Abstract
The investigation was carried out to study the effect of halopriming on NaCl and polyethylene glycol-6000 (PEG-6000) induced stress tolerance potential of three Vigna radiata (L.) Wilczek varieties, with varied abiotic stress tolerance potential. Halopriming is a seed priming technique in which the seeds were soaked in various salt solutions (in this study NaCl was used). The results of the study indicated that the application of stresses (both NaCl and PEG) induced retardation of growth attributes (measured in terms of shoot length, fresh weight, dry weight) and decrease in physiological attributes like total chlorophyll content, metabolites, photosynthetic and mitochondrial activity of the seedlings in all three V. radiata (L.) varieties. However, halopriming of the seeds could reduce the extent of decrease in these biological attributes. NaCl and PEG stress also caused increase in MDA content (a product of membrane lipid peroxidation) in all the varieties studied and this increase was significantly minimized under halopriming. From the present investigation it was evident that among the green gram varieties studied, Pusa Vishal, a NaCl tolerant variety showed enhanced tolerance to NaCl and PEG induced stress, when the seeds were subjected to halopriming followed by Pusa Ratna (stress sensitive variety). Pusa 9531 (drought tolerant variety) also showed positive halopriming effects but it was less significant when compared to other two varieties. It could be concluded that halopriming improved the drought and salinity stress tolerance potential of all varieties and it was significantly higher in the Pusa Vishal as compared to Pusa 9531 and Pusa Ratna.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-014-0234-6) contains supplementary material, which is available to authorized users.
Keywords: Abiotic stress, Drought, Salinity, Seed priming, Mitochondrial activity, Photochemical activity
Introduction
Plants are exposed to various adverse environmental conditions such as drought, salinity, extreme temperature, etc. These abiotic stresses adversely affect the plant growth and productivity. Among the various abiotic stresses, osmotic stress (drought and salinity) causes a variety of biochemical, physiological and metabolic changes in plants, ultimately reducing the yield (Xiong and Zhu 2002).
Green gram (Vigna radiata (L.) Wilczek) is a tropical legume which is an important dietary pulse crop of India enriched with lysine and protein. Green gram being a short duration crop is commonly cultivated as a rotation crop/intercrop in India. Various abiotic stresses, especially drought and salinity are major limitations to its production. Salt stress alone was found to cause reduction in germination percentage, shoot and root lengths, fresh weight and seedling vigour in green gram (Misra et al. 1996; Promila and Kumar 2000; Misra and Dwivedi 2004).
Seed priming has been developed as an indispensable method to produce tolerant plants against various stresses. It is a pre-germination treatment method which improves seed performance and provides faster and synchronized seed germination. In seed priming, the seed is either soaked in water (hydropriming), solutions of PEG (osmopriming), salt (CaCl2, CaSO4, NaCl etc.) or some definite chemicals prior to germination (Patade et al. 2009). Priming is believed to bring about some biochemical changes within the seed, which ultimately favours germination and further growth stages. According to Gurusinge et al. (1999), priming enhances the early events of germination. Even though some metabolic changes take place within the seed during priming, are not enough to induce radical protrusion (McDonald 2000). Due to the effect of some of these biochemical changes likely to take place prior to seed germination, plants raised from primed seeds showed various advantages over the non primed ones, such as sturdy and quick cellular defense response against abiotic stresses. The seedlings emerging from primed seeds also showed early and uniform germination. Moreover, the overall growth of plants was enhanced due to the effect of various seed priming treatments. The priming effects depend on the nature of priming agent, priming duration, concentration of priming agent and the plant (Jeong et al. 2000). Priming for enhanced resistance to abiotic stress is operating via various pathways involved in different metabolic processes (Jisha et al. 2013).
In halopriming, the seeds are immersed in different salt solutions (in this study NaCl was used) which facilitate the process of seed germination and subsequent seedling emergence even under adverse environmental conditions. In halopriming with NaCl, the seeds are treated in NaCl concentrations of tolerable limits. Halopriming is a simple and cheap agrotechnique and therefore found suitable to be recommended to the farmers owing to better synchrony of emergence and crop stand under various conditions of environment (Sedghi et al. 2010). Halopriming with NaCl was previously reported to bring about enhancement in germination and seedling establishment in milk thistle (Sedghi et al. 2010), enhancement in the salt tolerance of melon plants grown under salinity (Sivritepe et al. 2003, 2005). In sugarcane, halopriming with NaCl was found to be an effective pre germination practice for overcoming salinity and drought induced negative effects (Patade et al. 2009). Farhoudi and Sharifzadeh (2006) while working with canola reported salt priming-induced improvement in seed germination, seedling emergence and further growth under saline conditions. In green gram, pretreatment of the seeds with sub lethal dose of NaCl was found to ameliorate the injurious effects of NaCl stress (Saha et al. 2010).
The primary metabolism and associated biochemical changes occurring in seedlings emerging from primed and non primed seeds subjected to NaCl and polyethylene glycol-6000 (PEG-6000) stressed conditions was analyzed to study the effect of halopriming on osmotic stress tolerance potential of green gram varieties with varied tolerance potential to NaCl and drought.
Materials and methods
Materials
Green gram belongs to the family papilionaceae and is an important protein rich pulse crop. The seeds of V. radiata varieties were obtained from seed science and technology division, IARI, New Delhi. Pusa Ratna is generally considered as a sensitive variety to all kinds of abiotic stresses, especially to NaCl (Sehrawat et al. 2014). Therefore, in the present study Pusa Ratna is taken as a common sensitive variety to both NaCl and PEG stress. Pusa Vishal is a NaCl tolerant (Nazar et al. 2011) and Pusa 9531 is a drought tolerant (Dutta and Bera 2008) variety.
Methods
Halopriming technique
The seeds which were grown and harvested at uniform growth conditions were used for the present study. The seeds were stored in seed desiccators under vacuum condition for 3–6 months and within this period studies related to halopriming of seeds were carried out. To determine the effective NaCl concentration, which could bring about halopriming effect, the uniform sized seeds of three varieties V. radiata were soaked in various concentrations of NaCl (0, 20, 25, 30, 35, 40, 45, 50, 55, 60 mM) for 6 h in a screw cap bottle. The soaked seeds were further placed on a piece of clean filter paper, allowing dehydration under shade at 25 °C till the seeds retrieved the original moisture level as that of pre-priming stage. Seed weights were tested repeatedly at fixed intervals to ensure the seeds have attained the original dry weight. The untreated seeds were used as the control. After ~24 h dehydration, the seeds were germinated in light transparent plastic bottles (19 × 11 cm) containing absorbent cotton soaked with distilled water. The bottles were kept under a continuous light (120 μmol m−2 s−1) at 25 ± 2 °C. The growth and biochemical attributes of seedlings germinated from primed seeds were recorded on 7 d after germination.
To select stress imparting concentrations of NaCl and PEG-6000, the seeds of three V. radiata varieties were germinated in various concentrations of NaCl (0, 25, 50, 75, 100, 125 mM) and PEG (0, 5, 10, 15, 20, 25 %). The growth and biochemical attributes of seedlings germinated from primed and non-primed seeds were recorded on 7 d after germination. All the seed materials used for investigation were pre-washed for one min with 0.25 % Triton X-100 (Boehringer Mannheim Gmbh) to remove the dirt.
Morphological, physiological and biochemical studies
Shoot length was measured with the help of a meter scale in cm. For fresh weight measurements the seedlings were blotted and wrapped separately in pre-weighed labeled aluminium foils and was immediately weighed. For dry weight measurements, the same samples weighed for fresh weight were kept in an oven maintained at 80 °C. After 48 h the samples were transferred to a desiccator, allowed to cool and then weighed. The samples were reweighed as described above at regular intervals, until the weights became constant.
Total carbohydrate was estimated according to Dubois et al. (1956). Protein content of the plant material was estimated using Folin-ciocalteau reagent according to the method of Lowry et al. (1951). Proline content in the seedlings was estimated as per Bates et al. (1973). The MDA content estimation was done according to Heath and Packer (1968). The peroxidase (PER) assay was followed after Abeles and Biles (1991) with some minor modifications. Chlorophyll estimation was carried out by the method of Arnon (1949).
Thylakoids from leaves were isolated according to Puthur (2000) and the photochemical activities of the isolated thylakoids were assayed polarographically with a Clark-type oxygen electrode (DW1/AD, Hansatech, Norflok, UK) which was connected to a digital control box (OXYG1, Hansatech) at 4 °C as per the protocol of Puthur (2000). The light dependent O2 uptake/evolution was measured by irradiating the sample with saturating intensity of white light (1,800 μmol photons m−2 s−1), provided by a 100 W halogen lamp (LS2, Hansatech). The activity of Photosystem I (PS I) and Photosystem II (PS II) was expressed in terms of μmol of O2 consumed/evolved min−1 mg−1 chlorophyll.
Mitochondrial isolation from the seedlings was carried out according to Kolloffel (1967). Oxygen consumption by mitochondria was measured polarographically with the same Clark-type oxygen electrode at 25 °C as per the protocol of Schmitt and Dizengremel (1989). The mitochondrial activity was calculated in terms of μmol O2 consumed min−1 mg−1 protein.
Statistical analysis
Two way analysis of variance (ANOVA) was conducted taking varieties as one factor and treatments as the second factor and the treatment means were compared with LSD (least significant difference) wherever necessary in the data of Tables 1 and 2. One way ANOVA was conducted to find the variation in percentage increase in treatments of each variety (Figures) by using SPSS 17.0.
Table 1.
Shoot length, fresh weight and dry weight of three varieties of V. radiata seedlings under halopriming exposed to 0, NaCl and PEG-6000 stress. The data is an average of recordings from three independent experiments each with three replicates (i.e. n = 9). The data represent mean ± standard error
| Varieties | Shoot length /plant (cm) | Fresh weight/plant(g) | Dry weight/plant(g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 stress | NaCl stress | PEG stress | 0 stress | NaCl stress | PEG stress | 0 stress | NaCl stress | PEG stress | |
| Pusa Ratna | 18.47 ± 0.51 (17.83 ± 0.43) | 11.07 ± 0.60 (08.93 ± 0.31) | 10.73 ± 0.66 (07.30 ± 0.32) | 0.2911 ± 0.01 (0.2152 ± 0.01) | 0.1907 ± 0.01 (0.1297 ± 0.01) | 0.1588 ± 0.01 (0.1240 ± 0.01) | 0.0291 ± 0.01 (0.0261 ± 0.01) | 0.0192 ± 0.01 (0.0126 ± 0.01) | 0.0175 ± 0.01 (0.0155 ± 0.01) |
| Pusa 9531 | 17.00 ± 0.75 (13.53 ± 0.64) | 09.73 ± 0.43 (09.63 ± 0.32) | 03.93 ± 0.21 (03.87 ± 0.18) | 0.2180 ± 0.01 (0.1988 ± 0.01) | 0.2010 ± 0.01 (0.1660 ± 0.01) | 0.0750 ± 0.01 (0.0660 ± 0.01) | 0.0130 ± 0.01 (0.0100 ± 0.01) | 0.0170 ± 0.01 (0.0160 ± 0.01) | 0.0246 ± 0.01 (0.0200 ± 0.01) |
| Pusa Vishal | 18.67 ± 0.75 (17.57 ± 0.78) | 06.30 ± 0.51 (04.63 ± 0.38) | 06.97 ± 0.65 (05.93 ± 0.42) | 0.2921 ± 0.01 (0.2919 ± 0.01) | 0.1312 ± 0.01 (0.1107 ± 0.01) | 0.1585 ± 0.01 (0.1298 ± 0.01) | 0.0194 ± 0.01 (0.0179 ± 0.01) | 0.0154 ± 0.01 (0.0110 ± 0.01) | 0.0181 ± 0.01 (0.0139 ± 0.01) |
*The values in the parenthesis denote the value of respective parameters in the three varieties of V. radiata seedlings raised from non-primed seeds exposed to 0, NaCl and PEG-6000 stress. All the values were significantly different as estimated by two way ANOVA test (p < 0.01).
Table 2.
Photosynthetic pigment content of leaves of three varieties of V. radiata seedlings under halopriming exposed to 0, NaCl and PEG-6000 stress. The data is an average of recordings from three independent experiments each with three replicates (i.e. n = 9). The data represent mean ± standard error
| Varieties | Chlorophyll a (mg/g dw) | Chlorophyll b (mg/g dw) | Total chlorophyll (mg/g dw) | Carotenoids (mg/g dw) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 stress | NaCl stress | PEG stress | 0 stress | NaCl stress | PEG stress | 0 stress | NaCl stress | PEG stress | 0 stress | NaCl stress | PEG stress | |
| Pusa Ratna | 18.78 ± 0.86 (16.51 ± 0.95) | 12.00 ± 0.76 (12.00 ± 0.83) | 09.00 ± 0.36 (09.00 ± 0.45) | 05.99 ± 0.21 (05.15 ± 0.35) | 02.92 ± 0.11 (03.03 ± 0.21) | 02.81 ± 0.08 (02.24 ± 0.01) | 24.77 ± 1.66 (21.69 ± 1.34) | 14.51 ± 0.55 (15.01 ± 0.63) | 11.12 ± 0.55 (12.44 ± 0.31) | 08.69 ± 0.42 (06.79 ± 0.31) | 05.14 ± 0.32 (05.30 ± 0.21) | 03.72 ± 0.11 (04.07 ± 0.13) |
| Pusa 9531 | 18.51 ± 0.56 (15.98 ± 0.13) | 15.17 ± 0.85 (12.72 ± 0.65) | 05.23 ± 0.13 (03.23 ± 0.12) | 06.29 ± 0.42 (05.09 ± 0.32) | 04.50 ± 0.32 (04.02 ± 0.34) | 01.41 ± 0.01 (00.94 ± 0.01) | 24.74 ± 1.57 (21.03 ± 1.06) | 19.64 ± 0.75 (16.65 ± 0.65) | 08.33 ± 0.44 (04.17 ± 0.12) | 07.61 ± 0.31 (06.41 ± 0.22) | 06.02 ± 0.22 (05.25 ± 0.08) | 02.63 ± 0.12 (01.48 ± 0.06) |
| Pusa Vishal | 24.20 ± 1.06 (20.82 ± 0.95) | 09.96 ± 0.44 06.36 ± 0.83) | 11.55 ± 0.84 09.16 ± 0.54) | 07.76 ± 0.31 (06.31 ± 0.21) | 02.64 ± 0.09 (01.62 ± 0.01) | 03.64 ± 0.09 02.46 ± 0.02) | 31.93 ± 1.24 (27.03 ± 1.04) | 12.96 ± 0.14 (07.96 ± 0.23) | 18.72 ± 0.45 (11.59 ± 0.54) | 09.97 ± 0.42 (07.78 ± 0.31) | 04.46 ± 0.11 (03.36 ± 0.09) | 04.65 ± 0.07 (04.14 ± 0.08) |
*The values in the parenthesis denote the value of photosynthetic pigments in leaves of the three varieties of V. radiata seedlings raised from non-primed seeds exposed to 0, NaCl and PEG-6000 stress. All the values were significantly different as estimated by two way ANOVA test (p < 0.01).
Results
Selection of optimal concentration of NaCl for halopriming
After halopriming of three varieties of V. radiata, the seeds were germinated as described in materials and methods. The growth attributes (shoot length, fresh weight and dry weight) revealed that the most effective halopriming concentrations was 35 mM for Pusa Ratna, 50 mM for Pusa 9531 and Pusa Vishal respectively (supplementary table).
Selection of stress imparting concentration of NaCl and PEG
The concentration of NaCl and PEG which imparted 40–50 % retardation in various growth attributes (shoot length, fresh weight and dry weight) was selected as stress imparting concentrations of NaCl and PEG. It was 75 mM NaCl and 15 % PEG for Pusa Ratna; 75 mM NaCl and 20 % PEG for Pusa 9531; and 100 mM NaCl and 15 % PEG for Pusa Vishal (Supplementary Figs. i and ii).
Changes in growth parameters under halopriming
In general, the application of stress (both NaCl and PEG) retarded growth of seedlings raised from primed as well as non primed seeds. However, halopriming of the seeds could decrease extent of reduction in shoot length, fresh and dry weight of the seedlings as compared to the seedlings raised from non primed seeds. The results given below with respect to various parameters recorded in seedlings raised from primed seeds were obtained in comparison to the values recorded for each parameter of the seedlings raised from non primed seeds.
The NaCl primed Pusa Ratna recorded 24 % (NaCl stress) and 47 % increase (PEG stress) in shoot length when compared to respective non primed controls. Whereas NaCl primed Pusa 9531 and Pusa Vishal did not show significant increase in shoot length under stressed and non-stressed conditions (Table 1). Halopriming caused an increase of 35 % (0 stress), 47 % (NaCl stress) and 28 % (PEG stress) in fresh weight of the variety Pusa Ratna but the increase in fresh weight under halopriming was less significant in the other two varieties of V. radiata (Table 1). The NaCl primed Pusa Ratna recorded significant increase in dry weight only under NaCl stress (52 %) whereas in Pusa 9531 the dry weight increase on halopriming was observed under 0 stress (30 %). In Pusa Vishal, halopriming resulted in 40 % (NaCl stress) and 30 % (PEG stress) increase in dry weight (Table 1).
Biochemical and physiological changes under halopriming
Primary metabolites
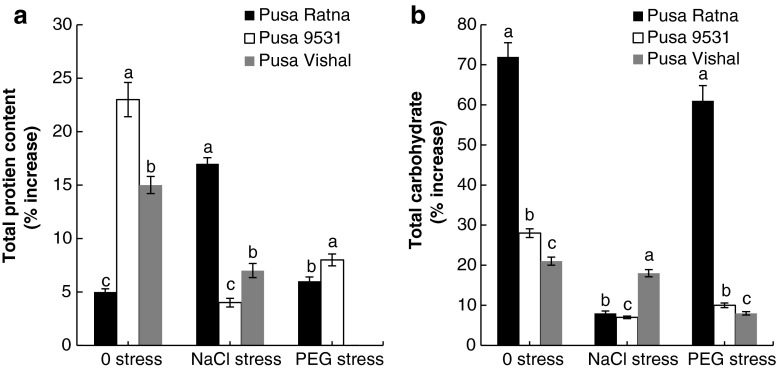
The percentage increase of total protein in the seedlings of all three varieties of V. radiata which were haloprimed and grown in non-stressed and stressed (NaCl and PEG) condition was to the extent of 3 to 23 % (Fig. 1a). In general, the accumulation of total carbohydrates in the seedlings of all the three varieties of V. radiata was at an increased level as compared to the accumulation of total proteins except in the haloprimed Pusa Ratna under NaCl stress. Haloprimed Pusa Ratna recorded 72 % (0 stress), 8 % (NaCl stress) and 61 % (PEG stress) increase in total carbohydrate. In Pusa 9531 and Pusa Vishal, the increase in total carbohydrate was less significant when compared to Pusa Ratna (Fig. 1b).
Fig. 1.
Percentage of increase in protein (a) and total carbohydrates (b) of seedlings raised from haloprimed seeds of three different varieties of V. radiata as compared to that of seedlings raised from non primed seeds. Total protein content in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl and PEG stress is 105, 231 and 186 mg/g dw, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 239, 241, 227 mg/g dw, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 258, 146 and 149 mg/g dw, respectively. Total carbohydrate content in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl and PEG stress is 145, 173, 187 mg/g dw, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 126, 124, 103 mg/g dw, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 161, 202, 204 mg/g dw, respectively. The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates. Values super scribed with same alphabet is homogenous for each stress at 1 % level significance (p < 0.01; ANOVA)
Proline and MDA
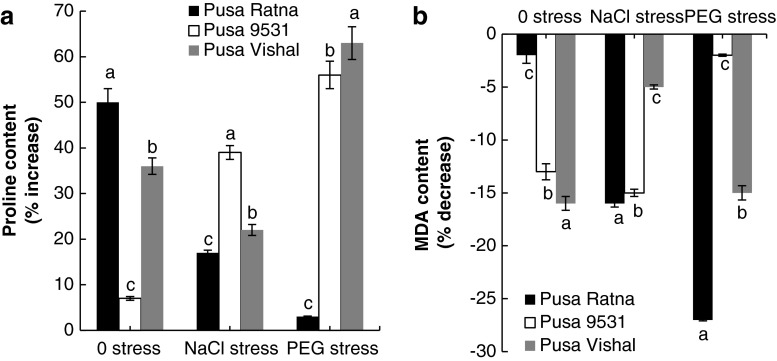
Among the three varieties of V. radiata, haloprimed Pusa Ratna recorded 50 % increase in proline content under unstressed condition while there was no significant increase in proline content under NaCl stress and PEG stress. At the same time, in Pusa 9531, halopriming resulted in proline accumulation under NaCl (39 %) and PEG (56 %) stress, but no significant increase of proline was recorded under unstressed condition. In Pusa Vishal, 36 % (0 stress), 22 % (NaCl stress) and 63 % (PEG stress) increase in proline content occurred as a result of halopriming (Fig. 2a). The MDA content was found to decrease (2–27 %) in the seedlings raised from haloprimed seeds as compared to seedlings from non primed seeds, either in the absence or presence of stress (NaCl and PEG) (Fig. 2b).
Fig. 2.
Percentage of change in proline (a) and MDA (b) of seedlings raised from haloprimed seeds of three different varieties of V. radiata as compared to that of seedlings raised from non primed seeds. Proline content in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl, PEG stress is 634, 641, 778 μg/g dw, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 793, 1,384, 3,149 μg/g dw, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 1,237, 1,912, 1,896 μg/g dw, respectively. MDA content in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl and PEG stress is 117, 127, 133 μmol/g dw, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 61, 67, 66 μmol/g dw, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 92, 104, 103 μmol/g dw, respectively. The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates. Values super scribed with same alphabet is homogenous for each stress at 1 % level significance (p < 0.01; ANOVA)
Guaiacol peroxidase (PER) activity
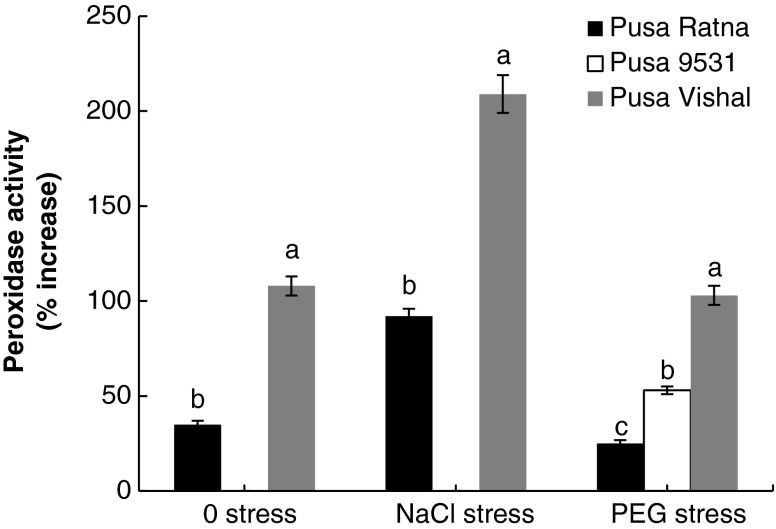
Accumulation of PER occurred especially in the NaCl tolerant variety Pusa Vishal, where 108 % (0 stress), 209 % (NaCl stress) and 103 % (PEG stress) increase was recorded. Pusa Ratna also accumulated PER in response to halopriming at the rate of 35 % (0 stress), 92 % (NaCl stress) and 25 % (PEG stress) respectively. The PER increase on halopriming was less significant in the drought tolerant variety Pusa 9531, where the increase occurred only under PEG stress (Fig. 3).
Fig. 3.
Percentage of increase in peroxidase activity of seedlings raised from haloprimed seeds of three different varieties of V. radiata as compared to that of seedlings raised from non primed seeds. Peroxidase activity in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl, PEG stress is 178, 433, 331 μmol guaiacol oxidized/min/g dw, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 473, 536, 963 μmol guaiacol oxidized/min/g dw, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 314, 353, 980 μmol guaiacol oxidized/min/g dw, respectively. The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates. Values super scribed with same alphabet is homogenous for each stress at 1 % level significance (p < 0.01; ANOVA)
Chlorophyll content and activity of photosystems
Halopriming enhanced the photosynthetic pigment content of Pusa Vishal as compared to Pusa 9531 and Pusa Ratna. Maximum increase in chlorophyll a was observed in the haloprimed Pusa Vishal and Pusa 9531 subjected to NaCl stress (57 %) and PEG stress (62 %) respectively. Chlorophyll b was found to be maximum in haloprimed Pusa Vishal (63 %) under NaCl stress and 48 % under PEG stress. Total Chlorophyll was also found to be maximum in haloprimed Pusa Vishal (63 %) under NaCl stress and 62 % under PEG stress. On halopriming, the carotenoids increased up to 78 % in Pusa 9531 under PEG stress and 33 % in Pusa Vishal under NaCl stress (Table 2).
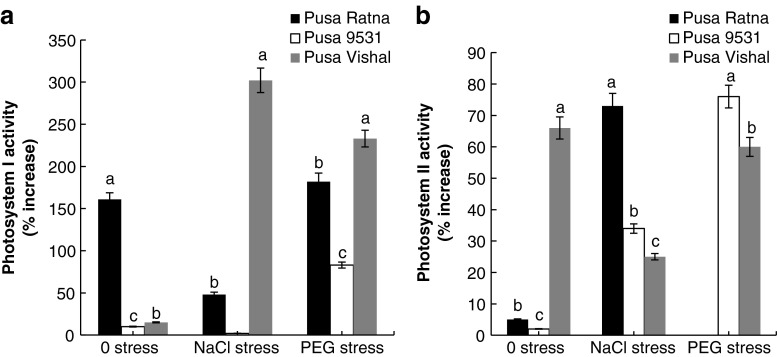
The activity of the PS I increased significantly in the leaves of Pusa Vishal seedlings raised from NaCl primed seeds under NaCl (302 %) and PEG (233 %) stress. While there was no significant increase in PS I activity under unstressed condition. But the increase in PS I activity was much lower and insignificant in Pusa 9531, with the exception of 95 % increase in seedlings exposed to PEG stress. In Pusa Ratna the increase in PS I activity was 161 % (0 stress), 48 % (NaCl stress) and 182 % (PEG stress) in seedlings raised from haloprimed seeds (Fig. 4a). In general, halopriming increased the PS II activity of all three varieties of V. radiata seedlings raised under non-stressed and stressed (NaCl and PEG) conditions as compared to their non primed controls. The seedlings raised from NaCl primed seeds of Pusa Vishal showed maximum increase in PS II activity, it was 67 % (0 stress), 25 % (NaCl stress) and 60 % (PEG stress) respectively. Whereas, in Pusa Ratna, the increase in PS II activity on halopriming was significant only under NaCl stress (73 %). Halopriming of Pusa 9531 brought about increase in PS II activity, the increase was 34 % (NaCl stress) and 76 % (PEG stress) (Fig. 4b).
Fig. 4.
Percentage of increase in PS I (a) and PS II (b) activity of seedlings raised from haloprimed seeds of three different varieties of V. radiata as compared to that of seedlings raised from non primed seeds. PS I activity in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl, PEG stress is 116, 155, 101 μmol of O2 consumed/mg chl/min, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 168, 165, 113 μmol of O2 consumed/mg chl/min, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 127, 103, 105 μmol of O2 consumed/mg chl/min, respectively. PS II activity in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl, PEG stress is 41, 14, 24 μmol of O2 evolved/mg chl/min, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 30, 28, 24 μmol of O2 evolved/mg chl/min, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 38, 26, 22 μmol of O2 evolved/mg chl/min, respectively. The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates. Values super scribed with same alphabet is homogenous for each stress at 1 % level significance (p < 0.01; ANOVA)
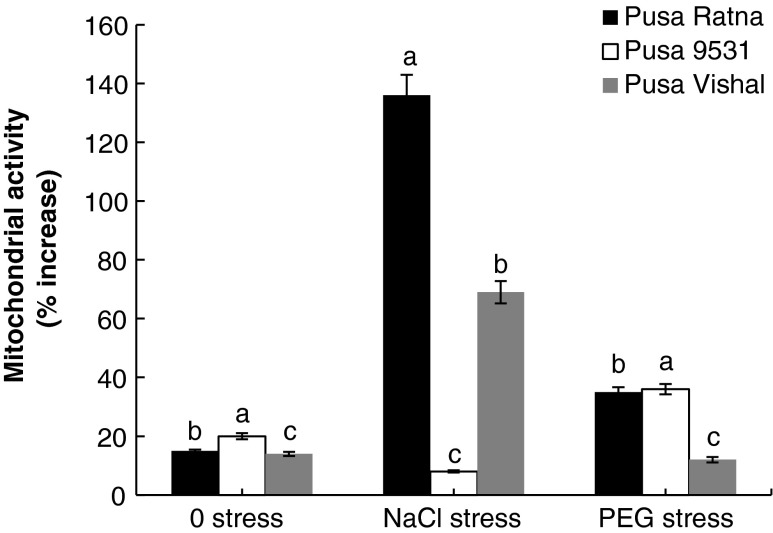
Mitochondrial activity
Pusa Ratna on halopriming resulted in 15 % (0 stress), 136 % (NaCl stress) and 35 % (PEG stress) increase in mitochondrial activity when compared to respective non primed controls. In Pusa 9531, halopriming resulted in 20 % (0 stress), 8 % (NaCl stress) and 36 % (PEG stress) increase in mitochondrial activity. In Pusa Vishal halopriming resulted in 14 % (0 stress), 69 % (NaCl stress) and 12 % (PEG stress) increase in mitochondrial activity (Fig. 5).
Fig. 5.
Percentage of increase in mitochondrial activity of seedlings raised from NaCl primed seeds of three different varieties of V. radiata as compared to that of seedlings raised from non primed seeds. Mitochondrial activity in seedlings raised from non primed seeds of Pusa Ratna in 0, NaCl, PEG stress is 44, 11, 27 μmol of O2 consumed/mg protein/min, respectively; for Pusa 9531 in 0, NaCl, PEG stress is 40, 37, 22 μmol of O2 consumed/mg protein/min, respectively and for Pusa Vishal in 0, NaCl, PEG stress is 40, 17, 30 μmol of O2 evolved/mg protein/min, respectively. The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates. Values super scribed with same alphabet is homogenous for each stress at 1 % level significance (p < 0.01; ANOVA)
Discussion
As the three varieties of V. radiata are known to exhibit different levels of tolerance towards NaCl, it became essential to check the optimal concentration of NaCl for halopriming and it was found that Pusa Ratna, a known abiotic stress sensitive variety was effectively haloprimed (in terms of better shoot length, fresh weight and dry weight) at a lower NaCl concentration (35 mM), whereas Pusa 9531(a known drought tolerant) and Pusa Vishal (a known NaCl tolerant) was effectively haloprimed at 50 mM concentration of NaCl.
As the three varieties selected had varied level of NaCl and PEG tolerance potential, the NaCl and PEG stress tolerance potential of the three V. radiata varieties was tested against a concentration which imparted 40–50 % growth retardation and it varied for each variety (75 mM NaCl and 15 % PEG for Pusa Ratna; 75 mM NaCl and 20 % PEG for Pusa 9531; and 100 mM NaCl and 15 % PEG for Pusa Vishal).
The beneficial effects of halopriming with NaCl were reported in many crops like sugar beet (Bourgne et al. 2000), melon (Sivritepe et al. 2003, 2005), wheat (Afzal et al. 2005), sugarcane (Patade et al. 2009), sunflower (Bajehbaj 2010), milk thistle (Sedghi et al. 2010) etc. The enhancement in the growth attributes like shoot length, fresh weight and dry weight after halopriming as compared to unprimed controls is the direct evidence of increased seedling vigour due to halopriming. Among the three varieties studied, Pusa Ratna showed maximum increase in shoot length and fresh weight under priming whereas the increase in seedling dry weight was highest in Pusa Vishal. This increase may be due to the metabolic changes associated with halopriming which resulted in better germination and better seedling establishment. Moreover, the halopriming enables the seedlings to cope with the stress imposed by NaCl or PEG. There are many reports that halopriming reduces the inhibitory effects of salinity on seed germination and seedling establishment. Halopriming significantly reduced the inhibitory effects of osmotic stress in melon (Sivritepe et al. 2003), canola (Farhoudi and Sharifzadeh 2006), chickpea (Sarwar et al. 2006) and sugar cane (Patade et al. 2009), which further resulted in better seedling establishment. In green gram the reduction in seedling growth under NaCl stress was ameliorated significantly by halopriming (Saha et al. 2010). The enhanced growth of seedlings indicates the stimulative role of priming solution (NaCl) in acclimation process of the seedlings as reported in soyabean (Umezawa et al. 2000) and rice (Djanaguiraman et al. 2006). Khan et al. (2009) reported that, halopriming was much efficient in improving germination and seedling growth of pepper seeds and our results also confirmed it. In earlier studies, it was observed that seedlings from NaCl primed canola seeds maintained greater mean seedling dry weights and cell membrane stability than untreated seedlings (Farhoudi et al. 2007). Halopriming has shown improved germination and growth of many crops under stressed conditions (Cayuela et al. 1996; Sivritepe et al. 2003; Farhoudi et al. 2007; Shahi et al. 2009).
There are reports for the accumulation of stress proteins and transcription factors under priming (Conrath et al. 2006). But in the present study there was no significant increase in total protein content on halopriming in all three varieties of V. radiata studied. It gives an indication that the major representative proteins (with storage and structural function) in the seedlings are not getting enhanced as a result of NaCl primimng. At the same time, It would be premature to cite any specific role for proteins in combating stress (NaCl /PEG) in these varieties, based on the insignificant enhancement of proteins in NaCl primed plants. Pusa Ratna showed highest increase in total carbohydrate under halopriming. The significant enhancement in the carbohydrates in NaCl primed plants on exposure to stress could be a probable mechanism of countering the stress by increasing the osmoticum. There are earlier reports that accumulations of soluble carbohydrates increase the tolerance to drought in plants (Kameli and Losel 1993). Increase in carbohydrate concentration under halopriming was also reported in muskmelon plants (Farhoudi et al. 2011). According to Cayuela et al. (1996), the increased salt tolerance of NaCl-primed seedlings seems to be the result of higher capacity for osmotic adjustment since plants from primed seeds have more Na+ and Cl− in roots and more sugars and organic acids in leaves than plants from non-primed seeds. The increased accumulation of osmolytes in the seedlings raised from primed seeds could facilitate the increased uptake of water resulting in a turgor which ultimately promotes the expansion of cells. It was earlier reported that improved seed performance under halopriming could be attributed partially to osmotic adjustment, metabolic repair processes or due to the buildup of metabolites necessary for germination (Haghpanah et al. 2009).
The elevated level of proline content observed in seedlings raised from seeds subjected to halopriming may be due to its prominent role in osmotic stress tolerance. Increase in the synthesis of proline is known as a common metabolic reaction of plants under stress (Behairy et al. 2012). Proline, in addition to its major role as compatible solute, provides carbon and nitrogen source for post stress recovery growth, stabilizes membranes and protein machinery, scavenges free radicals etc. From the difference in proline accumulation between the three varieties of V. radiata, it could be inferred that Pusa Vishal showed a higher dependence on proline accumulation for stress tolerance (both in NaCl and PEG) than Pusa Ratna and Pusa 9531. MDA content increased in seedlings raised from both NaCl primed and non-primed seeds under NaCl and PEG stress which indicates that peroxidation of membrane lipids occurred under these stresses. However, halopriming reduced the level of MDA in all the three varieties of V. radiata studied. The reduction in MDA content upon halopriming was more or less same in all the three varieties. The reduction in MDA content under halopriming was already reported in maize (Randhir and Shetty 2005), bitter gourd (Yeh et al. 2005) and mung bean (Saha et al. 2010).
A correlation between the antioxidant enzyme activities and osmotic stress tolerance was reported in many plants (Munns 2002; Ashraf and Ali 2008). From the present result it was clear that halopriming caused an increase in PER in seedlings of all the three varieties of V. radiata indicating that priming altered the metabolic pathway for the increased production of guaiacol peroxidase. Ashraf and Ali (2008) reported that enhanced antioxidant enzyme activities of catalase and peroxidase improved salt tolerance of canola seedlings.
The observed reduction in chlorophyll content under NaCl and PEG stress may be due to the degradation of chlorophyll pigments or interference in the synthesis of chlorophyll. Ashraf and Rasul (1988) reported that the reduction in chlorophyll content under osmotic stress is due to the suppression of enzymes required for chlorophyll synthesis. Moreover, salinity may cause destruction of chloroplast and instability of pigment protein complex (El-Samad et al. 2011). From the results it was clear that halopriming improved the photosynthetic efficiency in all the three varieties but it was significant in Pusa Vishal which was evident from the high chlorophyll content, high PS I and PS II activities in the primed seedlings. On halopriming, the rate of chlorophyll degradation was not to the extent as that observed in the case of non-primed seedlings. Therefore, the energy harvested by the chlorophyll would have effectively channelized into the photosystems, resulting in an enhanced PS I and PS II activities. The better photosynthetic capacity of Pusa Vishal is also reflected in the dry matter accumulated.
Under NaCl and PEG stress, the mitochondrial activity got reduced in all the varieties studied. Whereas the extent of reduction was lesser in seedlings emerged from seeds subjected to halopriming and it was lowest in Pusa Ratna followed by Pusa Vishal and then Pusa 9531. The reduction in mitochondrial activity under both NaCl and PEG stress could be due to the closure of stomata and the limited entry of CO2, which in turn leads to reduction in synthesis of photosynthates. The less availability of photosynthates reflects in the rate of mitochondrial activity by reduced respiration. The probable improvement in the mitochondrial intactness by maintaining the membrane integrity and concomitant increase in the number of mitochondria achieved through halopriming of the seed would have sufficed the increased energy demand of the establishing seedlings. The better growth parameters of the seedlings which have emerged from the haloprimed seeds are confirmation to our view. Seedlings of Pusa Ratna raised from haloprimed seeds showed enhanced growth vigour in terms of shoot length and fresh weight and coinciding with this result the mitochondrial activity in the same variety was least affected. Earlier studies suggest that salt stress negatively impacts mitochondria causing impaired electron transport activities and resulting release of reactive oxygen species in the mitochondria which ultimately causes lipid peroxidation (Mittova et al. 2003; Chen et al. 2009). Priming is known to improve the integrity of the outer membrane of mitochondria and even increases the number of mitochondria (Ashraf and Bray 1993; Benamar et al. 2003; Varier et al. 2010).
Conclusion
From the present investigation it can be concluded that among the green gram varieties studied, halopriming improved the PEG and NaCl stress tolerance potential of seedlings and it was significantly evident in the NaCl tolerant variety Pusa Vishal as compared to Pusa 9531(drought tolerant) and Pusa Ratna (abiotic stress sensitive). Therefore, by halopriming the seeds we can evidently make the NaCl tolerant variety more tolerant and the drought tolerant variety and sensitive variety better tolerant towards NaCl and PEG stress. Moreover, halopriming was found to be simple and cheap, and therefore could be recommended to the farmers, so they can get better synchrony of seedling emergence and better crop stand in V. radiata.
Electronic supplementary material
(DOCX 33 kb)
(DOCX 14 kb)
Acknowledgments
We thank Sh. Manjunath Prasad C. T., Scientist, Seed Science and Technology Division, IARI, New Delhi for providing seeds of V. radiata varieties. We are also thankful to Dr. Noufal K., for the proof reading and Dr. Jijeesh C. M., for assisting us in the statistical analysis.
References
- Abeles FB, Biles CL. Characterization of peroxidase in lignifying peach fruit endocarp. Plant Physiol. 1991;95:269–273. doi: 10.1104/pp.95.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal I, Basra SMA, Ahmad N, Farooq M. Optimization of hormonal priming techniques for alleviation of salinity stress in wheat (Triticum aestivum L.). Caderno de Pesquisa Se’r. Biogeosciences. 2005;17:95–109. [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–5 [DOI] [PMC free article] [PubMed]
- Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63:266–273
- Ashraf M, Bray CM (1993) DNA synthesis in osmoprimed leek (Allium porrum L) seeds. Seed Sci Res 3:15–23
- Ashraf M, Rasul E (1988) Salt tolerance of mungbean (Vigna radiata) at two growth stages. Plant Soil 110:63–67
- Bajehbaj AA. The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. Afr J Biotechnol. 2010;9:1764–1770. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Behairy RT, El-Danasoury M, Craker L. Impact of ascorbic acid on seed germination, seedling growth and enzyme activity of salt stressed fenugreek. J Medicinally Act Plants. 2012;1:106–113. [Google Scholar]
- Benamar A, Tallon C, Macherel D. Membrane integrity and oxidative properties of mitochondria isolated from imbibing pea seeds after priming or accelerated ageing. Seed Sci Res. 2003;13:35–45. doi: 10.1079/SSR2002122. [DOI] [Google Scholar]
- Bourgne S, Job C, Job D. Sugarbeet seed priming: solubilization of the basic subunit of 11S globulin in individual seeds. Seed Sci Res. 2000;10:153–161. [Google Scholar]
- Cayuela E, Perez-Alfocea F, Caro M, Bolarin MC. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol Plant. 1996;96:231–236. doi: 10.1111/j.1399-3054.1996.tb00207.x. [DOI] [Google Scholar]
- Chen X, Wang Y, Li J, Jiang A, Cheng Y, Zhang W. Mitochondrial proteome during salt stress-induced programmed cell death in rice. Plant Physiol Biochem. 2009;47:407–415. doi: 10.1016/j.plaphy.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B. Priming: getting ready for battle. Mol Plant Microbe Interact. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- Djanaguiraman M, Sheeba JA, Shanker AK, Devi DD, Bangarusamy U. Rice can acclimate to lethal level of salinity by pretreatment with sublethal level of salinity through osmotic adjustment. Plant Soil. 2006;284:363–373. doi: 10.1007/s11104-006-0043-y. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;26:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Dutta P, Bera AK. Screening of mungbean genotypes for drought tolerance. Legum Res. 2008;31:145–148. [Google Scholar]
- El-Samad HMA, Shaddad MAK, Barakat N. Improvement of plants salt tolerance by exogenous application of amino acids. J Med Plants Res. 2011;5:5692–5699. [Google Scholar]
- Farhoudi R, Sharifzadeh F (2006) The effects of NaCl priming on salt tolerance in canola (Brassica napus L.) seedlings grown under saline conditions. Indian J Crop Sci 1:74–78
- Farhoudi R, Sharifzadeh F, Poustini K, Makkizadeh MT, Kochakpor M (2007) The effects of NaCl priming on salt tolerance in canola (Brassica napus) seedlings grown under saline conditions. Seed Sci Technol 35:754–759
- Farhoudi R, Saeedipour S, Mohammadreza D. The effect of NaCl seed priming on salt tolerance, antioxidant enzyme activity, proline and carbohydrate accumulation of Muskmelon (Cucumis melo L.) under saline condition. Afr J Agric Res. 2011;6:1363–1370. [Google Scholar]
- Gurusinge SH, Cheng Z, Bradford KJ. Cell cycle activity during seed priming is not essential for germination advancement in tomato. J Expt Bot. 1999;50:101–106. doi: 10.1093/jxb/50.330.101. [DOI] [Google Scholar]
- Haghpanah A, Younesi O, Moradi A (2009) The effect of priming on seedling emergence of differentially matured sorghum (Sorghum bicolor L.) seeds. J Appl Sci Res 5:729–732
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I- Kinetics and stochiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Kang SM, Cho JL, Jeong YO. Germination of carrot, lettuce, onion and Welsh onion seeds are affected by priming chemicals at various concentrations. Korean J Hort Sci Tech. 2000;18:93–97. [Google Scholar]
- Jisha KC, Vijayakumari K, Puthur JT. Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant. 2013;35:1381–1396. doi: 10.1007/s11738-012-1186-5. [DOI] [Google Scholar]
- Kameli A, Losel DM. Carbohydrates and water status in wheat plants under water stress. New Phytol. 1993;125:609–614. doi: 10.1111/j.1469-8137.1993.tb03910.x. [DOI] [PubMed] [Google Scholar]
- Khan HA, Ayub CM, Pervez MA, Bilal RM, Shahid MA, Ziaf K (2009) Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ 28:81–87
- Kolloffel C (1967) Respiration rate and mitochondrial activity in the cotyledons of Pisum sativum during germination. Acta Bot Neerl 16:111–122
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McDonald MB. Seed priming. In: Black M, Bewley JD, editors. Seed technology and its biological basis. Sheffield: Sheffield Academic Press; 2000. pp. 287–325. [Google Scholar]
- Misra N, Dwivedi UN. Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci. 2004;166:1135–1142. doi: 10.1016/j.plantsci.2003.11.028. [DOI] [Google Scholar]
- Misra N, Murmu B, Singh P, Misra M. Growth and proline accumulation in mungbean seedlings as affected by sodium chloride. Biol Plant. 1996;38:531–536. doi: 10.1007/BF02890603. [DOI] [Google Scholar]
- Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856 [DOI] [PubMed]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Nazar R, Iqbal N, Sayeed S, Khan NA. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol. 2011;168:807–815. doi: 10.1016/j.jplph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Patade VY, Sujata B, Suprasanna P. Halopriming imparts tolerance to salt and PEG induced drought stress in sugarcane. Agric Ecosyst Environ. 2009;134:24–28. doi: 10.1016/j.agee.2009.07.003. [DOI] [Google Scholar]
- Promila K, Kumar S (2000) Vigna radiata seed germination under salinity. Biol Plant 43:423–426
- Puthur JT (2000) Photosynthetic events in Sesbania seban (L.) Merrill in relation to osmotic stress during different developmental stages. Ph.D. Thesis, Jamia Millia Islamia, New Delhi
- Randhir R, Shetty K. Developmental stimulation of total phenolics and related antioxidant activity in light-and dark-germinated corn by natural elicitors. Proc Biochem. 2005;40:1721–1732. doi: 10.1016/j.procbio.2004.06.064. [DOI] [Google Scholar]
- Saha P, Chatterjee P, Biswas AK (2010) NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J Exp Biol 48:593–600 [PubMed]
- Sarwar N, Yousaf S, Jamil FF. Induction of salt tolerance in chickpea by using simple and safe chemicals. Pak J Bot. 2006;38:325–329. [Google Scholar]
- Schmitt N, Dizengremel P. Effect of osmotic stress on mitochondria isolated from etiolated mung bean and sorghum seedlings. Plant Physiol Biochem. 1989;27:17–26. [Google Scholar]
- Sedghi M, Nemati A, Amanpour-Balaneji B, Gholipouri A (2010) Influence of different priming materials on germination and seedling establishment of milk thistle (Silybum marianum) under salinity stress. World Appl Sci J 11:604–609
- Sehrawat N, Yadav M, Bhat KV, Sairam RK, Jaiwal PK. Effect of salinity stress on mungbean [Vigna radiata (L.) Wilczek] in consecutive summer and spring season. Proceeddings of national conference on emerging horizons in science and technology. Fatehgarh Sahib: Sri Guru granth sahib world university; 2014. [Google Scholar]
- Shahi A, Farhoudi R, Mosavi M (2009) Effect of seed pretreatment on summer squash (Cucurbita pepo) seed germination and seedling characteristics under salinity condition. Seed Sci Biotechnol 3:5–11
- Sivritepe N, Sivritepe HO, Eris A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hortic. 2003;97:229–237. doi: 10.1016/S0304-4238(02)00198-X. [DOI] [Google Scholar]
- Sivritepe HO, Sivritepe N, Eris A, Turhan E. The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci Hortic. 2005;106:568–581. doi: 10.1016/j.scienta.2005.05.011. [DOI] [Google Scholar]
- Umezawa T, Shimizu K, Kato M, Ueda T. Enhancement of salt tolerance in soybean with NaCl pretreatment. Physiol Plant. 2000;110:59–63. doi: 10.1034/j.1399-3054.2000.110108.x. [DOI] [Google Scholar]
- Varier A, Vari AK, Dadlani M. The sub cellular basis of seed priming. Curr Sci. 2010;99:450–456. [Google Scholar]
- Xiong L, Zhu JK. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002;25:131–139. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Yeh YM, Chiu KY, Chen CL, Sung JM. Partial vacuum extends the longevity of primed bitter gourd seeds by enhancing their anti-oxidative activities during storage. Sci Hortic. 2005;104:101–112. doi: 10.1016/j.scienta.2004.08.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)
(DOCX 14 kb)