Abstract
The findings of a recent study associate LPHN3, a member of the latrophilin family, with an increased risk of developing attention deficit/hyperactivity disorder (ADHD), the most common psychiatric disorder in childhood and adolescence. Latrophilins comprise a new family of G protein-coupled receptors of unknown native physiological function that mediate the neurotoxic effects of α-latrotoxin, a potent toxin found in black widow spider venom. This receptor–toxin interaction has helped to elucidate the mechanistic aspects of neurotransmitter and hormone release in vertebrates. Such unprecedented discovery points to a new direction in the assessment of ADHD and suggest that further study of this receptor family may provide novel insights into the etiology and treatment of ADHD and other related psychiatric conditions.
Keywords: ADHD, LPHN3, latrophilin, G protein-coupled receptor, α-latrotoxin
INTRODUCTION
Living cells are capable of detecting external stimuli by means of receptor molecules present in the cytoplasm, the nucleus, or embedded in the plasma membrane. The nature of these stimuli may be physical (e.g., variation in potential difference across the membrane or its mechanical deformation), but in most cases chemical ligands signal a change in the external environment. Chemical ligands range broadly from complex macromolecules to small solutes, and it is the fine-tuning of their interaction with highly specific receptors that ultimately determines cell function and fate.
There are several classes of receptors: the predominant and best characterized are the histidine kinase sensors (HK) in prokaryote cells [Szurmant et al., 2007; Yamada and Shiro, 2008], the receptor tyrosine kinases (RTK) in metazoans [Dengjel et al., 2009; Hausott et al., 2009] and the G-protein coupled receptors (GPCR) found in all eukaryotes [Pierce et al., 2002; Cabrera-Vera et al., 2003]. These receptor classes have different membrane topologies that determine the mechanism of signal transduction. GPCRs have been the subject of intense research over the years; in fact, more than one-third of all current pharmaceuticals are directed at them. Such is their importance that 2009 was named “The Year in G-Protein Coupled Receptor Research” at the Endocrine Society's Annual Meeting ENDO 2009 [Millar and Newton, 2010], with 850 reviews and more than 8,000 original studies published.
In the last 10 years, latrophilins (LPHNs), a relatively new family of GPCRs, have received a great deal of attention as a potential target for drug discovery. LPHNs were discovered as receptors for α-latrotoxin (α-LTX), a potent neurotoxin isolated from black widow spider venom [Davletov et al., 1996; Lelianova et al., 1997]; this receptor family has since undergone extensive investigation involving regulated exocytosis of neurotransmitters and hormones [Davletov et al., 1998; Rahman et al., 1999; Silva et al., 2009b]. More recently, while studying the genetics of attention deficit/hyperactivity disorder (ADHD) our lab discovered linkage and association to one gene of the LPHN family. Common LPHN3 alleles at the marker and haplotype level were found to confer a major risk of susceptibility to developing ADHD in a genetic isolate from Colombia, South America, results that were successfully replicated in European and U.S. populations [Arcos-Burgos et al., 2010a].
In this review, we will update the current knowledge on LPHNs and discuss recent findings that for the first implicate this receptor class in the etiology of psychiatry disorders.
MOLECULAR STRUCTURE OF LATROPHILINS
All GPCRs are confined to the plasma membrane and share a common topology of seven transmembrane segments (7TM) [Gether et al., 2002; Pierce et al., 2002; Schoneberg et al., 2002]. Activation of G proteins upon ligand binding to the receptor transmits the signal to various intracellular effectors that eventually affect gene expression and/or metabolic reactions [Landry and Gies, 2002; Cabrera-Vera et al., 2003].
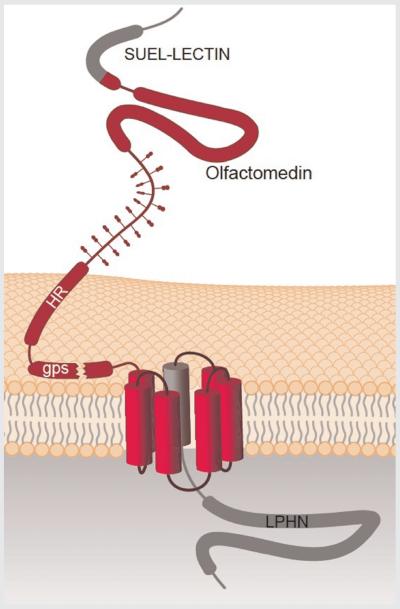
LPHNs represent a putative adhesion-class GPCR family [Fredriksson et al., 2003; Bjarnadottir et al., 2007] with large extracellular and intracellular domains [Sugita et al., 1998]. Long N-terminus GPCRs contain several cell adhesion modules (e.g., cadherin, IgG, laminin A, thrombospondin type 1, galactose lectin, EGF) and transmembrane segments resembling those of group B GPCRs [Hayflick, 2000; Stacey et al., 2000; Krasnoperov et al., 2002], which suggests that these receptors, now termed long N-terminus group B (LNB) GPCRs, are naturally occurring chimeras of cell adhesion molecules and signaling receptors capable of transducing cell–cell interactions into intracellular signals [Hamann et al., 1996; Stacey et al., 2002]. A sequence comparison analysis of LPHN extracellular region reveals some interesting features: a rhamnose-binding lectin domain (RBL); a region homologous to olfactomedins; a long region of homology shared with a protein family of unknown function, referred to as BAI1–3 (brain-specific angiogenesis inhibitors); and a short, cysteine-rich sequence (Fig. 1).
FIG. 1.
General structure of latrophilins. The long extracellular region (approx. 850 aa) comprises four domains and at least eight potential N-glycosylation sites. From N- to C-terminus, a SUEL LECTIN domain (rhamnose-binding lectin or RBL), a region homologous to olfactomedins and myocilin, a homology region (HR) with BAI1-3, a cysteine-rich GPCR proteolysis site (gps), and a C-terminal region (LPHN).
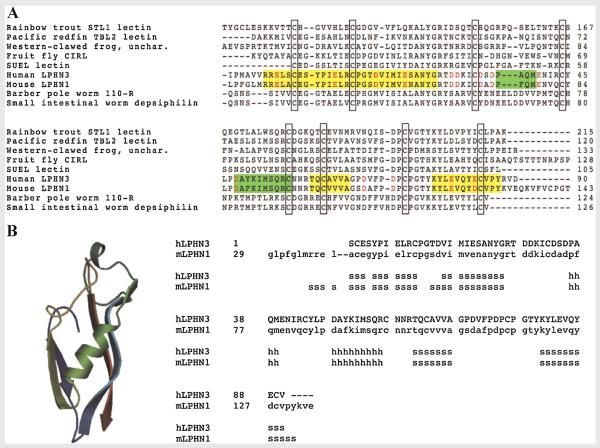
The RBL domain is a relatively rare domain first characterized from sea urchin egg lectins (SUEL). So far, mouse LPHN1 is the only latrophilin for which the tridimensional structure of the RBL domain and its rhamnose-binding properties have been determined [Vakonakis et al., 2008]. However, the fact that rhamnose is found only rarely in animals [Tymiak et al., 1993] makes it questionable as an endogenous ligand for LPHNs. Sequence analysis of the RBL domains of human LPHN3 and mouse LPHN1 (amino acids 35–124) using CrustalW algorithms (http://services.uniprot.org/) reveals a high degree of homology with other lectin-like proteins and the conservation of eight cysteine residues (Fig. 2), which is predictive of carbohydrate-binding properties of this domain in LPHNs. At least in mouse LPHN1, all eight cysteine residues are necessary for proper domain folding [Vakonakis et al., 2008].
FIG. 2.
A: Sequence comparison of RBL domains from lectins of different species. The open boxes show conserved cysteine residues across species. Green boxes, α-helices; yellow boxes, β-sheets; red letters: residues involved in carbohydrate binding in mouse LPHN1 RBL domain. B: Comparative modeling of human LPHN3 RBL domain based on mouse LPHN1 pdb file, using the SWISS-MODEL software (Peitsch, 1995; Guex and Peitsch, 1997; Schwede et al., 2003; Kopp and Schwede, 2004, 2006; Arnold et al., 2006). Sequence alignment reveals an 80% of identity. s, β-strands; h, α-helices.
Olfactomedins comprise a diverse family of secreted glycoproteins of unknown function (e.g., noelin, tiarin, and gliomedin) that are implicated in the development of the nervous system [Moreno and Bronner-Fraser, 2001; Tsuda et al., 2002; Eshed et al., 2005]. Secondary structure predictions confirm a more general helical structure of the N-terminal region in the olfactomedin-like sequences [Green and Klein, 2002], whereas LPHNs, on the other hand, are mostly strand-like, which suggests that the olfactomedin domain in LPHNs is likely the only domain related to olfactomedins.
The brain-specific angiogenesis inhibitors constitute another family of adhesion-class GPCRs, with three members identified so far, BAI1, 2 and 3. These genes are strongly expressed in brain [Shiratsuchi et al., 1997]. BAI1, a p53-inducible gene, has been implicated in the regulation of vascularization of malignancies including colorectal cancer, pulmonary adenocarcinoma, gastric cancer, and glioblastomas [Fukushima et al., 1998; Hatanaka et al., 2000; Lee et al., 2001; Kang et al., 2006]. Interestingly, a recent study reported an association between BAIAP2 (BAI associated protein 2, a left–right brain asymmetry-related gene) and ADHD in Spanish and German populations [Ribases et al., 2009].
The cysteine-rich sequence in LPHNs has a high homology with the sequences found in several GPCRs [Sugita et al., 1998; Krasnoperov et al., 2002]. LNB GPCRs have previously been found to undergo autocleavage at this cysteine-rich proteolysis site (GPS) that spans approximately 60 amino acids upstream of the first transmembrane segment [Gray et al., 1996; Krasnoperov et al., 1997; Nechiporuk et al., 2001] (Fig. 1). LPHNs are cleaved in the endoplasmic reticulum into an N-terminal fragment (p120) and a C-terminal fragment (p85), and this cleavage is necessary for efficient delivery to the plasma membrane and proper receptor function [Krasnoperov et al., 2002; Volynski et al., 2004; Krasnoperov et al., 2009; Silva et al., 2009a]. It was initially proposed that the GPS-dependent autoproteolysis of LPHNs was sufficient for receptor delivery to the cell surface, but recent evidence demonstrates that this sequence is also closely related to trafficking, possibly involving the interaction with auxiliary proteins along the secretory pathway [Deyev and Petrenko, 2010]. This proteolytic processing may be regarded as a mechanism of molecular compartmentalization of cell adhesion and G-protein activation functions.
The C-terminal cytoplasmic region of LPHNs does not show any particular domain structure, except that it contains multiple potential sites for palmitoylation and phosphorylation presumably implicated in the modulation of receptor activity, and several PEST sequences (rich in proline, glutamic acid, serine, and threonine) [Matsushita et al., 1999]. PEST sequences confer susceptibility to quick degradation by proteolysis [Rogers et al., 1986; Roth et al., 1998; Sekhar and Freeman, 1998], so those proteins containing these motifs may be considerably short lived. There are three putative PEST sequences in LPHN1, two in LPHN2 and one in LPHN3 from rat or bovine origin, which suggests that LPHN1 may be more susceptible to rapid degradation compared to LPHN2 and LPHN3.
LATROPHILINS AS α-LTX RECEPTORS
Black widow spider venom contains at least 86 toxins [Duan et al., 2006], but α-LTX is the only one of these specifically affecting vertebrates [Rosenthal and Meldolesi, 1989]. This toxin is capable of inducing the secretion of neurotransmitters and hormones in its target cells by stimulating exocytosis [Rosenthal et al., 1990]. α-LTX has been found to stimulate exocytosis largely by creating ion channels and triggering a massive influx of Ca2+ in the presynaptic membrane [Ceccarelli et al., 1979; Fesce et al., 1986]. Cation currents, however, do not explain all of the toxin's effects. In fact, α-LTX can stimulate small vesicle exocytosis in the absence of extracellular Ca2+ [Igarashi et al., 2000; Verhage et al., 2000; Deak et al., 2009]. Both Ca2+-dependent and -independent mechanisms rely on the toxin binding to specific surface receptors [Hlubek et al., 2000; Van Renterghem et al., 2000; Volynski et al., 2000]. To date, three receptors have been identified: neurexin, protein tyrosine phosphatase σ, and LPHNs [Sudhof, 2001; Ushkaryov et al., 2008]. LPHNs were identified simultaneously by two independent groups in bovine and rat brain extracts [Davletov et al., 1996; Krasnoperov et al., 1996; Lelianova et al., 1997], and it was the high affinity co-purification with α-LTX that gave the receptor its name. Since LPHN-mediated toxic effects do not require Ca2+ influx, LPHNs were also designated as Ca2+-independent receptors of α-LTX (CIRL). The precise mechanism of LPHN-mediated exocytosis in response to α-LTX is not well understood, but apparently the activation of intracellular G-protein signaling can be expendable. Transfection experiments in PC12 and chromaffin cells revealed that LPHN1 lacking its cytoplasmic tail still functions as an excellent α-LTX receptor [Sugita et al., 1998; Hlubek et al., 2000]. Likewise, α-LTX is capable of triggering massive exocytosis of β-hexosaminidase granules in LPHN1-transfected mast cells in the presence of extracellular Ca2+, but when the cells were deprived of the divalent cation release of the enzyme was dramatically reduced [Hiramatsu et al., 2010]. These results demonstrate that LPHN1-dependent signal transduction is not required in the presence of extracellular Ca2+ and suggest that in normal physiological conditions LPHNs may exert their major effects by simply recruiting α-LTX for membrane insertion near presynaptic active zones, without actually transducing any intracellular signals.
LATROPHILIN-MEDIATED INTRACELLULAR SIGNALING
The effects of α-LTX have been studied thoroughly in endocrine cells and the presynaptic apparatus of neurons. As discussed above, this toxin is capable of inducing receptor-mediated vesicle exocytosis through both Ca2+-dependent and -independent pathways. When LPHNs were identified as α-LTX receptors and further characterized as GPCRs, the moderate toxin's effects independent of extracellular Ca2+ made sense immediately. At that time, receptor-mediated activation of G proteins was a well-known mechanism implicated in regulated exocytosis in secretory cells, so LPHN-mediated signaling might explain extracellular Ca2+ expendability.
Neurons and endocrine cells are very similar with respect to the composition of their characteristic small vesicles and dense-core granules, besides having a variety of molecular, biochemical, and functional similarities. Such similarities in the molecular machinery for secretion suggest that processes responsible for secretion of neurotransmitters and hormones are likely to have evolved from the trafficking machinery that controls secretion in more simple systems such as yeast cells [Bennett and Scheller, 1993]. The fact that small G proteins are highly enriched at synapses is more than a mere coincidence [de Maturana and Sanchez-Pernaute, 2010]. Lelianova et al. [1997] found that LPHN1 co-purified with Gαo in rat and bovine brain extracts, and that this interaction was involved in the induction by α-LTX of inositol-3-phosphate and cyclic AMP in transfected COS-7 cells [Lelianova et al., 1997]. Shortly after, another group confirmed these results with the new finding that Gαq/11 was also linked to LPHN signaling [Rahman et al., 1999]. Coupling to Gαq/11 was able to activate phospholipase C followed by mobilization of intracellular Ca2+ stores and, eventually, norepinephrine small vesicle exocytosis. Moreover, activation of protein kinase C by LPHN-mediated diacylglycerol release was able to increase the Ca2+ sensitivity of the molecular machinery of vesicle fusion to accelerate insulin secretion in pancreatic β cells [Hu et al., 2006]. Another study performed in C. elegans showed that UNC-13, a major presynaptic diacylglycerol receptor essential for vesicle-mediated neurotransmitter release in mammals as well, is involved in LPHN-dependent regulation of exocytosis [Brose et al., 2000; Willson et al., 2004]. As aforementioned, LPHN1-expressing mast cells are also capable of responding, although moderately, to stimulation by α-LTX in the absence of extracellular Ca2+ [Hiramatsu et al., 2010], mechanism that is possibly mediated by phosphorylation of the soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins SNAP-23, syntaxin-4, and VAMP-8. β-hexosaminidase release was decreased upon inhibition of protein kinase C and phospholipase C signaling.
LPHN C-terminal region has also been found to interact with synaptic multidomain scaffolding proteins of the ProSAP/SSTRIP/Shank family [Kreienkamp et al., 2000, 2002]. These proteins play an important role in regulating the architecture of the cytoskeleton at synapses; thus, their interaction with LPHNs might control the vesicular fusion/docking process by affecting the architecture of the plasma membrane at the active zones. More recently, Popova et al. [2007] demonstrated that LPHN1 interacts with TRIP8b, a Rab8b-interacting adapter protein involved in regulated exocytosis. Since Rab8 has been shown to localize at or near recycling endosomes [Hattula et al., 2006; Roland et al., 2007], it is possible that TRIP8b is involved in regulating LPHN concentration on the cell surface.
EXPRESSION OF LATROPHILINS
LPHN expression studies have been performed mainly in rats, probably because of the feasibility of laboratory breeding and the bigger brain size compared to mice. Three highly homologous LPHNs, LPHN1–3, have been identified so far. LPHN1 is found predominantly in the brain, but low levels of mRNA are also found in most tissues [Sugita et al., 1998], which is suggestive of a widespread function not directly relevant to neuronal activity. LPHN2 is expressed primarily outside the brain (especially in liver and lung); although LPHN2 mRNA is detected in brain at low levels, the protein could not be detected by Western blot. LPHN3 expression is detectable mainly in brain [Sugita et al., 1998; Ichtchenko et al., 1999]. Matsushita et al. [1999] found by Northern blotting of RNA isolated from rat tissues that LPHN1 and 3 are almost exclusively brain-specific, although LPHN3 is much less abundant. LPHN1 mRNA was also found in very low amounts in the kidneys, lungs, and spleen.
Human studies reveal somewhat different findings from those described above. In a recent study, the GPCR expression pattern of human adult and fetal adrenal glands was compared using microarray analysis and confirmed by real-time PCR. The results showed that both LPHN2 and LPHN3 are highly expressed in this gland, but mRNA levels are significantly higher in fetal versus adult tissue [Xing et al., 2009]. Recently, we analyzed LPHN3 distribution in postmortem human brain tissue by Northern blot and revealed high-mRNA expression in amygdala, caudate nucleus, cerebellum, and prefrontal cortex. Lower expression was detected in corpus callosum, hippocampus, whole brain extract, occipital pole, frontal lobe, temporal lobe, and putamen. Expression was corroborated at the protein level using an anti-LPHN3 antibody. No expression was detected in thalamus, medulla, or spinal cord [Arcos-Burgos et al., 2010a].
LPHNs contain multiple splicing sites that result in different isoforms, with variations in both the extracellular and the intracellular domains. The positions of some of these sites seem to be conserved among LPHNs. A more detailed description of the splicing alternatives has been given [Matsushita et al., 1999]. Interestingly, the same LPHN can have a different number of isoforms across species (not all of them have been experimentally verified), which suggests an intricate physiological role for this receptor family. It is worth noting that human isoform 3 and mouse isoform 6 of LPHN3 might exist in soluble form. The amino acid sequences of these two isoforms correspond to only the extracellular portion of the proteins with no predicted transmembrane segments whatsoever. This phenomenon has been observed and well characterized for many other membrane receptors [Junttila et al., 2000; Jones et al., 2001; Suzuki et al., 2004; Tabata and Khurana Hershey, 2007; Secher, 2010]. Soluble counterparts of surface receptors may act as decoys that compete with the membrane-bound form for its specific ligand. The fact that LPHN1 and 3 possess highly conserved extracellular domains advocates for a more specialized function and may be indicative of selective pressure to maintain their ligand-binding specificity.
GENE–DRUG INTERACTIONS
Several studies focusing on a variety of genes have found interactions between LPHNs and several well-known drugs and chemicals (Table I). Most of these compounds mainly have an effect on LPHN mRNA expression, but for some others like 5-fluorouracil and methotrexate, which are cytostatics used in cancer treatment, LPHN proteins are capable of modifying the drugs' pharmacodynamic properties. Further interrogation of the brain-specific LPHN1 and LPHN3 might reveal important information about the pharmacodynamics of psychotropic drugs, with possible implications for the treatment of psychiatric disorders.
TABLE 1.
Drugs and Chemicals Known to Interact With Latrophilin Genes
| Gene | Drug | Organism | Interaction | Refs. |
|---|---|---|---|---|
| LPHN1 | Chlorine | R. norvegicus | Chlorine results in decreased expression of LPHN1 mRNA | Crosby et al. [2008] |
| Ozone | R. norvegicus | [Ozone co-treated with chlorine] results in increased expression of LPHN1 mRNA | Crosby et al. [2008] | |
| Dietary fats | M. musculus | Dietary fats results in increased expression of LPHN1 mRNA | de Wilde et al. [2008] | |
| Doxorubicin | H. sapiens | LPHN1 protein affects the chemical susceptibility to doxorubicin | Gyorffy et al. [2006] | |
| Ethinyl estradiol | R. norvegicus | Ethinyl estradiol results in decreased expression of LPHN1 mRNA | Heneweer et al. [2007] | |
| Ozone | R. norvegicus | [Ozone co-treated with chlorine] results in increased expression of LPHN1 mRNA | Crosby et al. [2008] | |
| Palm oil | M. musculus | Palm oil results in increased expression of LPHN1 mRNA | de Wilde et al. [2008] | |
| Paraquat | R. norvegicus | Paraquat affects the expression of LPHN1 mRNA | Tomita et al. [2004] | |
| Valproic acid | H. sapiens | Valproic acid results in increased expression of LPHN1 mRNA | Plant et al. [2009] | |
| Vinclozolin | R. norvegicus | Vinclozolin affects the expression of LPHN1 mRNA | Skinner et al. [2008] | |
| LPHN2 | Acetaminophen | M. musculus | Acetaminophen affects the expression of LPHN2 mRNA | Beyer et al. [2007] |
| Bisphenol A | R. norvegicus | Bisphenol A results in increased expression of LPHN2 mRNA | Naciff et al. [2002] | |
| Dietary fats | M. musculus | Dietary fats results in increased expression of LPHN2 mRNA | de Wilde et al. [2008] | |
| Ethanol | R. norvegicus | Ethanol results in increased expression of LPHN2 mRNA | Deaciuc et al. [2004] | |
| Fluorouracil | H. sapiens | LPHN2 protein affects the chemical susceptibility to fluorouracil | Gyorffy et al. [2006] | |
| Methotrexate | H. sapiens | LPHN2 protein affects the chemical susceptibility to methotrexate | Gyorffy et al. [2006] | |
| Mustard gas | R. norvegicus | Mustard gas affects the expression of LPHN2 mRNA | Dillman et al. [2005] | |
| Palm oil | M. musculus | Palm oil results in increased expression of LPHN2 mRNA | de Wilde et al. [2008] | |
| LPHN3 | Ethinyl estradiol | R. norvegicus | Ethinyl estradiol results in increased expression of LPHN3 mRNA | Naciff et al. [2005] |
| Methylselenic acid | H. sapiens | Methylselenic acid results in increased expression of LPHN3 mRNA | Zhang et al. [2005] |
LATROPHILINS IN NON-NEURONAL PROCESSES
Despite the increased attention LPHNs have received in the last few years, the number of research studies is still limited. Most of the work has tried to decipher the molecular mechanisms of presynaptic exocytosis in vertebrates, and has mainly implicated the brain-specific sisters LPHN1 and LPHN3. For the more ubiquitously expressed LPHN2 we were able to identify two publications that address non-neuronal functions of LPHNs. One of the studies found that LPHN2 is present in the primitive streak and atrioventricular canal at the time of epithelial–mesenchymal transition (EMT) in the embryonic chicken heart [Doyle et al., 2006]. The loss of LPHN2 message led to inhibition of the EMT by altering gene expression, which suggests a role for LPHN2 in facilitating Ca2+ signaling within the cell, as its closely related partner, LPHN1, has been shown to do. In the other study, LPHN2 was implicated in the development of breast cancer in humans. The authors found that aberrant expression of LPHN2 (then named LPHH1) due to loss of heterozygosity in chromosome 1p31.1 participates in maintaining a malignant phenotype [White et al., 1998].
A very recent study has implicated LPHN1 in C. elegans embryogenesis [Langenhan et al., 2009]. This study provides strong evidence that LPHN1 plays an essential role in the alignment of cell division planes during the early embryonic stages. The authors succeeded in demonstrating that LPHN1 affects the anterior-–posterior tissue polarity by interacting with a wnt spindle orientation pathway in the C. elegans embryo. Specifically, the RBL domain of LPHN1 was found to be indispensable to this interaction. LPHN1 expression was detected in the blastomeres in C. elegans early embryo and later in diverse tissues in the larval and adult stages, including the nervous system. The direct relevance of these results to vertebrate embryogenesis is remarkable, and it appears to support additional findings relating LPHNs to neuronal function in mammals.
LATROPHILINS IN NEURODEVELOPMENTAL PROCESSES
The brain-specific confinement of LPHN1 and LPHN3 and their function as GPCRs suggest possible roles in neuronal transmission and the regulation of neuronal viability. A previous study performed in rats showed that the differential expression of LPHNs in the brain affects susceptibility to ischemia-induced neuronal death. LPHN mRNAs were upregulated in CA1 neurons upon ischemic insult, whereas they were downregulated in CA3 neurons. Antisense oligonucleotides to LPHN1 and 3 mRNAs suppressed neuronal death associated with hypoxia in hippocampal and cortical cell cultures, suggesting a functional importance of these proteins in neurodegeneration [Bin Sun et al., 2002].
A striking behavioral finding was that mice lacking LPHN1 attend poorly to their offspring, which resulted in increased neonatal mortality [Tobaben et al., 2002]. In this study, homozygous mutant mice were indistinguishable in appearance from the wild type, and were fertile and viable for over 1 year, but the female knockout mice were less able than control mice to attend to their litters. As a consequence, when mouse pups were cared for by LPHN1-lacking females, most pups died within a week independently of the genotype. These data suggest that the LPHN1 deficiency does not significantly affect mouse brain function, but results in some behavioral effects. Additional behavioral analyses will be required to determine the extent of these behavioral changes.
LATROPHILINS IN PSYCHIATRIC CONDITIONS
For the first time, we found an association between LPHNs and ADHD [Arcos-Burgos et al., 2010a]. ADHD is the most common behavioral disorder of childhood affecting 8–12% of children worldwide [Biederman and Faraone, 2005]. In our study we found a significant allelic association to ADHD of several markers located inside the LPHN3 gene in chromosome 4q13.2. Iterative and expanded analyses of multiple generations of ADHD families provided a consistent and reliable presence of genetic association and linkage at the marker and haplotype level of LPHN3 variants, a finding that was strongly replicated in American, German, Spaniard, and Norwegian samples. Pooling of all samples narrowed the area of association to critical extracellular and transmembrane domains, and a region responsible for LPHN3 mRNA splicing. We also found that the dosage of the LPHN3 susceptibility haplotype varied inversely with the ratio of N-acetylaspartate/creatine (NAA/Cr), a measure of neuronal number known to be abnormal in ADHD.
The spatial and temporal expression of LPHN3 supports its role in the pathogenesis of ADHD. As mentioned above, LPHN3 is expressed in regions of the brain most affected in ADHD, that is, the amygdala, caudate nucleus, pontine nucleus, putamen, hippocampus, cerebral cortex, and cerebellar Purkinje cells [Krain and Castellanos, 2006; Arcos-Burgos et al., 2010a]. Most of these brain structures participate in three of the four main dopaminergic systems in the brain: the nigrostriatal, the mesolimbic, and the mesocortical systems. Also, LPHN3 appears to be expressed with increased intensity in more brain regions at earlier ages. For example, the 2-year-old individual we studied [Arcos-Burgos et al., 2010a] showed high levels of LPHN3 in the indusium griseum, a cerebral structure primarily involved in the embryological development of the limbic system. In addition, in situ hybridization experiments on rats have shown that the highest level of LPHN3 expression occurs in the forebrain immediately after birth and decreases during adult life [Ichtchenko et al., 1999]. Taken together, these data suggest that LPHN3 is strongly implicated in brain development at a time when ADHD is considered to arise, and provide new insights into the neurobiology and pathophysiology of human behavior.
Functional studies revealed that LPHN3 variants are expressed in key brain regions related to attention and activity. Further, these variants affect metabolism in neural circuits implicated in ADHD and are associated with response to stimulant medication. Preliminary studies suggest that incidence of ADHD in the general population would be reduced by about 9% if the effect of the LPHN3 variant that confers susceptibility to ADHD was controlled [Arcos-Burgos et al., 2010a].
Individuals with ADHD are at increased risk for substance use disorder (SUD) and disruptive behaviors [Molina et al., 1999; Palacio et al., 2004; Biederman et al., 2006; Szobot et al., 2007; Bukstein, 2008]. Children diagnosed with ADHD monitored during the transition into adolescence usually exhibit higher rates of alcohol, tobacco, and psychoactive drug use than children without ADHD [Biederman et al., 1995; Molina and Pelham, 2003]. The lifetime risk for SUD is approximately 50% in subjects with childhood ADHD persisting into adulthood [Biederman et al., 1995]. Similarly, a high ADHD prevalence is found in samples of adolescents with SUD [DeMilio, 1989; Horner and Scheibe, 1997; Kuperman et al., 2001]. In view of these findings, we decided to further interrogate markers within LPHN3 for ADHD comorbidities in several independent samples. In each sample, markers were associated with SUD and disruptive behavior disorders (Arcos-Burgos et al., 2010b).
These results, together with the previous findings implicating LPHN3 in the etiology of ADHD, strongly supports a broader role of LPHN3 in the etiology of psychiatric disorders.
CONCLUSIONS
There is sufficient accumulated data to confidently link LPHNs to brain physiology. Although the precise functions of these receptors are still unknown, the growing evidence implicating them in neurotransmitter exocytosis, their expression profile, and their unique structural properties strongly advocate for a role in interneuron communication. Our new findings relating LPHNs to psychiatric disorders will certainly provide new insights into their function in neuronal processes, especially during developmental stages.
LPHN3 seems to be a promising gene in the study of ADHD and other neurodevelopmental disorders. Comparative sequence analysis of this gene between ADHD affected and control individuals might reveal important functional variations responsible for the ADHD phenotype. Given the complexity of neuronal networks, the relevance of any mutations identified may not be immediately clear, especially because LPHN endogenous ligand(s) have not been discovered yet. So far, α-LTX and more recently the veterinary antihelmintic drug emodepside [Willson et al., 2004; Harder et al., 2005], are the only known ligands for LPHNs, but the fact that these two agents do not occur naturally in vertebrates renders these interactions physiologically irrelevant. Finding the LPHN3 endogenous ligand(s) will provide new insights into the possible role of LPHNs in the dopaminergic systems and will help us to understand, at least in part, the molecular basis of ADHD.
These results altogether open a new window into the evaluation of molecular substrates of ADHD and the development of new drugs targeted at specific brain genes and developmental pathways implicated in the disorder.
REFERENCES
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D, Domene S, Velez JI, Karkera JD, Balog J, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010a;15:1053–1066. doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Martinez AF, Acosta MT, Wallis D, de Leon J, Castellanos FX, Muenke M. Manuscript in preparation. 2010b. LPHN3 variants are associated to comorbid disruptive behavior disorders and substance use disorder. [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer RP, Fry RC, Lasarev MR, McConnachie LA, Meira LB, Palmer VS, Powell CL, Ross PK, Bammler TK, Bradford BU, et al. Multicenter study of acetaminophen hepatotoxicity reveals the importance of biological endpoints in genomic analyses. Toxicol Sci. 2007;99:326–337. doi: 10.1093/toxsci/kfm150. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Snyder LE, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychol Med. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Bin Sun H, Ruan Y, Xu ZC, Yokota H. Involvement of the calcium-independent receptor for alpha-latrotoxin in brain ischemia. Brain Res Mol Brain Res. 2002;104:246–249. doi: 10.1016/s0169-328x(02)00386-8. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Schioth HB. The adhesion GPCRs: A unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci. 2007;64:2104–2119. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Bukstein O. Substance abuse in patients with attention-deficit/hyperactivity disorder. Medscape J Med. 2008;10:24. [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Grohovaz F, Hurlbut WP. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J Cell Biol. 1979;81:163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby LM, Simmons JE, Ward WO, Moore TM, Morgan KT, Deangelo AB. Integrated disinfection by-products (DBP) mixtures research: gene expression alterations in primary rat hepatocyte cultures exposed to DBP mixtures formed by chlorination and ozonation/postchlorination. J Toxicol Environ Health A. 2008;71:1195–1215. doi: 10.1080/15287390802182581. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA. Isolation and biochemical characterization of a Ca2+-independent alpha-latrotoxin-binding protein. J Biol Chem. 1996;271:23239–23245. doi: 10.1074/jbc.271.38.23239. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Matsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA. Vesicle exocytosis stimulated by alpha-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+ EMBO J. 1998;17:3909–3920. doi: 10.1093/emboj/17.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maturana RL, Sanchez-Pernaute R. Regulation of corticostriatal synaptic plasticity by G protein-coupled receptors. CNS Neurol Disord Drug Targets. 2010;9:601–615. doi: 10.2174/187152710793361531. [DOI] [PubMed] [Google Scholar]
- de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW, de Groot P, Muller M, Mariman E, Smit E. Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics. 2008;32:360–369. doi: 10.1152/physiolgenomics.00219.2007. [DOI] [PubMed] [Google Scholar]
- Deaciuc IV, Arteel GE, Peng X, Hill DB, McClain CJ. Gene expression in the liver of rats fed alcohol by means of intragastric infusion. Alcohol. 2004;33:17–30. doi: 10.1016/j.alcohol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Deak F, Liu X, Khvotchev M, Li G, Kavalali ET, Sugita S, Sudhof TC. Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. J Neurosci. 2009;29:8639–8648. doi: 10.1523/JNEUROSCI.0898-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMilio L. Psychiatric syndromes in adolescent substance abusers. Am J Psychiatry. 1989;146:1212–1214. doi: 10.1176/ajp.146.9.1212. [DOI] [PubMed] [Google Scholar]
- Dengjel J, Kratchmarova I, Blagoev B. Receptor tyrosine kinase signaling: A view from quantitative proteomics. Mol Biosyst. 2009;5:1112–1121. doi: 10.1039/b909534a. [DOI] [PubMed] [Google Scholar]
- Deyev IE, Petrenko AG. Regulation of CIRL-1 proteolysis and trafficking. Biochimie. 2010;92:418–422. doi: 10.1016/j.biochi.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, Phillips CS, Dorsch LM, Croxton MD, Hege AI, Sylvester AJ, Moran TS, Sciuto AM. Genomic analysis of rodent pulmonary tissue following bis-(2-chloroethyl) sulfide exposure. Chem Res Toxicol. 2005;18:28–34. doi: 10.1021/tx049745z. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Scholz MJ, Greer KA, Hubbard AD, Darnell DK, Antin PB, Klewer SE, Runyan RB. Latrophilin-2 is a novel component of the epithelial–mesenchymal transition within the atrioventricular canal of the embryonic chicken heart. Dev Dyn. 2006;235:3213–3221. doi: 10.1002/dvdy.20973. [DOI] [PubMed] [Google Scholar]
- Duan ZG, Yan XJ, He XZ, Zhou H, Chen P, Cao R, Xiong JX, Hu WJ, Wang XC, Liang SP. Extraction and protein component analysis of venom from the dissected venom glands of Latrodectus tredecimguttatus. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:350–357. doi: 10.1016/j.cbpb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell–axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Fesce R, Segal JR, Ceccarelli B, Hurlbut WP. Effects of black widow spider venom and Ca2+ on quantal secretion at the frog neuromuscular junction. J Gen Physiol. 1986;88:59–81. doi: 10.1085/jgp.88.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Oshika Y, Tsuchida T, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Ueyama Y, Tamaoki N, Nakamura M. Brain-specific angiogenesis inhibitor 1 expression is inversely correlated with vascularity and distant metastasis of colorectal cancer. Int J Oncol. 1998;13:967–970. doi: 10.3892/ijo.13.5.967. [DOI] [PubMed] [Google Scholar]
- Gether U, Asmar F, Meinild AK, Rasmussen SG. Structural basis for activation of G-protein-coupled receptors. Pharmacol Toxicol. 2002;91:304–312. doi: 10.1034/j.1600-0773.2002.910607.x. [DOI] [PubMed] [Google Scholar]
- Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, Yarme A, Stetler-Stevenson MA, Siebenlist U, Kelly K. CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol. 1996;157:5438–5447. [PubMed] [Google Scholar]
- Green ML, Klein TE. A multidomain TIGR/olfactomedin protein family with conserved structural similarity in the N-terminal region and conserved motifs in the C-terminal region. Mol Cell Proteomics. 2002;1:394–403. doi: 10.1074/mcp.m200023-mcp200. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Gyorffy B, Surowiak P, Kiesslich O, Denkert C, Schafer R, Dietel M, Lage H. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 2006;118:1699–1712. doi: 10.1002/ijc.21570. [DOI] [PubMed] [Google Scholar]
- Hamann J, Vogel B, van Schijndel GM, van Lier RA. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF) J Exp Med. 1996;184:1185–1189. doi: 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder A, Holden-Dye L, Walker R, Wunderlich F. Mechanisms of action of emodepside. Parasitol Res. 2005;97:S1–S10. doi: 10.1007/s00436-005-1438-z. [DOI] [PubMed] [Google Scholar]
- Hatanaka H, Oshika Y, Abe Y, Yoshida Y, Hashimoto T, Handa A, Kijima H, Yamazaki H, Inoue H, Ueyama Y, et al. Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1) Int J Mol Med. 2000;5:181–183. doi: 10.3892/ijmm.5.2.181. [DOI] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpaa K, Laakkonen P, Peranen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- Hausott B, Kurnaz I, Gajovic S, Klimaschewski L. Signaling by neuronal tyrosine kinase receptors: Relevance for development and regeneration. Anat Rec (Hoboken) 2009;292:1976–1985. doi: 10.1002/ar.20964. [DOI] [PubMed] [Google Scholar]
- Hayflick JS. A family of heptahelical receptors with adhesion-like domains: A marriage between two super families. J Recept Signal Transduct Res. 2000;20:119–131. doi: 10.3109/10799890009150640. [DOI] [PubMed] [Google Scholar]
- Heneweer M, Houtman R, Poortman J, Groot M, Maliepaard C, Peijnenburg A. Estrogenic effects in the immature rat uterus after dietary exposure to ethinylestradiol and zearalenone using a systems biology approach. Toxicol Sci. 2007;99:303–314. doi: 10.1093/toxsci/kfm151. [DOI] [PubMed] [Google Scholar]
- Hiramatsu H, Tadokoro S, Nakanishi M, Hirashima N. Toxicon. 2010. Latrotoxin-induced exocytosis in mast cells transfected with latrophilin. (in press). DOI: 10.1016/j.toxicon.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Hlubek MD, Stuenkel EL, Krasnoperov VG, Petrenko AG, Holz RW. Calcium-independent receptor for alpha-latrotoxin and neurexin 1alpha [corrected] facilitate toxin-induced channel formation: Evidence that channel formation results from tethering of toxin to membrane. Mol Pharmacol. 2000;57:519–528. doi: 10.1124/mol.57.3.519. [DOI] [PubMed] [Google Scholar]
- Horner BR, Scheibe KE. Prevalence and implications of attention-deficit hyperactivity disorder among adolescents in treatment for substance abuse. J Am Acad Child Adolesc Psychiatry. 1997;36:30–36. doi: 10.1097/00004583-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Hu ZT, Zhao P, Liu J, Wu ZX, Xu T. Alpha-latrotoxin triggers extracellular Ca(2+)-dependent exocytosis and sensitizes fusion machinery in endocrine cells. Acta Biochim Biophys Sin (Shanghai) 2006;38:8–14. doi: 10.1111/j.1745-7270.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. Anovel ubiquitously expressed alpha-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem. 1999;274:5491–5498. doi: 10.1074/jbc.274.9.5491. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ohyama A, Ohbayashi K, Kozaki S, Komiya Y. The mechanism of the neurotransmitter release in growth cones. J Neurosci Res. 2000;60:743–753. doi: 10.1002/1097-4547(20000615)60:6<743::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: Mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: Selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc Med. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Kang X, Xiao X, Harata M, Bai Y, Nakazaki Y, Soda Y, Kurita R, Tanaka T, Komine F, Izawa K, et al. Antiangiogenic activity of BAI1 in vivo: Implications for gene therapy of humanglioblastomas. Cancer Gene Ther. 2006;13:385–392. doi: 10.1038/sj.cgt.7700898. [DOI] [PubMed] [Google Scholar]
- Kopp J, Schwede T. The SWISS-MODEL Repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32:D230–234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Schwede T. The SWISS-MODEL Repository: new features and functionalities. Nucleic Acids Res. 2006;34:D315–318. doi: 10.1093/nar/gkj056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Beavis R, Chepurny OG, Little AR, Plotnikov AN, Petrenko AG. The calcium-independent receptor of alpha-latrotoxin is not a neurexin. Biochem Biophys Res Commun. 1996;227:868–875. doi: 10.1006/bbrc.1996.1598. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, et al. alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 1997;18:925–937. doi: 10.1016/s0896-6273(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG. Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem. 2002;277:46518–46526. doi: 10.1074/jbc.M206415200. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Deyev IE, Serova OV, Xu C, Lu Y, Buryanovsky L, Gabibov AG, Neubert TA, Petrenko AG. Dissociation of the subunits of the calcium-independent receptor of alpha-latrotoxin as a result of two-step proteolysis. Biochemistry. 2009;48:3230–3238. doi: 10.1021/bi802163p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins. J Biol Chem. 2000;275:32387–32390. doi: 10.1074/jbc.C000490200. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ, Soltau M, Richter D, Bockers T. Interaction of G-protein-coupled receptors with synaptic scaffolding proteins. Biochem Soc Trans. 2002;30:464–468. doi: 10.1042/bst0300464. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Landry Y, Gies JP. Heterotrimeric G proteins control diverse pathways of transmembrane signaling, a base for drug discovery. Mini Rev Med Chem. 2002;2:361–372. doi: 10.2174/1389557023405945. [DOI] [PubMed] [Google Scholar]
- Langenhan T, Promel S, Mestek L, Esmaeili B, Waller-Evans H, Hennig C, Kohara Y, Avery L, Vakonakis I, Schnabel R, et al. Latrophilin signaling links anterior–posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev Cell. 2009;17:494–504. doi: 10.1016/j.devcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh JT, Shin BA, Ahn KY, Roh JH, Kim YJ, Kim KK. Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int J Oncol. 2001;18:355–361. [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem. 1997;272:21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: Multiply spliced G protein-coupled receptors with differential tissue distribution. FEBS Lett. 1999;443:348–352. doi: 10.1016/s0014-5793(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010;24:261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Smith BH, Pelham WE. Interactive effects of attention deficit hyperactivity disorder and conduct disorder on early adolescent substance use. Psychol Addict Behav. 1999;13:348–358. [Google Scholar]
- Moreno TA, Bronner-Fraser M. The secreted glycoprotein Noelin-1 promotes neurogenesis in Xenopus. Dev Biol. 2001;240:340–360. doi: 10.1006/dbio.2001.0472. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, Daston GP. Gene expression profile induced by 17alpha-ethynyl estradiol. bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68:184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Hess KA, Overmann GJ, Torontali SM, Carr GJ, Tiesman JP, Foertsch LM, Richardson BD, Martinez JE, Daston GP. Gene expression changes induced in the testis by transplacental exposure to high and low doses of 17{alpha}-ethynyl estradiol. genistein, or bisphenol A. Toxicol Sci. 2005;86:396–416. doi: 10.1093/toxsci/kfi198. [DOI] [PubMed] [Google Scholar]
- Nechiporuk T, Urness LD, Keating MT. ETL, a novel seven-trans-membrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J Biol Chem. 2001;276:4150–4157. doi: 10.1074/jbc.M004814200. [DOI] [PubMed] [Google Scholar]
- Palacio JD, Castellanos FX, Pineda DA, Lopera F, Arcos-Burgos M, Quiroz YT, Henao GC, Puerta IC, Ramirez DL, Rapoport JL, et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43:1506–1515. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- Peitsch MC. Protein modeling by E-mail. Bio/Technology. 1995;13:658–660. [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Plant KE, Anderson E, Simecek N, Brown R, Forster S, Spinks J, Toms N, Gibson GG, Lyon J, Plant N. The neuroprotective action of the mood stabilizing drugs lithium chloride and sodium valproate is mediated through the up-regulation of the homeodomain protein Six1. Toxicol Appl Pharmacol. 2009;235:124–134. doi: 10.1016/j.taap.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Popova NV, Plotnikov A, Deev IE, Petrenko AG. Interaction of calcium-independent latrotoxin receptor with intracellular adapter protein TRIP8b. Dokl Biochem Biophys. 2007;414:149–151. doi: 10.1134/s1607672907030155. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA. Norepinephrine exocytosis stimulated by alpha-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Philos Trans R Soc Lond B Biol Sci. 1999;354:379–386. doi: 10.1098/rstb.1999.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribases M, Bosch R, Hervas A, Ramos-Quiroga JA, Sanchez-Mora C, Bielsa A, Gastaminza X, Guijarro-Domingo S, Nogueira M, Gomez-Barros N, et al. Case–control study of six genes asymmetrically expressed in the two cerebral hemispheres: Association of BAIAP2 with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;66:926–934. doi: 10.1016/j.biopsych.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L, Meldolesi J. Alpha-latrotoxin and related toxins. Pharmacol Ther. 1989;42:115–134. doi: 10.1016/0163-7258(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal L, Zacchetti D, Madeddu L, Meldolesi J. Mode of action of alpha-latrotoxin: Role of divalent cations in Ca2(+)-dependent and Ca2(+)-independent effects mediated by the toxin. Mol Pharmacol. 1990;38:917–923. [PubMed] [Google Scholar]
- Roth AF, Sullivan DM, Davis NG. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J Cell Biol. 1998;142:949–961. doi: 10.1083/jcb.142.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneberg T, Schulz A, Gudermann T. The structural basis of G-protein-coupled receptor function and dysfunction in human diseases. Rev Physiol Biochem Pharmacol. 2002;144:143–227. [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher T. Soluble NCAM. Adv Exp Med Biol. 2010;663:227–242. doi: 10.1007/978-1-4419-1170-4_15. [DOI] [PubMed] [Google Scholar]
- Sekhar KR, Freeman ML. PEST sequences in proteins involved in cyclic nucleotide signalling pathways. J Recept Signal Transduct Res. 1998;18:113–132. doi: 10.3109/10799899809047740. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi T, Nishimori H, Ichise H, Nakamura Y, Tokino T. Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1) Cytogenet Cell Genet. 1997;79:103–108. doi: 10.1159/000134693. [DOI] [PubMed] [Google Scholar]
- Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y. Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem. 2009a;284:6495–6506. doi: 10.1074/jbc.M806979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Suckling J, Ushkaryov Y. Penelope's web: Using alpha-latrotoxin to untangle the mysteries of exocytosis. J Neurochem. 2009b;111:275–290. doi: 10.1111/j.1471-4159.2009.06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M, Lin HH, Gordon S, McKnight AJ. LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors. Trends Biochem Sci. 2000;25:284–289. doi: 10.1016/s0968-0004(00)01583-8. [DOI] [PubMed] [Google Scholar]
- Stacey M, Chang GW, Sanos SL, Chittenden LR, Stubbs L, Gordon S, Lin HH. EMR4, a novel epidermal growth factor (EGF)-TM7 molecule up-regulated in activated mouse macrophages, binds to a putative cellular ligand on B lymphoma cell line A20. J Biol Chem. 2002;277:29283–29293. doi: 10.1074/jbc.M204306200. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. alpha-Latrotoxin and its receptors: Neurexins and CIRL/latrophilins. Annu Rev Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Sudhof TC. alpha-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J Biol Chem. 1998;273:32715–32724. doi: 10.1074/jbc.273.49.32715. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ikeda T, Kuroyama H, Seki S, Kasai M, Utsuyama M, Tatsumi M, Uematsu H, Hirokawa K. Regulation of osteoclastogenesis by three human RANKL isoforms expressed in NIH3T3 cells. Biochem Biophys Res Commun. 2004;314:1021–1027. doi: 10.1016/j.bbrc.2003.12.191. [DOI] [PubMed] [Google Scholar]
- Szobot CM, Rohde LA, Bukstein O, Molina BS, Martins C, Ruaro P, Pechansky F. Is attention-deficit/hyperactivity disorder associated with illicit substance use disorders in male adolescents? A community-based case–control study. Addiction. 2007;102:1122–1130. doi: 10.1111/j.1360-0443.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Szurmant H, White RA, Hoch JA. Sensor complexes regulating two-component signal transduction. Curr Opin Struct Biol. 2007;17:706–715. doi: 10.1016/j.sbi.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: Breaking through the complexity. Curr Allergy Asthma Rep. 2007;7:338–345. doi: 10.1007/s11882-007-0051-x. [DOI] [PubMed] [Google Scholar]
- Tobaben S, Sudhof TC, Stahl B. Genetic analysis of alpha-latrotoxin receptors reveals functional interdependence of CIRL/latrophilin 1 and neurexin 1 alpha. J Biol Chem. 2002;277:6359–6365. doi: 10.1074/jbc.M111231200. [DOI] [PubMed] [Google Scholar]
- Tomita M, Okuyama T, Hidaka K, Ishikawa T, Adachi J, Nohno T. Early differential gene expression of rat lung after exposure to paraquat. Free Radic Res. 2004;38:821–829. doi: 10.1080/10715760410001713826. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Sasai N, Matsuo-Takasaki M, Sakuragi M, Murakami Y, Sasai Y. Dorsalization of the neural tube by Xenopus tiarin, a novel patterning factor secreted by the flanking nonneural head ectoderm. Neuron. 2002;33:515–528. doi: 10.1016/s0896-6273(02)00590-1. [DOI] [PubMed] [Google Scholar]
- Tymiak AA, Norman JA, Bolgar M, DiDonato GC, Lee H, Parker WL, Lo LC, Berova N, Nakanishi K, Haber E, et al. Physicochemical characterization of aouabain isomer isolated from bovine hypothalamus. Proc Natl Acad Sci USA. 1993;90:8189–8193. doi: 10.1073/pnas.90.17.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Rohou A, Sugita S. alpha-Latrotoxin anditsreceptors. Handb Exp Pharmacol. 2008;184:171–206. doi: 10.1007/978-3-540-74805-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakonakis I, Langenhan T, Promel S, Russ A, Campbell ID. Solution structure and sugar-binding mechanism of mouse latrophilin-1 RBL: A 7TM receptor-attached lectin-like domain. Structure. 2008;16:944–953. doi: 10.1016/j.str.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C, Iborra C, Martin-Moutot N, Lelianova V, Ushkaryov Y, Seagar M. alpha-latrotoxin formscalcium-permeable membrane pores via interactions with latrophilin or neurexin. Eur J Neurosci. 2000;12:3953–3962. doi: 10.1046/j.1460-9568.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Meunier FA, Lelianova VG, Dudina EE, Volkova TM, Rahman MA, Manser C, Grishin EV, Dolly JO, Ashley RH, et al. Latrophilin, neurexin, and their signaling-deficient mutants facilitate alpha-latrotoxin insertion into membranes but are not involved in pore formation. J Biol Chem. 2000;275:41175–41183. doi: 10.1074/jbc.M005857200. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Silva JP, Lelianova VG, Atiqur Rahman M, Hopkins C, Ushkaryov YA. Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C) EMBO J. 2004;23:4423–4433. doi: 10.1038/sj.emboj.7600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GR, Varley JM, Heighway J. Isolation and characterization of a human homologue of the latrophilin gene from a region of 1p31.1 implicated in breast cancer. Oncogene. 1998;17:3513–3519. doi: 10.1038/sj.onc.1202487. [DOI] [PubMed] [Google Scholar]
- Willson J, Amliwala K, Davis A, Cook A, Cuttle MF, Kriek N, Hopper NA, O'Connor V, Harder A, Walker RJ, et al. Latrotoxin receptor signaling engages the UNC-13-dependent vesicle-priming pathway in C. elegans. Curr Biol. 2004;14:1374–1379. doi: 10.1016/j.cub.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Xing Y, Nakamura Y, Rainey WE. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol. 2009;300:43–50. doi: 10.1016/j.mce.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Shiro Y. Structural basis of the signal transduction in the two-component system. Adv Exp Med Biol. 2008;631:22–39. doi: 10.1007/978-0-387-78885-2_3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dong Y, Zhao H, Brooks JD, Hawthorn L, Nowak N, Marshall JR, Gao AC, Ip C. Microarray Data Mining for Potential Selenium Targets in Chemoprevention of Prostate Cancer. Cancer Genomics Proteomics. 2005;2:97–9114. [PMC free article] [PubMed] [Google Scholar]