | - | 12 ± 1.50d | 3.3 ± 0.35d | 80 | 8.56 ± 0.95 |
| - | 3.0 | - | 18 ± 2.20a | 3.5 ± 2.20e | 95 | 10.05 ± 1.3 |
| - | 5.0 | - | 22 ± 2.75e | 3.5 ± 2.20e | 95 | 10.12 ± 1.25 |
| - | - | 1.0 | 9.5 ± 3.20f | 2.9 ± 0.35d | 30 | 7.55 ± 0.85 |
| - | - | 2.0 | 11 ± 2.77f | 2.9 ± 0.23d | 30 | 7.55 ± 0.85 |
| - | - | 3.0 | 12 ± 2.55f | 3.2 ± 0.35d | 35 | 8.05 ± 1.05 |
| - | - | 5.0 | 15 ± 3.3a | 3.9 ± 0.33 a | 35 | 8.55 ± 1.15 |
| 1.0 | 1.0 | - | 25 ± 2.25b | 4.2 ± 0.20a | 95 | 9.95 ± 1.40 |
| 1.0 | 5.0 | - | 28 ± 3.70c | 4.0 ± 0.35a | 100 | 10.75 ± 1.20 |
| 2.0 | 1.0 | - | 29 ± 2.70c | 5.0 ± 0.40b | 100 | 12.68 ± 1.3 |
| 3.0 | 2.0 | - | 30 ± 3.27c | 5.0 ± 0.35b | 100 | 12.58 ± 1.3 |
| 5.0 | 1.0 | - | 33 ± 2.70g | 5.5 ± 0.35c | 100 | 14.4 ± 1.15 |
| 1.0 | - | 1.0 | 22 ± 2.25e | 4.2 ± 0.20a | 95 | 8.55 ± 0.65 |
| 1.0 | - | 5.0 | 24.5 ± 3.70b | 4.0 ± 0.35a | 100 | 9.56 ± 1.05 |
| 2.0 | - | 1.0 | 24.5 ± 3.70b | 4.0 ± 0.40a | 100 | 12.23 ± 0.80 |
| 5.0 | - | 1.0 | 25 ± 3.25b | 4.2 ± 0.50a | 100 | 13.2 ± 1.25 |
Values are mean of 20 replicates ± SE. Means followed by same letters in each column are not significantly different (P = 0.05) using Duncan’s multiple range test. PGRs (Plant Growth regulators); BA (6-Benzyl Adenine); Kn (Kinetin); IAA (Indole-3-Acetic Acid); DW (Dry weight); WA (Withaferin A)
Table 2.
Effect of IAA, NAA and IBA on root induction, percentage rooting, root length and withaferin A (WA) production in regenerated shoots of W. ashwagandha cultured for five weeks on half strength MS medium
| PGR Concentration (μM) | Rooting percentage | Number of roots/shoot | Root length (cm) | WA (mg/g DW) | ||
|---|---|---|---|---|---|---|
| IBA | NAA | IAA | ||||
| 0.5 | - | - | 100 | 25 ± 2.7b | 2.80 ± 0.15a | 0.74 ± 0.06 |
| 1.0 | - | - | 100 | 29 ± 3.5c | 3.50 ± 0.35d | 0.80 ± 0.025 |
| 2.0 | - | - | 100 | 38 ± 3.8d | 4.50 ± 0.50e | 0.83 ± 0.06 |
| - | 0.5 | - | 65 | 18 ± 3.0a | 1.25 ± 0.50b | 0.73 ± 0.020 |
| - | 1.0 | - | 70 | 20 ± 3.5a | 1.80 ± 0.60c | 0.73 ± 0.09 |
| - | 2.0 | - | 70 | 19 ± 2.25a | 2.85 ± 0.45a | 0.75 ± 0.04 |
| - | - | 0.5 | 80 | 20 ± 2.5a | 1.65 ± 0.75c | 0.68 ± 0.022 |
| - | - | 1.0 | 80 | 20 ± 3.25a | 2.65 ± 0.60a | 0.70 ± 0.09 |
| - | - | 2.0 | 80 | 24 ± 3.50b | 2.75 ± 0.75a | 0.73 ± 0.045 |
| 1.0 | 1.0 | - | 100 | 30 ± 3.5c | 2.80 ± 0.15a | 0.82 ± 0.008 |
| 1.0 | 2.0 | - | 100 | 28 ± 4.3c | 2.80 ± 0.15a | 0.82 ± 0.009 |
| 2.0 | 0.5 | - | 100 | 38 ± 2.5d | 4.30 ± 0.50e | 0.83 ± 0.025 |
| - | 0.5 | 1.0 | 85 | 20 ± 2.5a | 1.65 ± 0.75c | 0.76 ± 0.022 |
| - | 1.0 | 2.0 | 80 | 22 ± 2.5a | 1.65 ± 0.75c | 0.73 ± 0.025 |
| 1.0 | - | 1.0 | 100 | 29 ± 3.3c | 3.60 ± 0.25d | 0.81 ± 0.065 |
| 2.0 | - | 1.0 | 100 | 38 ± 4.5d | 4.50 ± 0.50e | 0.82 ± 0.065 |
| 2.0 | - | 2.0 | 100 | 36 ± 5.3d | 4.50 ± 0.50e | 0.78 ± 0.090 |
Values are mean of 20 replicates ± SE. The differences in the number of roots and root length among the treatments are significant (P < 0.05). Means followed by same letters in each column are not significantly different (P = 0.05) using Duncan’s multiple range test. NAA (Naphthalene acetic acid); IBA (Indole Butyric Acid); IAA (Indole-3-Acetic Acid)
Explants preparation and establishment of the shoot cultures
Nodal explants of 30-day-old seedlings were used for the induction of shoot cultures. The explants were washed thoroughly under running tap water for 30 min and soaked in 2 % (v/v) solution of Tween-20 (Sigma-Aldrich, St. Louis, USA) for 15 min followed by washing under running tap water for 1 h to remove the dust particles. Further they were treated with Bavistin (1 % w/v) for 10 min followed by treatment with 0.1 % (w/v) HgCl2 for 2 min. Finally, the explants were rinsed three times in sterilized double-distilled water and placed on MS solid media supplemented with different concentrations and combinations of BA (0.5-10 μM), Kinetin (Kn) (1-5 μM) and IAA (1-5 μM) for induction of shoot cultures and quantification of WA (Table 1). After proliferating shoots, micro-shoots were transferred to fresh medium every 5–6 weeks. The total number of shoots, and their length, were recorded after 5 weeks of fifth subculture, using at least 20 explants per medium. The shoots (both in vitro and ex vitro) were analyzed for WA quantification. After successful establishment in the MS solid media the shoots were placed in 250 ml Erlenmeyer flasks containing 50 ml of MS medium supplemented with BA (0.5 μM) and Kn (1.0 μM), the medium that showed the maximum shoot proliferation and WA accumulation in the static medium.
Rooting of shoots, plant transfer into soil and establishment of root cultures
For rooting, excised shoots were separated and transferred into half or full-strength MS medium, with NAA, IAA and IBA in concentrations ranging from (0.5-2.0 μM) (Table 2). The root tissues from both media combinations were also analyzed for WA analysis. Half strength MS medium showed comparatively better response in root number and length however there were no significant differences in the content of WA. Roots were further cultured in 50 ml culture tube containing 20 ml of culture medium. The rooting percentage, and the number and length of roots, were recorded after 5 weeks of culture. Healthy plantlets with well-developed roots were subsequently placed in plastic pots containing a mixture of sterile soil and sand (1:1 v/v) with 15 % vermiculite, and covered with glass beakers for 5–10 days. The pots were irrigated with tap water every second day. These plants were maintained in the green house (25 ± 2 ºC) photoperiod conditions for acclimatization ex vitro for 3–4 weeks. The survival frequency was recorded once plants had hardened and acclimatized.
Some of the roots were cultured in 100 ml Erlenmeyer flasks containing 40 ml of half strength liquid MS medium supplemented with IBA (2.0 μM), on a rotary shaker at 80 rpm, in darkness, and sub cultured to fresh medium every 6 weeks. The root biomass from 5–7 passages of stable root cultures, maintained in MS half strength liquid medium fortified with IBA (2.0), was collected for WA analyses and compared with ex vitro and naturally grown plant root biomass.
Withaferin A extraction and quantification
Root and shoot samples of in vitro, ex vitro and field grown mother plants were separately oven dried at 45-50 ºC for 48–50 h. Dried and powdered samples were percolated separately four times with ethanol: water (1:1) at room temperature. Extracts were centrifuged and concentrated to 1/8th of the original volume under reduced pressure at 50 ± 5 ºC. The concentrated residues were extracted with chloroform. Thereafter, chloroform was distilled off under reduced pressure. The residue was weighed and dissolved in HPLC grade methanol (Ranbaxy Fine Chemicals) and subjected to HPLC for detection and quantification of WA as described previously (Mir et al. 2014a). HPLC was carried out on a Shimadzu HPLC system consisting of a Pump LC-10ATVP, an automatic sampling unit (auto-sampler), SIL-10ADVP, a column oven CTO-10ASVP, a diode array detector, SPD-M10AVP and SCL-10AVP Version 5.40 (Shimadzu). Class VP software (Version 6.10) was used for data analysis and processing using a C18 phenomenex column (5 μm, 250 × 4.0 mm I.D., PN 00G-4252-EO) by UV detector at 237 nm. The samples were eluted with MeOH-H2O (60:40) at a flow rate of 0.7 ml min−1.
Statistical analysis
All experiments were repeated a minimum of three times with 20 explants per treatment. The proliferation of shoots and rooting were subjected to one-way analysis of variance (ANOVA), followed by Duncan’s POST-HOC test. WA was quantified as percent dry weight (DW). A two-sided P value of 0.05 was used to determine statistical significance. All analyses were conducted using SPSS software. Data are presented as mean ± standard error (SE).
Results and discussion
Shoot proliferation and establishment
In the face of indiscriminate exploitation for bioactive withanolides an effective propagation protocol of W. ashwagandha for conservation, usage in traditional medicine, and for commercial exploitation was developed. Nodal explants of an elite genotype of W. ashwagandha (WA02) were inoculated on MS medium supplemented with various combinations and concentration of PGRs (Table 1). Shoot multiplication was observed at all the concentrations of PGRs after five weeks. However, the average number of shoots per explants and ex-plant responses varied significantly among the treatments (Table 1). Maximum number of shoots and length of the shoots (33.0 ± 2.70 and 5.5 ± 0.35 cm, respectively) were observed in explants inoculated on MS medium fortified with 5.0 μM (BA) and 1.0 μM (Kn) (Table 1 & Fig. 1a). It was very interesting to observe that cultures initiated on MS medium containing BA and Kn showed higher number of multiple shoots. Cytokinins are a class of plant growth substances that are active in promoting cell division as well as being active in numerous physiological processes, including plant cell growth and differentiation (Mahesh and Jeyachandran, 2013). The stimulating effect of BA alone or in combination with Kn on bud breaking and in vitro multiple shoot formation has been reported in W. somnifera (Ray and Jha, 2001; Sharada et al. 2007; Ahuja et al. 2009; Dewir et al. 2010; Mir et al. 2014a), Trichodesma indicum (Mahesh and Jeyachandran, 2013) and Drimia robusta (Baskaran et al. 2013) and many other species (Kumar et al. 2005; Baskaran et al. 2012; Thiem et al. 2013). The positive effect of BA was observed only unto a threshold of 5.0 μM as the further increase in the concentration of BA alone or in combination with Kn did not elicit any greater response in the parameters studied. Higher concentrations of BA were also observed to be conducive for callus formation. Sporadic and very limited callus formation occurred at the base of the shoot following culture initiation (first two subcultures). Similar results have been reported for common species W. somnifera of genus Withania (Sangwan et al. 2007a, b) and other genera (Sliva et al. 2010; Baskaran et al. 2013). In vitro-derived shoot-tip explants exhibited high regeneration potential with no evidence of decline even after 8–10 subcultures of mass propagation. Profuse flowering of cultures growing on Kn (5.0 μM) and BA (1.0 μM) medium was observed after eight weeks and the flowers were fully functional (Fig. 1b).
Fig. 1.
a-f Plant regeneration of W. ashwagandha from nodal ex plants: a) Multiple shoots developed from nodal ex plant on MS medium with 5.0 μM BA after 5 weeks; b) In vitro flowering; c) Rooting of shoots on semi solid MS half strength medium containing 2.0 μM IBA; d) Roots grown in liquid medium; e) Acclimatized plants in plastic pots in green house after six weeks; f) Field growing flowering plant
In an attempt to maximize production of bioactive withanolides and to circumvent the decline in shoot proliferation due to solid medium, the effect of growing the shoot cultures in liquid MS medium was investigated. Among the various combinations of PGRs used, the liquid medium supplemented with BA (5.0 μM) and Kn (1.0 μM) has been selected for mass production of shoots and WA production due its positive response on shoot multiplication and WA synthesis. The shoots derived from five week old explants of W. ashwagandha were further cultured in 50 ml liquid MS media supplemented with BA (5.0 μM) and Kn (1.0 μM). Shoot biomass in MS liquid medium was significantly increased after five weeks and reached to 30.5 ± 2.15 g FW (3.5 ± 0.08 g DW) compared to 23.0 ± 3.5 g FW (2.3 ± 0.10 g DW) in static cultures (data not provided). In liquid medium, high frequency of multiple shoot formation coupled with enhanced biomass and withanolide production was reported (Mir et al. 2014a).
Rooting of shoots and acclimatization ex vitro
Rooting and subsequent acclimation of in vitro plantlets are the crucial steps in micro-propagation. Following 8 weeks of culture on the proliferating medium, some of the W. ashwagandha shoots spontaneously formed roots. The remaining in vitro raised cut shoots were transferred for rooting to half and full strength MS media supplemented with various concentrations of NAA, IAA and IBA (Table 2). The half strength MS liquid medium showed better results with regard to number of roots and root length; however percentage of rooting was similar in both the treatments as described previously in W. somnifera (Mir et al. 2014a). Hence, only half strength media compositions were highlighted as shown in Table 2. IBA the most widely employed auxin for rooting (Ahuja et al. 2009; Baskaran et al. 2013) yielded the optimum response both in terms of percentage of adventitious root induction and average number of roots per shoot. NAA and IAA induced callusing prior to rooting. Hundred percent rhizogenesis was observed in MS half-strength medium supplemented with 2.0 μM IBA (Fig. 1c-d & Table 2). The medium also showed the highest root length of 4.5 ± 0.50 cm with 38 ± 3.8 number of roots per shoot. The roots formed were initially whitish, but subsequently changed to brown when the shoots had matured possibly due to the increased concentration of secondary metabolites. The roots were further grown in half strength liquid MS medium. The root growth was initially very slow, but became rapid after 3 weeks of culture. As with plantlets, the colour of the roots produced in liquid culture was age dependent; the first roots produced were initially white, but changed in colour to brown (Fig. 1d). These changes in colour may be related to an accumulation of secondary compounds (Thiem et al. 2013). The survival rate of regenerated plants was 90 % and they grew normally after planting into plastic pots containing a mixture of sterile soil and sand (1:1 v/v) with 15 % vermiculite followed by the maintenance in the green house (Fig. 1e). Ex vitro (green house) plants derived from in vitro micro-propagated ones were phenotypically similar to those of natural/field grown plants and no detectable differences in their growth characteristics were observed. Further studies are needed for deeper insights into the withanolide dynamics at various developmental stages. The field grown organs were directly taken from the ex plant source at maturity stage for comparative studies as shown in Table 3.
Table 3.
Comparative analysis of contents of withaferin A in different plant parts of W. ashwagandha from in vitro (static and liquid), green house and field grown plants
| Source of Plant material | Tissue type (Organ) | Treatments | Withaferin A (mg/g DW) |
|---|---|---|---|
| Field grown plant | Leaves | - | 12.75 ± 0.60 |
| Roots | - | 0.82 ± 0.07 | |
| In vitro culture* | Shoots | MS + 5.0 μM (BA) and 1.0 μM (Kn) | 14.4 ± 1.15 |
| Roots | MS + 2.0 μM (IBA) | 0.83 ± 0.06 | |
| In vitro culture (liquid) ** | Shoots | MS + 5.0 μM (BA) and 1.0 μM (Kn) | 14.82 ± 1.50 |
| Roots | MS + 2.0 μM (IBA) | 0.945 ± 0.10 | |
| Green house*** | Shoots | MS + 5.0 μM (BA) and 1.0 μM (Kn) | 14.6 ± 0.85 |
| Roots | MS + 2.0 μM (IBA) | 0.835 ± 0.15 |
*Plants grown on MS agar medium
**Shoots and roots were transferred in flasks containing 50 ml liquid culture medium and harvested after five weeks
***Green house plants were grown in green house in poly bags
Withaferin A (WA) estimation and accumulation
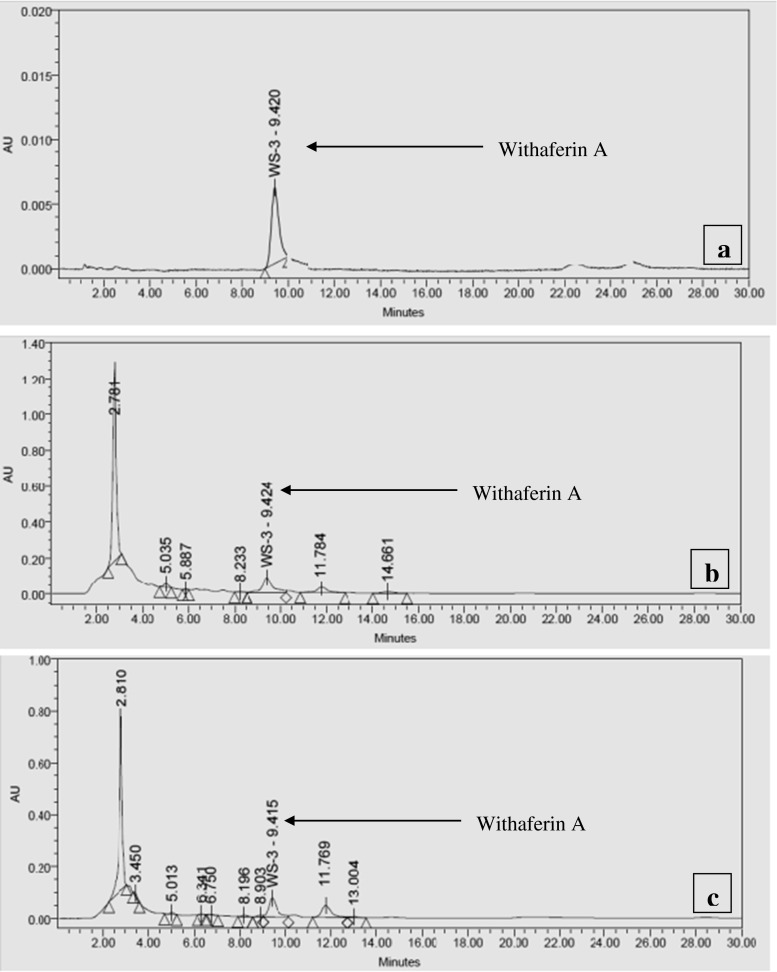
WA content in the shoot and root tissues of in vitro, green house and naturally/field grown plants were quantified using HPLC. Representative HPLC chromatograms of mother plants and in vitro cultured shoots together with standard pure molecule are given in Fig. 2. It was also noted that different concentrations and combinations of PGRs in the culture medium affected the WA accumulation in both shoots and roots indicating a strong influence of PGRs on withanolide accumulation (Table 1 and 2). The media combinations containing BA or Kn accumulated higher contents of WA compared to field grown plants whereas, IAA elicited least WA content. The highest production of WA (14.4 ± 1.15 mg/g DW) was observed in MS medium supplemented with 5.0 μM 6-benzyladenine (BA) and 1.0 μM Kinetin (Kn). Comparative analysis showed that the production and accumulation of WA was higher in the in vitro and ex vitro derived shoots and root tissues as compared to field grown mother plants (Table 1, 2 and 3). The elicitation of other bioactive withanolides was also observed in this study, however they were not quantified. Green house acclimatized plants synthesized the highest content of WA (14.866 ± 0.85 mg/g DW) and probably other secondary compounds compared to in vitro grown plants (Table 3). This could be explained by different environmental conditions that may influence the synthesis of withanolides. This finding was consistent with previous reports of withanolide production by shoot cultures of the common species, W. somnifera (Ray and Jha, 2001; Sharada et al. 2007; Ahuja et al. 2009; Mir et al. 2014a). The shoots further cultured in MS liquid medium containing BA (5.0 μM) and Kn (1.0 μM) were monitored for WA content. A steady increase in WA was observed from the first week of inoculation to the fifth week and after five weeks it was observed to be 14.82 ± 1.50 mg/g of DW (Table 3). The enhanced production of WA is in line with our previous studies in W. somnifera (Mir et al. 2014a).
Fig. 2.
a-c Representative HPLC chromatograms of shoot extract of in vitro and naturally grown W ashwagandha; a) Standard withaferin A marker; b) Mother plant leaf extract; c) Shoot culture in vitro after 5 weeks. Withaferin A (WA)
The root tissues, which contain minor quantity of WA, did not show any significant variations between in vitro, green house and field grown plants, however, roots of green house plants showed slightly higher accumulation (0.835 ± 0.15 mg/g DW) (Table 2 and 3). The bioactive content in the present study was thus varied and tissue dependent probably due to the de novo synthesis as reported in the previous studies (Sangwan et al. 2007a, b; Baskaran and Jayabalan 2008; Dewir et al. 2010; Baskaran et al. 2012, 2013). Dynamics of WA in the in-vitro roots were also investigated in the liquid medium containing 2.0 μM of IBA. The highest accumulation of the compound (0.945 ± 0.10 mg/g) in the root cultures was reported in five weeks time. Further, WA was most dominant in the shoots from both greenhouse and in vitro grown plants and minor in roots. The present study has successfully demonstrated an enhancement of WA production by in vitro liquid cultures, it is therefore, anticipated that liquid cultures would serve as a cost-effective alternative for the production of WA. It also offers the prospect of developing a continuous bioreactor system for greatly improved productivity (Gawde and Paratkar, 2012; Mir et al. 2014a).
In conclusion, this protocol of in vitro propagation and WA production in W. ashwagandha, besides useful in the conservation could also provide an alternative to environmentally and economically unwise random harvesting of plants from the wild for the production of bioactive withanolides. The present study, first of its own kind in this high value medicinal plant reported higher WA accumulation in in-vitro and ex-vitro plants compared to field grown plants. It enhances the prospects for establishment of cost effective bioreactor for the large scale production of bioactive withanolides. The in vitro mass propagation protocol can also be useful in traditional medicine as an alternative to natural populations.
Acknowledgments
We acknowledge the Director, Indian Institute of Integrative Medicine (IIIM), Jammu for providing facilities to carry out this work. Authors also acknowledge Prof. Don A. Cowan, Director, CMEG and Genomics Research Institute (GRI), University of Pretoria for support and help. We also acknowledge Mr. Prabhu Datt, Natural Product Chemistry Division of IIIM for HPLC analysis. The assistance, support and guidance of Dr. Arun Kumar (Senior TO, IIIM) and Mrs. Manju Sambyal are highly acknowledged. Thanks are also due to the University of Pretoria and National Research Foundation (NRF), South Africa for Vice-Chancellor and Freestanding Postdoctoral Fellowships to BAM.
Contribution by the authors
B.A.M. designed and performed the experiments and wrote the first draft of the manuscript. S.K. and B.A.M. analyzed data and organized it in figures and tables. S.K. and B.A.M. edited the final version of the manuscript.
Conflict of interest
No conflict of interest declared.
Abbreviations
- BA
6-Benzyladenine
- DW
Dry weight
- FW
Fresh weight
- HPLC
High Performance Liquid Chromatography
- IAA
Indole-3-acetic acid
- IBA
Indole-3-butyric acid
- Kn
Kinetin
- MS
Murashige and Skoog medium
- NAA
Naphthalene acetic acid
- PGRs
Plant growth regulators
- WA
Withaferin A
References
- Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, Hoda MN, Islam F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Human Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- Ahuja A, Kaur D, Sharada M, Kumar A, Suri KA, Dutt P. Glycowithanolides accumulation in vitro shoot cultures of Indian ginseng (Withania somnifera Dunal) Nat Prod Comm. 2009;4:479–482. [PubMed] [Google Scholar]
- Baskaran P, Jayabalan N. Effect of growth regulators on rapid micropropagation and psoralen production in Psoralea corylifolia L. Acta Physiol Plant. 2008;30:345–451. doi: 10.1007/s11738-007-0129-z. [DOI] [Google Scholar]
- Baskaran P, Ncube B, Van Staden J. In vitro propagation and secondary product production by Merwilla plumbea (Lindl.) Speta. Plant Growth Regul. 2012;67:235–245. doi: 10.1007/s10725-012-9682-6. [DOI] [Google Scholar]
- Baskaran P, Singh S, Staden JV. In vitro propagation, proscillaridin A production and antibacterial activity in Drimia robusta. Plant Cell Tiss Organ Cult. 2013;114:259–267. doi: 10.1007/s11240-013-0322-2. [DOI] [Google Scholar]
- Dewir YH, Chakrabarty D, Lee SH, Hahn EJ, Paek KY. Indirect regeneration of Withania somnifera and comparative analysis of withanolides in in vitro and greenhouse grown plants. Biologia Plantarum. 2010;54:357–360. doi: 10.1007/s10535-010-0063-6. [DOI] [Google Scholar]
- Gawde AJ, Paratkar GT. Production and Enhancement of Wedelolactone in Shoot Cultures of Eclipta alba. J Herbs Spices Med Plants. 2012;18:203–209. doi: 10.1080/10496475.2010.499305. [DOI] [Google Scholar]
- Hahm ER, Lee J, Singh SV. Role of Mitogen-Activated Protein Kinases and Mcl-1 in Apoptosis Induction by Withaferin A in Human Breast Cancer Cells. Molecular Carcinogenesis. 2013 doi: 10.1002/mc.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, De Keukeleire D, Essawi T, Haegeman G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mir BA, Sehgal D, Dar TH, Koul S, Kaul MK, Raina SN, Qazi GN. Utility of multidisciplinary approach for genome diagnostics of cultivated and wild germplasm resources of medicinal Withania somnifera, and status of new species, W. ashwagandha, in the cultivated taxon. Plant Syst Evol. 2011;291:141–151. doi: 10.1007/s00606-010-0372-4. [DOI] [Google Scholar]
- Kumar R, Sharma K, Agrawal V. In vitro clonal propagation of Holarrhena antidysentrica L. wall through nodal explants from mature trees. In Vitro Cell Dev Biol- Plant. 2005;41:137–144. doi: 10.1079/IVP2004624. [DOI] [Google Scholar]
- Mahesh A, Jeyachandran R. Influence of plant growth regulators on micropropagation and in in vitro flowering of Trichodesma indicum (Linn) R. Br. Plant biosystems. 2013;147:493–499. doi: 10.1080/11263504.2012.727876. [DOI] [Google Scholar]
- Mayola E, Gallerne C, Esposti DD, et al. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- Mir BA, Koul S, Kumar A, Kaul MK, Soodan AS, Raina SN. Intraspecific variation in the Internal Transcribed Spacer (ITS) regions of rDNA in Withania somnifera (L) Dunal. I J Biotechnol. 2010;9:325–328. [Google Scholar]
- Mir BA, Khazir J, Mir NA, Dar TH, Koul S (2012) Botanical, chemical and pharmacological review of Withania somnifera (Indian ginseng): an ayurvedic medicinal plant. Ind J Drugs Diseases 6:147–160
- Mir BA, Koul S, Soodan AS. Reproductive biology of Withania ashwagandha sp. novo. Indus Crops Prod. 2013;45:442–446. doi: 10.1016/j.indcrop.2012.12.023. [DOI] [Google Scholar]
- Mir BA, Khazir J, Hakeem KR, Koul S, Cowan DA. Enhanced production of withaferin-A in shoot cultures of Withania somnifera (L) Dunal. DOI. 2014 [Google Scholar]
- Mir BA, Khazir J, Hakeem KR, Kumar A, Koul S. Withanolides array of Withania ashwagandha sp. novo populations from India. Indus Crops Prod. 2014;59:9–13. doi: 10.1016/j.indcrop.2014.04.024. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiolgia Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Ray S, Jha S. Production of withaferin A in shoot cultures of Withania somnifera. Planta Med. 2001;67:432–436. doi: 10.1055/s-2001-15811. [DOI] [PubMed] [Google Scholar]
- Sangwan RS, Chaurasia ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Qazi GN, Tuli R. Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha) Curr Sci. 2007;86:461–465. [Google Scholar]
- Sangwan RS, Chaurasiya ND, Lal P, Misra L, Uniyal GC, Tuli R, Sangwan NS. Withanolide A biogeneration in in vitro shoot cultures of Ashwagandha (Withania somnifera Dunal), a main medicinal plant in Ayurveda. Chem Pharm Bull. 2007;55:1371–1375. doi: 10.1248/cpb.55.1371. [DOI] [PubMed] [Google Scholar]
- Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria RK, Verma V, Kumar A. Withanolide production by in vitro cultures of Withania somnifera and its association with differentiation. Biol Plant. 2007;51:161–164. doi: 10.1007/s10535-007-0031-y. [DOI] [Google Scholar]
- Sliva S, Viehmannova I, Vitamvas J. Micropropagation and morphogenesis of Arracacha (Arracacia xanthorrhiza Bancroft) Agricult Trop et Subtrop. 2010;43:206–211. [Google Scholar]
- Thiem B, Kikowska M, Krawczyk A, Wieckowska B, Sliwinska E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss Organ Cult. 2013;114:197–206. doi: 10.1007/s11240-013-0315-1. [DOI] [Google Scholar]
- Tuli R, Sangwan RS, Kumar S, Bhattacharya S, Misra LN, Trivedi PK, Tewari SK, Misra P, Chaturvedi P, Sangwan NS, Nair KN, Ojha SK, Mehrotra S, Khajuria A, Suri KA. Ashwagandha (W. somnifera) A model Indian Medicinal Plant. India: CSIR Publications; 2009. p. 294. [Google Scholar]