Abstract
Schizencephaly (SCH) is a clinically and etiologically heterogeneous cerebral malformation presenting as unilateral or bilateral hemispheric cleft with direct connection between the inner and outer liquor spaces. The SCH cleft is usually lined by gray matter, which appears polymicrogyric implying an associated impairment of neuronal migration. The majority of SCH patients are sporadic, but familial SCH has been described. An initial report of heterozygous mutations in the homeobox gene EMX2 could not be confirmed in 52 patients investigated in this study in agreement with two independent SCH patient cohorts published previously. SCH frequently occurs with additional cerebral malformations like hypoplasia or aplasia of the septum pellucidum or optic nerve, suggesting the involvement of genes important for the establishment of midline forebrain structures. We therefore considered holoprosencephaly (HPE)-associated genes as potential SCH candidates and report for the first time heterozygous mutations in SIX3 and SHH in a total of three unrelated patients and one fetus with SCH; one of them without obvious associated malformations of midline forebrain structures. Three of these mutations have previously been reported in independent patients with HPE. SIX3 acts directly upstream of SHH, and the SHH pathway is a key regulator of ventral forebrain patterning. Our data indicate that in a subset of patients SCH may develop as one aspect of a more complex malformation of the ventral forebrain, directly result from mutations in the SHH pathway and hence be considered as yet another feature of the broad phenotypic spectrum of holoprosencephaly.
Introduction
Schizencephaly [SCH (MIM 269160)] is a distinct congenital cerebral malformation presenting as transcerebral mantle cleft between lateral ventricle and pial surface. Further subgroups have been introduced based on unilateral or bilateral, mostly symmetric occurrence and separated (termed open-lip SCH) or closed cleft walls (termed closed-lip SCH) (Guerrini and Filippi 2005). SCH may be found at any position of the cerebral hemispheres, but is most frequently located in the perisylvian area. Histologically, the gray matter lining the cleft may either appear as polymicrogyria or as disorganized cortex with islands of gray matter, potentially indicating earlier or later regional disruption of cerebral development (Hayashi et al. 2002). Clinical features correlate with extent and severity of the cleft and associated cortical malformations. Frequently observed neurological symptoms include motor deficits such as hemi- or tetraparesis, and epileptic seizures (Lopes et al. 2006).
In 1996, putative causal de novo heterozygous mutations in the EMX2 gene were reported in three of eight patients with open-lip SCH, one of them a 1-bp insertion within the homeo domain sequence (Brunelli et al. 1996). Two other patients carried a heterozygous splice mutation, and altered splicing was demonstrated in transfected 3T3 cells. The same group reported de novo EMX2 mutations in a second cohort of six patients in 1997 (Faiella et al. 1997). In contrast, negative results of an EMX2 mutation analysis in two additional patient cohorts have been reported recently and extensively discussed (Tietjen et al. 2007; Merello et al. 2008).
Interestingly, Barkovich (1995) reported that up to one-third of SCH patients present with the additional features of agenesis of the septum pellucidum and optic nerve hypoplasia, also classified as septo-optic dysplasia (SOD). Likewise, systematic evaluation of MR images in a series of 35 SCH patients confirmed a high proportion of associated anomalies of forebrain midline structures, including hypo- or aplasia of the corpus callosum (20%) or septum pellucidum (69%) as well as small chiasm and optic nerve (48%) (Hayashi et al. 2002). These anomalies of midline forebrain structures, including SOD, may also occur and hence be considered as part of the wide phenotypic spectrum of the distinct developmental anomaly holoprosencephaly [HPE (MIM 236100)], the most common malformation of the human forebrain (Dubourg et al. 2007). Seventeen percent of the SCH cases reported by Hayashi et al. (2002) presented with a traversing sylvian fissure extending to the top of the hemisphere on para-sagittal images, which is also one of the characteristic features in patients with syntelencephaly, also known as middle interhemispheric fusion variant (MIHV) of HPE (Barkovich and Quint 1993). This reported substantial proportion of SCH patients with specific midline anomalies clearly rules out a casual association between those two developmental brain anomalies and instead suggests an underlying common developmental defect prior to the termination of neuronal migration, predisposing to both SOD/HPE as well as SCH in a subset of SCH patients. Furthermore, Barkovich et al. 2002 reported abnormal cortical formation in 12 of 96 HPE patients, indicating that in a subset of HPE patients the forebrain malformation is associated with disturbed neuronal migration.
The clinical term HPE, like SCH, refers to a clinically and genetically heterogeneous entity. A causal mutation in one of the three major genes SHH (Sonic hedgehog homolog), ZIC2 (Zic family member 2/odd-paired homolog), and SIX3 (Sine oculis homeobox homolog 3) can be identified in up to 25% of probands with non-syndromic HPE and normal chromosomes (Dubourg et al. 2007). Most of these mutations as well as rare mutations in additional HPE-associated genes have been related to alteration of the SHH signaling pathway. The functional consequences of the identified mutations are remarkable in several ways: (1) Under the assumption of an autosomal dominant inheritance the penetrance is reduced (about 80%). (2) An extremely wide phenotypic variation is observed even between heterozygous mutation carriers of one family ranging from single median maxillary central incisor (SMMCI) to the most severe type of HPE incompatible with postnatal life (Roessler et al. 1996; Hehr et al. 2004; Solomon et al. 2009). (3) Furthermore, the identification of more than one mutation in anecdotal patients as well as evidence for teratogenic factors suggest a significant modifying effect of both genetic as well as environmental factors. Consequently, a “multi-hit” hypothesis was proposed for the pathogenesis of HPE (Ming and Muenke 2002).
Considering the key function of SHH and its interaction partners for the patterning and development of ventral forebrain structures, the characterization of additional human phenotypes resulting from impaired SHH signaling is crucial and could be an important tool to further dissect the function of HPE-associated genes during brain development. In addition to classical HPE the currently known phenotypic HPE spectrum includes a variety of distinct heterogeneous entities like isolated SMMCI (Nanni et al. 2001), non-syndromic colobomatous microphthalmia (Schimmenti et al. 2003) or neural tube defects (Brown et al. 2002).
Given this broad phenotypic spectrum of HPE and the considerable phenotypic overlap between SCH and HPE, we hypothesized that in some patients SCH may develop as one aspect of a more complex malformation of the ventral forebrain and result from mutations in genes commonly associated with HPE.
Patients and methods
Blood or DNA samples from 52 individuals with unilateral or bilateral SCH were analyzed with informed consent for potential sequence variations in the coding regions and flanking splice sites of the EMX2 gene (reference sequence NM_004098). Available DNA samples from a subgroup of 49 schizencephaly patients of the initial patient cohort were then tested for potential sequence variations in the coding regions and flanking splice sites of the genes SHH (reference sequence NM_000193.2), SIX3 (reference sequence NM_005413.2), and ZIC2 (reference sequence NM_007129.2) by direct sequencing (EMX2: 52 patients; SHH: 2 patients; SIX3: 30 patients; ABI Prism BigDye Terminator Cycle Sequencing Kit version 1.1, ABI 3100 Avant sequencer; Applied Biosystems, Foster City, CA, USA) and/or high-resolution DNA melting (SHH: 47 patients, SIX3: 19 patients, ZIC2: 49 patients; HRM Master Mix and LightCycler 480; Roche Applied Science). Reaction protocols have previously been described (Hehr et al. 2004; Roessler et al. 2009a, b); further details are available on request. In addition, multiplex ligation-dependent probe amplification (SALSA MLPA KIT P187 Holoprosencephaly Version 03; MRC Holland, Amsterdam, the Netherlands) was performed for a subset of 27 patients of the initial patient cohort with available fresh or frozen blood samples according to the manufacturer's recommendations.
Results and discussion
Mutation analysis of the coding sequence of the EMX2 gene in 52 patients with unilateral or bilateral SCH did not reveal any potentially disease-related sequence variants. In combination with the previous data of two additional patient cohorts (Tietjen et al. 2007; Merello et al. 2008), these findings raise doubts about the functional significance of EMX2 mutations as a common cause for schizencephaly.
In order to test a potential involvement of HPE-associated genes in the pathogenesis of SCH, we then prospectively analyzed a subset of 27 SCH patients by MLPA without abnormal results. Sequence analysis of 49 SCH patients for mutations in the HPE-associated genes SHH, SIX3, and ZIC2 revealed sequence variants within the SIX3 and SHH gene in five independent SCH patients; four of them were considered as pathogenic (Table 1). For the fetus of family 1, a detailed ultrasound examination at 27 weeks of gestation revealed the presence of a complex cerebral malformation, including microbrachycephaly, alobar holoprosencephaly with monoventricle, and a large unilateral SCH cleft of the left posterior cerebral hemisphere (Fig. 1a). The pregnancy was terminated. Mild associated craniofacial features, reported at autopsy, included hypotelorism and a hypoplastic nasal bone. A heterozygous SIX3 nonsense mutation (Fig. 1b: c.385G>T; p.Glu129X) was identified in the fetal DNA sample, but not in DNA samples of both parents, confirming its de novo occurrence (Fig. 1c). This mutation has been previously reported in patients with a wide variety of HPE spectrum findings and subsequently demonstrated to result in loss of function in two different zebrafish biosensor assays (Domené et al. 2008; Lacbawan et al. 2009).
Table 1.
Genetic data for fetus/patients 1–5 with identified heterozygous sequence alterations
| Patient | Gene | Mutation |
Occurence | References | |
|---|---|---|---|---|---|
| cDNA | Protein | ||||
| Fetus 1 | SIX3 | c.385G>T | p.Glul29X | De novo | This study and Lacbawan et al. (2009) |
| Patient 2 | SIX3 | c.109G>T | p.Gly37Cys | Paternal | This study and Lacbawan et al. (2009) |
| Patient 3 | SIX3 | c.499G>T | p.Ala167Ser | NA | This study |
| Patient 4 | SIX3 | ac.618C>A + c.621G>A | ap.Gly206Gly + p.Glu207Glu | Paternal | This study |
| Patient 5 | SHH | c.869G>A | p.Gly290Asp | Maternal | This study/Schell-Apacik et al. (2009), and Roessler et al. (2009a) |
NA not assessed
Uncertain functional significance
Fig. 1.
Fetus of family 1 (a–c), and patient 2 (d–f) with identified SIX3 mutations. a Re-evaluation of fetus 1 by prenatal sonography in the 30th week of gestation with microcephaly, monoventricle, and absent corpus callosum showing a large SCH cleft on the left posterior region (arrows); b heterozygous base substitution c.385G>T in codon 129 of the SIX3 gene; c BcuI-RFLP of both parents and a normal control to confirm de novo occurrence of the resulting heterozygous nonsense mutation p.Glu129X. d Axial T1-weighted image of patient 2 showing unilateral SCH. e Sagittal T2-weighted image with characteristic thinning of the corpus callosum corresponding to the site of the cleft but without additional obvious midline anomalies; f heterozygous SIX3 missense mutation p.Gly37Cys (c.109G>T) in patient 2
The heterozygous SIX3 missense mutation p.Gly37Cys (Fig. 1f: c.109G>T) was identified in the N-terminal polyglycine stretch in a 3-year-old girl (patient 2) with unilateral schizencephaly of the left hemisphere. Cerebral MR imaging revealed characteristic thinning and kinking of the corpus callosum at the site of the cleft, thought to represent reduced transcallosal connections. But no obvious additional cerebral abnormalities were observed; in particular, there was no indication for HPE-associated malformations of the midline forebrain structures (Fig. 1d, e). Her mother first noted less frequent use of her right arm around the age of 3 months. Under continuous physiotherapy her developmental milestones were all reached in time (unaided sitting at 6 months, crawling at 9 months, and free walking at 14 months). At the age of 3 years, development of a pes equinovarus on the right side was noted. Currently, at the age of 8 years, she attends the second grade at an elementary school, and her grades are overall excellent. Neurological examination revealed a slight spastic paresis of the right leg, but was otherwise normal. Repeated electroencephalogram was normal. The missense mutation p.Gly37Cys was also identified in her father, paternal grandfather, and aunt. Clinical examination of the father did not reveal any abnormalities or craniofacial minor signs of the holoprosencephaly spectrum. Gly37Cys is currently the most N-terminal located known SIX3 missense mutation; in zebrafish biosensor assays a moderate functional effect was demonstrated (Domené et al. 2008). Together with the observation of other hypomorphic SIX3 alleles in independent HPE patients as well as the description of p.Gly37Cys in HPE patients of three further unrelated families and additional of their less-severely affected relatives, a functional and clinical relevance of this SIX3 missense mutation can also be assumed for patient 2 (Domené et al. 2008; Lacbawan et al. 2009).
A novel missense variant c.499G>T (p.Ala167Ser) within the highly conserved SIX domain of the SIX3 cDNA was identified in the first child of non-consanguineous German parents (patient 3; Fig. 2a). A preexisting maternal diabetes mellitus was well controlled during pregnancy by an insulin pump with HbA1c between 6.5 and 7%, but still needs to be considered as exogenous cofactor for fetal brain malformations. Routine ultrasound examination in week 32 of gestation indicated a cerebral malformation. The girl was born by cesarian section at 35 + 6 weeks of gestation and presented with presumable diabetic macrosomia. Postnatal MR confirmed a complex brain malformation with extended occipitotemporal open-lip SCH on the left side and large cystic formation within the left hemisphere. In silico functional sequence analysis (http://neurocore.charite.de/MutationTaster/) indicated p.Ala167Ser to be presumably disease causing. Clinical relevance of p.Ala167Ser is further supported by its location immediately adjacent to one of two putative eh1-like motifs (aa 155–166) within the highly conserved SIX domain as well as the observation of other hypomorphic missense mutations in close vicinity (p.Phe157Ile, p.Ala172Val, p.His173Pro, and p.Tyr174His) in additional independent HPE patients (Domené et al. 2008).
Fig. 2.
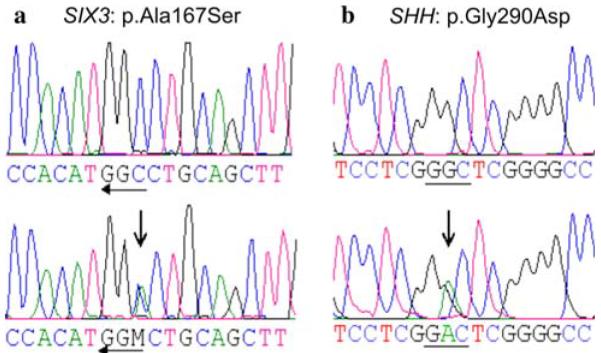
a Reverse sequence of patient 3 with heterozygous c.499G>T of the SIX3 gene (p.Ala167Ser); b heterozygous c. 869G>A of the SHH gene in patient 5 (p.Gly290Asp)
A SIX3 sequence variant of uncertain functional significance was identified in a 5 5/12-year-old male patient of non-consanguineous Syrian parents with global developmental delay (patient 4). He was found to be heterozygous for two novel adjacent synonymous base substitutions in codons 206 and 207 (c.618C>A + c.621G>A; p.Gly206-Gly + p.Glu207Glu) affecting the first two codons of the homeodomain of the SIX3 gene product, respectively. Both heterozygous substitutions were also found in the phenotypically normal father. At the age of 3 months cerebral MR imaging confirmed for patient 4 a complex brain malformation with microcephaly, left-sided open-lip SCH, enlarged ventricles, and polymicrogyric cortical malformation not only lining the cleft but also extending over both hemispheres. At the age of 5 3/12 years, a first seizure was reported without certain epilepsy characteristic activity in the subsequent electroencephalogram (EEG). Actual neuropediatric evaluation confirmed a global developmental delay, pronounced spastic tetraparesis, and muscular hypotonia. Body weight (13.1 kg), height (98 cm) as well as head circumference (43 cm) at the age of 5 5/12 years were all below the third percentile. In silico splice site prediction analysis (http://www.fruitfly.org/seq_tools/splice.html; http://www.es.embnet.org/*mwang/assp.html) did not reveal any obvious splicing effects for p.Gly206Gly + p.Glu207Glu. However, further web-based exonic splicing enhancer prediction analysis (http://rulai.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi?process=home) suggested for the mutant allele with both substitutions the creation of the consensus sequence motif for an additional SF2/ASF (score 2.71378; threshold 1.956) as well as SRp40 (score 2.98263; threshold 2.67) splice enhancer. Both consensus sequences are recognized by the human proteins SF2/ASF or SRp40, respectively, and are important for the recognition and utilization of regular as well as alternative splice sites (Wang et al. 2005).
The novel sequence variants, identified in patients 3 and 4, have not been observed in 200 control chromosomes. However, due to the lack of ethnically matched controls, in particular the presence of p.Gly206Gly + p.Glu207Glu as polymorphic allele in the Syrian population cannot be excluded.
SIX3 has originally been characterized as one of the earliest genes expressed during development of the anterior forebrain and acts directly upstream of SHH (Geng et al. 2008). Furthermore, a functional association of SIX3 with members of the groucho co-repressor sub-family was identified as an important regulator of growth and regional patterning of the vertebrate forebrain. In addition, the interaction of SIX3 and Geminin was shown to modulate the cellular decisions between proliferation and differentiation of neuroepithelial cells (Del Bene et al. 2004). Recent functional analysis in two biosensor zebrafish assays suggested loss of function, presumably through alteration of its BMP repressor function, for almost 90% of the tested 46 SIX3 mutations previously identified in HPE patients, including both SIX3 mutations p.Gly37Cys and p.Glu129X reported in this manuscript (Domené et al. 2008).
In addition, we recently identified a heterozygous SHH missense change p.Gly290Asp (Fig. 2b) in patient 5 with hypoplasia of the corpus callosum, optic atrophy, and bilateral closed-lip SCH as well as in his phenotypically normal mother. Detailed MR findings and clinical data of this patient have been reported by Schell-Apacik et al. (2009). This SHH missense mutation has also been reported before in three apparently independent patients with HPE (Roessler et al. 2009a).
Schizencephaly, like holoprosencephaly is a clinically and etiologically heterogeneous brain malformation. In contrast to porencephaly, the cleft is usually lined by a malformed cortex, suggesting an associated temporally and regionally restricted true migration defect. Furthermore, the majority of SCH patients are sporadic. More than 15% of SCH patients present with distinct additional extra-cranial abnormalities, e.g. gastroschisis, known to be associated with vascular disruption, suggesting an underlying local vascular event as the major cause of sporadic SCH (Curry et al. 2005). In some patients SCH has also been associated with intrauterine infection, e.g. cytomegalovirus infections (Iannetti et al. 1998).
However, several aspects suggest an underlying genetic etiology for a relevant subset of SCH cases: (1) Symmetric clefts of both hemispheres (e.g. patient 5 of this report) are observed in about one-third of patients. (2) About one-third of patients with unilateral SCH present with polymicrogyria in a symmetric position in the contralateral hemisphere. (3) Most importantly, recurrent SCH in sibs of a SCH patient has occasionally been observed (Hilburger et al. 1993), including one pair of affected brothers in this SCH patient cohort.
Our negative results of an EMX2 mutation screen in conjunction with two other groups (Tietjen et al. 2007; Merello et al. 2008) now increase the total number to 175 SCH patients of mixed ethnic origin without any evidence of EMX2 sequence variants.
Furthermore, we report here four heterozygous mutations and one potentially relevant sequence variant in the HPE-associated genes SIX3 and SHH in four patients and one fetus with SCH with or without associated anomalies of the cerebral midline.
Insights from the Sonic Hedgehog pathway are important for several reasons: (1) The reduced penetrance and extremely broad phenotypic variability resulting from mutations in associated genes suggest an efficient, highly redundant and complex backup system, maintaining or “hedging” an appropriate early craniofacial and brain development even under suboptimal conditions. On a genetic level this may in part be realized through rescue of mutant gene products by other members of the same or interrelated signaling pathways, and is even more obvious in mouse models, where for six3 as well as shh and several other of its interaction partners a characteristic and strain dependant brain phenotype only results from homozygous mutations, while heterozygous animals are normal (Schachter and Krauss 2008). (2) From an evolutionary point of view it is tempting to speculate that the higher diversity and function of higher mammalian brains may at least in part have been realized through loss of some of this redundancy. (3) But at the same time this may occur at the expense of a higher susceptibility to alterations by both genetic as well as exogenous factors. Consequently and in line with the “multi-hit” hypothesis (Ming and Muenke 2002), phenotypic consequences of heterozygous hypomorphic or even null alleles of SHH or other members of the SHH pathway may only emerge in the presence of additional cofactors and affect very different aspects of early craniofacial and brain development. This hypothesis would also offer a possible explanation, why mutations in this signaling cascade clinically manifest in many, but not all, human mutation carriers with so different phenotypes like holoprosencephaly, singular median maxillary incisor or the here presented example of schizencephaly, which in some patients may develop as one aspect of the broad holoprosencephaly spectrum.
Acknowledgments
The authors wish to thank the families and their physicians for their participation in this study. The authors also thank C. Mai, C. Gross, Z. Kowalczyk and T. Friedrich for excellent technical assistance, and B.H. Weber for his continuous support and encouragement.
Footnotes
Parts of the article were created within the capacity of a US Governmental Employment and therefore are in the public domain in the United States of America.
References
- Barkovich AJ. Pediatric neuroimaging. Raven Press; New York: 1995. [Google Scholar]
- Barkovich AJ, Quint D. Middle interhemispheric fusion: an unusual variant of holoprosencephaly. Am J Neuroradiol. 1993;14:431–440. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Simon EM, Clegg NJ, Kinsman SL, Hahn JS. Analysis of the cerebral cortex in holoprosencephaly with attention to the sylvian fissures. Am J Neuroradiol. 2002;23:143–150. [PMC free article] [PubMed] [Google Scholar]
- Brown LY, Hodge SE, Johnson WG, Guy SG, Nye JS, Brown S. Possible association of NTDs with a polyhistidine tract polymorphism in the ZIC2 gene. Am J Med Genet. 2002;108:128–131. doi: 10.1002/ajmg.10221. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Faiella A, Capra V, Nigro V, Simeone A, Cama A, Boncinelli E. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nat Genet. 1996;12:94–96. doi: 10.1038/ng0196-94. [DOI] [PubMed] [Google Scholar]
- Curry CJ, Lammer EJ, Nelson V, Shaw GM. Schizencephaly: heterogeneous etiologies in a population of 4 million California births. Am J Med Genet. 2005;137A:181–189. doi: 10.1002/ajmg.a.30862. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Tessmar-Raible K, Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature. 2004;427:745–749. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- Domené S, Roessler E, El-Jaick KB, Snir M, Brown JL, Vélez JI, Bale S, Lacbawan F, Muenke M, Feldman B. Mutations in the human SIX3 gene in holoprosencephaly are loss of function. Hum Mol Genet. 2008;17:3919–3928. doi: 10.1093/hmg/ddn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8–21. doi: 10.1186/1750-1172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiella A, Brunelli S, Granata T, D'Incerti L, Cardini R, Lenti C, Battaglia G, Boncinelli E. A number of schizencephaly patients including 2 brothers are heterozygous for germline mutations in the homeobox gene EMX2. Eur J Hum Genet. 1997;5:186–190. [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Filippi T. Neuronal migration disorders, genetics, and epileptogenesis. J Child Neurol. 2005;20:287–299. doi: 10.1177/08830738050200040401. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Tsutsumi Y, Barkovich AJ. Morphological features and associated anomalies of schizencephaly in the clinical population: detailed analysis of MR images. Neuroradiology. 2002;44:418–427. doi: 10.1007/s00234-001-0719-1. [DOI] [PubMed] [Google Scholar]
- Hehr U, Gross C, Diebold U, Wahl D, Beudt U, Heidemann P, Hehr A, Mueller D. Wide phenotypic variability in families with holoprosencephaly and a sonic hedgehog mutation. Eur J Pediatr. 2004;163:347–352. doi: 10.1007/s00431-004-1459-0. [DOI] [PubMed] [Google Scholar]
- Hilburger AC, Willis JK, Bouldin E, Henderson-Tilton A. Familial schizencephaly. Brain Dev. 1993;15:234–236. doi: 10.1016/0387-7604(93)90072-g. [DOI] [PubMed] [Google Scholar]
- Iannetti P, Nigro G, Spalice A, Faiella A, Boncinelli E. Cytomegalovirus infection and schizencephaly: case reports. Ann Neurol. 1998;43:123–127. doi: 10.1002/ana.410430122. [DOI] [PubMed] [Google Scholar]
- Lacbawan F, Solomon BD, Roessler E, El-Jaick K, Domené S, Vélez JI, Zhou N, Hadley D, Balog JZ, Long R, Fryer A, Smith W, Omar S, McLean SD, Clarkson K, Lichty A, Clegg NJ, Delgado MR, Levey E, Stashinko E, Potocki L, Vanallen MI, Clayton-Smith J, Donnai D, Bianchi DW, Juliusson PB, Njølstad PR, Brunner HG, Carey JC, Hehr U, Müsebeck J, Wieacker PF, Postra A, Hennekam RC, van den Boogaard MJ, van Haeringen A, Paulussen A, Herbergs J, Schrander-Stumpel CT, Janecke AR, Chitayat D, Hahn J, McDonald-McGinn DM, Zackai EH, Dobyns WB, Muenke M. Clinical spectrum of SIX3-associated mutations in holoprosencephaly: correlation between genotype, phenotype and function. J Med Genet. 2009;46:389–398. doi: 10.1136/jmg.2008.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CF, Cendes F, Piovesana AM, Torres F, Lopes-Cendes I, Montenegro MA, Guerreiro MM. Epileptic features of patients with unilateral and bilateral schizencephaly. J Child Neurol. 2006;21:757–760. doi: 10.1177/08830738060210090501. [DOI] [PubMed] [Google Scholar]
- Merello E, Swanson E, De Marco P, Akhter M, Striano P, Rossi A, Cama A, Leventer RJ, Guerrini R, Capra V, Dobyns WB. No major role for the EMX2 gene in schizencephaly. Am J Med Genet A. 2008;146A:1142–1150. doi: 10.1002/ajmg.a.32264. [DOI] [PubMed] [Google Scholar]
- Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–1032. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Du Y, Hall RK, Aldred M, Bankier A, Muenke M. SHH mutation is associated with solitary median maxillary central incisor: a study of 13 patients and review of the literature. Am J Med Genet. 2001;102:1–10. doi: 10.1002/1096-8628(20010722)102:1<1::aid-ajmg1336>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Roessler E, Lacbawan F, Dubourg C, Paulussen A, Herbergs J, Hehr U, Bendavid C, Zhou N, Ouspenskaia M, Bale S, Odent S, David V, Muenke M. The full spectrum of holoprosencephaly-associated mutations within the ZIC2 gene in humans predicts loss-of-function as the predominant disease mechanism. Hum Mutat. 2009a;30:E541–E554. doi: 10.1002/humu.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, El-Jaick KB, Dubourg C, Vélez JI, Solomon BD, Pineda-Alvarez DE, Lacbawan F, Zhou N, Ouspenskaia M, Paulussen A, Smeets HJ, Hehr U, Bendavid C, Bale S, Odent S, David V, Muenke M. The mutational spectrum of holoprosencephaly-associated changes within the SHH gene in humans predicts loss-of-function through either key structural alterations of the ligand or its altered synthesis. Hum Mutat. 2009b;30:E921–E935. doi: 10.1002/humu.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter KA, Krauss RS. Murine models of holoprosencephaly. Curr Top Dev Biol. 2008;84:139–170. doi: 10.1016/S0070-2153(08)00603-0. [DOI] [PubMed] [Google Scholar]
- Schell-Apacik CC, Ertl-Wagner B, Panzel A, Klausener K, Rausch G, Muenke M, von Voss H, Hehr U. Maternally inherited heterozygous sequence change in the sonic hedgehog gene in a male patient with bilateral closed-lip schizencephaly and partial absence of the corpus callosum. Am J Med Genet A. 2009;149A:1592–1594. doi: 10.1002/ajmg.a.32940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmenti LA, de la Cruz J, Lewis RA, Karkera JD, Manligas GS, Roessler E, Muenke M. Novel mutation in sonic hedgehog in non-syndromic colobomatous microphthalmia. Am J Med Genet A. 2003;116A:215–221. doi: 10.1002/ajmg.a.10884. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Lacbawan F, Jain M, Domené S, Roessler E, Moore C, Dobyns WB, Muenke M. A novel SIX3 mutation segregates with holoprosencephaly in a large family. Am J Med Genet A. 2009;149A:919–925. doi: 10.1002/ajmg.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen I, Bodell A, Apse K, Mendonza AM, Chang BS, Shaw GM, Barkovich AJ, Lammer EJ, Walsh CA. Comprehensive EMX2 genotyping of a large schizencephaly case series. Am J Med Genet A. 2007;143A:1313–1316. doi: 10.1002/ajmg.a.31767. [DOI] [PubMed] [Google Scholar]
- Wang J, Smith PJ, Krainer AR, Zhang MQ. Distribution of SR protein exonic splicing enhancer motifs in human protein-coding genes. Nucleic Acids Res. 2005;33:5053–5062. doi: 10.1093/nar/gki810. [DOI] [PMC free article] [PubMed] [Google Scholar]



