Abstract
Ephedra foliata Boiss. & Kotschy ex Boiss., (family – Ephedraceae), is an ecologically and economically important threatened Gymnosperm of the Indian Thar Desert. A method for micropropagation of E. foliata using nodal explant of mature female plant has been developed. Maximum bud-break (90 %) of the explant was obtained on MS medium supplemented with 1.5 mg l−1 of benzyl adenine (BA) + additives. Explant produces 5.3 ± 0.40 shoots from single node with 3.25 ± 0.29 cm length. The multiplication of shoots in culture was affected by salt composition of media, types and concentrations of plant growth regulators (PGR’s) and their interactions, time of transfer of the cultures. Maximum number of shoots (26.3 ± 0.82 per culture vessel) were regenerated on MS medium modified by reducing the concentration of nitrates to half supplemented with 200 mg l−1 ammonium sulphate {(NH4) 2SO4} (MMS3) + BA (0.25 mg l−1), Kinetin (Kin; 0.25 mg l−1), Indole-3-acetic acid (IAA; 0.1 mg l−1) and additives. The in vitro produced shoots rooted under ex vitro on soilrite moistened with one-fourth strength of MS macro salts in screw cap bottles by treating the shoot base (s) with 500 mg l−1 of Indole-3-butyric acid (IBA) for 5 min. The micropropagated plants were hardened in the green house. The described protocol can be applicable for (i) large scale plant production (ii) establishment of plants in natural habitat and (iii) germplasm conservation of this endemic Gymnosperm of arid regions.
Keywords: Ephedra foliata, Fleshy fruit bract, In vitro propagation, Modified MS medium, Ex vitro rooting
Introduction
Ephedra foliata Boiss. & Kotschy ex Boiss., which belongs to the evolutionarily advanced family Ephedraceae, is widely distributed in Afghanistan, Syria, Pakistan and India. In India, it is locally known as Unth phog. E. foliata represents single species of gymnosperm in arid and semiarid areas of the Thar Desert in India (Bhandari 1990; Shekhawat et al. 2012). E. foliata is a dioecious climber and female plant bears semi-transparent, nutritious edible berry like seed cones (or “fruits”) which have pleasant sweet taste due to presence of fleshy bract (Fig. 2b). The fleshy bracts of the fruits used as emergency food during the times of scarcity in arid regions (Bhandari 1990; Kotia 2008). Fruits of E. foliata can be explored as nutraceutically active food ingredient at commercial level, as these can be harvested and stored for many years at 10 ± 2˚C. E. foliata is a new world species of genus Ephedra (Caveney et al. 2001) which contains trace amount of ephedrine and pseudoephedrine alkaloid (Ramawat 1978; O’Dowd et al. 1993). It also contains non protein amino acid with cyclopropyl ring structure and several nitrogenous secondary compounds (Kynurenates) which are known to show strong pharmacological actions on sympathetic nervous system of mammals (Caveney et al. 2001). Conventionally, this plant is propagated through seeds; however, E. foliata exhibit high ratio of male over female plants so absence of proper seed setting is the major problem, further-more the seeds damaged by rodents and birds and hence natural propagation through seeds is significantly affected (Singh 2004). The gender of the seed-raised plant is known only after it bears cones. High value of the plant in nutraceutical market (Kamboj 2000) is the cause of its unsustainable exploitation by which set adverse pressure on its wild stock. Extensive habitat destruction, very slow growth, poor reproductive system and over grazing are other causes by which status of this plant is drastically affected. This endemic plant has now become rare or endangered species (Khan et al. 2003; Singh 2004). Therefore, a large scale propagation of this plant is a pre-requisite to meet the pharmaceutical and nutraceuticals needs and for its consevation. The in vitro work carried on E. foliata has been related to alkaloid production from callus (Ramawat and Arya 1979) and somatic embryogenesis (Dhiman et al. 2010). Survey of literature reveals that no attempt has been made to develop a protocol for in vitro propagation of E. foliata using nodal explant through axillary shoot proliferation, which is advantageous (over callus-derived indirect regeneration) for maintain genetic integrity and least susceptible to genetic modifications that occurs under in vitro conditions (Singh et al. 2012) as well as produce large number of plants in a short period of time. In the present communication, we utilized nodal explants first time for in vitro shoot propagation, ex vitro rooting and acclimatization/hardening of female E. foliata for large scale plant production and conservation of this fruit bearing Gymnosperm.
Fig. 2.
In vitro propagation of E. foliata (a).Mature female plant used as source of explant (b).Different stages of female cones and bract during maturation (c).Shoot bud induction from nodal explant on MS medium + 1.5 mg l−1 BA + additives (d) & (e). Repeated transfer of mother explant with in vitro shoot buds on MMS2 medium + 0.75 mg l−1 BA + 0.25 mg l−1 Kin + additives (f).Shoot multiplication on MMS3 medium + 0.25 mg l−1 BA + 0.25 mg l−1 Kin + additives (g).Healthy shoots multiplied on MMS3 medium + 0.25 mg l−1 BA + 0.25 mg l−1 Kin + 0.1 mg l−1 IAA + additives (h). Ex vitro rooted shoot after treatment with 500 mgl−1 IBA (i). Hardened and well acclimatized plant of E. foliata in black polybag (Scale bar = 1 cm)
Materials and methods
Plant material and surface sterilization
Mature female plant of E. foliata grown in Botanical Garden of Jai Narain Vyas University, Jodhpur, Rajasthan (India) was used as source of explant (Fig. 2a). Various explants such as apical shoot tip, below apical nodal segment, fresh hard green phylloclade nodal segment, and woody nodal segment were collected during different seasons for culture establishment. The harvested shoots were brought to laboratory and processed and cut into segment of 5–6 cm length having single node. The cut ends of explant were given quick dip in molten (liquid) wax for sealing and treated with systemic fungicide 0.1 % (w/v) Bavistin (BASF India Limited, Mumbai, India) for 17–18 min followed by surface sterilization with 0.1 % (w/v) solution of HgCl2 (Hi-Media, India) for 3–4 min. These were washed thoroughly with sterilized water for 6–7 times and then kept in chilled, sterile aqueous solution of antioxidants (0.1 % of ascorbic acid and 0.05 % citric acid) for 8–10 min.
Nutrient media and culture conditions
MS medium (Murashige and Skoog 1962) with 3 % (w/v) sucrose and 0.8 % (w/v) agar–agar (Bacteriological grade, Qualigens Fine Chemicals, Mumbai, India) and additives (50 mg l−1 ascorbic acid, 25 mg l−1 each of adenine sulphate, arginine and citric acid) was used as the basal medium for culture initiation and further shoot multiplication. Nutrient media were adjusted to pH 5.8 with 1 N KOH or 1 N HCl before autoclaving at 121 ° C and 15 psi for 15 min. Initially the cultures were kept under diffuse light (10–20 μmol m−2 s−1 PFD) and 32 ± 2 ° C for 3–5 days and thereafter transferred to culture room at 28 ± 2 ºC and 60 % RH under 40–50 μmol m−2 s−1 PFD. Before inoculation, the ends of surface sterilized explant were cut using sterile scissors in order to remove wax coating then they were inoculated vertically on MS medium with different concentration of BA (0.5-2.5 mg l−1) and Kin (0.5–2.5 mg l−1) separately for activation of axillary meristem. Medium without PGR’s served as control.
Shoot multiplication
For shoot proliferation, two-step process was used i) Repeated transfer of mother explant along with in vitro produced axillary shoots on fresh medium ii) Subculture of excised differentiated shoot segments in form of shoot clumps (4–5 shoots with 1–2 nodes) on fresh medium. In repeated transfer, mother explant were transferred to MS medium with various concentrations (0.25–1.0 mg l−1) of BA or Kin alone or combination of BA (0.25-1.0 mg l−1) with Kin (0.25 mg l−1) for 5 passages, each passage with interval of 3-weeks. After third passage of repeated transfer, induced multiple shoots were excised into clumps (2–4 shoots) and transferred to MS medium containing various concentrations of BA (0.1–1.0 mg l−1) in combination with either Kin (0.25 mg l−1) alone or Kin (0.25 mg l−1) and IAA (0.1–0.5 mg l−1) or Kin (0.25 mg l−1) and NAA (0.1–0.25 mg l−1) for induction of more number of shoots. Subcluturing was done every 25–30 days. To check/reduce necrosis of shoot tip, premature abscission of shoots and early senescence as well as to evaluate the effect of different concentration of nitrates and sulphate on shoot multiplication, MS medium as well as three MMS media (MMS1, MMS2, MMS3) were used during repeated transfer and sub-culturing of in vitro produced shoots (Table 1).
Table 1.
Various culture media used for shoot amplification with different concentrations (mg l−1) of nitrates and (NH4)2SO4
| MS salt | MS | MMS1 | MMS2 | MMS3 |
|---|---|---|---|---|
| NH4NO3 | 1,650 | 1,650 | 1,650 | 825 |
| KNO3 | 1,900 | 1,900 | 1,900 | 950 |
| (NH4)2SO4 | 0 | 100 | 200 | 200 |
Ex vitro rooting of micro shoots
The in vitro produced microshoots were excised carefully and washed thoroughly with sterile water in order to remove the adhered nutrient agar and then treated for 5 min with different concentrations of IBA (100–700 mg l−1) and NAA (100–700 mg l−1) separately under the green house environment. The auxin-treated shoots were transferred to bottles containing autoclaved soilrite (a mixture of horticulture grade perlite with Irish peatmoss and exfoliated vermiculite supplied by Kel Perlite, Bangalore, India) moistened with one-fourth strength of MS macro salts solutions to facilitate ex vitro rooting. Once root formation initiated in auxin treated micro shoots, these were subjected to acclimatization.
Acclimatization of in vitro regenerated microshoots
The plantlets rooted ex vitro were placed in the greenhouse initially, near the pad section at 80–90 % RH and 28 ± 2 ºC (relatively high humidity and low temperature). For acclimatization, the translucent polycarbonate caps of bottles containing rooted shoots were gradually loosened over a period of 15 days and then finally removed to allow gradual exposure of the bottled plantlets to external environment. The rooted plantlets were gradually shifted from the pad section of the greenhouse towards the fan section at 60-70 % RH and 32 ± 2 ºC (relatively low humidity and high temperature) for further acclimatization. After 40–50 days, the hardened plantlets were transferred to the black poly-bags containing mixture of organic manure, garden soil, soil-rite and sand (1:1:1:3) and kept under the green house conditions. For acclimatization experiments, 20 replicates were taken per treatment and experiments were carried out three times.
Experimental design, data collection and statistical analysis
The experiments were set up in completely randomized block design (RBD; Compton and Mize 1999) and repeated thrice. All the experiments were conducted with a minimum of 20 replicates per treatment. One replicate means one culture vessel. The observations on number of shoots, height of shoots and percentage of rooted shoots were recorded after a regular time interval of 1 week. The significance of differences among means was carried out using Duncan’s multiple range tests (DMRT) at P < 0.05. The results are expressed as means ± SD of three experiments and subjected to one-way analysis of variance (ANOVA; Gomez and Gomez 1984) using SPSS v.17 (SPSS, Chicago, USA).
Results and discussion
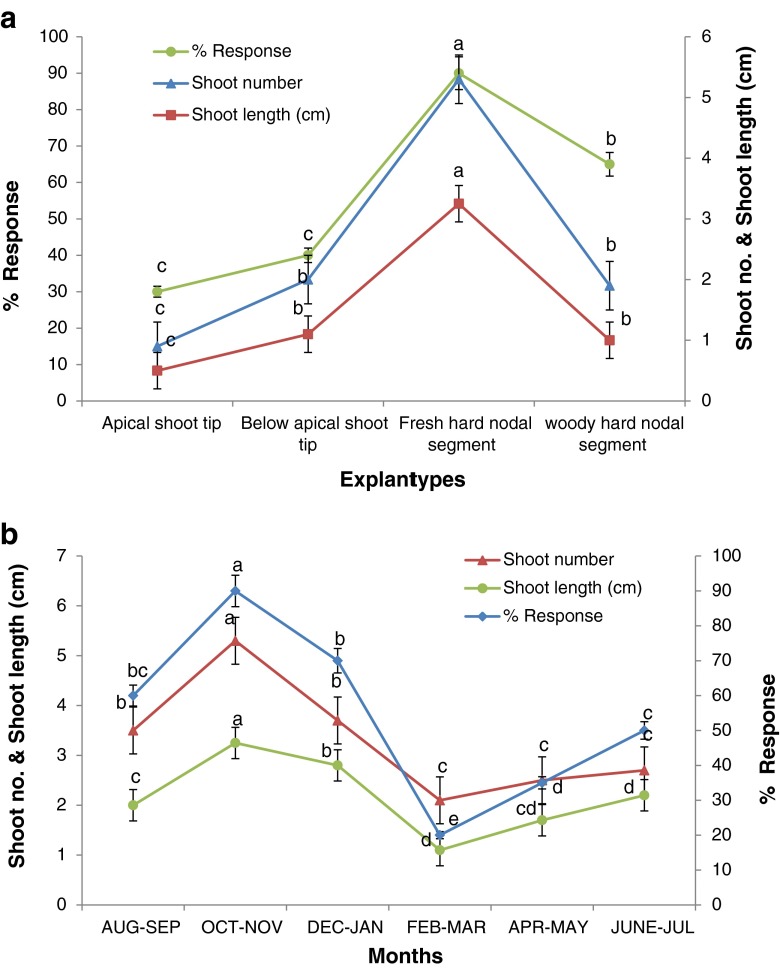
In gymnosperms, types of explant and timing of explant excision are important factors for in vitro culture establishment (Bonga et al. 2010). Among different types of explants evaluated during different seasons fresh, hard, thick, green, phylloclade nodal segments (Fig. 1a) collected during month of October-November was found most suitable for culture establishment. The explant showed variations in their morphogenic response during different months of the year (Fig. 1b). It was recorded that woody and apical juvenile or below apical nodal segments are less responsive in terms of shoot induction than fresh hard thick green nodal segments even if these were harvested during October-November because woody branches of plant harbours a variety of recalcitrant microbes. It is also assumed that some zones are existing within plants that are more morphogenetically competent than other parts of the plant (Bonga et al. 2010). Apical juvenile or below apical nodal segments were sensitive to surface sterilization and prone to deterioration. Seasonal morphogenic response and effect of explant types on culture establishment has also been reported in many plants like Leptadenia reticulata (Rathore et al. 2013a) and Lawsonia inermis (Ram and Shekhawat 2011). The explants i.e. fresh hard nodal segments of E. foliata are hollow, thus the surface sterilization with HgCl2 cause damage and death of the tissue. To check this, the cut ends of the nodal explant were sealed with the liquid wax and then surface sterilized, this minimized the toxic effect of HgCl2 (Shekhawat et al. 2011). The problem of phenolic exudation was overcome by treating the nodal explant with antioxidant solution of ascorbic acid and citric acid (Phulwaria et al. 2012). Pre-treatment with 0.1 % of bavistin for 17–18 min and surface sterilization with 0.1 % of HgCl2 for 3–4 min was sufficient for eliminating fungal and bacterial contamination up to 90 %.
Fig. 1.
a) Response of different explant types of E. foliata in terms of shoot number and shoot length b) Response of nodal explants of E. foliata in terms of shoot number and shoot length in different months of the year. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Influence of PGR’s on shoot bud induction
Irrespective of the types and concentrations of cytokinins in MS medium, shoot bud induction from the nodal explant occurred within 5–7 days of inoculation. MS medium supplemented with 1.5 mg l−1 BA was found most effective for shoot bud induction. On this medium, maximum number of shoot (5.3 ± 0.40 per node) and highest shoot length (3.25 ± 0.29 cm) were achieved after 2 weeks (Fig. 2c; Table 2) of inoculation. Shoot bud induction was also observed on MS medium without PGRs, however, response was delayed (15–20 days) in comparison to medium containing PGRs. At higher concentration (2.0-2.5 mg l−1) or lower concentrations (0.5-1.0 mg l−1) of BA, the number of shoots were significantly low. Kin was found less effective as compared to BA for multiple shoot induction. BA is very effective for reinvigoration and shoot bud induction from mature tissues in Pinus radiata (Zhang et al. 2010), Terminalia bellirica (Phulwaria et al. 2012), Caralluma edulis (Patel et al. 2014) possibly due to stability of BA and as it is easily metabolized by plant tissue (Letham and Palni 1983). Cultures were initially placed in diffused light conditions (10–20 μmol m−2 s−1 PFD) at 32 ± 2 ºC for bud break and thereafter transferred to culture room having high light intensity (40–50 μmol m−2 s−1 PFD). Morphogenesis can be enhanced if explants are kept in diffused light for certain period. Incubation of explants under diffuse light promoted early axillary meristem activation and bud breaking, such observation was also reported in Rauvolfia serpentina (Kataria and Shekhawat 2005) and Leptadenia reticulata (Rathore et al. 2013a).
Table 2.
Effect of cytokinins (BA or Kin) on shoot bud induction from nodal explant of E. foliata
| BA (mg l−1) | Kin (mg l−1) | Response (%) | Number of shoots (mean ± SD) | Shoot length (cm) (mean ± SD) |
|---|---|---|---|---|
| 0.0 | 0.0 | 0.0 | 0.7 ± 0.44g | 0.18 ± 0.40g |
| 0.5 | 0.0 | 45.3 | 2.7 ± 0.75d | 1.81 ± 0.07d |
| 1.0 | 0.0 | 67.2 | 3.6 ± 0.48c | 2.43 ± 0.20b |
| 1.5 | 0.0 | 90.0 | 5.3 ± 0.40a | 3.25 ± 0.29a |
| 2.0 | 0.0 | 80.0 | 4.5 ± 0.42b | 2.48 ± 0.41b |
| 2.5 | 0.0 | 70.2 | 3.8 ± 0.65c | 2.24 ± 0.22bc |
| 0.0 | 0.5 | 30.0 | 1.1 ± 0.93fg | 0.55 ± 0.23fg |
| 0.0 | 1.0 | 33.3 | 1.8 ± 0.28f | 0.95 ± 0.23f |
| 0.0 | 1.5 | 37.0 | 2.3 ± 0.57e | 1.29 ± 0.14e |
| 0.0 | 2.0 | 45.7 | 2.9 ± 0.86cd | 1.76 ± 0.24d |
| 0.0 | 2.5 | 50.0 | 2.8 ± 0.79d | 2.07 ± 0.18c |
Medium: MS + additives. Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05. Shoot regeneration scored after 2 weeks of culture initiation
Shoot multiplication and maintenance of cultures
Repeated transfer of original explant
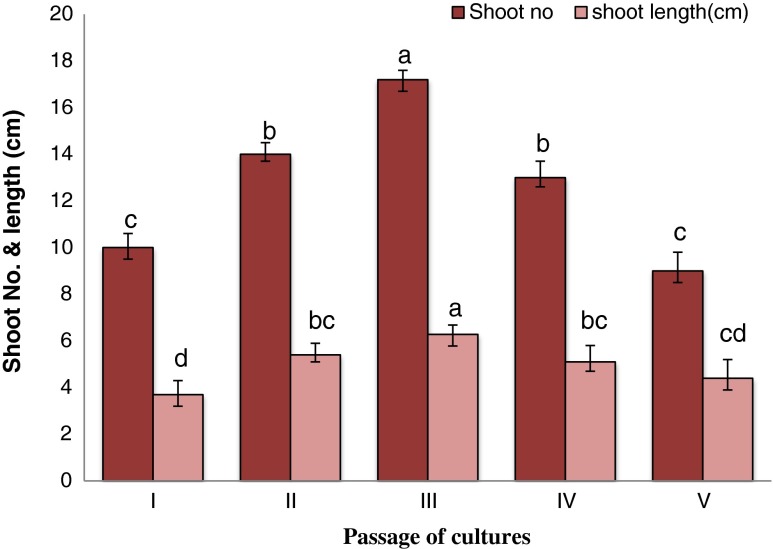
In vitro regenerated shoots from nodal explant were multiplied by repeated transferring of mother explant along with in vitro produced axillary shoots to fresh MS medium containing lower concentration of cytokinin (BA or Kin) than previously used for shoot bud induction alone or with their combination for 5 passages each with 3 weeks of time interval. Of the various PGRs tested, the combination of 0.75 mg l−1 BA and 0.25 mg l−1 Kin proved to be optimum for shoot multiplication. However, the shoots differentiated on medium supplemented with only cytokinins were weak and exhibited low vigor during transfer for different passages. To overcome these problems, differentiated shoots were transferred to the medium modified with different nitrogen source i.e. NH4NO3 and KNO3 combination with (NH4) 2 SO4 (Table 1). Out of three different medium compositions tested, MMS2 medium was found best for promoting growth and vigor of shoots. A maximum number of 19.9 ± 0.99 shoots per culture vessel (each 5.48 ± 0.66 cm long) was achieved on this medium after 3rd passage of culture (Fig. 2d, e; Table 3) and reduced thereafter (Fig. 3). Due to induction of basal dormant meristametic tissue of mother explants, repeated transfer was an efficient technique for rejuvenation and reinvigoration and to increase shoot number of in vitro cultures, which has been adopted by many workers on different plants (Panwar et al. 2012; Phulwaria et al. 2012). According to Preece (1995) the correct and balanced nutrient level in the medium removes the stress in explants and leads to dramatically improved in vitro performance, which cannot be achieved solely by the use of PGRs. Addition of (NH4) 2 SO4 improved the growth of shoots without affecting the shoot number. There are many reports of using (NH4)2 SO4 in MS medium for improving shoot growth and vigor in vitro (Ram and Shekhawat 2011; Rathore et al. 2013b).
Table 3.
Effect of cytokinins (BA and Kin) on shoot multiplication through repeated transfer of mother explants of E. foliata
| BA (mg l−1) | Kin (mg l−1) | Number of shoots (mean ± SD) | Shoot length (cm) (mean ± SD) |
|---|---|---|---|
| 0.00 | 0.00 | 1.5 ± 0.53j | 0.70 ± 0.48i |
| 0.25 | 0.00 | 4.5 ± 0.9 hi | 1.92 ± 0.90gh |
| 0.50 | 0.00 | 5.1 ± 0.74h | 2.24 ± 0.84fg |
| 0.75 | 0.00 | 9.4 ± 0.97f | 2.81 ± 0.42ef |
| 1.00 | 0.00 | 14.2 ± .79e | 3.13 ± 0.62de |
| 0.00 | 0.25 | 5.2 ± 0.42h | 1.45 ± 0.59h |
| 0.00 | 0.50 | 7.3 ± 0.78g | 1.86 ± 0.77h |
| 0.00 | 0.75 | 6.9 ± 0.87g | 2.37 ± 0.99fg |
| 0.00 | 1.00 | 8.7 ± 0.67f | 2.86 ± 0.90ef |
| 0.25 | 0.25 | 15.1 ± 0.99d | 3.64 ± 0.93cd |
| 0.50 | 0.25 | 18.2 ± 0.92b | 4.57 ± 0.63b |
| 0.75 | 0.25 | 19.9 ± 0.99a | 5.48 ± 0.66a |
| 1.00 | 0.25 | 17.3 ± 0.94c | 4.13 ± 0.31bc |
Medium: MMS2 + additives. Passage of repeated transfer of mother explants: 3rd. Data were recorded after 4 weeks of culture. Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Fig. 3.
Effect of passages with interval of 4 weeks of repeated transfer of cultures on MMS2 medium with additives, 0.75 mg l−1 BA + 0.25 mg l−1 Kin. Values followed by different letters are significantly different at 5 % level as determined by Duncan’s test (DMRT)
Subculturing of in vitro regenerated shoots
To improve shoot multiplication in the subsequent cultures, after the 3rd passage of repeated transfer, multiple shoots were excised into clumps (2–4 shoots) and sub cultured on MS and various MMS media containing different combinations of PGRs. In the present study, MMS 3 medium was found best among various modified media used. Maximum number of shoots (26.3 ± 0.82 shoots per culture vessel) with average length of 6.29 ± 0.47 cm were obtained on MMS3 medium supplemented with BA (0.25 mg l−1), Kin (0.25 mg l−1), IAA (0.1 mg l−1) and additives (50 mg l−1 ascorbic acid, 25 mg l−1 each of adenine sulphate, arginine and citric acid) after 4 weeks of culture (Fig. 2g; Table 4). A significant (P < 0.05) improvement in shoot multiplication during subculture in terms of average shoot number and shoot length were observed when low concentration of auxin (IAA) was added in medium containing a combination of BA and Kin. Presence of only cytokinin is less effective than combination with auxin (Fig. 2f). NAA was less effective auxin than IAA. A similar synergistic effect of cytokinin–auxin combination towards shoot multiplication has also been demonstrated in a number of cases (Rathore et al. 2013b; Patel et al. 2014).
Table 4.
Effect of BA and auxin (IAA or NAA) with Kin 0.25 mgl−1 on shoot multiplication of E. foliata
| BA (mg l−1) | IAA (mg l−1) | NAA (mg l−1) | Number of shoots (mean ± SD) | Shoot length (cm) (mean ± SD) |
|---|---|---|---|---|
| 0.00 | 0.00 | 0.0 | 2.7 ± 0.48k | 1.13 ± 0.41f |
| 0.10 | 0.00 | 0.0 | 12.6 ± 0.51i | 3.62 ± 0.71e |
| 0.25 | 0.00 | 0.0 | 17.7 ± 0.48g | 5.11 ± 0.57c |
| 0.50 | 0.00 | 0.0 | 20.5 ± 0.53d | 5.44 ± 0.58bc |
| 0.75 | 0.00 | 0.0 | 22.4 ± 0.52c | 5.72 ± 0.72b |
| 1.00 | 0.00 | 0.0 | 18.3 ± 0.48f | 4.42 ± 0.54d |
| 0.10 | 0.10 | 0.0 | 16.3 ± 0.48h | 5.44 ± 0.49bc |
| 0.25 | 0.10 | 0.0 | 26.3 ± 0.82a | 6.29 ± 0.47a |
| 0.50 | 0.10 | 0.0 | 24.2 ± 0.42b | 5.80 ± 0.51b |
| 0.25 | 0.25 | 0.0 | 19.4 ± 0.51e | 5.15 ± 0.34c |
| 0.25 | 0.50 | 0.0 | 16.7 ± 0.67h | 4.54 ± 0.58d |
| 0.25 | 0.00 | 0.1 | 12.8 ± 0.63i | 4.46 ± 0.62d |
| 0.25 | 0.00 | 0.25 | 10.5 ± 0.53j | 3.87 ± 0.51e |
Medium: MMS3 + additives. Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
The shoots obtained on BA + Kin, either alone or in combination with IAA, were thin and exhibited shoot tip necrosis, premature abscission of shoot and early senescence during the present investigation. Such reports also exist for some other plant species mentioning the occurrence of early leaf fall and low vigor of shoots (Rathore et al. 2013b) during in vitro shoot multiplication. The premature abscission as well as shoot tip and shoot base necrosis could effectively be controlled by the use of half strength of nitrates and incorporation of (NH4)2 SO4 in medium. Sulphate ion promotes absorption of nitrates and buffers the culture medium (Ivanova and van Staden 2008; Kopriva et al. 2009). Ogura-Tsujita and Okubo (2006) demonstrated that reduced concentration of nitrates (NH4NO3 and KNO3) influences endogenous ethylene and auxin-cytokinin level in medium. Results of this study showed that low concentration of nitrates reduces endogenous ethylene and thus prevent premature abscission and promotes shoot growth in vitro. There are many reports of using reduced nitrate concentration for improving shoot quality (Shirdel et al. 2011; Rathore et al. 2013b). Subculturing period is critical for the survival and multiplication of the culture. When the cultures were kept on medium for more than 30 days, they showed decline in growth.
Ex vitro rooting of micro shoots
Out of the two root inducing growth hormones (IBA and NAA) tested, IBA was found to be the most suitable, considering all the parameters for rooting like, rooting percentage, number and nature of roots and shoot growth. Induction of roots from in vitro produced shoots is a critical step and also a limiting factor when mature trees and gymnosperms are to be cloned. About 80 % in vitro raised shoots rooted (5.5 ± 0.53 roots per shoot with 2.4 ± 0.52 cm length) ex vitro within 2–3 weeks when the base of the shoot (1.0–3.0 mm) was treated with IBA 500 mg l−1 for 5 min and transferred to soilrite and grown under the green house conditions (Fig. 2h; Table 5). Poor and delayed rooting performance was recorded on all concentrations of NAA as compared to IBA. The promoting effect of IBA on rooting has also been reported in Arnebia hispidissima (Phulwaria and Shekhawat 2013), Leptadenia reticulata (Rathore et al. 2013a). IBA is now used as a commercial rooting growth hormone for many plant species worldwide (Epstein and Luduig-Muller 1993). In vitro produced shoots rooted under ex vitro environment were hardened with ease; required less cost, time, labour and were better adapted to natural climate. By this approach shoots regenerated were stronger and exhibited higher number of roots which were morphologically similar to naturally developed roots. The main advantage of ex vitro rooting is that root damage during transplantation to soil is minimized so ex vitro rooted plantlets exhibit higher rate of survival when transferred to field. Ex vitro rooted plants have greater and improved resistance to types of stresses experienced during hardening (Yan et al. 2010; Phulwaria and Shekhawat 2013; Patel et al. 2014).
Table 5.
Effect of auxin (IBA or NAA) on ex vitro rooting of regenerated shoots of E. foliata
| IBA (mg l−1) | NAA (mg l−1) | Response (%) | Number of roots (mean ± SD) | Root length (cm) (mean ± SD) |
|---|---|---|---|---|
| 0.0 | 0.0 | 0.0 | 0.0 ± 0.0 h | 0.0 ± 0.00g |
| 100 | 0.0 | 47.0 | 1.4 ± 0.52e | 0.81 ± 0.03ef |
| 200 | 0.0 | 56.0 | 2.4 ± 0.69d | 1.2 ± 0.42c |
| 300 | 0.0 | 68.0 | 3.4 ± 0.69c | 1.8 ± 0.43b |
| 500 | 0.0 | 80.0 | 5.5 ± 0.53a | 2.4 ± 0.52a |
| 700 | 0.0 | 70.0 | 4.5 ± 0 .70b | 1.9 ± 0.32b |
| 0.0 | 100 | 33.0 | 0.67 ± 0.12g | 0.57 ± 0.06f |
| 0.0 | 200 | 39.0 | 0.84 ± 0.05fg | 0.73 ± 0.05ef |
| 0.0 | 300 | 53.0 | 1.3 ± 0.48ef | 0.86 ± 0.09de |
| 0.0 | 500 | 61.0 | 2.1 ± 0.32d | 0.97 ± 0.05cde |
| 0.0 | 700 | 43.0 | 2.2 ± 0.63d | 1.1 ± 0.21cd |
Auxin pulse treatment duration: 5 min. Means in each column followed by same letters are not significantly different according to DMRT at P < 0.05
Acclimatization of in vitro propagated plantlets
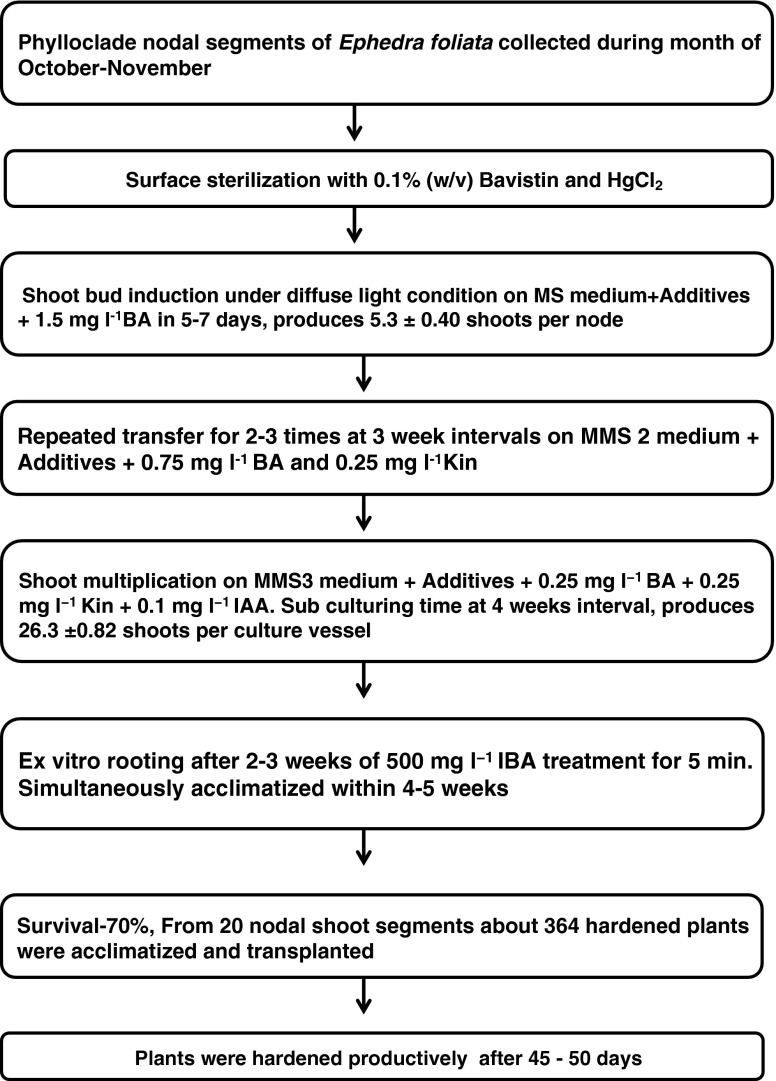
The micropropagated plants were hardened productively after a period of 45–50 days of hardening and acclimatization. The hardened and acclimatized plantlets were then successfully transferred to black polybags containing a (1:1:1:3) (w/w/w/w) ratio of organic manure, garden soil, soilrite and sand. The plants in black polybags were transferred to the nursery after 60 days of hardening in the green house (Fig. 2i). The percentage of survival of plantlets during acclimatization was about 70 %. Following this protocol about 364 hardened plants were acclimatized and transplanted within 10–12 weeks starting with 20 nodal shoot segments. The micropropagated plants were morphologically uniform and exhibited similar growth characteristics and vegetative morphology to the mother plant. A flow chart for micropropagation protocol of E. foliata is showing in Fig. 4.
Fig. 4.
Flow-chart of the defined protocol for mass-scale production of plants of E. foliata
To the best of our knowledge, this study reports for the first time, a successful method for efficient and rapid in vitro propagation of E. foliata from nodal explants. Use of ex vitro rooting technique for plant production serves as a more economical option in terms of labor cost and saving time. In conclusion, using procedure described in the present study could be employed for large scale multiplication within a short period of time which providing suitable planting material for cultivation at commercial level and minimizing male over female plant ratio, as well as ex situ conservation of germplasm of this endemic and threatened Gymnosperm species of arid area.
Acknowledgments
Author (DL) gratefully acknowledge the financial support from Council of Scientific and Industrial Research (CSIR), New Delhi in the form of Junior and Senior Research Fellowship. Author (NR) wish to acknowledge the support of University Grants Commission (UGC), New Delhi for the award Junior and Senior Research Fellowship. We thank Department of Biotechnology, Government of India, and New Delhi for establishing Micropropagation and Hardening Unit used for the present research
Abbreviations
- BA
6-Benzyl adenine
- IAA
Indole-3 acetic acid
- IBA
Indole-3-butyric acid
- Kin
Kinetin
- MMS
Modified Murashige and Skoog medium
- MS
Murashige and Skoog medium
- NAA
α- Naphthalene acetic acid
- PFD
Photon flux density
- PGRs
Plant growth regulators
- RH
Relative humidity
Contributor Information
Deepika Lodha, Email: deeplodha113@gmail.com.
N. S. Shekhawat, Email: biotechunit@gmail.com
References
- Bhandari MM. Flora of Indian Desert. Jodhpur: MPS Reports; 1990. [Google Scholar]
- Bonga JM, Klimaszewska KK, von Aderkas P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Org Cult. 2010;100:241–254. doi: 10.1007/s11240-009-9647-2. [DOI] [Google Scholar]
- Caveney S, Charlet DA, Freitag H, Maier-stolte M, Starratt AN (2001) New observations on the secondary chemistry of world Ephedra (Ephedraceae). American J Bot 88:1199–1208 [PubMed]
- Compton ME, Mize CW (1999) Statistical considerations for in vitro research: I-Birth of an idea to collecting data. In Vitro Cell Dev Biol Plant 35:115–121
- Dhiman M, Sharma V, Moitra S (2010) Somatic Embryogenesis and Plant Regeneration in Ephedra foliata (Boiss.); a non Coniferous Gymnosperm. Plant Tissue Cult Biotech 20:133–143
- Epstein E, Luduig-Muller J. Indole-3-butyric acid in plants: occurrence, synthesis, metabolism and transport. Physiol Plant. 1993;88:382–389. doi: 10.1111/j.1399-3054.1993.tb05513.x. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedure for agricultural research. New York: Wiley; 1984. [Google Scholar]
- Ivanova M, van Staden J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoot of Aloe polyphylla. Plant Cell Tissue Org Cult. 2008;92:227–231. doi: 10.1007/s11240-007-9311-7. [DOI] [Google Scholar]
- Kamboj VP. Herbal medicine. Curr Sci. 2000;78:35–39. [Google Scholar]
- Kataria V, Shekhawat NS. Cloning of Rouvolfia serpentina - endangered medicinal plant. J Sustain For. 2005;20:53–65. doi: 10.1300/J091v20n01_04. [DOI] [Google Scholar]
- Khan TI, Dular AK, Solomon DM. Biodiversity conservation in the Thar Desert; with emphasis on endemic and medicinal plants. The Environmental. 2003;23:137–144. [Google Scholar]
- Kopriva S, Mugford SG, Matthewman C, Koprivova A. Plant sulfate assimilation genes: redundancy versus specialization. Plant Cell Rep. 2009;28:1769–1780. doi: 10.1007/s00299-009-0793-0. [DOI] [PubMed] [Google Scholar]
- Kotia A. Threatened Plants and Their Habitats in Indian Thar Desert. In: Rawat GS, editor. Special Habitats and Threatened Plants of India. Dehradun: ENVIS Bulletin: Wildlife and Protected Areas Wildlife Institute of India; 2008. pp. 115–121. [Google Scholar]
- Letham DS, Palni LMS. The Biosynthesis and Metabolism of Cytokinins. Ann Rev Plant Physiol. 1983;34:163–197. doi: 10.1146/annurev.pp.34.060183.001115. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- O’Dowd NA, McCauley PG, Richardson DHS, Wilson G. Callus production, suspension culture and in vitro alkaloid yields of Ephedra. Plant Cell Tissue Org Cult. 1993;34:149–155. doi: 10.1007/BF00036095. [DOI] [Google Scholar]
- Ogura-Tsujita Y, Okubo H (2006) Effects of low nitrogen medium on endogenous changes in ethylene, auxins and cytokinins in in vitro shoot formation from rhizomes of Cymbidium kanran. In Vitro Cell Dev Biol Plant 42:614–616
- Panwar D, Ram K, Harish, Shekhawat NS (2012) In vitro propagation of Eulophia nuda Lindl., an endangered orchid. Sci Hortic 139:46–52
- Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS (2014) In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: An endemic and endangered edible plant species of the Thar Desert. Sci Hortic 165:175–180
- Phulwaria M, Shekhawat NS. An efficient in vitro shoot regeneration from immature inflorescence and ex vitro rooting of Arnebia hispidissima (Lehm). DC. - A red dye (Alkannin) yielding plant. Physiol Mol Biol Plants. 2013;19:435–441. doi: 10.1007/s12298-013-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulwaria M, Rai MK, Harish, Gupta AK, Ram K, Shekhawat NS (2012) An improved micropropagation of Terminalia bellirica from nodal explant of mature tree. Acta Physiol Plant 34:299–305
- Preece JE. Can nutrient salts partially substitute for plant growth regulators? Plant Tissue cult Biotechnol. 1995;1:26–37. [Google Scholar]
- Ram K, Shekhawat NS. Micropropagation of commercially cultivated Henna (Lawsonia inermis) using nodal explants. Physiol Mol Biol Plant. 2011;17:281–289. doi: 10.1007/s12298-011-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramawat KG (1978) On the growth of Ephedra tissues in culture with special references to secondary products. PhD thesis, Department of Botany, Jai Narain Vyas University, Jodhpur, India
- Ramawat KG, Arya HC. Alkaloid content of Ephedra in vivo and in vitro. Indian J Exp Bot. 1979;17:106–107. [Google Scholar]
- Rathore MS, Rathore MS, Shekhawat NS. Ex vivo implications of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight. & Arn.-An endangered plant. Environ Exp Bot. 2013;86:86–93. doi: 10.1016/j.envexpbot.2010.05.009. [DOI] [Google Scholar]
- Rathore NS, Rathore N, Shekhawat NS (2013b) In vitro propagation and micromorphological studies of Cleome gynandra: a C4 model plant closely related to Arabidopsis thaliana. Acta Physiol Plant 35:2691–2698
- Shekhawat MS, Shekhawat NS, Harish, Ram K, Phulwaria M, Gupta AK (2011) High Frequency Plantlet Regeneration from Nodal Segment Culture of Female Momordica dioica (Roxb). J Crop Sci Biotechnol 14:133–137
- Shekhawat NS, Phulwaria M, Harish, Rai MK, Kataria V, Shekhawat S, Gupta AK, Rathore NS, Vyas M, Rathore N, Vibha JB, Choudhary SK, Patel AK, Lodha D, Modi R (2012) Bioresearches of Fragile Ecosystem/Desert. Proc Natl Acad Sci India 82:319–334
- Shirdel M, Motallebi-Azar A, Masiha S, Mortazavi N, Matloobi M, Sharafi Y. Effects of inorganic nitrogen source and NH4+: NO3− ratio on proliferation of dog rose (Rosa canina) J Med Plant Res. 2011;5:4605–4609. [Google Scholar]
- Singh AK. Endangered economic species of Indian desert. Genet Resour Crop Evol. 2004;51:371–380. doi: 10.1023/B:GRES.0000023452.91250.52. [DOI] [Google Scholar]
- Singh SK, Rai MK, Sahoo L. An improved and efficient micropropagation of Eclipta alba through transverse thin cell layer culture and assessment of clonal fidelity using RAPD analysis. Indust Crop Prod. 2012;37:328–333. doi: 10.1016/j.indcrop.2011.12.005. [DOI] [Google Scholar]
- Yan H, Liang C, Yang L, Li Y. In vitro and ex vitro rooting of Siratia grosvenorii, a traditional medicinal plant. Acta Physiol Plant. 2010;32:115–120. doi: 10.1007/s11738-009-0386-0. [DOI] [Google Scholar]
- Zhang H, Horgan KJ, Reynolds PH, Jameson PE. 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol. 2010;30:514–526. doi: 10.1093/treephys/tpp130. [DOI] [PubMed] [Google Scholar]