Abstract
The sudden onset of atrial fibrillation (AF) is often associated with rapid irregular palpitations, chest pain, shortness of breath and considerable anxiety. If a patient presents shortly after the onset of the arrhythmia the physician may adopt initially an expectant “wait and see” policy, perhaps with the help of mild sedation and drug therapy to reduce the ventricular rate. If the arrhythmia does not terminate spontaneously and has been present for less than 24-48 hours restoration of sinus rhythm by cardioversion should be considered. This manuscript reviews the option of electrical cardioversion versus pharmacologic and the data for, the role of, and the status of vernakalant with respect to the latter.
Keywords: Atrial fibrillation, amiodarone, cardioversion, vernakalant.
INTRODUCTION
The sudden onset of atrial fibrillation (AF) is often associated with rapid irregular palpitations, chest pain, shortness of breath and considerable anxiety. If a patient presents shortly after the onset of the arrhythmia the physician may adopt initially an expectant “wait and see” policy, perhaps with the help of mild sedation and drug therapy to reduce the ventricular rate. If the arrhythmia does not terminate spontaneously and has been present for less than 24-48 hours restoration of sinus rhythm by cardioversion should be considered. If the patient remains symptomatic and if no transient causative factors remain, the best option is early cardioversion. If the arrhythmia persists more than 48 hours and the patient is not anticoagulated rate control should be continued until the patient has been effectively anticoagulated for at least three weeks, or transesophageal echocardiography has shown no thrombus in the left atrium. At this point a decision on long term rate or rhythm control should be made. Generally patients who are symptomatic, active, young, and have less underlying cardiovascular disease are selected for rhythm control whereas those who are asymptomatic old, sedentary and have substantial underlying heart disease may be treated with rate control. Should rhythm control be selected early cardioversion is recommended.
ELECTRICAL VERSUS PHARMACOLOGICAL CARDIOVERSION
Electrical cardioversion may be easily accomplished by DC cardioversion. With relatively recent onset AF the technique is almost always successful, although recurrences of the arrhythmia are common. In an urgent setting the technique has the disadvantages of requiring a fasted patient andan anaesthesiologist. If both of these conditions are not met pharmacological cardioversion should be considered. In other settings the patient or physician may prefer the use of antiarrhythmic therapy, which can be easily administered and rapidly effective. Physician preference for the pharmacological approach varies widely and largely depends on previous experience, local tradition and national regulations, such as the compulsory attendance of an anesthesiologist. In theory the pharmacological technique has the advantage of providing a continuing antiarrhythmic effect immediately following cardioversion to ward off AF recurrence, and testing the efficacy of a drug which might then be used for long term control of the arrhythmia although effects re: cardioversion and suppression of recurrence can be discordant.
DRUGS FOR CARDIOVERSION
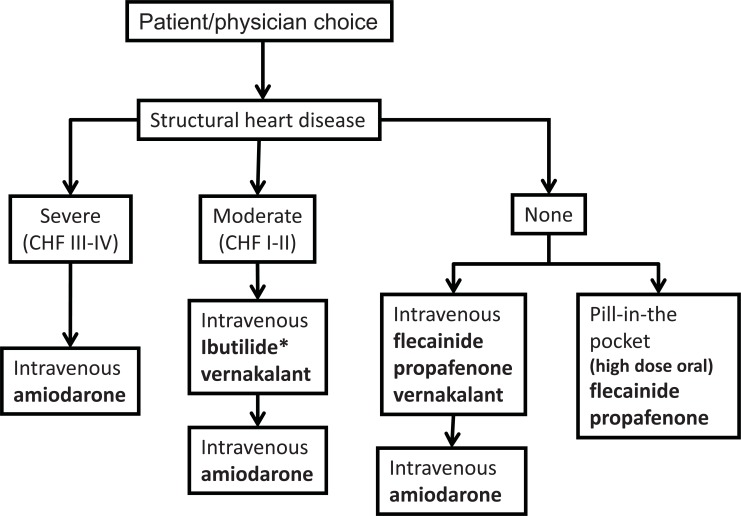
There is a wide range of antiarrhythmic agents that might be used for conversion of AF to sinus rhythm [1-15] (Table 1). Flecainide and propafenone may be given orally as a large single dose – the “pill-in-the-pocket” technique. Usually, however, antiarrhythmic agents are given intravenously as prolonged injections or slow infusions. Amiodarone, flecainide, propafenone and ibutilide are the most usual, but sotalol has also been used. The choice of drug depends on the availability of the drug, the presence of underlying moderate or severe structural heart disease, the urgency of the situation and the experience of the physician. An approach to cardioversion of AF, and the choice of drug is illustrated in (Fig. 1).
Table 1.
Drugs used for cardioversion of AF. Vernakalant is not approved in the USA and ranolazine is not approved anywhere for this indication. Ibutilide is only sporadically approved outside the USA.
| Drug | Dose | Efficacy | Acute Side Effects |
|---|---|---|---|
| Flecainide | 200-300 mg p.o. stat 2mg/kg i.v. over 10 min |
55-85% | Hypotension, rapid AFL, QRS widening |
| Propafenone | 450-600 mg p.o. stat 2 mg/kg i.v. over 10 min |
52-85% | Hypotension, rapid AFL, QRS widening |
| Amiodarone | 150 mg i.v. bolus, or 5 mg/kg i.v. over 1 hour | 35-90% (delayed by 8-24 h) | Hypotension, bradycardia, QT prolongation (low risk of TdP), phlebitis |
| Ibutilide | 1 mg i.v. over 10 min, repeat if necessary | 25-50% | QT prolongation, TdP, bradycardia |
| Vernakalant | 3 mg/kg i.v. over 10 minutes, then, if needed, after 15 minutes 2mg/kg over 10 minutes | 48-62% | Hypotension (esp. in heart failure), QT prolongation (low risk of TdP) |
| Ranolazine | 2 g p.o. stat | ~60-70% | Well-tolerated, but limited data |
Not all drugs which are widely approved are available everywhere. TdP: torsades de pointes, AFl: atrial flutter. Flecainide and propafenone are not available i.v. in the U.S.
Fig. (1).
Choice of pharmacological agent for cardioversion in an acute setting. Choice of an antiarrhythmic drug is dependent on availability and underlying structural heart disease. Optimum drug choices are indicated in bold. CHF = congestive heart failure.
VERNAKALANT
Vernakalant is a novel antiarrhythmic agent which is available in many parts of the world, but only as an intravenous formulation. It is not currently available in the USA. The drug is designed for rapid termination of acute onset AF in a patient with no or minimal heart disease and some forms of structural heart disease including stable coronary heart disease, left ventricular hypertrophy or mild heart failure [3-8, 12-21].
ELECTROPHYSIOLOGICAL PROPERTIES OF VERNAKALANT
Vernakalant [3-8, 12-21] is a novel amino-cyclohexyl ether drug, which has a unique ion channel-blocking profile amongst drugs used clinically for cardioversion of AF. It exerts a frequency- and voltage-dependent INa block, including inhibition of the late sodium current, which is probably the most important of its electrophysiological effects with regard to termination of AF. The powerful effect on intra-atrial conduction at fast rates is due to blockade of this current. However it also inhibits the early activating K+ channels (IKur; Ito) and IKACh. IKur and IKACh are currents which are specific to the atrium and cause prolongation of atrial rather than ventricular refractoriness and may contribute to the efficacy of the drug. The effect of vernakalant on these currents was responsible for its original classification as an ARDA (atrial repolarization delaying agent). However, vernakalant also blocks the rapidly activating potassium current IKr which accounts for mild QT prolongation. QRS widening due to INa blockade also contributes to QT prolongation.
Other drugs such as antazoline [10, 11], a first generation antihistamine, and ranolazine [1], an agent used predominantly for the treatment of chronic stable angina, have similar electrophysiological properties and ranolazine in particular shares many of the properties of vernakalant including blockade of the late sodium current.
VERNAKALANT CLINICAL TRIAL PROGRAM [3-8, 12-21]
Clinical electrophysiological studies demonstrated that vernakalant had a differential effect on cardiac tissue refractoriness, prolonging atrial much more than ventricular refractory periods. Vernakalant also prolonged AV nodal refractoriness and lengthened the Wenckebach point. Sinus node recovery was prolonged. Although the ventricular QRS duration increased at fast cycle lengths there was no measurable effect on atrial paced or spontaneous QRS or QT durations.
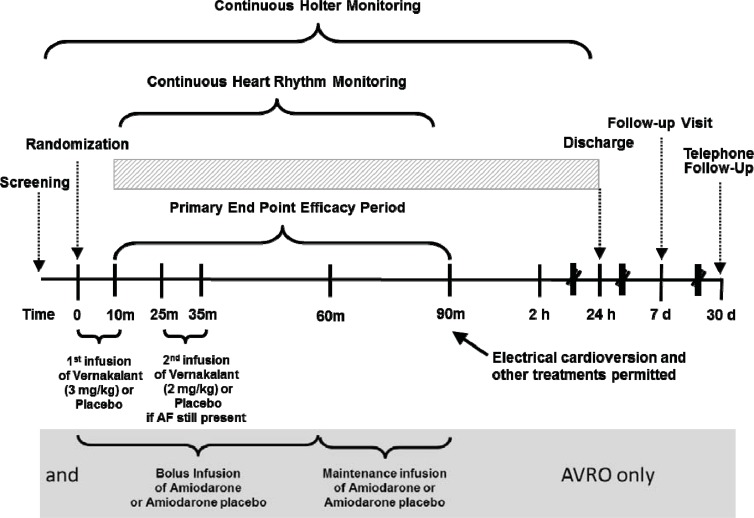
CRAFT (Controlled Randomised Atrial Fibrillation Trial) was a dose ranging study which compared two regimens involving two sequential infusions over 10 minutes spaced by 15 minutes if atrial arrhythmia had not terminated following the initial infusion (see Fig. 2). The efficacy of 2 mg/kg followed by 3 mg/kg was inferior to 0.5 mg/kg followed by 1 mg/kg in terms of termination of AF (placebo: 1/19, low dose: 2/18 high dose: 11/18 [61%]). Since there was a substantial increase in termination after the second infusion the order of dosing was reversed and 3 mg/kg followed by 2 mg/kg was selected as the dose regimen for all subsequent clinical trials.
Fig. (2).
Schematic of the clinical trials for the development of vernakalant. All trial used sinus rhythm at 90 minutes after the start of the vernakalant infusion as the primary endpoint. In CRAFT the infusion doses were reversed. In CRAFT, ACT I, II and III patients were randomised to blinded vernakalant or placebo. In AVRO patients received either amiodarone or vernakalant (or appropriate placebos) in a in a double-blind, double-dummy design. ACT IV was not controlled, but contributed to the safety database of vernakalant.
The pivotal clinical trials (Table 2) were all known as ACT (Arrhythmia Conversion Trials). ACT I and II were controlled randomised (2:1 vernakalant:placebo) trials of AF termination in patients with AF 3 hours to 45 days duration. The primary endpoint in this and all other trials in the program was the percentage of patients in sinus rhythm at 90 minutes after the start of the vernakalant infusion. Patients with no heart disease or mild heart failure, or stable coronary artery disease were enrolled. Some were already on treatment with antiarrhythmic drugs. Patients with baseline QT prolongation (QT>440 msec), hypotension, moderate to severe heart failure (NHYA II or IV) or acute coronary syndrome within 30 days were excluded. The basic trial design remained similar to that of the CRAFT study and was the template for all other studies (Fig. 2). Over 500 patients were enrolled in these two studies.
Table 2.
Clinical trial program for vernakalant. All trials included patients with atrial fibrillate except Scene 2 which enrolled patients with atrial flutter.
| Study | Design | Number of patients | AF duration | Primary endpoint |
|---|---|---|---|---|
| CRAFT | Double-blind, dose-ranging, placebo-controlled, phase II | 56 | 3-72 h | Proportion of patients converted to SR during or within 30 minutes after the last infusion |
| ACT I | Double-blind, placebo controlled, phase III | 336 | 3 h - 45 days stratified by 3 h - 7 days and 8-45 days |
Proportion of patients converted to SR within 90 minutes of drug initiation in AF 3 h - 7 days |
| ACT II | Double-blind, placebo controlled, phase III | 160 | 3-72 h 24 h - 7 days after cardiac surgery |
Proportion of patients converted to SR within 90 minutes of drug initiation in AF 3 h - 7 days |
| ACT III | Double-blind, placebo controlled, phase III | 262 | 3 h - 45 days stratified by 3 h - 7 days and 8-45 days |
Proportion of patients converted to SR within 90 minutes of drug initiation in AF 3 h - 7 days |
| ACT IV | Open-label, safety, phase III/IV | 167 | AF 3 h - 45 days | Proportion of patients converted to SR within 90 minutes of drug initiation in AF 3 h - 7 days |
| AVRO | Double-blind, active-controlled (i.v. amiodarone), phase III | 232 | AF 3 - 48 h | Proportion of patients converted to SR within 90 minutes of drug initiation |
| Scene 2 | Double-blind, controlled, phase II/III | 54 | Atrial flutter 3 h - 45 days | Proportion of patients converted to SR within 90 minutes of drug initiation |
ACT II recruited 160 patients who developed AF, between 3 and 72 hours duration occurring between 24 hours and 7 days after cardiac surgery. ACT IV was an uncontrolled open-label safety study that recruited 167 patients.
AVRO (A Phase III Prospective, Randomized, Double-Blind, Active-Controlled, Multi-Center, Superiority Study of Vernakalant Injection versus Amiodarone in Subjects with Recent Onset Atrial Fibrillation) was an active comparator study that was required by European regulators. The comparator was amiodarone, which was chosen because it was the market leader for this indication (most popular drug for AF conversion), available in all the countries in which the study took place and approved for use in patients with underlying heart disease. Other antiarrhythmics, such as flecainide, were considered but rejected for obvious reasons. However, comparisons with class 1C agents are now needed. Since the universal endpoint in the vernakalant development program was the percentage of patients in sinus rhythm at 90 minutes this was also the primary endpoint in the AVRO study. Because of the fundamentally different methods of intravenous administration of amiodarone and vernakalant a double-blind, double-dummy technique was employed (see Fig. 2). Patient recruitment was similar to that in the ACT I and III trials except that less hypotensive patients (blood pressure less than 110 mmHg rather than 100 mmHg) were excluded.
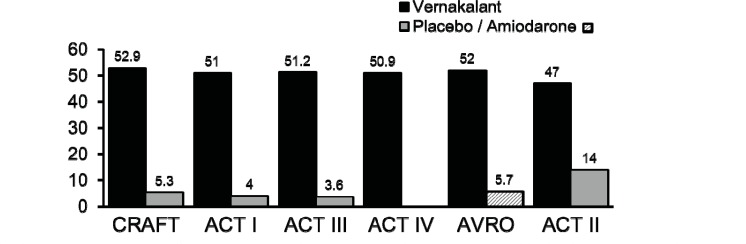
The results of all studies were remarkably consistent – approximately 50% of the patients who received vernakalant reverted to sinus rhythm before 90 minutes compared with approximately 5% with placebo or amiodarone (Fig. 3). In ACT II 14% of placebo treated patients reverted to sinus rhythm which was not unexpected. The efficacy difference between amiodarone and vernakalant was remarkable but anticipated. Although amiodarone slows the ventricular rate promptly and therefore reduces symptoms, AF conversion is generally late and closer to 24 hours than 90 minutes.
Fig. (3).
Termination of recent onset AF following intravenous infusion of vernakalant compared with placebo (ACT I, II and III) or amiodarone (AVRO). The median time to termination was between 8 and14 minutes. 75-80% converted after the first dose.
Modelling studies based on the date from the ACT studies suggest that the conversion rate is critically dependent on the duration of the AF episode, and that approximately 75% of AF lasting less than 24 hours should terminate in response to vernakalant infusion. Meta-analysis shows that most patients respond similarly except those with heart failure who are more resistant. Indirect comparisons show that vernakalant is at least as good, if not superior to flecainide and propafenone. The effect of vernakalant infusion is durable in that AF recurs within 30 days in less than 5%.
Of note: in each of these studies, despite efficacy for AF, vernakalant was no better than placebo for terminating atrial flutter.
Adverse effects were few – only one case of torsades de pointes could possibly have been related to vernakalant infusion and in that case an ibutilide infusion had also been administered (a protocol violation). Hypotension and sinus bradycardia were the most significant cardiovascular adverse events. Hypotension can be minimised by ensuring adequate hydration prior to administration of vernakalant. A constellation of dysgeusia, paraesthesia and nausea occurred in between 10 - 20% of patients, but these effects were neither prolonged nor serious.
POST APPROVAL STUDIES
Since approval in Europe and elsewhere vernakalant has been used in a variety of settings. Urgent and near immediate cardioversions have been performed in the emergency room, catheter laboratory, electrophysiology laboratory, coronary care unit and intensive care unit. The drug has also been used electively in patients with minimal or modest heart disease. Most of these small but relevant studies have not yet been fully reported but the results have been favorable and the investigators have been enthusiastic in their endorsement of this new drug. Few if any proarrhythmic effects other than transient ventricular arrhythmias and 1:1 atrial flutter have been reported. Importantly there are not yet any report of torsade de pointes.
TERMS OF APPROVAL IN THE EUROPEAN UNION
In 2010 vernakalant was approved by the European Union for cardioversion of AF which was less than 7 days in duration, or for post-operative AF less than 3 days in duration. The drug is contraindicated when systolic blood pressure is less than 100 mm Hg, when severe aortic stenosis, or heart failure (class NYHA III and IV) is present, when acute coronary syndrome has occurred within the previous 30 days, if there is QT interval prolongation (baseline > 440 msec), or when intravenous Class I/III antiarrhythmic dugs have been administered within the previous 4 hours. Before its use patients should be adequately hydrated and ECG and hemodynamic monitoring should be used. It was noted that the infusion can be followed by DC cardioversion if necessary.
The terms of the approval are very strictly aligned to the evidence base for vernakalant. Of the drugs previously approved for cardioversion only ibutilide had a comparably sized database and the safety of ibutilide was compromised by the risk of torsade de pointes which had not surfaced as a problem during the development of vernakalant. Regulators were able to label the drug to avoid the majority of other adverse effects of vernakalant offered an alternative to amiodarone in patients with mild or moderate heart disease. Although amiodarone achieves conversion in a high proportion of patients its action is relatively delayed. A clear unmet need could potentially be filled by this new antiarrhythmic drug. The efficacy of vernakalant versus its placebo for atrial fibrillation conversion was greater in its clinical trials than that of ibutilide versus placebo in its clinical trials, whereas the opposite was true for atrial flutter.
Vernakalant has been marketed in some, but not all European countries, both within and outside the European Union. Other countries, notably in Asia have also used the drug after appropriate approval.
WHAT IS THE SITUATION IN THE USA?
When vernakalant appeared before an FDA advisory panel it was recommended for approval, but the FDA continued to have questions about the safety of the drug, and to some extent about the need for pharmacological cardioversion. In any event it was agreed with the FDA that a further study with any drug should be conducted (ACT V) designed not only to bolster efficacy data, but most importantly to further explore safety and to strengthen the safety database. Unfortunately the study ran into difficulty early when a single patient died following the infusion of vernakalant without certainty that the drug was not involved. Although full details of this event have not been made public the FDA did not favor the continuation of the study without further protocol revisions. The study was then discontinued. Despite further discussion and negotiations the sponsor and the agency were unable to agree on any further study that would satisfactorily clarify the benefit-risk ratio of intravenous vernakalant for AF cardioversion. Thus, the FDA remained unconvinced that vernakalant was worthy of approval.
Although an on-going post-approval database was being steadily accumulated outside of the U.S. and plans were made for development of the oral formulation of vernakalant, the then sponsor of vernakalant decided that reluctance from the FDA to license the intravenous administration of vernakalant for AF termination rendered further development unattractive and its further development was halted.
Meanwhile the drug remains marketed in many other parts of the world and is generally considered useful for the rapid termination of recent onset AF, provided that patients are appropriately selected, both electrocardiographic and hemodynamic monitoring are in place, and the drug is infused in accordance with its approved label. In the future other sponsors may elect to apply to the FDA for further consideration for U.S. approval. Meanwhile other drugs, old and new with similar electrophysiological properties continue to be investigated and developed.
The difference between regulatory opinions on both sides of the Atlantic is disturbing and perplexing. Agencies in Europe and the USA came to different conclusions when examining the same evidence-base. Thus, neither opinion can be entirely or easily accepted. Such differences lead to implausible and ridiculous positions that cannot be defended. The importance of the USA “market” is such that the decisions of the FDA have an influence that is more widespread than its jurisdiction or mandate should allow. Sponsors abandon drugs that do not have the blessing of the FDA and leave the world in general at a loss to influence progress.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
Professor Camm has been an advisor and consultant for Cardiome and Merck.
REFERENCES
- 1.Burashnikov A, Antzelevitch C. Role of Late Sodium Channel Current Block in the Management of Atrial Fibrillation. Cardiovasc Drugs Ther. 2013;27(1):79–89. doi: 10.1007/s10557-012-6421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EU Summary of Product Characteristics. BRINAVESS MSD. 2010 [Google Scholar]
- 3.de Riva-Silva M, Montero-Cabezas JM, Salgado-Aranda R, López-Gil M, Fontenla-Cerezuela A, Arribas-Ynsaurriaga F. 1:1 Atrial Flutter After Vernakalant Administration for Atrial Fibrillation Cardioversion. Rev Esp Cardiol. 2012;65(11):1062–4. doi: 10.1016/j.recesp.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Bash LD, Buono JL, Davies GM , et al. Systematic review and meta-analysis of the efficacy of cardioversion by vernakalant and comparators in patients with atrial fibrillation. Cardiovasc Drugs Ther. 2012;26(2):167–79. doi: 10.1007/s10557-012-6374-4. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A, Pourrier M, Gibson JK, Lynch JJ, Antzelevitch C. Rate-dependent effects of vernakalant in the isolated non-remodeled canine left atria are primarily due to block of the sodium channel: comparison with ranolazine and dl-sotalol. Circ Arrhythm Electrophysiol. 2012;5(2):400–8. doi: 10.1161/CIRCEP.111.968305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camm AJ, Toft E, Torp-Pedersen C , et al. Efficacy and safety of vernakalant in patients with atrial flutter: a randomized. double-blind.placebo-controlled trial.Europace . 2012; 14(6):804–9. doi: 10.1093/europace/eur416. [DOI] [PubMed] [Google Scholar]
- 7.Buccelletti F, Iacomini P, Botta G , et al. Efficacy and safety of vernakalant in recent-onset atrial fibrillation after the European medicines agency approval: systematic review and meta-analysis. J Clin Pharmacol. 2012;52(12):1872–8. doi: 10.1177/0091270011426876. [DOI] [PubMed] [Google Scholar]
- 8.Torp-Pedersen C, Camm AJ, Butterfield NN, Dickinson G, Beatch GN. Vernakalant: Conversion of atrial fibrillation in patients with ischemic heart disease. Int J Cardiol. 2013;166(1):147–51. doi: 10.1016/j.ijcard.2011.10.108. [DOI] [PubMed] [Google Scholar]
- 9.Heldal M, Atar D. Pharmacological conversion of recent-onset atrial fibrillation: A systematic review. Scand Cardiovasc J. 2013;47(1):2–10. doi: 10.3109/14017431.2012.740572. [DOI] [PubMed] [Google Scholar]
- 10.Farkowski MM, Maciag A, Dabrowski R, Pytkowski M, Kowalik I, Szwed H. Clinical efficacy of antazoline in rapid cardioversion of paroxysmal atrial fibrillation -- a protocol of a single center. randomzed. double-blind., placebo-controlled study (the AnPAF Study). Trials. 2012;13:162. doi: 10.1186/1745-6215-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline SR, Dreifus LS, Watanabe Y, McGarry TF, Likoff W. Evaluation of the antiarrhythmic properties of antazoline.A preliminary study. Am J Cardiol. 1962;9:564–7. doi: 10.1016/0002-9149(62)90075-9. [DOI] [PubMed] [Google Scholar]
- 12.Friederich P, Pfizenmayer H. The novel Kv1. channel blocker vernakalant for successful treatment of new-onset atrial fibrillation in a critically ill abdominal surgical patient. Br J Anaesth. 2011;107(4):644–5. doi: 10.1093/bja/aer278. [DOI] [PubMed] [Google Scholar]
- 13.Torp-Pedersen C, Raev DH, Dickinson G, Butterfield NN, Mangal B, Beatch GN. A randomized. placebo-controlled study of vernakalant (oral) for the prevention of atrial fibrillation recurrence after cardioversion. Circ Arrhythm Electrophysiol. 2011;4(5):637–43. doi: 10.1161/CIRCEP.111.962340. [DOI] [PubMed] [Google Scholar]
- 14.Bechard J, Gibson JK, Killingsworth CR , et al. Vernakalant selectively prolongs atrial refractoriness with no effect on ventricular refractoriness or defibrillation threshold in pigs. J Cardiovasc Pharmacol. 2011;57(3):302–7. doi: 10.1097/FJC.0b013e3182073c94. [DOI] [PubMed] [Google Scholar]
- 15.Camm AJ, Capucci A, Hohnloser SH , et al. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol. 2011;57(3):313–21. doi: 10.1016/j.jacc.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Pratt CM, Roy D, Torp-Pedersen C , et al. Atrial Arrhythmia Conversion Trial (ACT-III) Investigators.Usefulness of vernakalant hydrochloride injection for rapid conversion of atrial fibrillation. Am J Cardiol. 2010;106(9):1277–83. doi: 10.1016/j.amjcard.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Stiell IG, Roos JS, Kavanagh KM, Dickinson G. A multicenter. open-label study of vernakalant for the conversion of atrial fibrillation to sinus rhythm. Am Heart J. 2010;159(6):1095–101. doi: 10.1016/j.ahj.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Kowey PR, Dorian P, Mitchell LB , et al. Atrial Arrhythmia Conversion Trial Investigators.Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a random zed.double-blind., placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009; 2(6):652–9. doi: 10.1161/CIRCEP.109.870204. [DOI] [PubMed] [Google Scholar]
- 19.Roy D, Pratt CM, Torp-Pedersen C , et al. Atrial Arrhythmia Conversion Trial Investigators.Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phae 3.randomized., placebo-controlled trial. . Circulation. 2008; 117(12):1518–25. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 20.Dorian P, Pinter A, Mangat I, Korley V, Cvitkovic SS, Beatch GN. The effect of vernakalant (RSD1235). an investigational antiarrhythmic aent.on atrial electrophys-iology in humans. . J Cardiovasc Pharmacol. 2007;50(1):35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- 21.Roy D, Rowe BH, Stiell IG , et al. A randomized. controlled trial of RSD 235.a novel anti-arrhythmic agent., in the treatment of recent onset atrial fibrillation. . J Am Coll Cardiol . 2004; 44(12):2355–61. doi: 10.1016/j.jacc.2004.09.021. [DOI] [PubMed] [Google Scholar]