Abstract
An efficient protocol was developed for the rapid in vitro multiplication of an endemic and critically endangered medicinal herb, Ceropegia noorjahaniae Ans., via enhanced axillary bud proliferation from nodal explants. The effects of phytohormones [6-benzylaminopurine (BAP), kinetin (Kin) thidiazuron (TDZ), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) or α-naphthalene acetic acid (NAA)] on in vitro regeneration were investigated. The highest number of shoots (18.3 ± 1.3), maximum shoot length (10.1 ± 0.8 cm) and the highest response of shoot induction (95 %) were recorded on MS medium supplemented with 2.0 mg/l BAP. Rooting was best achieved on half-strength MS medium augmented with IBA (1.0 mg/l). Half-strength MS medium supplemented with BAP (4 mg/l) and sucrose (5 %, w/v) produced an average of 5.6 flower buds per microshoots with highest (90 %) flower bud induction response. The plantlets regenerated in vitro with well-developed shoot and roots were successfully established in pots containing sterile sand and coco peat (1:1) and grown in a greenhouse with 85 % survival rate. The regenerated plants did not show any detectable morphological variation. The developed method can be successfully employed for large-scale multiplication and conservation of C. noorjahaniae.
Keywords: Ceropegia noorjahaniae, Endangered, Endemic, Micropropagation, In vitro, Nodal explant
Introduction
Ceropegia noorjahaniae Ans. (Apocynaceae) is one of endemic and critically endangered plants of Western Ghats, India (Nayar and Sastry 1987). The plant is well known for its edible tubers and ornamental flowers. The plant can easily be identified by its 2.7 cm long, glabrous and attractive corolla. Most of the Ceropegia species have morphologically unique flowers with beautiful architecture and a few of the species are ornamentals and are cultivated in Europe and United States (Hodgkiss, 2004; Reynolds, 2006). The root tubers of Ceropegia species are storehouse of starch, sugars, gum, albuminoids, fats, crude fiber and other valuable phytoconstituents. The starchy tubers are a good source of nutritive tonic (Kirtikar and Basu, 1935). The pharmacological importance of the genus Ceropegia is also due to the presence of ‘cerpegin’ a pyridine alkaloid (Sukumar et al. 1995).
Being endemic, scarcity of pollinators and poor seed setting are the major causes in its dwindling numbers leading to a continuous depletion of its natural population (Yadav and Kamble, 2006). The plant is found in its natural habitat in the state of Maharashtra, especially in Satara district and the extent of its occurrence is estimated to be less than 400 sq. km. with fragmented populations. In 1996, only 19 mature individuals were located at Jarandeshwar hills of Satara district (Mishra and Singh 2001). A typical habitat of this plant is found along ghat slopes in well drained rocky-gravelly soil above 1,000 m altitude.
High frequency multiplication through plant tissue culture technique would minimize the damage to remnant populations. There are some reports on micropropagation studies of Ceropegia species using different explants (Patil 1998; Beena et al. 2003; Chavan et al. 2011a; Phulwaria et al. 2013; Dhir and Shekhawat 2013; Chavan et al. 2013; Chavan et al. 2014; Dhir and Shekhawat 2014). However, no such studies have been reported in C. noorjahaniae. In this context, an efficient micropropagation protocol has been developed for rapid multiplication and conservation of C. noorjahaniae. Moreover, attempts were also made for in vitro flowering of C. noorjahaniae.
Materials and methods
Nodal segments of young C. noorjahaniae were collected from mature plants grown in the botanical garden of Shivaji University, Kolhapur. The explants were washed in running tap water for at least 25 min and then soaked in a 5 % (v/v) detergent, labolene (Qualigens, India) for 10 min. Surface sterilization of the explant was done with 0.1 % HgCl2 (w/v) for 5 min and rinsed thoroughly (4 times) with sterile distilled water. Shoot segments cut into a single node explants were cultured on Murashige and Skoog’s medium (Murashige and Skoog, 1962) supplemented with various phytohormones viz. BAP (0.5 – 2.5 mg/l), Kin (0.5 – 2.5 mg/l), TDZ (0.5 – 2.5 mg/l), IAA (0.5 – 2.0 mg/l) and IBA (0.5 – 2.0 mg/l). Rooting of in vitro derived shoots was accomplished on ½ MS medium supplemented with various concentrations and combinations of auxins viz. IBA (0.5 – 2.5 mg/l), IAA (0.5 – 2.5 mg/l) and NAA (0.5 – 2.5 mg/l). Sucrose (3 %, w/v) was used as carbon source and the pH of the medium was adjusted to 5.8 prior to the addition of gelling agent (Clarigel, 0.2 %, Himedia, India). About 10–15 ml medium was poured in the culture tubes and the medium were autoclaved at 121 °C tempreature and 15 lbs pressure for 20 min. Apical buds from MS medium supplemented with BAP (0.5 mg/l) were taken out and cultured on MS medium amended with BAP (4.0 mg/l) and varied concentration of sucrose (3–8 %) for in vitro flowering (Chavan et al. 2011a). All the cultures were maintained at 25 ± 1 °C with 16 h light/8 h dark photoperiod cycle with 45 μmol m−2 S−1 irradiation (Philips, India). All the experiments were repeated twice with 20 replicates per treatment. The cultures were observed periodically and morphological changes were recorded after 4 weeks of culture. The results were analyzed statistically and significance of differences among means were analyzed using Dunnett’s multiple range test.
Results and discussion
The morphogenetic responses of nodal segment explants to various concentrations and combinations of phytohormones are summarized in Table 1. MS medium without phytohormones (control) failed to induce shoots from nodal explants. The multiplication rate and shoot numbers are variable with type and concentration of phytohormones. All concentrations and combinations of phytohormones facilitated shoot bud differentiation. Swelling of the axillary bud took place within seven days and then differentiation into multiple shoots occurred within four weeks. Among the various cytokinins tested, BAP was found to be more efficient than others with respect to initiation and subsequent proliferation of shoots (Table 1). Maximum numbers of shoots (18.3 ± 1.3), highest shoot length (10.1 ± 0.8) and maximum shoot induction response (95 %) were achieved on MS medium fortified with BAP (2.0 mg/l) within 28 days (Table 1, Fig. 1a, b). Upon lowering or higher concentrations of BAP, a reduction in the number of shoots per culture was recorded (Table 1). The effect of BAP on multiple shoot bud differentiation has been demonstrated in number of cases using nodal explant (Patil 1998; Faisal et al. 2007; Chavan et al. 2011a, Bisht et al. 2012). In the present case, BAP alone proved to be more effective than other phytohormones and their combinations. Similar observations were made in Ceropegia jainii (Patil 1998) and Ceropegia attenuata (Chavan et al. 2011a).
Table 1.
Effect of different phytohormones on shoot induction and multiplication from nodal explants of C. noorjahaniae
| Sr. No. | Phytohormones | Concentration (mg/l) | Response (%) ± SE | Shoot number ± SE | Shoot length ± SE |
|---|---|---|---|---|---|
| 1 | Hormone free | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 | BAP | 0.5 | 70 | 6.3 ± 0.9** | 7.8 ± 1.2** |
| 3 | 1.0 | 75 | 10.5 ± 1.4** | 8.8 ± 1.2** | |
| 4 | 1.5 | 80 | 11.3 ± 1.3** | 9.6 ± 1.1** | |
| 5 | 2.0 | 95 | 18.3 ± 1.3** | 10.1 ± 0.8** | |
| 6 | 2.5 | 85 | 13.9 ± 1.4** | 8.2 ± 0.9** | |
| 7 | Kin | 0.5 | 60 | 1.2 ± 0.2ns | 3.7 ± 0.7** |
| 8 | 1.0 | 70 | 3.8 ± 0.5** | 4.2 ± 0.7** | |
| 9 | 1.5 | 75 | 2.3 ± 0.4** | 3.8 ± 0.5** | |
| 10 | 2.0 | 55 | 2.2 ± 0.4** | 3.2 ± 0.7** | |
| 11 | 2.5 | 50 | 1.8 ± 0.5* | 3.3 ± 0.8** | |
| 12 | TDZ | 0.5 | 90 | 1.8 ± 0.2* | 5.9 ± 0.5** |
| 13 | 1.0 | 75 | 2.7 ± 0.4** | 4.8 ± 0.7** | |
| 14 | 1.5 | 75 | 2.1 ± 0.3** | 4.6 ± 0.6** | |
| 15 | 2.0 | 65 | 1.7 ± 0.3* | 2.8 ± 0.5** | |
| 16 | 2.5 | 60 | 0.9 ± 0.2ns | 2.3 ± 0.5* | |
| 17 | BAP + Kin | 2.0 + 0.5 | 60 | 4.8 ± 0.9** | 2.9 ± 0.6** |
| 18 | 2.0 + 1.0 | 75 | 6.2 ± 0.8** | 3.5 ± 0.6** | |
| 19 | 2.0 + 1.5 | 65 | 3.9 ± 0.7** | 4.2 ± 0.7** | |
| 20 | 2.0 + 2.0 | 65 | 2.2 ± 0.4* | 3.7 ± 0.7** | |
| 21 | BAP + TDZ | 2.0 + 0.5 | 50 | 3.3 ± 0.7** | 3.1 ± 0.7** |
| 22 | 2.0 + 1.0 | 55 | 2.7 ± 0.6** | 4.4 ± 0.6** | |
| 23 | 2.0 + 1.5 | 45 | 1.1 ± 0.3ns | 2.6 ± 0.8* | |
| 24 | 2.0 + 2.0 | 40 | 0.7 ± 0.2ns | 2.1 ± 0.6ns | |
| 25 | BAP + IBA | 2.0 + 0.5 | 90 | 10.5 ± 0.9** | 8.3 ± 0.7** |
| 26 | 2.0 + 1.0 | 80 | 4.2 ± 0.6** | 7.4 ± 1.0** | |
| 27 | 2.0 + 1.5 | 85 | 3.2 ± 0.4** | 7.6 ± 0.9** | |
| 28 | 2.0 + 2.0 | 75 | 1.9 ± 0.3* | 7.2 ± 0.7** | |
| 29 | BAP + IAA | 2.0 + 0.5 | 70 | 1.9 ± 0.3* | 6.5 ± 1.0** |
| 30 | 2.0 + 1.0 | 85 | 3.1 ± 0.3** | 6.7 ± 0.9** | |
| 31 | 2.0 + 1.5 | 80 | 3.6 ± 0.4** | 5.6 ± 0.7** | |
| 32 | 2.0 + 2.0 | 80 | 4.4 ± 0.6** | 4.8 ± 0.7** |
Mean ± S.E. of 20 replicates per treatment. The values are significantly different at ns-non significant, *P < 0.05 and **P < 0.01 level when compared by Dunnett multiple comparisons test
Fig. 1.
In vitro plant regeneration of C. noorjahaniae. a–b: Shoot multiplication (MS + BAP 2.0 mg/l); c: In vitro rooting (½MS + IBA 1.0 mg/l); d: In vitro flowering (MS + BAP 4.0 mg/l + sucrose 5 %); e–f: Hardened plantlet after transferred into pot containing sterile sand and coco peat
MS medium supplemented with Kin (1.0 mg/l) produced an average of 3.8 ± 0.5 shoots per nodal explant. However, TDZ did not enhance shoot induction significantly. Similar observations were made in case of Ceropegia attenuata (Chavan et al. 2011a). A combination of BAP (2.0 mg/l) and IBA (0.5 mg/l) produced an average 10.5 ± 0.9 shoots with 90 % multiplication response. The synergistic effect of BAP in combination with an auxin has been reported for some other Apocynaceae members such as Hemidesmus indicus (Sreekumar et al. 2000), Holostemma ada-kodien (Martin 2002), Ceropegia candelabrum (Beena et al. 2003), and Ceropegia panchganiensis (Chavan et al. 2013). In the present study, the shoot multiplication rate was highest on MS medium fortified with BAP (2.0 mg/l) hence, used for the subsequent subcultures and large-scale multiplication of C. noorjahaniae. Increased shoot numbers were observed upto the 3rd subculture (upto 25 shoots/nodal explant) and then declined afterwards. Enhanced shoot multiplication in subsequent culture is in accordance with other Apocynaceae plants like Gymnema sylvestre (Komalavalli and Rao 2000), Holostemma ada-kodien (Martin 2002), Ceropegia spiralis (Chavan et al. 2011b), and Ceropegia santapaui (Chavan et al. 2014)
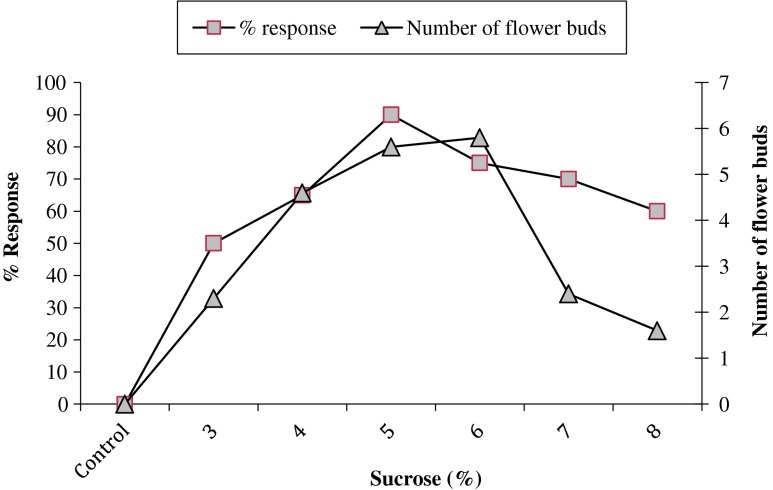
The tissue culture of C. noorjahaniae was not only favored rapid multiplication but was also characterized by in vitro flowering that synchrony with the plants growing in the wild. Phytohormone free MS media (control) failed to induce the flower buds and shoots on this medium remains stunted. The flower bud induction results are summarized in Fig. 2. In the present case, 5.6 flower buds/shoot were observed in MS medium supplemented with BAP (4.0 mg/l) and sucrose (5 %) with 90 % response (Figs. 1d and 2). Similarly, few other Ceropegia species showed the in vitro flowering ability in BAP and sucrose supplemented MS medium (Nair et al. 2007; Chavan et al. 2011a, b).
Fig. 2.
Effect of different concentrations of sucrose (%) with BAP (4.0 mg/l) on in vitro flowering of C. noorjahaniae
To optimize the rooting response of plantlets raised in vitro, different auxins (IAA, IBA, and NAA) were tested at various concentrations and combinations (Table 2). The rooting was significantly affected by the concentrations of IBA. Half-strength MS medium fortified with IBA (1.0 mg/l) was found most effective for induction of healthy roots. In this medium maximum root number (8.1 ± 0.7), maximum root length (4.0 ± 0.2) and highest rooting response (100 %) was also achieved (Table 2, Fig. 1c). The success of IBA in promoting efficient root induction has been reported for Hemidesmus indicus (Sreekumar et al. 2000), Holostemma ada-kodien (Martin 2002), Ceropegia candelabrum (Beena et al. 2003), and Ceropegia attenuata (Chavan et al. 2011a). The roots on this medium were found to be thicker which has an added advantage during planting. NAA and IAA were poor for induction of roots in C. noorjahaniae. However, they showed significant root induction when combined with IBA.
Table 2.
Effect of different phytohormones on root induction of C. noorjahaniae on half strength MS medium
| Sr. No. | Phytohormones | Concentration (mg/l) | Response (%) | Number of roots/shoot ± SE | Root length (cm) ± SE |
|---|---|---|---|---|---|
| 1 | Hormone free | 00 | 0.0 | 0.0 | |
| 2 | IBA | 0.5 | 80 | 3.2 ± 0.5** | 2.2 ± 0.3** |
| 3 | 1.0 | 100 | 8.1 ± 0.7** | 4.0 ± 0.2** | |
| 4 | 1.5 | 90 | 5.2 ± 0.6** | 3.0 ± 0.3** | |
| 5 | 2.0 | 80 | 4.1 ± 0.6** | 2.6 ± 0.3** | |
| 6 | 2.5 | 75 | 1.8 ± 0.3ns | 2.6 ± 0.4** | |
| 7 | NAA | 0.5 | 55 | 0.7 ± 0.2ns | 1.6 ± 0.4* |
| 8 | 1.0 | 60 | 1.3 ± 0.3* | 2.2 ± 0.4** | |
| 9 | 1.5 | 70 | 2.7 ± 0.6** | 2.6 ± 0.6** | |
| 10 | 2.0 | 65 | 1.8 ± 0.4** | 2.1 ± 0.4** | |
| 11 | 2.5 | 65 | 1.1 ± 0.2ns | 1.9 ± 0.4** | |
| 12 | IAA | 0.5 | 70 | 1.7 ± 0.5** | 1.8 ± 0.4** |
| 13 | 1.0 | 70 | 2.3 ± 0.4** | 2.1 ± 0.4** | |
| 14 | 1.5 | 75 | 2.0 ± 0.4** | 1.9 ± 0.3** | |
| 15 | 2.0 | 65 | 1.1 ± 0.2ns | 1.7 ± 0.4** | |
| 16 | 2.5 | 60 | 0.9 ± 0.2ns | 1.1 ± 0.3ns | |
| 17 | IBA + NAA | 1.0 + 0.5 | 80 | 6.1 ± 0.9** | 3.2 ± 0.4** |
| 18 | 1.0 + 1.0 | 75 | 4.3 ± 0.6** | 2.7 ± 0.4** | |
| 19 | 1.0 + 1.5 | 80 | 4.0 ± 0.6** | 2.4 ± 0.3** | |
| 20 | 1.0 + 2.0 | 80 | 2.6 ± 0.4** | 2.2 ± 0.3** | |
| 21 | IBA + IAA | 1.0 + 0.5 | 75 | 1.1 ± 0.2* | 2.1 ± 0.5** |
| 22 | 1.0 + 1.0 | 90 | 2.3 ± 0.3** | 2.6 ± 0.3** | |
| 23 | 1.0 + 1.5 | 85 | 3.7 ± 0.5** | 2.6 ± 0.3** | |
| 24 | 1.0 + 2.0 | 80 | 2.2 ± 0.3** | 2.3 ± 0.4** |
Mean ± S.E. of 20 replicates per treatment. The values are significantly different at ns-non significant, *P < 0.05 and **P < 0.01 level when compared by Dunnett multiple comparisons test
The period of transition during the process of hardening after transfer from the in vitro to ex vitro conditions is considered to be the most important step in micropropagation technique. Hardening of in vitro regenerated plantlets of C. noorjahaniae was carried out by following the method of Chavan et al. (2011a). Shoots with well roots were transferred to small plastic pots containing sterile sand and coco peat (1:1). In this planting substrate, 85 % survival rate of the plantlets was recorded after field transfer. Plants were transferred subsequently to field conditions where they grew well and exhibited morphological characters similar to parent plants (Fig. 1e–f).
In conclusion, the successful micropropagation system described here provides an effective means for the rapid propagation and conservation of an endemic, critically endangered and potential ornamental plant, C. noorjahaniae. The increased multiplication rate and cost effectiveness make this protocol highly advantageous. Furthermore, this developed protocol can be applied for mass multiplication and conservation of other Ceropegia species.
Acknowledgments
Senior research fellowship (SRF) to JJC and financial support for this work from Department of Biotechnology (DBT), Govt. of India, New Delhi is gratefully acknowledged. We also thank Dr V. A. Bapat (Emeritus Scientist), Department of Biotechnology, Shivaji University, Kolhapur for critical comments during manuscript preparation.
References
- Beena MR, Martin KP, Kirti PB, Hariharan M. Rapid in vitro propagation of medicinally important Ceropegia candelabrum. Plant Cell Tissue Organ Cult. 2003;72:285–289. doi: 10.1023/A:1022395809204. [DOI] [Google Scholar]
- Bisht S, Bisht NS, Bhandari S. In vitro micropropagation in Polygonatum verticillatum (L.) All. an important threatened medicinal herb of Northern India. Physiol Mol Biol Plants. 2012;18:89–93. doi: 10.1007/s12298-011-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan JJ, Nimbalkar MS, Adsul AA, Kamble SS, Gaikwad NB, Dixit GB, Gurav RV, Bapat VA, Yadav SR. Micropropagation and in vitro flowering of endemic and endangered plant Ceropegia attenuata Hook. J Plant Biochem Biotechnol. 2011;20:276–282. doi: 10.1007/s13562-011-0059-0. [DOI] [Google Scholar]
- Chavan JJ, Nimbalkar MS, Gaikwad NB, Dixit GB, Yadav SR. In vitro propagation of Ceropegia spiralis Wight-an endemic and rare potential ornamental plant of peninsular India. Proc Natl Acad Sci India Sect B. 2011;81:120–126. [Google Scholar]
- Chavan JJ, Gaikwad NB, Yadav SR. High multiplication frequency and genetic stability analysis of Ceropegia panchganiensis, a threatened ornamental plant of Western Ghats: Conservation implications. Sci Hort. 2013;161:134–142. doi: 10.1016/j.scienta.2013.06.042. [DOI] [Google Scholar]
- Chavan JJ, Gaikwad NB, Umdale SD, Kshirsagar PR, Bhat KV, Yadav SR. Efficiency of direct and indirect shoot organogenesis, molecular profiling, secondary metabolite production and antioxidant activity of micropropagated Ceropegia santapaui. Plant Growth Regul. 2014;72:1–15. doi: 10.1007/s10725-013-9830-7. [DOI] [Google Scholar]
- Dhir R, Shekhawat GS. Production, storability and morphogenic response of alginate encapsulated axillary meristems and genetic fidelity evaluation of in vitro regenerated Ceropegia bulbosa: A pharmaceutically important threatened plant species. Ind Crop Prod. 2013;47:139–144. doi: 10.1016/j.indcrop.2013.02.005. [DOI] [Google Scholar]
- Dhir R, Shekhawat GS. Ecorehabilitation and biochemical studies of Ceropegia bulbosa Roxb.: a threatened medicinal succulent. Acta Physiol Plant. 2014 [Google Scholar]
- Faisal M, Ahmad N, Anis M. An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep. 2007;1:155–161. doi: 10.1007/s11816-007-0025-4. [DOI] [Google Scholar]
- Hodgkiss RJ (2004) http://www.succulent-plant.com/ceropg.html
- Kirtikar KR, Basu BD (1935) Indian medicinal plants 3, (M/s Bishen Singh Mahendrapal Singh, New Delhi), India (1935), p 1638
- Komalavalli N, Rao MV. In vitro micropropagation of Gymnema sylvestre – a multipurpose medicinal plant. Plant Cell Tissue Organ Cult. 2000;61:97–105. doi: 10.1023/A:1006421228598. [DOI] [Google Scholar]
- Martin KP. Rapid propagation of Holostemmaada-kodien Schult -A rare medicinal plant through axillary bud multiplication and indirect organogenesis. Plant Cell Rep. 2002;21:112–117. doi: 10.1007/s00299-002-0483-7. [DOI] [Google Scholar]
- Mishra DK, Singh NP. Endemic and threatened flowering plants of Maharashtra. Calcutta: Botanical Survey of India; 2001. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays for tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nair AK, Naik DD, Pandit SS. High frequency in vitro flowering in six species of Ceropegia. J Plant Biol. 2007;50:374–377. doi: 10.1007/BF03030670. [DOI] [Google Scholar]
- Nayar MP, Sastry ARK. Red data book of Indian plants, Vol. 1 & 2. Kolkata: Botanical Survey of India; 1987. [Google Scholar]
- Patil VM. Micropropagation studies in Ceropegia spp. In Vitro Cell Dev Biol Plant. 1998;34:240–243. doi: 10.1007/BF02822714. [DOI] [Google Scholar]
- Phulwaria M, Shekhawat NS, Rathore JS, Singh RP. An efficient in vitro regeneration and ex vitro rooting of Ceropegia bulbosa Roxb - A threatened and pharmaceutical important plant of Indian Thar Desert. Ind Crop Prod. 2013;42:25–29. doi: 10.1016/j.indcrop.2012.05.013. [DOI] [Google Scholar]
- Reynolds S (2006) http://www.sagereynolds.com/cero/clist.com
- Sreekumar S, Seeni S, Pushpangadan P. Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy 4-methoxy benzaldehyde. Plant Cell Tissue Organ Cult. 2000;62:211–218. doi: 10.1023/A:1006486817203. [DOI] [Google Scholar]
- Sukumar E, Gopal RH, Rao RB, Viswanathan S, Thirugnanasambantham P, Vijayasekaran V. Pharmacological actions of cerpegin, a novel pyridine alkaloid from Ceropegia juncea. Fitoterapia. 1995;66:403–406. [Google Scholar]
- Yadav SR, Kamble MY (2006) Threatened Ceropegias of the Western Ghats and strategies for their conservation, http://www2.wii.gov.in/envis/threatened-plants/Chapter-18.htm