Abstract
Peripartum cardiomyopathy (PPCM) is a form of idiopathic dilated cardiomyopathy affecting women in late pregnancy or early puerperium. Although initially described in the late 1800s, it has only recently been recognized as a distinct cardiac condition. The reported incidence and prognosis varies according to geography. The clinical course varies between complete recovery to rapid progression to chronic heart failure, heart transplantation or death. In spite of significant improvements in understanding the pathophysiology and management of the PPCM many features of this unique disease are poorly understood, including incidence, etiology, epidemiology, pathophysiology, predictors of prognosis and optimal therapy. The present article revisits these concepts and recent advances in PPCM.
Keywords: Peripartum cardiomyopathy, review.
INTRODUCTION
Peripartum cardiomyopathy (PPCM) is usually defined as development of heart failure secondary to left ventricle systolic dysfunction towards the end of pregnancy or in the months following delivery, without other identifiable causes for dysfunction of the heart [1, 2]. PPCM can be difficult to recognize because symptoms of heart failure such as orthopnea, dyspnea, edema (most commonly in the legs and ankles), palpitations, chest pain and cough may be present in women during the last months of a normal pregnancy and early puerperium.
HISTORICAL PERSPECTIVE OF DEFINITION IN PPCM
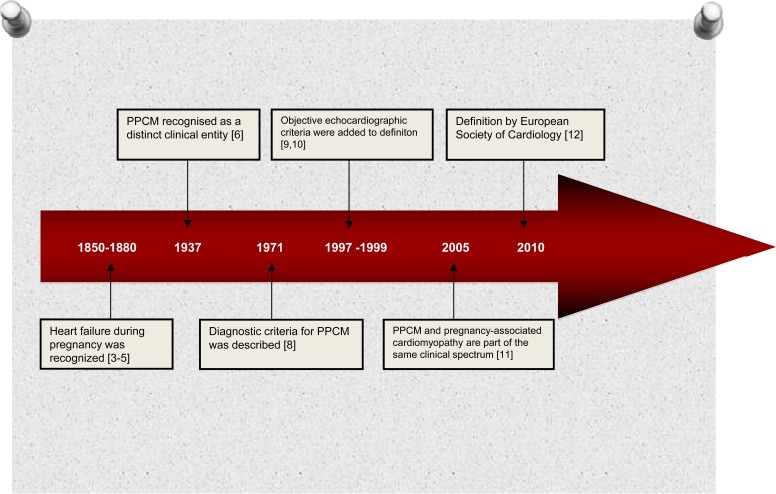
The association between cardiac failure and the puerperium was first noted in the 19th century [3-5] but PPCM was first described by Gouley and colleagues in 1937 who were described the clinical and pathological features of 7 pregnant women with fatal heart failure [6] (Fig. 1). One year late Gouley, Hull and Hidden described 80 patients with this condition and defined it ‘Postpartal Heart Failure’ [7]. In 1971, Demakis et al. were first to describe the diagnostic criteria of PPCM [8] as the development of new onset heart failure within the last month of pregnancy or first 5 months postpartum, in the absence of any identifiable cause for heart failure, and any recognisable heart disease before the last month of pregnancy. In 1997, the National Heart, Lung, and Blood Institute (NHLBI) and the Office of Rare Diseases ofthe National Institutes of Health (NIH) of the United States, added echocardiographic criteria, such as depressed left ventricular shortening fraction (<30%) or left ventricular ejection fraction (<45%) to Demakis’s definition of PPCM [9]. In 1999, Hibbard has also proposed that additional criteria should include objective echocardiographic measurements of left ventricular dysfunction (ejection fraction less than 45%, fractional shortening of 30% or both and end-diastolic dimension of greater than 2.7 cm/m² body surface area) (10]. Uri Elkayam and colleagues showed that although the majority of patients with PPCM are diagnosed in the peripartum period, early presentation during pregnancy is not uncommon [11]. They evaluated 23 cases with pregnancy-associated cardiomyopathy diagnosed between the 17th and 36th weeks of gestation found them to be indistinguishable from 100 women meeting classic criteria for PPCM [11].
Fig. (1).
Historical perspective of definition in peripartum cardiomyopathy.
These findings indicate that PPCM and pregnancy-associated cardiomyopathy are part of the same clinical spectrum and some patients may present with PPCM symptoms earlier than the last gestational month. A recent position statement from a European Society of Cardiology (ESC) working group on PPCM has therefore expanded the definition of PPCM to “an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricle systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found” [12].
EPIDEMIOLOGY
The true incidence of PPCM is unknown due to incomplete detection and reporting of cases and lack of systematic epidemiological studies. Most studies were single-centre case series and reflect a variance in the incidence of PPCM in different parts of the world, suggesting a geographical and etnic disparity. Most of these studies were performed in the USA, Haiti and South Africa. From the available literature, the incidence of PPCM appears to be around 1 in 1149–4350 in the USA [13-16], 1 in 1000 in South Africa [17-19], and 1 in 299 in Haiti [20, 21]. Data from the rest of the world including European countries are much more limited.
ETIOLOGY
The etiology and pathogenesis of PPCM is poorly understood. Multiple etiologies have been proposed for PPCM including inflammation, viral myocarditis, abnormal immune or hemodynamic response to pregnancy, apoptosis, hormonal abnormalities, impaired angiogenesis, increased oxidative stress, malnutrition, cardiomyocyte-specific deletion of the transcription factor signal transducer and activator of transcription 3 (STAT3) protein and genetic factors. Studies supporting these etiologies are discussed in more detail below.
INFLAMMATION
In a single-centre, prospective study of 100 patients with newly diagnosed PPCM, Sliwa and colleagues reported that the baseline levels of C-reactive protein (CRP) correlated positively with baseline left ventricle diameters and inversely with left ventricle ejection fraction and baseline Fas/Apo-1 levels predicted mortality [18]. In another study, serum markers of inflammation, including CRP, interferon γ and interleukin-6, have been found to be elevated in PPCM patients compared with healthy female control subjects [22].
MYOCARDITIS
Some previous studies suggested that PPCM might be caused by viral infections. Reported incidence of myocarditis in peripartum cardiomyopathy varies from 9% to 62% [23-27].
Bultmann and colleagues performed molecular investigation of endomyocardial biopsy specimens from 26 patients diagnosed with PPCM [24]. Eight patient specimens (30.7%) demonstrated viral genomes from a wide variety of pathogens, including parvovirus B19, human herpes simplex virusn 6, Epstein Barr virus, and human cytomegalovirus. However, the prevalence of these viruses was virtually identical in 33 women control subjects (30.3%) without evidence of myocardial tissue inflammation who had endomyocardial biopsy for the exclusion of myocarditis or medication-associated cardiac disease. Felker and colleagues studied a population of 42 women with PPCM, all of whom had undergone an endomyocardial biopsy, and right heart catheterization [27]. In this study, 62% of the study subjects had clinical histologic evidence of myocarditis or borderline myocarditis.
Cenac et al. found a correlation between anti-Chlamydia pneumoniae antibodies and PPCM in and suggested that these antibodies were prognostic indicators for PPCM; with higher levels of the antibodies being associated with a poorer prognosis [28, 29]. Although these findings suggest an association between myocarditis and PPCM, no causal relationship has yet been determined. Depression of immune function during pregnancy might contribute to triggering the reactivation of a latent virus and result in PPCM.
ABNORMAL IMMUNOLOGIC AND HEMODYNAMIC RESPONSES TO PREGNANCY
Fetal microchimerism refers to the presence of very low numbers of fetal cells in a woman who is, or has been, pregnant [30]. It has been estimated that the number of fetal cells in maternal peripheral blood during the second trimester is around 1–6 cells/ml [31].
Increased fetomaternal transfer of cells occurs after termination of pregnancy, and women with a history of fetal loss have been reported to exhibit a high frequency of fetal cell microchimerism [30, 31]. Ansari and colleagues suggested that fetal microchimerism may trigger an exaggerated autoimmune response in the postpartum period [32]. This could explain the higher incidence of PPCM in twin pregnancies and recurrences in subsequent pregnancies.
Physiological changes during pregnancy facilitate the adaptation of the cardiovascular system to the increased metabolic needs of the mother [33]. These changes cause a reversible hypertrophy and dilatation of the left ventricle which resolves shortly after birth in a normal pregnancy. PPCM patients may have an abnormal left ventricular recovery response to physiological changes [34].
PROLACTIN
STAT3 is thought to be involved in cardiac protection from pregnancy induced oxidative stress by inhibition of the reactive oxygen species. Recently, Hilfiker-Kleiner et al. demonstrated that female mice with cardiomyocyte-specic deletion of STAT3 developed PPCM phenotype [35]. A reduction in STAT-3 leads to increased oxidative stress and activation of cathepsin D, which cleaves prolactin into its antiangiogenic and proapoptotic 16 kDa fragment. The 16 kDa fragment inhibits endothelial cell proliferation and migration, induces endothelial cell apoptosis and impairs cardiomyocyte function [35].
GENETIC FACTORS
PPCM has been classified as a nongenetic form of dilated cardiomyopathy [36]. However, a few previous case reports have reported familial clustering suggesting a potential genetic basis [37-40]. Familiality and genetics of PPCM were characterized in two recent studies. Morales et al. performed a systematic search of 110 women from 520 families of patients with nonischemic dilated cardiomyopathy and identified 45 patients with PPCM [41]. Nineteen patients underwent genetic sequencing for genes known to be associated with dilated cardiomyopathy. Familial mutations were identified in MYH7, SCN5A, and PSEN2 genes and sporadic mutations in MYH6 andTNNT2 genes of peripartum cardiomyopathy patients. Similarly van Spaendonck-Zwarts et al. reviewed a database of 90 dilated cardiomyopathy families, and identified that 5 (6%) of 90 families included patients with PPCM [42]. Genetic analyses showed a mutation in the gene encoding cardiac troponin C (TNNC1) in one dilated cardiomyopathy family with members of PPCM. These findings suggest that PPCM may have a genetic predisposition.
RISK FACTORS FOR PPCM
Though the exact cause of PPCM is unknown, several potential risk factors have been identified. Advanced maternal age, black race, multiparity, multiple gestations, obesity, smoking, being unmarried, prolonged tocolysis, malnutrition, cocaine abuse, low socioeconomic status, twin pregnancies, history of hypertension, preeclampsia, and eclampsia are each associated with a higher incidence of PPCM, but without a direct causal association in large prospective studies [43-52].
In a recent study, Patten et al. showed that mice that lack cardiac PGC-1α, a major regulator of angiogenesis, develop PPCM [53]. PGC-1α contributes to angiogenesis by regulating the expression of vascular endothelial growth factor (VEGF) and by indirectly preventing the cleavage of prolactin into a smaller anti-angiogenic protein. The authors found that female PGC-1α knockout mice showed cardiac dilation and dysfunction after birth, whereas control females and male mice displayed none of these effects. The authors detected high levels of soluble VEGF receptor Flt-1 (sFlt-1) both in women with PPCM and in women with preeclampsia or multiple gestations. sFlt-1 has anti-angiogenic properties and induced severe heart failure when administered to PGC-1α knockout mice even in the absence of pregnancy. These results showed that insufficient angiogenesis causes PPCM and point towards sFtl-1 as a possible therapeutic target. These data may also explain how late pregnancy poses a threat to cardiac homeostasis, and why preeclampsia and multiple gestation are important risk factors for the development of PPCM.
CLINICAL PRESENTATION AND DIAGNOSIS
PPCM is usually diagnosed in the first 6 months postpartum and in some cases, prepartum. Presentation of PPCM is similar to that of patients presenting with heart failure due to other causes. However, diagnosis of PPCM is often missed or delayed because most of the signs and symptoms of normal pregnancy are similar to those of heart failure [54].
Patients usually present with palpitations, peripheral edema, fatigue, shortness of breath at rest or on exertion, cough, hemoptysis, chest pain, and/or paroxysmal nocturnal dyspnea [54, 55].
Physical examination often reveals tachycardia, tachypnea, jugular venous distension, displaced apical impulse, right ventricular heave, murmurs of mitral and tricuspid regurgitation, third heart sound (S3), pulmonary rales, hepatosplenomegaly, ascites, and peripheral edema. Blood pressure is frequently increased but it may be normal or decreased [54, 55].
PPCM should be diagnosed after careful exclusion of other medical conditions such as anemia, thyroid disorders, idiopathic dilated cardiomyopathy, accelerated hypertension, myocardial infarction, sepsis, severe preeclampsia, valve disease, postpartum depression, pulmonary vasculitides, amniotic fluid embolism and pulmonary embolism [55].
IMAGING
The resting 12-lead electrocardiogram (ECG) is the first-line diagnostic tool in the assessment of patients with suspected PPCM. Although a normal ECG does not rule out the diagnosis, most women suffering from PPCM have an abnormal ECG. The most common abnormalities on the ECG are ST- or T-wave abnormalities, p-wave abnormality, bundle branch block, left ventricular hypertrophy, ventricular or supraventricular arrhythmias, and QRS-axis deviation [56-58]. Radiological signs of heart failure such as cardiomegaly, Kerley B lines, prominent pulmonary vasculature, pulmonary edema, and pleural effusion can be found at chest X-ray.
Echocardiography is the most important diagnostic tool in PPCM and is without adverse effects. The finding of left ventricular systolic dysfunction is essential in the diagnosis, and other criteria include a left ventricular ejection fraction less than 45 %, fractional shortening of less than 30 % on M-mode echocardiography, or both, and a left ventricular end-diastolic dimension of greater than 2.7 cm/m2 of body surface area [59, 60]. Tranthoracic echocardiography can also detect mural thrombus, mitral or tricuspid regurgitation, right ventricular systolic dysfunction, and pericardial effusion [61]. To our knowledge, there are no studies that have validated the usefulness of new echocardiographic techniques such as tissue Doppler imaging, three dimensional echocardiography, speckle tracking and/or strain rate imaging in PPCM.
Magnetic resonance imaging (MRI) has been widely used to evaluate obstetrical, placental, and fetal abnormalities in pregnant patients. Intravenous gadolinium is contraindicated in pregnancy, and should only be used if absolutely essential, and only after discussion of risks and benefits with the patient. Although safety concerns have been raised with respect to both mother and fetus, to date, there has been no reports that the use of clinical MR imaging without gadolinium during pregnancy had resulted in deleterious effects [62-64]. Still, MRI is suggested to be avoided during pregnancy, particularly during the first trimester, but may be preferable to other studies using ionizing radiation. Lactating women who receive iodinated contrast or gadolinium can continue breast feeding without interruption [62-64]. The role of cardiac MRI has been investigated in a limited number of studies with small study populations of PPCM [65-68].
Marmursztejn et al. described the cardiac MRI images of two patients with PPCM [66]. The first patient had nogadolinium enhancement with cardiac MRI at baseline, and regained normal cardiac function at clinical follow-up. The second patient had several areas of myocardial late gadolinium enhancement, and had persistent left ventricular dysfunction on clinical follow-up. Mouquet et al. reported that in a study of 8 patients diagnosed with PPCM, except for depressed ejection fraction, the patients did not exhibit a specific myocardial MRI pattern or myocardial late enhancement [68]. Differences in cardiac MRI appearances of the patients with PPCM may be releted to differences in pathophysiology, myocardial inflammation, myocardial involvement, disease severity or duration between patients.
BIOMARKERS
There are limited data regarding the use of biomarkers in the diagnosis and therapy of PPCM. Hu et al. prospectively evaluated 106 newly diagnosed PPCM patients and measured Troponin T concentration within 2 weeks of the onset of the disease [69]. They found that an initial Troponin T concentration of >0.04 ng/ml predicted persistent left ventricular dysfunction at 6-months follow-up. Fett et al. showed that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity CRP levels were markedly increased in patients with acute PPCM compared to controls [70]. Forster et al. showed that, baseline CRP levels were similar in patients with complete recovery and nonrecovery, whereas median serum levels of NT-proBNP were significantly higher in nonrecovered patients [22].
Invasive evaluation, such as cardiac catheterization or endomyocardial biopsy, is usually unnecessary for diagnosis or treatment. Coronary arteries are usually normal in PPCM and the endomyocardial biopsy usually reflects nonspecific edema, inflammation, hypertrophy, and fibrosis, and thus is not warranted.
MANAGEMENT
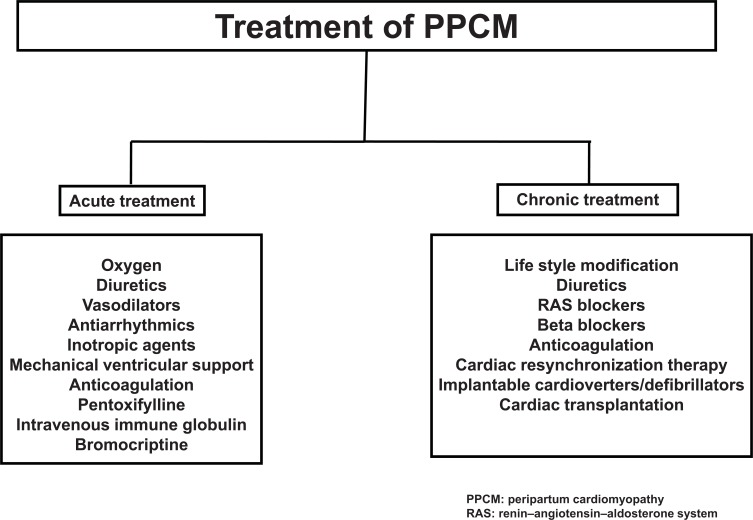
The management of patients with PPCM is similar to that of other forms of non-ischaemic dilated cardiomyopathy but must be individualized based on the patient's clinical presentation. For optimal management of PPCM, a multidisciplinary team approach involving physicians (cardiologist, obstetrician, anaesthetist and paediatrician) and several allied health care disciplines are essential. Therapeutic aspects of PPCM management can be evaluated in two steps: acute stabilization and chronic therapy (Fig. 2).
Fig. (2).
Management of peripartum cardiomyopathy.
ACUTE THERAPY
The goals of treatment in PPCM patients presenting with acute heart failure are to relieve symptoms and signs, optimize volume status, prevent hospital admissions, improve quality of life, increase functional capacity and improve survival. Heart failure should be treated according to the guidelines for management of acute or chronic heart failure [71]. Immediate delivery is recommended for all women with significant hemodynamic instability, regardless of gestational age, to prevent potential maternal and fetal complications. Hospital admission is recommended for patients with acute decompensated heart failure [71]. Supplemental oxygen may be administered if hypoxemia is present. Initial therapy of acute decompensated heart failure usually includes a diuretic such as furosemide. Combination of a vasodilator such as nitroglycerin, angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and beta blockers should be used in the acute setting in patients without marked bradycardia or marked hypotension. Inotropic agents should usually be reserved for patients with low cardiac output with compromise of vital organ perfusion.
Dopamine, dobutamine, milrinone and levosimendan can be used for inotropic support. Beneficial effects of levosimendan therapy, a promising new inotrope, have been described in five case reports previously [72-76]. However, in a prospective, randomized study we treated 12 PPCM patients with conventional heart failure therapy and 12 patients with levosimendan in addition to the conventional therapy. After a mean follow-up period of 21 months addition of levosimendan to conventional therapy did not improve outcome in patients with PPCM [77].
In addition to the above-mentioned standard therapeutic options for heart failure, specific targeted agents have been advocated for treatment of PPCM. In a retrospective study of women with PPCM, patients treated with high dose immune globulin had a greater improvement in ejection fraction during early follow-up than patients treated conventionally [78]. Pentoxifylline, a vasodilator drug that also inhibits tumour necrosis factor α, has been shown to improve outcome in a small study when added to conventional treatment [79]. Recently, inhibition of prolactin release by bromocriptine or cabergoline has been reported as a promising specific therapeutic approach to treat patients with PPCM and to prevent development of cardiomyopathy in PPCM patients with subsequent pregnancies [80-84].
In a prospective, single center, randomized study, Sliwa et al. [85] reported that the addition of bromocriptine to standard heart failure therapy in women with PPCM, resulted in significantly greater improvements in functional capacity and left ventricle function, than with standard therapy alone. However, bromocriptine therapy is not without risk. There have been a few case reports of acute thromboembolic events such as myocardial infarction and central retinal vein occlusion in women receiving bromocriptine for the suppression of postpartum lactation [86-88].
Therefore, bromocriptine may be applied in combination with anticoagulants such as low-dose heparin. Larger, randomized studies are needed in order to confirm the beneficial effects of bromocriptine in PPCM. Similar to bromocriptine, all other specific treatment modalities such as IV immunoglobulin, pentoxifylline mentioned above were studied in small-scale or uncontrolled trials. Their efficacy and safety will need to be validated in large scale randomized controlled trials to be considered as definitive and beneficial treatment options for PPCM.
In patients with refractory decompensated heart failure despite optimal medical therapy, implantation of a mechanical assist device or cardiac transplantation may need to be considered, especially in patients who are dependent on inotropes or intra-aortic balloon pump counterpulsation. Recent studies have demonstrates that most PPCM patients treated with conventional therapy will survive, and more than 50% will have complete recovery of left ventricular systolic function [2, 11]. Therefore, cardiac transplantation or mechanical circulatory support, should be considered as the last resort for patients with PPCM, when all other treatments have failed.
CHRONIC MANAGEMENT
For the management of PPCM prepartum, the pregnancy status of the patient is important. Possible adverse effects on the fetus must be considered when prescribing medications for patients presenting with PPCM during pregnancy. Therefore, in the following section, management of chronic heart failure in PPCM patients will be summarized separately for theantepartum and postpartum periods.
1. Antepartum Management
Angiotensin converting enzyme inhibitors and ARBs are contraindicated during pregnancy and breast feeding; however, they are recommended for management of PPCM in the postpartum period [89] for mothers who are not breastfeeding. Hydralazine and nitrates can be used instead of ACE inhibitors/ARBs during pregnancy. Digoxin and diuretics may be used safely during pregnancy and while breast-feeding. While some beta blockers may be considered fairly safe for use during pregnancy, beta blockers may cause certain risks to the fetus if taken during late pregnancy.
Some of these risks may include a very low heart rate, low blood sugar levels, growth retardation and/or decreased breathing [90-92]. The U.S. Food and Drug Administration (FDA) uses a pregnancy category system to classify the possible risks to a fetus when a specific medicine is taken during pregnancy. Pregnancy Category B is given to medicines that have not been adequately studied in pregnant humans, but do not appear to cause harm to the fetus in animal studies and these include beta blockers like acebutolol, pindolol and sotalol. Pregnancy Category C is given to medicines that have not been studied in pregnant humans, but do appear to cause harm to the fetus in animal studies. Also, medicines that have not been studied in any pregnant women or animals are automatically given a pregnancy Category C rating. These include most of the beta blockers used in heart failure such as carvedilol, metoprolol and bisoprolol. Pregnancy Category D is a classification given to medicines that have been shown to present a risk to the fetus in studies of pregnant women, but may still offer benefits that outweigh the risks the drug presents. A pregnancy Category D medicine may still be given to a pregnant woman if the healthcare provider believes that the benefits to the woman outweigh the possible risks to the unborn child. Only one beta blocker, atenolol is officially a pregnancy Category D medication [93].
Spironolactone is thought to have antiandrogenic effects in the first trimester and has been assigned to pregnancy category C by the United States Food and Drug Administration (FDA) and should be avoided during pregnancy and breastfeeding.
Pregnancy itself is a hypercoaguable state associated with increased levels of coagulant factors and decreased levels of natural anticoagulants [94, 95]. Left ventricular dysfunction, in addition to a hypercoagulable state during pregnancy, may increase the risk of thromboembolic events in patients with PPCM [96, 97]. Anticoagulation should be considered in patients with evidence of a systemic embolism, severe left ventricular dysfunction, paroxysmal or persistent atrial fibillation, documented mural thrombus, and in patients receiving bromocriptine therapy. Choosing a specific anticoagulation regimen for a pregnant woman is challenging due to the potential teratogenic effects of warfarin and dosing complexities of the various agents. Warfarin freely crosses the placental barrier, can harm the fetus, can cause miscarriage, stillbirth, birth defects, or fatal bleeding in an unborn baby and is classified a category X (contraindicated) drug during pregnancy by the FDA [98].
However, warfarin is “relatively safe” during the second and third trimesters but must be stopped and switched to a heparin several weeks before delivery [99]. Unfractionated heparin and low-molecular weight heparin do not cross the placenta and are considered safe during pregnancy. When low-molecular weight heparin is used, anti-Xa levels should be monitored [100]. Anticoagulation therapy should be continued until left ventricular function has improved. Both warfarin and heparin are not secreted into breast milk, and are therefore safe during breast-feeding [100].
2. Postpartum Management
Management of heart failure after delivery should follow standard heart failure guidelines with regard to pharmacological and device therapy [71]. Lifestyle changes, such as exercising, reducing the salt in diet, managing stress, treating depression, losing weight, can improve quality of life. Bed rest is not recommended due to the risk of venous thromboembolism, unless unstable hemodynamics are present. Beta blocker treatment is indicated for all patients with heart failure, if tolerated.
Although bromocriptine was shown to be effective during the acute phase of PPCM by small studies as described above, its effects on the subacute or chronic phase of PPCM is unknown. Recent studies showed favorable results with wearable cardiac defibrillator [101], cardiac resynchronization therapy [102], and cardiac transplantation [103] in PPCM patients.
MANAGEMENT OF LABOUR AND DELIVERY
Timing and mode of delivery depends on the patient's presentation and clinical status. A multidisciplinary team approach including an obstetrician, cardiologist, anesthesiologist, and pediatrician is crucial to adequate management of patients with PPCM at the time of labor and delivery. Early delivery is usually not indicated in clinically stable patients, but may be considered in patients who become hemodynamically unstable. Patients with class III or IV symptoms and moderate to severe left ventricular dysfunction in the first or second trimester can be considered for termination of pregnancy. Spontaneous labour and vaginal delivery is generally acceptable in stable patients. Cesarean delivery is generally reserved for obstetrical indications. However, planned caesarean section is usually the preferred delivery mode in unstable patients who are critically ill and in need of inotropic therapy or mechanical support.
BREASTFEEDING
There are no randomized studies showing any detrimental effects with breastfeeding in patients with PPCM. Recent observations of a potential association of prolactin and its derivates with detrimental cardiovascular effects; have created a debate about prevention of lactation. These observations include increased serum levels of endothelial microparticles, activated cathepsin D, total prolactin, and the cleaved 16 kDa prolactin fragments in patients with acute PPCM [104].
The 16 kDa prolactin fragment has been shown to have detrimental cardiovascular actions including inhibition of endothelial cell proliferation and migration, induction of endothelial cell apoptosis, destroying cardiac microvasculature, lowering cardiac function, and promoting ventricular dilatation in experimental models [35]. Although there is no clear data showing adverse cardiac effects from breast feeding by women with PPCM, ESC working group on PPCM raised the question whether breast feeding should be avoided because of the potential effects of prolactin subfragments [12]. Recent ESC guidelines on the management of cardiovascular diseases during pregnancy also suggests preventing lactation in patients with cardiomyopathies and heart failure due to high metabolic demands of lactation and breastfeeding, with an indication of Class IIb [89]. If the decision to breastfeed is made, careful attention should be paid to fetal safety and to excretion of drug or drug metabolites in breast milk. In summary, ACE inhibitors, ARBs, atenolol, statins, aldosterone antagonists, and renin inhibitors should be avoided during pregnancy and breastfeeding. However, heparin, warfarin, beta blockers except atenolol, digoxin and some ACE inhibitors (quinapril, captopril and enalapril) can be administered during breast feding [89, 105].
DURATION OF THERAPY
Currently, there is no clear consensus on the appropriate duration of heart failure drug therapy and its prophylactic role in subsequent pregnancies. It is also unknown when to discontinue heart failure medications in recovered PPCM patients or whether there is any deterioration in left ventricular function after an initial recovery in these patients. Amos et al. reported a lack of deterioration of left ventricular function during an average follow-up period of 29 months in 15 patients with full recovery who stopped taking medication [106].
On the other hand, Goland et al. demonstrated spontaneous deterioration of left ventricular function in three patients [107]. Recently we have published results of 42 prospectively followed PPCM patients [108]. Twenty patients (47.6%) recovered completely, 10 died (23.8%), and 12 (28.6%) had persistent left ventricular dysfunction. Average time to complete recovery was 19.3 months after initial diagnosis (3–42 months). Early recovery was observed only in six patients (30%) within six months of diagnosis, whereas delayed recovery, was observed in 14 out of 20 patients (70%) beyond 6 months. Four patients (2 patients with complete recovery and 2 patients with partial recovery) showed delayed deterioration (12, 24, 26, and 34 months after diagnosis) during the study period. Of the four patients with spontaneous deterioration of left ventricular function, two patients with partial recovery were receiving full-dosage heart failure treatment, but two patients with complete recovery stopped taking their medications after recovery, and had delayed deterioration at 24 and 34 months. The findings of late deterioration are very important and indicate the need for a close follow-up with periodic determination of cardiac function in women in whom medications are discontinued after complete recovery.
Due to probability of either delayed recovery or deterioration of left ventricular function in PPCM, long-term follow-up may be needed not only in non-recovered patients, but also in patients with complete recovery. After clinical and echocardiographic evidence of full recovery, it may be acceptable to gradually taper the drug doses over a period of 12 to 24 months; however, we suggest that ACE inhibitors and beta blockers should be continued for at least 2 years after complete recovery.
SUBSEQUENT PREGNANCY
Women with PPCM have a high risk of relapse in subsequent pregnancies, and terminating pregnancy may not prevent the onset of PPCM [109]. A subsequent pregnancy carries a recurrence risk for PPCM of 30–50% [12, 43]. Therefore, all patients with a history of PPCM should be informed that subsequent pregnancy may reduce cardiac function, result in the development of heart failure, and lead to death. Women with an ejection fraction <2 5 % at diagnosis or in whom the ejection fraction has not normalized should be advised against another pregnancy. However, it should be kept in mind that women with prior PPCM in whom left ventricular function has returned to normal (ejection fraction >50%) still remain at significant risk for morbidity and mortality with a subsequent pregnancy [109, 110].
PROGNOSIS
Available data suggest that the prognosis of PPCM vary geographically [111]. Most of the studies have been performed in the USA, Haiti, Turkey and South Africa. Most reports from the USA reflect the lowest mortality rates (0%–9.6%) across the continents [14, 106]. However, interestingly Modi et al. reported that the recovery and survival rates of PPCM patients in the USA can be very similar to those reported from Haiti and South Africa, with a mortality rate of 15.9% [112].
In this cohort, 87.5% of the patients were African Americans, suggesting an association of race and ethnicity with poorer outcomes. In South Africa, case series have demonstrated that mortality rates have slowly improved over time, but 6-month and 2-year mortality rates still remain high at 10 and 28%, respectively [19]. Single center studies from Brazil and Haiti report mortality rates of 14–16% over 6 months [20, 113]. In Turkey, we reported a mortality rate of 30% over a 4-year follow-up in our first cohort [114]. In a second prospective cohort of 24 patients [78] we reported a mortality rate of 25% over an average follow up of 20.9 ± 9 months. In the last study of a prospective cohort of 42 patients from three different tertiary centers in Turkey, we again reported a similar mortality rate of 23.8% [108].
Several predictors of poor prognosis have been reported in the previous studies including increased maternal age, higher parity, later onset of symptoms following delivery, non-Caucasian and delayed diagnosis [107, 115]. Factors associated with favorable prognosis include small left ventricle diastolic dimension (<5.5 to 6.0 cm), elevated systolic function (ejection fraction >30% to 35% and fractional shortening >20%) at the time of diagnosis, absence of troponin elevation, absence of left ventricular thrombus and non-African American ethnicity [113-118].
THE FUTURE
PPCM is still a relatively unknown disease. Due to the rarity of PPCM, large scale multicenter studies have not been conducted, and the optimal initial and long term specific therapeutic approaches have not been well defined. With an intend to better characterize the disease and develop understanding into the mechanisms, two large registries have been e formed in USA/Canada and Europe in 2009. The first one is the PPCM Network, a National Institutes of Health-sponsored North American registry of PPCM, linking more than 30 medical centers.
The second one is the Study Group on PPCM which was formed by the Heart Failure Association of the ESC and consists of experts from cardiology, basic sciences, intensive care, obstetrics and gynecology, imaging, genetics and other disciplines. It is anticipated that both of these registries will provide important information and insights into the mechanisms and pathophyiology as well as natural history of PPCM. There is a great need for future large scale studies to determine the triggering factors involved in development of PPCM, the clinical and biological predictors of outcomes, the efficacy and safety of targeted therapies in PPCM such as prolactin inhibition with bromocriptine. Such future preclinical, controlled, randomized clinical studies and registries may lead to improved understanding of the pathogenesis of and development of specific treatment modalities for PPCM.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Güder G, Brenner S, Angermann CE, Störk S. Peripartum cardiomyopathy. An update. Minerva Ginecol. 2012;64(5):361–73. [PubMed] [Google Scholar]
- 2.Elkayam U, Jalnapurkar S, Barakat M. Peripartum cardiomyopathy. Cardiol Clin. 2012;30(3):435–40. doi: 10.1016/j.ccl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie C. Clinical contribution to the patho-diagnosis and treatment of certain chronic diseases of the heart. Edinburgh Med J. 1850;2:2. [PMC free article] [PubMed] [Google Scholar]
- 4.Virchow R. Sitzing der Berliner Geburtshilflisher Gersellskhalt cited by Porak C De l'influence réciproque de la grossesse et des maladies du. Coeur thesis Paris. . 1880 [Google Scholar]
- 5.Porak C. De l'influence réciproque de la grossesse et des maladies du. Coeur thesis Paris. . 1880 [Google Scholar]
- 6.Gouley B, McMillan T, Bellet S. Idiopathic myocardial degeneration associated with pregnancy and especially the puerperium. Am J Med Sci. 1937;19:185–99. [Google Scholar]
- 7.Hull E, Hidden E. Postpartal heart failure. South Med J. 1938;31:265–70. [Google Scholar]
- 8.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–8. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- 9.Pearson GD, Veille JC, Rahimtoola S , et al. Peripartum cardiomyopathy National Heart Lung and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 10.Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94(2):311–6. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 11.Elkayam U, Akhter MW, Singh Hetal. Pregnancy-associated cardiomyopathy clinical characteristics and a comparison between early and late presentation. Circulation. 2005;11:2050 –5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 12.Sliwa K, Hilfiker-Kleiner D, Petrie MCetal. Current state of knowledge on aetiology diagnosis management and therapy of peripartum cardiomyopathy a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 13.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy an omnious diagnosis. Am J Obstet Gynecol. 1997;176:182–8. doi: 10.1016/s0002-9378(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 14.Chapa JB, Heiberger HB, Weinert L, DeCara J, Lang R, Hibbard JU. Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol. 2005;105:1303–8. doi: 10.1097/01.AOG.0000161382.30233.ba. [DOI] [PubMed] [Google Scholar]
- 15.Mielniczuk LM, Williams L, Davis DRetal. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97:1765–8. doi: 10.1016/j.amjcard.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Brar SS, Khan SS, Sandhu GKetal. Incidence mortality and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100:302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 17.Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy experiences at King Edward VIII Hospital Durban. South Africa and a review of the literature Trop Doct. 1995;25:118–23. doi: 10.1177/004947559502500310. [DOI] [PubMed] [Google Scholar]
- 18.Sliwa K, Forster O, Libhaber Eetal. Peripartum cardiomyopathy inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 19.Sliwa K, Forster O, Tibazarwa Ketal. Long-term outcome of Peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol. 2011;147(2):202–8. doi: 10.1016/j.ijcard.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Fett JD, Carraway RD, Dowell DL, King ME, Pierre R. Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. Am J Obstet Gynecol. 2002;186:1005–10. doi: 10.1067/mob.2002.122423. [DOI] [PubMed] [Google Scholar]
- 21.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 22.Forster O, Hil?ker-Kleiner D, Ansari AAetal. Reversal of IFN-gamma oxL DL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008;10:861–8. doi: 10.1016/j.ejheart.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Melvin KR, Richardson PJ, Olsen EG, Daly K, Jackson G. Peripartum cardiomyopathy due to myocarditis. N Engl J Med. 1982;307(12):731–4. doi: 10.1056/NEJM198209163071207. [DOI] [PubMed] [Google Scholar]
- 24.Bultmann BD, Klingel K, Nabauer M, Wallwiener D, Kan-dolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193(2):363–5. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Rizeq MN, Rickenbacher PR, Fowler MB, Billingham ME. Incidence of myocarditis in peripartum cardiomyopathy. Am J Cardiol. 1994;74(5):474–7. doi: 10.1016/0002-9149(94)90906-7. [DOI] [PubMed] [Google Scholar]
- 26.Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL. Peripartum myocarditis and cardiomyopathy. Circulation. 1990;81(3):922–8. doi: 10.1161/01.cir.81.3.922. [DOI] [PubMed] [Google Scholar]
- 27.Felker GM, Jaeger CJ, Klodas E , et al. Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J. 2000;140:785–91. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- 28.Cenac A, Gaultier Y, Devillechabrolle A, Moulias R. Enterovirus infection in peripartum cardiomyopathy. Lancet. 1988;2:968–9. doi: 10.1016/s0140-6736(88)92641-4. [DOI] [PubMed] [Google Scholar]
- 29.Cenac A, Djibo A, Chaigneau C, Velmans N, Or Wla J. Are anti-Chlamydia pneumoniae antibodies prognosis indicators for peripartum cardiomyopathy?. J Cardiovasc Risk. 2003;10:195–9. doi: 10.1097/01.hjr.0000065925.57001.3b. [DOI] [PubMed] [Google Scholar]
- 30.Lissauer D, Piper K, Moss P, Kilby M. Persistence of fetal cells in the mother: friend or foe?. BJOG. 2007;114:1321–32. doi: 10.1111/j.1471-0528.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi DW, Farina A, Weber W , et al. Signi?cant fetal-maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184:703–6. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- 32.Ansari AA, Fett JD, Carraway RE , et al. Autoimmune mechanisms as the basis for human peripartum cardiom yopathy. Clin Rev Allergy Immunol. 2002;23:301–24. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]
- 33.Desai DK, Moodley J, Naidoo DP. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstetrics Gynecology. 2004;104(1):20–9. doi: 10.1097/01.AOG.0000128170.15161.1d. [DOI] [PubMed] [Google Scholar]
- 34.Johnson-Coyle L, Jensen L, Sobey A. American College of Cardiology Foundation, American Heart Association.Peripartum cardiomyopathy: review and practice guidelines. Am J Crit Care. 2012;21(2):89–98. doi: 10.4037/ajcc2012163. [DOI] [PubMed] [Google Scholar]
- 35.Hil?ker-Kleiner D, Kaminski K, Podewski E , et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Elliott P, Andersson B, Arbustini E , et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myo-cardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 37.Pearl W. Familial occurrence of peripartum cardiomyopathy. Am Heart J. 1995;129:421–2. doi: 10.1016/0002-8703(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 38.Fett JD, Sundstrom BJ, King ME , et al. Mother–daughter peripartum cardiomyopathy. Int J Cardiol. 2002;86(2-3):331–2. doi: 10.1016/s0167-5273(02)00357-1. [DOI] [PubMed] [Google Scholar]
- 39.Baruteau AE, Leurent G, Schleich JM, Gervais R, Daubert JC, Mabo P. Can peripartum cardiomyopathy be familial?. Int J Cardiol. 2009;137(2):183–5. doi: 10.1016/j.ijcard.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Meyer GP, Labidi S, Podewski E, Sliwa K, Drexler H, Hilfiker-Kleiner D. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep. 2010;4:80. doi: 10.1186/1752-1947-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales A, Painter T, Li R , et al. Rare variant mutations in pregnancy-associated or peripartumcardiomyopathy. Circulation. 2010;121(20):2176–82. doi: 10.1161/CIRCULATIONAHA.109.931220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ , et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121(20):2169–75. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 43.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–93. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 44.Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM. Peripartum cardiomyopathy: a comprehensive review. Int J Cardiol. 2007;118(3):295–303. doi: 10.1016/j.ijcard.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Karaye KM, Sani MU. The impact of income on the echocardiographic pattern of heart diseases in Kano. Nigeria. Niger J Med. 2008;17(3):350–5. doi: 10.4314/njm.v17i3.37409. [DOI] [PubMed] [Google Scholar]
- 46.Kamiya CA, Kitakaze M, Ishibashi-Ueda H , et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders -Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J. 2011;75(8):1975–81. doi: 10.1253/circj.cj-10-1214. [DOI] [PubMed] [Google Scholar]
- 47.Gentry MB, Dias JK, Luis A, Petel R, Thornton J, Reed GL. African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol. 2010;55:654–9. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homans DC. Peripartum cardiomyopathy. N Engl J Med. 1985;312(22):1432–7. doi: 10.1056/NEJM198505303122206. [DOI] [PubMed] [Google Scholar]
- 49.Ntusi NBA, Mayosi BM. Aetiology and risk factors of peripartum cardiomyopathy: a systematic review. Int J Cardiol. 2009;131:168–79. doi: 10.1016/j.ijcard.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 50.Fett JD, Ansari AA, Sundstrom JB, Combs GF. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol. 2002;86:311–6. doi: 10.1016/s0167-5273(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 51.Cenac A, Simonoff M, Moretto P, Djibo A. A low plasma selenium is a risk factor for peripartum cardiomyopathy.A comparative study in Sahelian Africa. Int J Cardiol. 1992;36:57–9. doi: 10.1016/0167-5273(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 52.Sebillotte CG, Deligny C, Hanf M , et al. Is African descent an independent risk factor of peripartum cardiomyopathy?. Int J Cardiol. 2010;145(1):93–4. doi: 10.1016/j.ijcard.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 53.Patten IS, Rana S, Shahul S , et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart. 2007;93(10):1176–83. doi: 10.1136/hrt.2007.127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhakta P, Biswas BK, Banerjee B. Peripartum cardiomyopathy: review of the literature. Yonsei Med J. 2007;48(5):731–47. doi: 10.3349/ymj.2007.48.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tibazarwa K, Lee G, Mayosi B, Carrington M, Stewart S, Sliwa K. The 12-lead ECG in peripartum cardiomyopathy. Cardiovasc J Afr. 2012;23(6):322–9. doi: 10.5830/CVJA-2012-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labidi S, Hilfiker-Kleiner D, Klein G. Left bundle branch block during pregnancy as a sign of imminent peripartum cardiomyopathy. Eur Heart J. 2011;32(9):1076. doi: 10.1093/eurheartj/ehq487. [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto O, Matsuda M, Nakamoto K , et al. Peripartum cardiomyopathy presenting with syncope due to Torsades de pointes: a case of long QT syndrome with a novel KCNH2 mutation. Intern Med. 2012;51(5):461–4. doi: 10.2169/internalmedicine.51.5943. [DOI] [PubMed] [Google Scholar]
- 59.Pyatt JR, Dubey G. Peripartum cardiomyopathy: current understanding. comprehensive management review and new developments. Postgrad Med J. 2011;87(1023):34–9. doi: 10.1136/pgmj.2009.096594. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharyya A, Basra SS, Sen P, Kar B. Peripartum cardiomyopathy: a review. Tex Heart Inst J. 2012;39(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- 61.Karaye KM. Right ventricular systolic function in peripartum and dilated cardiomyopathies. Eur J Echocardiogr. 2011;12(5):372–4. doi: 10.1093/ejechocard/jer024. [DOI] [PubMed] [Google Scholar]
- 62.Kanal E, Shellock FG. Policies. guidelnes. and recommendations for MR imaging safety and patient management. SMRI Safety Committee. J Magn Reson Imaging . 1992; 2(2):247–8. doi: 10.1002/jmri.1880020222. [DOI] [PubMed] [Google Scholar]
- 63.De Wilde JP, Rivers AW, Price DL. A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the fetus. Prog Biophys Mol Biol. 2005;87:335–53. doi: 10.1016/j.pbiomolbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Kanal E, Barkovich AJ, Bell C , et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–74. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 65.Renz DM, Röttgen R, Habedank D , et al. New insights into peripartum cardiomyopathy using cardiac magnetic resonance imaging. Rofo. 2011;183(9):834–41. doi: 10.1055/s-0031-1281600. [DOI] [PubMed] [Google Scholar]
- 66.Marmursztejn J, Vignaux O, Goffinet F, Cabanes L, Duboc D. Delayed-enhanced cardiac magnetic resonance imaging features in peripartum cardiomyopathy. Int J Cardiol. 2009;137(3):e63–4. doi: 10.1016/j.ijcard.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 67.Baruteau AE, Leurent G, Martins RP , et al. Peripartum cardiomyopathy in the era of cardiac magnetic resonance imaging: first results and perspectives. Int J Cardiol. 2010;144(1):143–5. doi: 10.1016/j.ijcard.2008.12.153. [DOI] [PubMed] [Google Scholar]
- 68.Mouquet F, Lions C, de Groote P , et al. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18(12):2765–9. doi: 10.1007/s00330-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 69.Hu CL, Li YB, Zang JM , et al. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart. 2007;93:488–90. doi: 10.1136/hrt.2006.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fett JD, Dowell DL, Carraway RD, Sundstrom JB, Ansari AA. One hundred cases of peripartum cardiomyopathy.. and counting: what is going on?. . Int J Cardiol. 2004;97(3):571–3. doi: 10.1016/j.ijcard.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 71.McMurray JJ, Adamopoulos S, Anker SD , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology.Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 72.Benlolo S, Lefoll C, Katchatouryan V, Payen D, Mebazaa A. Successful use of levosimendan in a patient with peripartum cardiomyopathy. Anesth Analg. 2004;98(3):822–4. doi: 10.1213/01.ane.0000099717.40471.83. [DOI] [PubMed] [Google Scholar]
- 73.Benezet-Mazuecos J, de la Hera J. Peripartum cardiomyopathy: a new successful setting forlevosimendan. Int J Cardiol. 2008;123(3):346–7. doi: 10.1016/j.ijcard.2006.11.171. [DOI] [PubMed] [Google Scholar]
- 74.Uriarte-Rodríguez A, Santana-Cabrera L, Sánchez-Palacios M. Levosimendan use in the emergency management of decompensated peripartum cardiomyopathy. J Emerg Trauma Shock. 2010;3(1):94. doi: 10.4103/0974-2700.58651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brogly N, Guasch E, Puertas L, Alsina E, López T, Gilsanz F. Acute early postpartum cardiac failure associated with dilatedcardiomyopathy: successful treatment with intra-aortic balloon counter-pulsation and levosimendan. Ann Fr Anesth Reanim. 2010;29(11):807–10. doi: 10.1016/j.annfar.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen HD, Mc Keown B. Levosimendan for post-partum cardiomyopathy. Crit Care Resusc. 2005;7:107–10. [PubMed] [Google Scholar]
- 77.Biteker M, Duran NE, Kaya H , et al. Effect of levosimendan and predictors of recovery in patients withperipartum cardiomyopathy. a randomized clinical trial. Clin Res Cardiol. 2011;100(7):571–7. doi: 10.1007/s00392-010-0279-7. [DOI] [PubMed] [Google Scholar]
- 78.Bozkurt B, Villaneuva FS, Holubkov R , et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol. 1999;34:177–80. doi: 10.1016/s0735-1097(99)00161-8. [DOI] [PubMed] [Google Scholar]
- 79.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2002;4:305–9. doi: 10.1016/s1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 80.de Jong JS, Rietveld K, van Lochem LT, Bouma BJ. Rapid left ventricular recovery after cabergoline treatment in a patient withperipartum cardiomyopathy. Eur J Heart Fail. 2009;11(2):220–2. doi: 10.1093/eurjhf/hfn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilfiker-Kleiner D, Struman I, Hoch M, Podewski E, Sliwa K. 16-kDa Prolactin and Bromocriptine in Postpartum Cardiomyopathy. Curr Heart Fail Rep. 2012;9(3):174–82. doi: 10.1007/s11897-012-0095-7. [DOI] [PubMed] [Google Scholar]
- 82.Emmert MY, Prêtre R, Ruschitzka F, Krähenmann F, Falk V, Wilhelm MJ. Peripartum cardiomyopathy with cardiogenic shock: recovery afterprolactin inhibition and mechanical support. Ann Thorac Surg. 2011;91(1):274–6. doi: 10.1016/j.athoracsur.2010.06.110. [DOI] [PubMed] [Google Scholar]
- 83.Biteker M, Duran NE, Ozkan M. The role of bromocriptine in peripartum cardiomyopathy. Am J Obstet Gynecol. 2009;201(2):e13. doi: 10.1016/j.ajog.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 84.Biteker M. Current therapeutic perspectives in peripartum cardiomyopathy. Ann Thorac Surg. 2011;91(1):331–2. doi: 10.1016/j.athoracsur.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 85.Sliwa K, Blauwet L, Tibazarwa K , et al. Evaluation of bromocriptine in the treatment of acute severe peripartumcardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–73. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 86.Nagaki Y, Hayasaka S, Hiraki S, Yamada Y. Central retinal vein occlusion in a woman receiving bromocriptine. Ophthalmologica. 1997;211(6):397–8. doi: 10.1159/000310840. [DOI] [PubMed] [Google Scholar]
- 87.Dutt S, Wong F, Spurway JH. Fatal myocardial infarction associated with bromocriptine for postpartum lactation suppression. Aust N Z J Obstet Gynaecol. 1998;38(1):116–7. doi: 10.1111/j.1479-828x.1998.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 88.Pop C, Metz D, Matei M, Wagner E, Tassan S, Elaerts J. Postpartum myocardial infarction induced by Parlodel. Arch Mal Coeur Vaiss. 1998;91(9):1171–4. [PubMed] [Google Scholar]
- 89.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C , et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(24):3147–97. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 90.Gelson E, Curry R, Gatzoulis MAetal. Effect of Maternal Heart Disease on Fetal Growth. Obstet Gynecol. 2011;117(4):886–91. doi: 10.1097/AOG.0b013e31820cab69. [DOI] [PubMed] [Google Scholar]
- 91.Siu SC, Colman JM, Sorensen Setal. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation. 2002;105:2179–84. doi: 10.1161/01.cir.0000015699.48605.08. [DOI] [PubMed] [Google Scholar]
- 92.Nakhai-Pour HR, Rey E, Bérard A. Antihypertensive medication use during pregnancy and the risk for major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B Dev Reprod Toxicol. 2010;89:147–54. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 93.Frishman WH, Chesner M. Beta-adrenergic blockers in pregnancy. Am Heart J. 1988;115:147–152. doi: 10.1016/0002-8703(88)90530-3. [DOI] [PubMed] [Google Scholar]
- 94.Comp PC, Thurnau GR, Welsh J, Esmon CT. Functional and immunologic protein S levels are decreased during pregnancy. Blood. 1986;68:881–5. [PubMed] [Google Scholar]
- 95.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–82. [PubMed] [Google Scholar]
- 96.Kim DY, Islam S, Mondal NT, Mussell F, Rauchholz M. Biventricular thrombi associated with peripartum cardiomyopathy. J Health Popul Nutr. 2011;29(2):178–80. doi: 10.3329/jhpn.v29i2.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koç M, Sahin DY, Tekin K, Cayli M. Development of biventricular large apical thrombi and cerebral embolism in a young woman with peripartum cardiomyopathy. Kardiyol Dern Ars. 2011;39(7):591–4. doi: 10.5543/tkda.2011.01534. [DOI] [PubMed] [Google Scholar]
- 98.Howie PW. Anticoagulants in pregnancy. Clin Obstet Gynaecol. 1986;13:349. [PubMed] [Google Scholar]
- 99.Bonow RO, Carabello BA, Chatterjee Ketal. 2006 Writing Committee Members American College of Cardiology/American Heart Association Task Force.2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) endorsed by the Society of Cardiovascular Anesthesiologists Society for Cardiovascular Angiography and Interventions and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 100.Goland S, Elkayam U. Anticoagulation in pregnancy. Cardiol Clin. 2012;30(3):395–405. doi: 10.1016/j.ccl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Saltzberg MT, Szymkiewicz S, Bianco NR. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail. 2012;18(1):21–7. doi: 10.1016/j.cardfail.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Mouquet F, Mostefa Kara M, Lamblin Netal. Unexpected and rapid recovery of left ventricular function in patients withperipartum cardiomyopathy: impact of cardiac resynchronization therapy. Eur J Heart Fail. 2012;14(5):526–9. doi: 10.1093/eurjhf/hfs031. [DOI] [PubMed] [Google Scholar]
- 103.Rasmusson K, Brunisholz K, Budge Detal. Peripartum cardiomyopathy post-transplant outcomes from the United Network for Organ Sharing Database. J Heart Lung Transplant. 2012;31(2):180–6. doi: 10.1016/j.healun.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 104.Walenta K, Schwarz V, Schirmer SHetal. Circulating microparticles as indicators of peripartum cardiomyopathy. Eur Heart J. 2012;33(12):1469–79. doi: 10.1093/eurheartj/ehr485. [DOI] [PubMed] [Google Scholar]
- 105.Ghuman N, Rheiner J, Tendler BE, White WB. Hypertension in the postpartum woman clinical update for the hypertension specialist. J Clin Hypertens (Greenwich). 2009;11:726–33. doi: 10.1111/j.1751-7176.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–13. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 107.Goland S, Modi K, Bitar Fetal. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–50. doi: 10.1016/j.cardfail.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 108.Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail. 2012;14(8):895–901. doi: 10.1093/eurjhf/hfs070. [DOI] [PubMed] [Google Scholar]
- 109.Elkayam U, Tummala PP, Rao Ketal. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–71. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 110.Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. J Am Coll Cardiol. 2011;58(4):337–50. doi: 10.1016/j.jacc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 111.Biteker M. Peripartum cardiomyopathy in Turkey. Int J Cardiol. 2012;158(3):e60–1. doi: 10.1016/j.ijcard.2011.10.138. [DOI] [PubMed] [Google Scholar]
- 112.Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009;201:171e1–5. doi: 10.1016/j.ajog.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 113.Carvalho A, Brandao A, Martinez EE , et al. Prognosis in peripartum cardiomyopathy. Am J Cardiol. 1989;64:540–2. doi: 10.1016/0002-9149(89)90438-4. [DOI] [PubMed] [Google Scholar]
- 114.Duran N, Günes H, Duran I, Biteker M, Ozkan M. Predictors of prognosis in patients with peripartum cardiomyopathy. Int J Gynaecol Obstet. 2008;101(2):137–4. doi: 10.1016/j.ijgo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 115.Ravikishore AG, Kaul UA, Sethi KK, Khalilullah M. Peripartum cardiomyopathy: prognostic variables at initial evaluation. Int J Cardiol. 1991;32:377–80. doi: 10.1016/0167-5273(91)90301-5. [DOI] [PubMed] [Google Scholar]
- 116.Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154(1):27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 117.Goland S, Bitar F, Modi K , et al. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011;17(5):426–30. doi: 10.1016/j.cardfail.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 118.Ro A, Frishman WH. Peripartum cardiomyopathy. Cardiol Rev. 2006;14(1):35–42. doi: 10.1097/01.crd.0000174805.68081.f7. [DOI] [PubMed] [Google Scholar]