Abstract
A great bulk of evidence supports the concept that regular exercise training can reduce the incidence of coronary events and increase survival chances after myocardial infarction. These exercise-induced beneficial effects on the myocardium are reached by means of the reduction of several risk factors relating to cardiovascular disease, such as high cholesterol, hypertension, obesity etc. Furthermore, it has been demonstrated that exercise can reproduce the “ischemic preconditioning” (IP), which refers to the capacity of short periods of ischemia to render the myocardium more resistant to subsequent ischemic insult and to limit infarct size during prolonged ischemia. However, IP is a complex phenomenon which, along with infarct size reduction, can also provide protection against arrhythmia and myocardial stunning due to ischemia-reperfusion. Several clues demonstrate that preconditioning may be directly induced by exercise, thus inducing a protective phenotype at the heart level without the necessity of causing ischemia. Exercise appears to act as a physiological stress that induces beneficial myocardial adaptive responses at cellular level. The purpose of the present paper is to review the latest data on the role played by exercise in triggering myocardial preconditioning.
Keywords: Cardioprotection, cardiovascular disease, heart, ischemia, remote preconditioning, warm-up.
INTRODUCTION
The notion that regular exercise is cardioprotective is supported by numerous human epidemiological studies. According to the World Health Organization, cardiovascular diseases will become the major cause of death in the world as a whole by the year 2020 and will exact human and economic costs that will be unparalleled by any other single disease [1]. The fact that physical activity is cardioprotective and that physical inactivity is a risk factor for these pathologies is supported by a great quantity of scientific research. Furthermore, there is overwhelming proof that an individual’s exercise capacity is a strong predictor of increased risk of death from any cause in both healthy subjects and in those with cardiovascular diseases [2].
The concept that regular exercise confers protection against coronary disease can be traced to the seminal work of Morris and co-workers [3] and it has been extensively investigated since in a number of studies which have demonstrated that regular exercise is beneficial for the cardiovascular apparatus since it decreases the incidence of myocardial infarction and increases the chances of survival after coronary events [4-16]. Regular physical activity, assessed over a mean of 2.4 years, was associated with a 27% reduction in total mortality and a 31% reduction in cardiac mortality [17]. Moreover, it is a well-established and useful tool in rehabilitation for stable coronary insufficiency and after infarction [18-20], and the evidence supporting the beneficial effect of physical training in patients with coronary disease is good [21]. Yet, the relative risk of coronary artery disease hasbeen estimated to be circa 2 fold higher for inactive subjects compared with physically active individuals [22].
However, regular exercise protects against chronic and acute coronary disease through mechanisms which have not yet been completely elucidated. It is believed that exercise operates by the reduction of several risk factors related to cardiovascular pathologies, including high blood pressure, dis-lipidemia, obesity, insulin resistance, and autonomic dis-regulation [18, 23, 24].
Moreover, physical training can also reduce vascular resistance and induce structural adaptations in the coronary tree (i.e. increased number of capillaries and number and size of arteries and arterioles), thereby enhancing the blood transport capacity at this level [25]. Much attention has also been paid to the capacity of exercise to improve functions of the vascular endothelium. Indeed, the vasculature is the largest organ in the body and endothelial cells are important in regulating some key functions in homeostasis such as platelet aggregation, immune responses, and vascular permeability. Moreover, endothelium maintains vascular tone, thereby determining blood flow distribution to each tissue [26], and produces numerous substances, including nitric oxide (NO), which is important in regulating vasomotor function and in maintaining the health of the vascular wall [27]. It is now established that exercise can improve endothelial functions and endothelial-dependent vasodilatation as well as increase gene expression for endothelial NO synthase (eNOS) [28-31]. Finally, it has recently been found both in humans and in animals that physical exercise can also mimic the “Ischemic Preconditioning” (IP) phenomenon, which refers to the capacity of short episodes of ischemia to render the myocardium more resistant to subsequent more prolonged ischemic events [32-35]. These recent findings further strengthen the potential benefit of exercise on chronic and acute coronary disease.
The purpose of this article is to review the latest data on the potential role of exercise in inducing the preconditioning phenomenon. The general features of ischemic preconditioning are briefly reviewed in the first part, whilst the second deals with the potential role of exercise in triggering the cardioprotection afforded by preconditioning.
ISCHEMIC PRECONDITIONING: GENERAL CONCEPTS
The heart is able to change its phenotype in response to various kinds of stress in order to become more resistant to subsequent injury. This is well testified by phenomena such as stunning, hibernation, and preconditioning [36]. This latter condition identifies the capacity of various kinds of stimuli such as ischemia, rapid pacing, heat stress, exercise, and drugs to put the heart in a defensive status which is more resistant to ischemia.
The classical form of preconditioning is ischemic preconditioning (IP), which refers to the capacity of brief sub-lethal episodes of myocardial ischemia to protect against cellular damage due to more sustained periods of ischemia able to induce infarction. IP was first reported by Murry and co-workers [37] in dogs, where it was found that IP could greatly reduce infarction size. This necrosis-sparing effect was remarkable and reduced necrosis from an expected 30% to only 7% of the risk area. Moreover, it is to be emphasized that protection was unrelated to the development of collateral flows. Importantly, in the years that followed this original observation in dogs, the infarct-sparing effect of IP was reproduced in several mammalian species, including the rat, the rabbit, the swine, and the goat [38-42]. Since then the phenomenon has been recognized as “the strongest form of in vivo protection against myocardial ischemic injury other than early reperfusion” [36].
Along with the necrosis-sparing effect, IP has been demonstrated to protect the heart against damage caused by ischemia-reperfusion such as ventricular arrhythmias and myocardial stunning [43-46]. Moreover, IP can affect also coronary reactivity, as after preconditioning maneuvers the coronary vascular bed was found to respond to brief coronary occlusions with a reduced reactive hyperemic flow and a more rapid vasodilatation. The latter phenomenon was likely achieved through an increase in endothelial release of NO. Therefore, along with myocardial cell protection, IP can also exert beneficial effects on vascular endothelium responsiveness of the coronary tree [41].
Since experimentally-induced infarction in humans is not a feasible option, in vivo research to test directly whether or not IP can effectively reduce infarct size in humans is not possible. However, in vitro studies in isolated cultured human atrial myocytes have demonstrated that contractility recovery time after sustained ischemia was shorter if ischemia was preceded by a preconditioning maneuver [47, 48]. Furthermore, studies conducted in the course of coronary angioplasty have reported that the electrocardiographic (ST segment elevation) and clinical (chest pain) signs of myocardial ischemia were attenuated after the first balloon inflation, which can be considered as a preconditioning maneuver [49-51]. Yet, it is well established that if myocardial infarction is preceded by angina, which can be considered a form of ischemic preconditioning, then patients have smaller infarct size and better outcomes after thrombolytic therapy than patients without pre-infarction angina [52-56]. Therefore, these findings taken together support the concept that, like in animals, even human cardiomyocytes can be preconditioned. This fact suggests that IP can be induced in humans.
Finally, the “warm-up” phenomenon (i.e improved exercise performance and reduced severity of angina exhibited by some patients with coronary artery disease a few minutes after a previous effort) has also been considered a sort of ischemic preconditioning, mainly because the warm-up time course is consistent with that of IP. However, it should be taken into account that the opening of collateral flows together with some metabolic adaptations may in part be responsible for the phenomenon [57-62]. Hence, it is unclear at this point whether other mechanisms apart from preconditioning are involved in the warm-up.
As concerns the temporal characteristics, it is now established that IP is a biphasic phenomenon with an early phase of protection, commonly known as the first window of protection (FWOP), and a second phase, termed the second window of protection (SWOP). While FWOP is active immediately after preconditioning ischemia and lasts for about 2-3 hours, SWOP starts 12-24 hours after the initial ischemia and lasts 72-90 hours and confers a delayed protection [63, 64]. This late phase of protection is particularly attractive in a clinical perspective because of its sustained duration. Apart from the duration, other differences between FWOP and SWOP exist. Indeed, SWOP is more effective than FWOP in attenuating myocardial stunning, whereas FWOP exerts a more pronounced effect in reducing infarct size [65, 66].
ISCHEMIC PRECONDITIONING: RECEPTORS, MEDIATORS AND EFFECTORS
The chain of cellular events that confers resistance to ischemia is not completely understood. It has been reported that the accumulation of intracellular adenosine, produced from ATP degradation during the brief ischemic periods of preconditioning preceding the sustained ischemia, interacts with adenosine receptors A1 and A2, thus activating a complex metabolic cascade that eventually induces myocardial protection [38, 67]. The fact that adenosine agonist administration confers protection, whilst A1 receptor blockers eliminate the preconditioning effects bears witness with the concept that adenosine plays a role in the cardioprotection of IP [38, 68]. The role of adenosine has also been confirmed in vivo preconditioning of the human myocardium. Indeed, it was found that adenosine infusion could attenuate the ischemic signs due to balloon inflation during angioplasty maneuvers, thus strengthening the role played by this metabolite in IP [69, 70].
In addition to adenosine, the ischemic heart also releases other end-products and metabolites, such as reactive oxygen species (ROS), reactive nitrogen species (RNS), bradykinin, and opioids, which are all considered to be capable of inducing preconditioning [71-74]. All these substances seem to work in parallel by means of interaction with their specific receptors which activate the protein kinase C (PKC) [67]. Probably, a threshold for IP exists and the accumulation of each single metabolite alone is insufficient to trigger the threshold required for protection, i.e. their effects can sum to exceed the threshold to initiate IP and if any of the metabolites is eliminated by an antagonist, the hypothesized threshold can no longer be reached [67].
A particular role is played by ROS and RNS. Both ROS and RNS are involved in normal cell regulation in which oxidants and redox status are important in signal transduction [75-78]. Several studies report that ROS/RNS are among the principle responsible for the reversible protein kinase activation observed after ischemia, as their production may induce either reversible or irreversible activation of proteins/enzymes [79-81]. Conversely, the ROS/RNS scavengers block preconditioning cardioprotection [78, 82-84]. Although ROS/RNS production (redox stress) may be detrimental, from these studies it appears that their production may be also beneficial (redox signalling) [78, 85]. In fact, excessive ROS and RNS formation during reperfusion that follows infarcting ischemia may enhance cell death, while low concentrations of ROS/RNS modulate cell signalling processes during reperfusion and they are essential for the cardioprotection induced by IP.
Hence, PKC activation appears to be the common path along which all preconditioning stimuli converge. It is noteworthy that oxygen-free radicals can directly activate PKC, without the need to interact with a specific receptor [42]. PKC eventually phosphorylates some unknown effectors responsible for ischemic resistance. The metabolic events following PCK activation are only partially understood. Other kinases besides PKC have been identified as being potentially involved in the IP metabolic chain. Among others, tyrosine kinase seems to be downstream of PCK in this cascade [86]. Moreover, several mytogen activated protein kinases (MAPK) seem to exist downstream of PKC and play a role in cardioprotection. However, this topic is a much debated chapter in the history of IP [42] and exhaustive attention to it was not foreseen in this review.
Notwithstanding the debate over the metabolic cascade following PCK activation, there is general consensus that, at least for FWOP, the final effectors of the cardioprotection induced by IP are the ATP-sensitive potassium (KATP) channels, although it should be considered that it remains still unknown the exact mechanism which leads to cellular resistance to ischemia. These channels are normally closed in the non-ischemic heart, i.e. when the cellular level of ATP is normal. However, when ischemia occurs the fall in intracellular ATP level open these channels. Moreover, they are also modulated by ADP, NO, pH, fatty acid, G-proteins and various ligands such as adenosine, acetylcholine etc. [87, 88].
The fact that the opening of KATP channels was involved in the IP phenomenon was first proposed by Gross and co-workers [89]. Since then, it has also been demonstrated that KATP blockers can abolish IP-induced protection [90, 91] and that channel openers such as cromakalin, bimakalin, or pinacidil can mimic IP protection [92, 93]. Moreover, the importance of KATP channels also results from the clinical observation that diabetic patients in treatment with glibencamide and other sulfonylureas, which block these channels, exhibit increased mortality from cardiovascular causes and have poorer outcomes at the time of myocardial infarction [94, 95]. Actually, the opening of KATP channels may trigger several actions against cellular damage. Among others, it may induce depolarization of the cellular membrane and shortening of the action potential, thus preventing Ca2+ entry and overload, a phenomenon that may lead to cell death. Furthermore, the shortening of action potential spares energy. All these effects are potentially beneficial and may in part explain the protective action of KATP channels opening during ischemia.
Recent findings showing that cardiac cells contain at least two different types of KATP channels, sarcolemmal (sKATP) and mitochondrial (mKATP), have contributed to a greater understanding of IP. It has recently been proposed that the mKATP channels are the true final effectors of IP. In fact, several findings argue against a role of sKATP channels: for example, bimakalim was found to reduce infarct size at a dose that did not affect action potential duration [96], and this action clearly excludes the sKATP channels’ involvement in the phenomenon; in simulated ischemia models of cultured cardiomyocytes, which were quiescent and did not generate any action potential, it was still possible to induce protection through the administration of KATP channel openers [97], and this fact means that action potential shortening is not mandatory for cardioprotection; finally, and perhaps most importantly, the use of diazoxide, a KATP channel opener 1000 to 2000 times more potent in opening mKATP than sKATP channels [98], has been demonstrated able to induce cardioprotection in the micromolar range without shortening the action potential, thereby suggesting that the opening of sKATP channels is not mandatory; yet the specific blocker of mKATP 5-hydroxidecaonate blocks the cardioprotection induced by mKATP openers [98, 99]. All these facts strongly suggest that mKATP channels are the real effectors of the cellular metabolic cascade that confers preconditioning and make it necessary to reconsider the role of sKATP channels as effectors of preconditioning. However, it is still unclear why opening mKATP channels is cardioprotective and several not mutually exclusive hypotheses have been developed [100-107].
It is to be noted that the early moments of reperfusion after sustained ischemia are crucial since significant reversible and irreversible organ damage is triggered. This period can lead to reperfusion injury such as arrhythmias, transient mechanical dysfunction, microvascular injury, and inflammatory response [85, 108]. Recently, regulators of mitochondrial membrane permeability have been reported to play a critical role in the IP phenomenon [78, 85, 109]. In detail, mitochondrial permeability transition pores (mPTP) have been found involved in the reperfusion injury following the ischemic insult. These pores open in the reperfusion period, when cellular pH increases after its reduction occurring in the ischemic phase. The consequence of long-lasting mPTP opening is the collapse of mitochondrial membrane potential, matrix swelling, rupture of mitochondrial membrane, and ultimately apoptosis. It has found that IP can delay the post-ischemic recovery of intracellular pH, thereby preventing mPTP opening directly and indirectly [85, 110]. Hence, it is conceivable to hypothesize that IP, by counteracting mPTP opening, can protect the heart from damage due to the ischemia/reperfusion period.
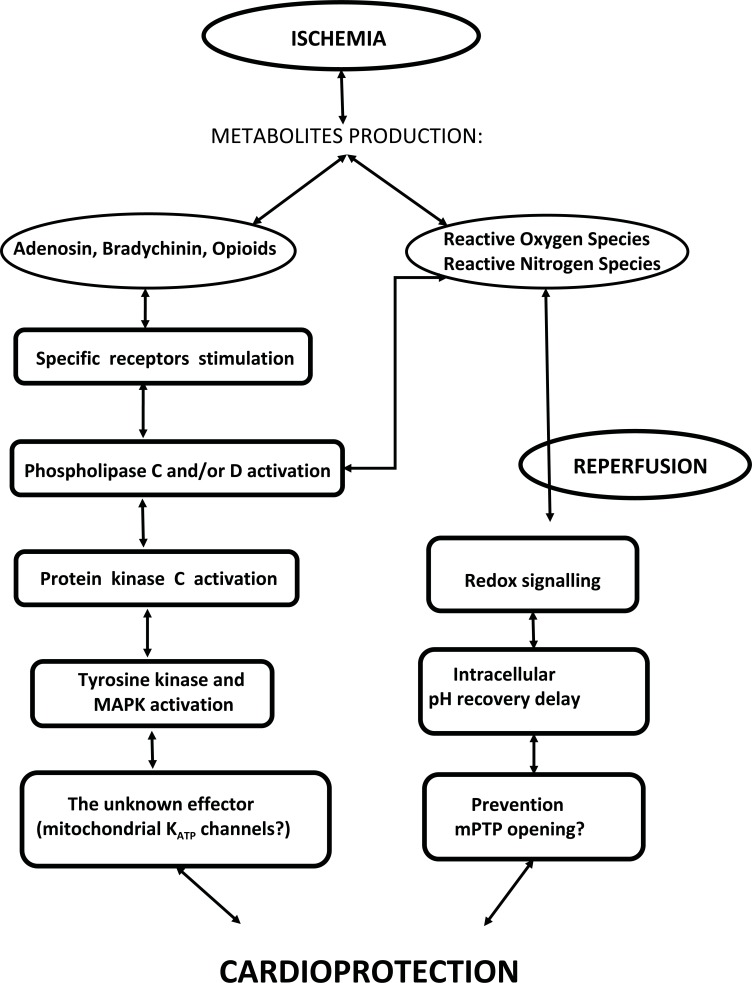
The putative mechanisms of IP cascade during the FWOP period are schematically illustrated by (Fig. 1).
Fig. (1).
Putative mechanisms of IP cascade during the first window of ischemic protection. Ischemia causes the production of some metabolites that, in turn, activate Protein Kinase C. This leads to activation of several cell kinases, such as Tyrosine kinase and MAPK. An unknown effector (possibly mitochondrial KATP channels) is downstream of this cascade. Redox signalling due to reactive oxygen and nitrogen species production also contributes by preventing mPTP opening during reperfusion. See text for more details.
If the molecular cascade of FWOP is complex and only partially understood, the cellular events leading to delayed protection, i.e. the SWOP period, are even more complex and less understood. As previously stated, SWOP shows a particular time course, as it starts several hours after FWOP has ceased, i.e. about 12-24 hours after the preconditioning stimuli, and lasts 72-90 hours. This fact is generally interpreted by taking into account that gene expression and new protein synthesis are mandatory for the protection afforded by SWOP [65, 111]. Similarly to what has been described for FWOP, there are also putative triggers, mediators, and final effectors for SWOP. However, exhaustive attention to this particular topic was not foreseen in the present manuscript and readers should refer to other papers for a detailed review of this issue [42, 65, 66]. Here, we believe that elucidating the general features of SWOP should suffice to understand the phenomenon.
As for FWOP, adenosine [112-114], bradykinin [115, 116], oxygen free radicals [117-119], and Opioids [120, 121] are all believed to initiate the cascade which leads to SWOP. Thus, the same stimuli that initiate FWOP can also induce SWOP. It is widely accepted that the cellular kinases that open mKATP channels in early preconditioning can also activate the transcription of genes that codify some cardioprotective proteins. These probably include inducible NO synthase (iNOS) [122-125], Cyclooxygenase-2 [126, 127], Aldose Reductase [128], Super Oxide Dismutases [129, 130], and Heat Shock Proteins (HSPs) [131-134].
The final effectors of delayed preconditioning are still unknown. Delayed preconditioning appears to be of a polygenic nature and that the heart shifts to a defensive phenotype requiring a complex coordinated activation of multiple genes [65]. Hence, there is probably no single end-effector of protection, but probably the contemporary expression of the same proteins may induce the protective status through several different actions.
Some evidence suggests that mKATP channel opening may also be a component of delayed preconditioning. In animal model of IP (rats and rabbits) it has been found that diazoxide applied 24 hours before ischemia is able to induce protection against infarction [135] and that this protection can be eliminated by treatment with the mKATP channels selective blockers 5-hydroxydecanoate [136]. Recently, it has also been reported that delayed preconditioning in humans requires the opening of mKATP channels [137]. Thus, it appears that mKATP channels are a component of SWOP, even though the specific importance of their involvement requires further clarification.
A particular role in the SWOP phenomenon is played by NO as it participates in the signaling pathway from FWOP to SWOP as well as acting as a trigger for SWOP. In detail, during the preconditioning maneuvers preceding FWOP, the reduction in pH that occurs in the coronary vessel activates a kininogenase which provokes the production of bradykinin which, in turn, through endothelial B2 receptors interaction, stimulates NO production by eNOS [66, 124]. The NO generated by eNOS activates PKC and, in turn, the kinases responsible for the transcription of genes which codify the cardioprotective proteins of SWOP, including iNOS [138], which appears to be an essential mediator for delayed preconditioning [65, 122, 125, 139]. As mentioned above, NO also appears to act as a trigger for SWOP since it can directly open mKATP channels, thus rendering the cell more resistant to ischemia [140]. Bolli and co-workers [141] proposed a scenario where the NO generated early by eNOS initiates the preconditioning cascade that confers immediate protection and activates the iNOS that produces the NO involved in delayed protection. It is noteworthy that both in animals and in humans pre-treatment with NO donor under normoxic or ischemic conditions can mimic the effects of SWOP [35, 142-144]. In our opinion, it is useful to emphasize that delayed preconditioning is probably a universal mechanism whereby the heart responds to general stress, and that it can be activated by several situations such as heat shock, hibernation, pacing, and physical exercise [36, 65, 145].
EVIDENCE THAT EXERCISE CAN INDUCE PRECONDITIONING
Much evidence shows that exercise can induce preconditioning and protect the heart from ischemic insults without the necessity of a previous ischemia. Several studies conducted both in the animal and in the human setting strongly suggest that exercise provides myocardial protection against damage due to ischemia.
ANIMAL STUDIES
Increasing myocardial O2 consumption with tachycardia may confer cardioprotection in animal models of IP [32, 146, 147]. This result was achieved without ischemia, thus suggesting that tachycardia alone may directly induce preconditioning. This finding leads to speculation that exercise-induced tachycardia might exert the same effect. However, the infarct-sparing effect due to exercise in conscious dogs was greater than that observed due to tachycardia alone in anesthetized dogs, thus suggesting that during exercise, stimuli other than increased myocardial O2 consumption may activate the cascade that induces preconditioning [32, 147]. Moreover, in isolated hearts from exercise-trained rats it was found that the recovery of cardiac function after global ischemia was greater compared with hearts obtained from sedentary animals [148]. These results are in accordance with findings from other investigations in which it was reported that endurance exercise training enhances myocardial performance during ischemic/reperfusion maneuvers [149, 150].
Overall, the mentioned studies have repeatedly shown that both short-term and long-term exercise training decrease ischemia reperfusion-induced cardiac injury.
HUMAN STUDIES
In the human setting, recent observations during repeated exercise tests performed in patients with stable angina suggest that exercise can trigger the preconditioning phenomenon. As previously illustrated in the introduction, the warm-up phenomenon, which refers to the enhanced exercise performance and the reduced severity of angina shown by some patients with coronary disease a few minutes after a previous effort, has been attributed to a preconditioning-like effect. This mainly because the warm-up time course is consistent with that of FWOP. Furthermore, it has been demonstrated that, if the patient had performed previous exercise, the degree of myocardial stunning following exercise-induced ischemia may be attenuated [151] and that myocardial oxygen consumption was reduced during the warm-up compared to the first effort [152], thereby indicating that the warm-up might improve myocardial performance and metabolism during subsequent efforts. Nevertheless, the opening of collateral flows may potentially play a role in the warm up, thus confounding the interpretation of data. Indeed, warm-up angina has been related to coronary vasodilation despite the fact that arterial vasodilators have little effect on exercise tolerance [153]. Moreover, it has never been explained why enhanced myocardial performance afforded by the second effort appears after a rest period of at least 2-5 min following the first effort, but no more than 30-60 min [154], which is consistent with the FWOP time course. Lambiase and co-workers [33] have demonstrated that the protection due to IP is independent of collateral recruitment. Taken together, these facts support the concept that warm-up is an IP mechanism related phenomenon.
It should be underscored that improved exercising capacity and reduced signs of ischemia can still be present 24 to 48 hours after ischemic exercise, i.e. during the SWOP period, when collateral flows should already be closed. In detail, Lambiase and co-workers [33] were the first to demonstrate that 24 hours after exercise-induced ischemia there is enhanced resistance to further ischemia caused by exertion. These findings were then confirmed by Crisafulli and co-workers in patients with stable angina [35]. These authors showed that, together with the reduced ischemic signs, global hemodynamics was also improved during exercise performed 48 hours after a previous exercise-induced ischemia and this fact provides clues that in the human being, along with FWOP (which is possibly coincidental with the warm-up phenomenon), SWOP can also be induced by efforts leading to ischemia.
Besides, Michaelides and co-workers [34] showed that most patients (37/50) with coronary insufficiency who underwent a sequence of repetitive exercise had improved myocardial performance measured with thallium-scintigraphy during the last of the sequential tests. Authors concluded that repetitive exercise induced cardiac adaptations, which may represent an aspect of ischemic preconditioning.
Moreover, by employing a sequence of repeated treadmill efforts, it has recently been reported that in the FWOP period (i.e. 30 minutes after a previous ischemia-inducing exercise) signs of the presence of cardioprotection (reduced ST segment depression, increased rate pressure product and longer onset of chest pain) were present. However, these signs disappeared 6 hours later, during the non-protecting period between the fading of FWOP and the appearance of SWOP, to return after 24 hours (i.e. during SWOP) [154]. Nevertheless, it should be mentioned that one study failed to demonstrate the possibility of inducing SWOP in humans by exercise-induced ischemia [155].
Therefore, it appears that the human heart may be preconditioned by exercise, although direct proof (the necrosis-sparing effect) that exercise is cardioprotective is still lacking. Furthermore, it should be highlighted that all the evidence demonstrating the possibility of preconditioning the human heart with exercise derives from studies on patients with coronary disease, in whom the preconditioning effect is triggered by exercise-induced myocardial ischemia. On the contrary, the attempt to enhance cardiac performance in healthy subjects by means of a sequence of maximal tests on a cycle-ergometer and by pharmacological-induced SWOP failed, thereby suggesting that ischemia must occur to detect any beneficial cardiac effect [156]. Thus, to the best of our knowledge, the capability of exercise to recruit the preconditioning phenomenon without the occurrence of ischemia is still to be demonstrated in humans, even though the aforementioned studies carried out on animals provide evidence supporting this possibility.
It should also be considered that the quoted investigation in humans focused on the preconditioning effects due to exercise-induced ischemia while, as yet, no studies have dealt with the effects of regular exercise training programs. This kind of exercise are normally conducted at sub-ischemic threshold intensity. As a consequence, no information is available on thresholds for exercise duration and severity to reach the beneficial effects of preconditioning induced by exercise. In this light, there is a compelling need for studies which investigate on the possibility of preconditioning the human myocardium with exercise and further establish what kind and at which intensity effort should be performed to trigger the preconditioning phenomenon.
EXERCISE AND PRECONDITIONING: POSSIBLE MECHANISMS
Although the mechanisms responsible for exercise-induced cardioprotection remain elusive, some exercise-induced cellular adaptations are likely to be involved in the phenomenon. Probably, exercise can activate the multiple downstream kinase cascade responsible for cardioprotection. Actually, studies performed on animals have demonstrated a necrosis-sparing effect and improved myocardial function after ischemia following exercise training [157-160]. However, at this stage, the precise mechanism through which exercise operates is only speculative and several possible mechanisms have been postulated.
As previously described, exercise-induced tachycardia alone may trigger preconditioning. Furthermore, several metabolites such as adenosine, bradykinin, and opioids are released during exercise. These metabolites are known to induce classic preconditioning, and this fact alone may explain the capability of exercise to recruit the preconditioning phenomenon. Moreover, the possibility that exercise may induce preconditioning by directly causing myocardial hypoxia cannot be ruled out. However, it has been found that a single episode of moderate sub-maximal physical exercise unlikely to induce hypoxia could confer cardioprotection on the rat heart. This protection has been explained through a PKC-mediated mechanism, since pharmacological inhibition of this enzyme during exercise resulted in the abrogation of protection [161]. These results suggest that, for the activation of the preconditioning cascade, hypoxia is not a prerequisite and that the PCK activity may be directly modulated by exercise.
Acute exercise generates ROS and does so in an intensity and duration-dependent manner [162]. The old view of exercise is a potential source of harmful oxidative damage has been changed. In fact, muscle derived ROS produced during prolonged inactivity contribute to muscle atrophy whereas the same stimulus coming from working fibers is essential for adaptations induced by training. This apparent paradox may be explained by the hormesis theory: chemical and toxic substances that are deleterious at high doses can have at low dose beneficial effects. Thus, increases in ROS due to exercise could induce beneficial adaptations [162]. It is noteworthy that antioxidant supplementation can reverse beneficial exercise adaptations [163, 164] The formation of ROS during exercise may be another potential exercise-related mechanism underlying cardioprotection [165, 166] since ROS production can directly activate PKC, without the need to interact with a specific receptor. Furthermore, physical training could up-regulate myocardial anti-oxidant capacity in order to overcome the oxidative stress caused by exercise. Indeed, myocardial Manganese Super Oxide Dismutase and extracellular Superoxide Dismutase levels have been reported to be increased in several studies [34, 160, 167]. The up-regulation of Super Oxide Dismutases is believed to be involved in SWOP, thus providing another mechanism through which exercise can act to protect the heart from ischemic insults.
A key role in cardioprotection due to physical exercise is probably played by NO production. It is well known that exercise increases shear stress within vessels. Shear stress is considered one of the most important mechanical stimuli leading to NO production and, actually, exercise enhances NO production [25, 29]. As stated in the previous paragraph, NO acts both as a trigger and mediator of SWOP. In fact, exposure to NO donors can reproduce the effects of delayed preconditioning in the absence of ischemia [35, 144]. Furthermore, NO is also believed to participate in FWOP protection, although conflicting results exist on whether NO action during FWOP exerts a beneficial effect or simply triggers SWOP [124]. Thus, it is possible to speculate that exercise-induced increase NO production is a further mechanism by which physical training acts in order to induce cardioprotection. It is also to be considered that skeletal muscle also generates RNS including NO or nitrite ion [162, 168], which at high doses may cause nitrosative stress and tissue damage, but at low doses exerts beneficial effects in signalling transduction, as previously described.
Exercise can act also by increasing the expression of cardiac stress proteins, which are likely to be involved in myocardial protection [169, 170]. In this regard, HSPs have been extensively studied and it has been found that the myocardial level of these proteins can increase up to threefold in response to exercise [169]. It is believed that HSPs can protect cells from a variety of stresses including ischemia-reperfusion injury [138]. However, their contribution to the cardioprotection afforded by exercise remains controversial since the cardioprotection lasts longer than HSP half time [171].
Finally, the phenomenon known as “remote preconditioning”, which refers to the possibility of preconditioning the heart by causing ischemia in a remote organ, should also be taken into account. This particular kind of preconditioning has been demonstrated by inducing transient ischemia in several organ and tissue such as the small intestine, kidney, and skeletal muscle [172-175] and it has been shown to induce both early and delayed cardioprotection [176-178]. Probably, remote preconditioning is initiated by humoral factors released into the blood and transferred to the heart where they trigger protection [179]. In the genesis of the remote preconditioning key molecules appear to be adenosine and bradykinin, although the exact mechanisms involved are still unknown [175, 180].
It must also be considered that amount of products deriving from the anaerobic metabolism (such as lactate, ADP, adenosine etc.) is released into the blood during exercise, even in non-ischemic conditions [15, 181, 182], i.e. without the flow reduction setting used to cause remote preconditioning in the aforementioned experiments. It is therefore conceivable to hypothesize that these substances produced during exercise may trigger preconditioning, but to the best of our knowledge this hypothesis has never been verified.
It has been recently demonstrated that dialysated plasma from humans undergoing high-intensity exercise reduced infarct size in isolated rabbit hearts after ischemia-reperfusion injury. This phenomenon was also present with plasma from humans exposed to remote ischemic preconditioning [183]. Authors of the quoted study concluded that exercise-induced cardioprotection was at least partially mediated by systemic release of one or more humoral factors. A similar outcome was also obtained in the mice heart perfused with dialysated plasma from highly trained humans (swimmers) undergoing a protocol of ischemia-reperfusion to trigger the remote preconditioning phenomenon. In this study, along with the infarct reduction effect in the mice heart, the remote ischemic preconditioning maneuvers were also able to enhance the athletic performance during swimming [184]. The fact that remote preconditioning is able to improve exercise performance has been also demonstrated by a series of studies reporting that IP can improve oxygen uptake, maximal power output, blood lactate accumulation, and time to exhaustion in tests performed in the laboratory setting [185-193]. Furthermore, remote ischemic preconditioning has been found able to improve endothelium-dependent function after strenuous exercise [188]. The effect of remote ischemic preconditioning on the endothelium has been attributed to humoral mechanisms that lead to increased activation of ATP-sensitive potassium channels and increased concentration of NO [189-191]. Yet, a recent investigation reported that the ischemic preconditioning induced by intermittent upper-arm ischemia done before primary percutaneous coronary intervention could attenuate reperfusion injury in patients with evolving myocardial infarction, thereby resulting in increased myocardial salvage [192]. Collectively, these data strongly suggest that remote ischemic preconditioning can act as a mechanism whereby the heart can be preconditioned by exercise.
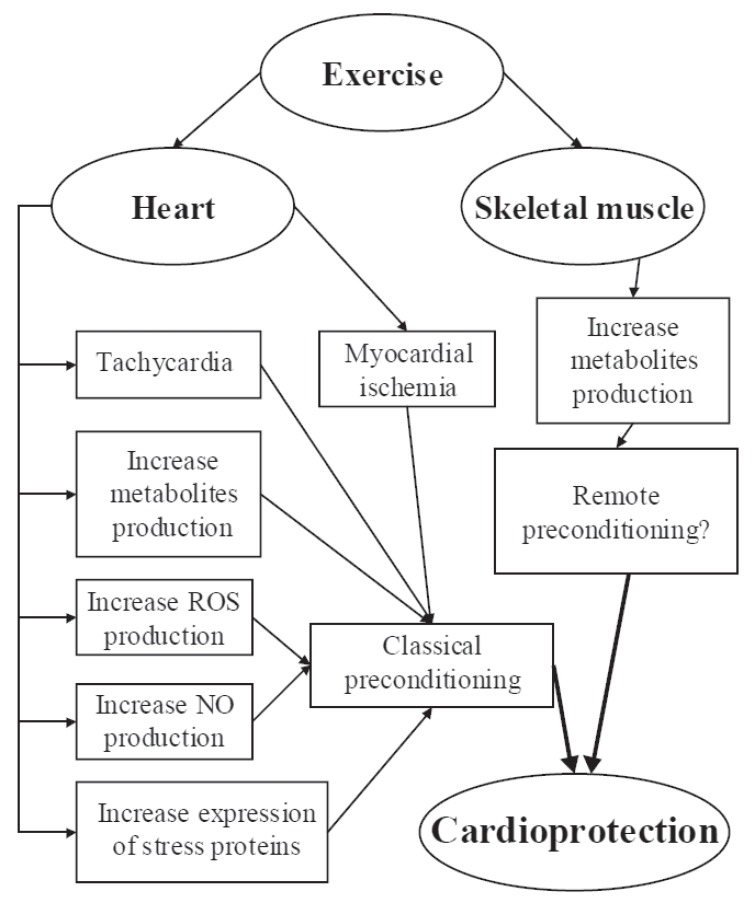
The major putative mechanisms responsible for the exercise-induced preconditioning discussed in the present paragraph are shown by (Fig. 2).
Fig. (2).
Putative mechanisms responsible for exercise-induced preconditioning: exercise directly affects the heart by means of ischemia, tachycardia, NO, ROS, other metabolites, and stress protein production. Remote preconditioning is a further possible mechanism by which affects the heart. See text for more details.
CONCLUSIONS
Although the exact mechanisms underlying exercise-induced cardioprotection against ischemia still need to be elucidated, there are continuing advances in the understanding of the phenomenon. From the cited studies it appears that exercise acts as a physiological stress that, similar to that due to sub-lethal ischemia, heat, caloric restriction, and hypoxia can enhance cellular defense [193] and induce a defensive phenotype in the heart, which probably depends on the contemporary production of several molecules and proteins. These changes at cellular level render the heart more tolerant to ischemic insult and damage. However, the mechanisms responsible for the phenomenon are only partially understood and require further investigation. In humans, the direct demonstration of the possibility of preconditioning the heart of healthy individuals are still lacking, notwithstanding a preconditioning effect due to ischemia has been observed in patients suffering from coronary disease (i.e. the “warm-up”). Numerous unanswered questions still remain such as. In particular, describing the specific pathways and mechanisms that are involved in exercise-induced cardioprotection is crucial for therapeutic intervention. Moreover, the characterization of exercise intensity and duration required to trigger protection due to ischemic preconditioning is another important topic that needs to be investigated. This is particularly important for patients suffering from coronary heart disease, as it is generally accepted that exercise training should be conducted below the threshold for myocardial ischemia. However, patients may be deprived of the potential benefits of more intense exercise by the use of such a protocol. In controlled conditions, ischemic exercise training does not seem to be harmful and it may afford protection induced by IP [194]. Thus, it is possible to speculate that introducing short periods of ischemic training in these patients may be effective in inducing the IP phenomenon.
Further research is warranted to address all these issues. In any case, it appears that regular exercise should be recommended not only to obtain the beneficial effects on conventional cardiovascular risk factors, but also to achieve the preconditioning status [195, 196]. Currently, given the lack of a therapeutic target, the only available practical method of inducing ischemic preconditioning at the heart level is exercise training [197].
ACKNOWLEDGEMENTS
This study was supported by the University of Cagliari and the Italian Ministry of Scientific Research. The authors wish to thank Mr. Barry Mark Wheaton for his editorial assistance.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative protections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. New Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 3.Morris JN, Heady JA, Raffle P, Roberts CG, Parks JW. Coronary heart disease and physical activity of work. Lancet. 1953;265:1053–7. doi: 10.1016/s0140-6736(53)90665-5. [DOI] [PubMed] [Google Scholar]
- 4.McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation. 1978;57:958–62. doi: 10.1161/01.cir.57.5.958. [DOI] [PubMed] [Google Scholar]
- 5.Froelicher V, Battler A, McKirnam MD. Physical activity and coronary heart disease. Cardiology. 1980;65:153–90. doi: 10.1159/000170805. [DOI] [PubMed] [Google Scholar]
- 6.Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2:1207–10. doi: 10.1016/s0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 7.Eichner ER. Exercise and heart disease. Am J Med. 1983;75:1008–23. doi: 10.1016/0002-9343(83)90882-3. [DOI] [PubMed] [Google Scholar]
- 8.Paffenbarger RS, Hyde RT, Wing A, Hsieh C. Physical activity. all-cause mortaity.and longevity of college alumni. N Eng J Med. 1986; 314:605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 9.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Ann Rev Public Health. 1987;8:253–87. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 10.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: combined experience of randomized clinical trials. JAMA. 1988;260:945–50. [PubMed] [Google Scholar]
- 11.O’Connor GT, Buring JE, Yusaf S, Goldhaber SZ, Olmstead EM. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–44. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 12.Blair SN, Bouchard C, Shephard RJ, Stephens T, editors. Champaign IL:: Human Kinetics; 1994. Physical activity. fitess.and coronary heart disease. In Physical activity fitness and health. ; pp. 579–90. [Google Scholar]
- 13.Sandvick L, Erikssen J, Thoulaw WE, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy and middle aged Norwegian men. N Engl J Med. 1993;328:5343–7. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 14.Hull SS Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during myocardial ischemia. Circulation. 1994;89:548–52. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 15.Myers J. Exercise and cardiovascular health. Circulation. 2003;107:e2–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 16.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–18. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Kar S, Fay WP. Thrombosis. physical actiity.and acute coronary syndromes. J Appl Physiol . 2011; 111:599–605. doi: 10.1152/japplphysiol.00017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–72. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 19.Leon AS. Exercise following myocardial infarction. Sports Med. 2000;29:301–11. doi: 10.2165/00007256-200029050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hambrecht R, Walther C, Möbius-Winkler S , et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease. Circulation. 2004;109:1371–8. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16 (S1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 22.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- 23.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol (Heart Circ Physiol) 2009;297:H1171–93. doi: 10.1152/ajpheart.00534.2009. [DOI] [PubMed] [Google Scholar]
- 24.McCartney N. Role of resistance training in heart disease. Med Sci Sports Exerc. 1998;30(10 Suppl):S396–402. doi: 10.1097/00005768-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol. 1992;73(6):2209–25. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin MH, Joseph B. Wolfe Memorial lecture.Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc. 2004;36(3):352–62. doi: 10.1249/01.mss.0000117114.02875.5c. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Alamillo C, Juncos LA, Cases A, Haas JA, Romero C. Interactions between vasoconstrictors and vasodilators in regulating hemodynamics of distinct vascular beds. Hypertension. 2003;42 (part2):831–6. doi: 10.1161/01.HYP.0000088854.04562.DA. [DOI] [PubMed] [Google Scholar]
- 28.Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol. 1997;273:H2575–9. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- 29.Gattullo D, Pagliaro P, Marsh N, Losano G. New insight into nitric oxide and coronary circulation. Life Sci. 1999;65:2167–74. doi: 10.1016/s0024-3205(99)00299-4. [DOI] [PubMed] [Google Scholar]
- 30.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hägg U, Wandt B, Bergström G, Volkmann R, Gan LM. Physical exercise capacity is associated with coronary and peripheral vascular function in healthy young adults. Am J Physiol (Heart Circ Physiol) 2005;289:H1627–34. doi: 10.1152/ajpheart.00135.2005. [DOI] [PubMed] [Google Scholar]
- 32.Domenech R, Macho P, Schwarze H, Sanchez G. Exercise induces early and late myocardial preconditioning in dogs. Cardiovasc Res. 2002;55:561–6. doi: 10.1016/s0008-6363(02)00334-6. [DOI] [PubMed] [Google Scholar]
- 33.Lambiase P, Edwards RJ, Cusack MR, Bucknall CA, Redwood SR, Marber MS. Exercise-induced ischemia initiates the second window of protection in humans inde-pendent of collateral recruitment. J Am Coll Cardiol. 2003;41:1174–82. doi: 10.1016/s0735-1097(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 34.Michaelides AP, Andrikopoulos GK, Oikonomou EV , et al. Improved myocardial performance during repetitive exercise testing: the role of extracellular superoxide dismutase activity in a model of exercise-induced myocardial preconditioning. Am Heart J. 2003;146:160–7. doi: 10.1016/S0002-8703(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 35.Crisafulli A, Melis F, Tocco F , et al. Exercise-induced and nitroglycerin-induced myocardial preconditioning improves hemodynamics in patients with angina. Am J Physiol (Heart Circ Physiol) 2004;287:H235–42. doi: 10.1152/ajpheart.00989.2003. [DOI] [PubMed] [Google Scholar]
- 36.Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. and participants.Medical and cellular implications of stuning.hibernation., and preconditioning. Circulation. 1998; 97:1848–67. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- 37.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 38.Liu GS, Thornton J, Van Winkle DM, Stanley AWH, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–6. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Downey JM. Ischemic preconditioning protects against infarction in rat heart. Am J Physiol. 1992;263:H1107–12. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- 40.Vahlhaus C, Schultz R, Post H, Onallah R, Heusch G. No prevention of ischemic preconditioning by the protein kinase C inhibitor staurosporine in swine. Circ Res. 1996;79:407–14. doi: 10.1161/01.res.79.3.407. [DOI] [PubMed] [Google Scholar]
- 41.Gattullo D, Linden RJ, Losano G, Pagliaro P, Westerhof N. Ischemic preconditioning changes the pattern of coronary reactive hyperaemia in the goat: role of adenosine and nitric oxide. Cardiovasc Res. 1999;42:57–64. doi: 10.1016/s0008-6363(98)00319-8. [DOI] [PubMed] [Google Scholar]
- 42.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 43.Shiki K, Hearse DJ. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am J Physiol. 1987;272:H1470–6. doi: 10.1152/ajpheart.1987.253.6.H1470. [DOI] [PubMed] [Google Scholar]
- 44.Asimakis GK, Innerd-McBridge K, Medellin G, Conti VR. Ischemic preconditioning attenuates acidosis and postischemic dysfunction in isolated rat heart. Am J Physiol. 1992;263:H887–94. doi: 10.1152/ajpheart.1992.263.3.H887. [DOI] [PubMed] [Google Scholar]
- 45.Parratt J, Vegh A. Pronounced antiarrhythmic effects of ischemic preconditioning. Cardioscience. 1994;5:9–18. [PubMed] [Google Scholar]
- 46.Cohen MV, Yang XM, Downey JM. Smaller infarct after preconditioning does not predict extent of early functional improvement of reperfused heart. Am J Physiol. 1999;277:H1754–61. doi: 10.1152/ajpheart.1999.277.5.H1754. [DOI] [PubMed] [Google Scholar]
- 47.Speecly-Dick ME, Grover GH, Yellon DM. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K+ channel?.Studies of contractile function after simulated ischemia in an atrial in vitro model. Circ Res. 1995;77:1030–5. doi: 10.1161/01.res.77.5.1030. [DOI] [PubMed] [Google Scholar]
- 48.Carr CS, Hill RJ, Masamune H , et al. Evidence for a role of both A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischemia. Cardiovasc Res. 1997;36:52–9. doi: 10.1016/s0008-6363(97)00160-0. [DOI] [PubMed] [Google Scholar]
- 49.Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrman HC, Lashey WL. Adaptation to ischemia during percutaneous transluminal coronary angioplasty: clinical. hemodynmic.and metabolic features. . Circulation . 1990; 82:2044–51. doi: 10.1161/01.cir.82.6.2044. [DOI] [PubMed] [Google Scholar]
- 50.Tomai F, Crea F, Gaspardone A , et al. Mechanisms of cardiac pain during coronary angioplasty. J Am Coll Cardiol. 1993;22:1892–6. doi: 10.1016/0735-1097(93)90775-v. [DOI] [PubMed] [Google Scholar]
- 51.Laskey WK. Beneficial impact of preconditioning during PTCA on creatine kinase release. Circulation. 1999;99:2085–9. doi: 10.1161/01.cir.99.16.2085. [DOI] [PubMed] [Google Scholar]
- 52.Kloner RA, Shook T, Przyklenk K , et al. Previous angina alters in-hospital outcome in TIMI-4. Circulation. 1995;91:37–47. doi: 10.1161/01.cir.91.1.37. [DOI] [PubMed] [Google Scholar]
- 53.Ottani F, Galvani M, Ferrini D , et al. Prodromal angina limits infarct size: a role for ischemic preconditioning. Circulation. 1995;91:291–7. doi: 10.1161/01.cir.91.2.291. [DOI] [PubMed] [Google Scholar]
- 54.Andreotti F, Pasceri V, Hackett DR, Davies GJ, Harder AW, Maseri A. Pre-infarction angina as a predictor of more rapid coronary thrombolysis in patients with acute myocardial infarction. N Engl J Med. 1996;334:7–12. doi: 10.1056/NEJM199601043340102. [DOI] [PubMed] [Google Scholar]
- 55.Ishihara M, Sato H, Tateishi H , et al. Implications of prodromal angina pectoris in anterior wall acute myocardial infarction: acute angiographic findings and long-term prognosis. J Am Coll Cardiol. 1997;30:970–5. doi: 10.1016/s0735-1097(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 56.Noda T, Minatoguchi S, Fujii K , et al. Evidence for the delayed effect in human ischemic preconditioning: prospective multicenter study for preconditioning in acute myocardial infarction. J Am Coll Cardiol. 1999;34:1966–74. doi: 10.1016/s0735-1097(99)00462-3. [DOI] [PubMed] [Google Scholar]
- 57.Marber MS, Joy MD, Yellon DM. Is warm-up in angina ischemic preconditioning?. Br Heart J. 1994;72:213–5. doi: 10.1136/hrt.72.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maybaum SM, Ilan MM, Mogilevsky JMetal. Improvement in ischemic parameters during repeated exercise testing a possible model for myocardial preconditioning. Am J Cardiol. 1996;78:, 1087–91. doi: 10.1016/s0002-9149(96)90057-0. [DOI] [PubMed] [Google Scholar]
- 59.Kay P, Kittelson J, Stewart RAH. Relation between duration and intensity of first exercise and “warm up” in ischemic heart disease. Heart. 2000;83:17–21. doi: 10.1136/heart.83.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bilinska M, Rudnicki S, Beresewicz A. Delayed attenuation of myocardial ischemia with repeated exercise in subjects with stable angina a possible model for the second window of protection?. Basic Res Cardiol. 2000;95:418–23. doi: 10.1007/s003950070042. [DOI] [PubMed] [Google Scholar]
- 61.Kelion AD, Webb TP, Gardner MA. The warm-up effect protects against ischemic left ventricular dysfunction in patients with angina. J Am Coll Cardiol. 2001;37:705–10. doi: 10.1016/s0735-1097(00)01182-7. [DOI] [PubMed] [Google Scholar]
- 62.Tomai F. Warm-up phenomenon and preconditioning in clinical practice. Heart. 2002;87:99–100. doi: 10.1136/heart.87.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuzuya T, Hoshida S, Yamashita Netal. Delayed effects of sub-lethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 64.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 65.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 66.Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001;69:1–15. doi: 10.1016/s0024-3205(01)01113-4. [DOI] [PubMed] [Google Scholar]
- 67.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Ann Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 68.Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pre-treatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–65. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 69.Leesar MA, Stoddard M, Ahmed M, Broadbent J, Bolli R. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation. 1997;95:2500–7. doi: 10.1161/01.cir.95.11.2500. [DOI] [PubMed] [Google Scholar]
- 70.Leesar MA, Stoddard MF, Xuan YT, Tang XL, Bolli R. Non-electrocardiographic evidence that both ischemic preconditioning and adenosine preconditioning exist in hu-mans. J Am Coll Cardiol. 2003;42:437–45. doi: 10.1016/s0735-1097(03)00658-2. [DOI] [PubMed] [Google Scholar]
- 71.Parrat JR. Protection of the heart by ischemic preconditioning: mechanisms and possibilities for pharmacological exploitation. Trends Pharmacol Sci. 1994;15:19–25. doi: 10.1016/0165-6147(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 72.Goto M, Liu Y, Yang X-M, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611–21. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- 73.Schultz JEJ, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibencamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100–4. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- 74.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Moll Cell Cardiol. 1997;29:207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 75.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61(3):461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 76.Pagliaro P, Moro F, Tullio F, Perreli MG, Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid Redox Signal. 2011;14:833–50. doi: 10.1089/ars.2010.3245. [DOI] [PubMed] [Google Scholar]
- 77.Pagliaro P, Gattullo D, Penna C. Nitroglycerine and sodium trioxodinitrate: from the discovery to the preconditioning effect. J Cardiovasc Med (Hagerstown) 2013;14(10):698–704. doi: 10.2459/JCM.0b013e3283621ac6. [DOI] [PubMed] [Google Scholar]
- 78.Tullio F, Angotti C, Perrelli MG, Penna C, Pagliaro P. Redox balance and cardioprotection. Basic Res Cardiol. 2013;108(5):371. doi: 10.1007/s00395-013-0392-7. [DOI] [PubMed] [Google Scholar]
- 79.Clerk A, Michael A, Sudgen PH. Stimulation of myltiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein. HSP2/27.in neonatal ventricular myocytes. Biochem J. 1998; 333:581–9. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2011;88:305–12. doi: 10.1161/01.res.88.3.305. [DOI] [PubMed] [Google Scholar]
- 81.Pham FH, Sugden PH, Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res. 2000;86:1252–8. doi: 10.1161/01.res.86.12.1252. [DOI] [PubMed] [Google Scholar]
- 82.Pagliaro P, Chiribiri A, Mancardi D, Rastaldo R, Gattullo D, Losano G. Coronary endothelial dysfunction after ischemia and reperfusion and its prevention by ischemic preconditioning. Ital Heart J. 2003;4:383–94. [PubMed] [Google Scholar]
- 83.Skyschally A, Schulz R, Gres P, Korth HG, Heush G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals of ascorbic acid. Am J Physiol Heart Circ Physiol. 2003;284:698–703. doi: 10.1152/ajpheart.00693.2002. [DOI] [PubMed] [Google Scholar]
- 84.Tritto I, Ambrosio G. Role of oxidants in the signaling pathway of preconditioning Antioxid Redox Signal 2001 3 3-10. Erratum in Antioxid Redox Signal. 2001;3(5):937. doi: 10.1089/152308601750100425. [DOI] [PubMed] [Google Scholar]
- 85.Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury cardioprotective mechanism Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baines CP, Wang L, Cohen MV, Downey JM. Protein tyrosine kinases is downstream of protein kinase C for ischemic preconditioning’s anti-infarct effect in the rabbit heart. J Mol Cell Cardiol. 1998;30:383–92. doi: 10.1006/jmcc.1997.0601. [DOI] [PubMed] [Google Scholar]
- 87.Hiraoka M, Furukawa T. Functional modulation of cardiac ATP-sensitive K+ channels. News Physiol Sci. 1998;13:131–7. doi: 10.1152/physiologyonline.1998.13.3.131. [DOI] [PubMed] [Google Scholar]
- 88.Edwards G, Weston AH. KATP-fact or artefact New thoughts on the mode of action of the potassium channel openers. Cardiovasc Res. 1994;28:735–7. doi: 10.1093/cvr/28.6.735. [DOI] [PubMed] [Google Scholar]
- 89.Gross GJ, Auchampach JA. Role of ATP-dependent potassium channels in myocardial ischemia. Cardiovasc Res. 1992;26:1011–6. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
- 90.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–33. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 91.Van Winkle DM, Chien GL, Woff RA, Soifer BE, Kuzume K, Davis RF. Cardioprotection provided by adenosine receptor activation is abolished by blockade of the KATP channel. Am J Physiol (Heart Circ Physiol). 1994;266:H829–39. doi: 10.1152/ajpheart.1994.266.2.H829. [DOI] [PubMed] [Google Scholar]
- 92.Grover GJ. Protective effects of ATP-sensitive potassium-channel openers in experimental myocardial ischemia. J Cardiovasc Pharmacol. 1994;24:S18–27. [PubMed] [Google Scholar]
- 93.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–9. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 94.Klimt CR, Knatterud GL, Meinert CL, Prout TE. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. Diabetes. 1970;19:747–30. [PubMed] [Google Scholar]
- 95.Rytter L, Troelsen S, Beck-Nielsen H. Prevalence and mortality of acute myocardial infarction in patients with diabetes. Diabetes Care. 1985;8:230–4. doi: 10.2337/diacare.8.3.230. [DOI] [PubMed] [Google Scholar]
- 96.Yao Z, Gross GJ. Effects of the KATP channel opener bimakalim on coronary blood flow. monophasic action potential duraion.and infarct size in digs. Circulation . 1994; 89:1769–75. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]
- 97.Armstrong SC, Kao R, Gao W , et al. Comparison of in vitro preconditioning responses of isolated pig and rabbit cardiomyocytes: effect of a protein phosphate inhibitor. fostriecin. J Moll Cell Cardiol. 1997;29:3009–24. doi: 10.1006/jmcc.1997.0507. [DOI] [PubMed] [Google Scholar]
- 98.Garlid KD, Paucek P, Yarov-Yarovoy V , et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels.Possible mechanism of cardioprotection. . Circ Res. 1997;81:1072–82. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 99.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–9. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 100.O’Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–55. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- 101.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid K. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol (Heart Circ Physiol) 2001;280:H649–57. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 102.Dos Santos P, Kowaltoski A, Laclau MN , et al. Mechanisms by which opening the mitochondrial ATP-sensitive K+ channel protects the ischemic heart. Am J Physiol (Heart Circ Physiol) 2002;283:H284–95. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 103.Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca++ overload in rat mitochondria. J Physiol. 1999;519:347–60. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pain T, Yang XM, Critz SD , et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–6. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 105.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–8. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 106.Cohen MV, Yang X-M, Liu GS, Heusch G, Downey JM. Acetylcholine. bradyknin.opioids., and phenylephrine., but not adenosine., trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. . Circ Res . 2001;89:273–8. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 107.Krenz M, Oldenburg O, Wimpee H , et al. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002;97:365–73. doi: 10.1007/s003950200045. [DOI] [PubMed] [Google Scholar]
- 108.Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal. 2007;9:1373–81. doi: 10.1089/ars.2007.1689. [DOI] [PubMed] [Google Scholar]
- 109.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–9. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 110.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–8. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yellon DM, Baxter GF. “A second window of protection” or delayed preconditioning phenomenon: future horizons for myocardial protection?. J Moll Cell Cardiol. 1995:1023–4. doi: 10.1016/0022-2828(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 112.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 113.Baxter GF, Yellon DM. Time course of delayed myocardial protection after transient adenosine A1-receptor activation in the rabbit. J. Cardiovasc Pharmacol. 1997;29:631–8. doi: 10.1097/00005344-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 114.Kudo M, Wang Y, Xu M , et al. Adenosine A1 receptor mediates late preconditioning via activation of PKC-d signaling pathway. Am J Physiol. 2002;283:H296–301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- 115.Ebrahim Z, Yellon DM, Baxter GF. Bradykinin induces delayed cardioprotection in the rat heart via nitric oxide dependent mechanism. Am J Physiol (Heart Circ Physiol) 2001;281:H1458–64. doi: 10.1152/ajpheart.2001.281.3.H1458. [DOI] [PubMed] [Google Scholar]
- 116.Kostitprapa C, Ockaili RA, Kukreja RC. Bradychinin B2 receptor is involved in the late phase of preconditioning in rabbit heart. J Mol Cell Cardiol. 2001;33:1355–62. doi: 10.1006/jmcc.2000.1396. [DOI] [PubMed] [Google Scholar]
- 117.Sun J-Z, Tang X-L, Park S-W, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–76. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou XB, Zhai XL, Ashraf M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocites. Circulation. 1996;93:1177–84. doi: 10.1161/01.cir.93.6.1177. [DOI] [PubMed] [Google Scholar]
- 119.Kaeffer N, Richard V, Thuillez C. Delayed coronary endothelial protection 24 hours after preconditioning: role of free radicals. Circulation. 1997;96:2311–6. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- 120.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999;84:846–51. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 121.Patel HH, Moore J, Hsu AK, Gross GJ. Bw373u86. a delta opioid agoist.partially mediates delayed cardioprotection via a free radical mechanism that is independent of opioid receptor stimulation. J Moll Cell Cardiol . 2001;33:1455–65. doi: 10.1006/jmcc.2001.1408. [DOI] [PubMed] [Google Scholar]
- 122.Guo Y, Jones WK, Xuan YT , et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br J Pharmacol. 1999;126:701–8. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pagliaro P, Gattullo D, Rastaldo R, Losano G. Involvement of nitric oxide in ischemic preconditioning. Ital Heart J. 2001;2:660–8. [PubMed] [Google Scholar]
- 125.Xi L, Tekin D, Gursoy E , et al. Evidence that NOS2 acts as a trigger and a mediator of late preconditioning induced by acute systemic hypoxia. Am J Physiol (Heart Circ Physiol) 2002;283:H5–12. doi: 10.1152/ajpheart.00920.2001. [DOI] [PubMed] [Google Scholar]
- 126.Shinmura K, Tang X-L, Wang Y , et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo Y, Bao W, Wu W-J, Shinmura K, Tang X-L, Bolli R. Evidence for an essential role of cycooxigenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:480–5. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shinmura K, Liu S-Q, Tang X-L , et al. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circulation. 2000;102 (Suppl. II ):II–120. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- 129.Yamashita N, Nishida M, Hoshida S , et al. Induction of manganese superoxide dismutase in rat cardiac myocytes increases tolerance to hypoxia 24 hours after preconditioning. J Clin Invest. 1994;94:2193–9. doi: 10.1172/JCI117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoshida S, Yamashita N, Otsu K , et al. The importance of manganese superoxide dismutase in delayed preconditioning: involvement of reactive oxygen species and cytokines. Cardiovasc Res. 2002;55:495–505. doi: 10.1016/s0008-6363(02)00337-1. [DOI] [PubMed] [Google Scholar]
- 131.Locke M, Tanguay R, Klabunde R , et al. Enhanced post-ischemic myocardial recovery following induction of HSP72. Am J Physiol. 1995;38:H320–5. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- 132.Plumier JCL, Ross BM, Currie RW , et al. Transgenic mice expressing the human heat-shock protein 70 have improved postischemic myocardial recovery. J Clin Invest. 1995;95:1854–60. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dana A, Skarli M, Papakrivopoulou J, Yellon DM. Adenosine A1 receptor induced delayed preconditioning in rabbits: induction of p38 MAPK activation and Hsp27 phosporylation via tyrosine kinase and protein kinase C-dependent mechanism. Circ Res. 2000;86:989–97. doi: 10.1161/01.res.86.9.989. [DOI] [PubMed] [Google Scholar]
- 134.Powers SK, Locke M, Demirel HA. Exercise. heat shock protins.and myocardial protection from I-R injury. Med Sci Sports Exerc. 2001;33:386–92. doi: 10.1097/00005768-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Takashi E, Wang Y, Ashraf M. Activation of mitochondrial KATP channels elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–53. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 136.Bernardo NL, D’Angelo M, Okubo S, Joy A, Kukreja RC. Delayed ischemic preconditioning is mediated by opening of ATP-sensitive potassium channels in the rabbit heart. Am J Physiol (Heart Circ Physiol) 1999;276:H1323–30. doi: 10.1152/ajpheart.1999.276.4.H1323. [DOI] [PubMed] [Google Scholar]
- 137.Loubani M, Hassouna A, Galinanes M. Delayed preconditioning on the human myocardium: signal transduction and clinical implications. Cardiovasc Res. 2004;61:600–9. doi: 10.1016/j.cardiores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 138.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann N Y Acc Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 139.Xi L, Tekin D, Gursoy E, Salloum F, Levasseur JE, Kukreja RC. Evidence that NOS2 acts as a trigger and mediator of late preconditioning induced by acute systemic hypoxia. Am J Physiol (Heart Circ Physiol) 2002;283:H5–12. doi: 10.1152/ajpheart.00920.2001. [DOI] [PubMed] [Google Scholar]
- 140.Sasaki N, Sato T, Ohler A, O’Rourke B, Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–45. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 141.Bolli R, Dawn B, Tang X-L , et al. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–38. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leesar MA, Stoddard M, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–41. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 143.Hill MF, Takano H, Tang XL, Kodani E, Shirk G, Bolli R. Nitroglycerin induces late preconditioning against myocardial infarction in conscious rabbits despite development of nitrate tolerance. Circulation. 2001;104:694–9. doi: 10.1161/hc3201.092218. [DOI] [PubMed] [Google Scholar]
- 144.Jneid H, Chandra M, Alshaher M , et al. Delayed preconditioning-mimetic actions of nitroglycerin in patients undergoing exercise tolerance tests. Circulation. 2005;111:2565–71. doi: 10.1161/CIRCULATIONAHA.104.515445. [DOI] [PubMed] [Google Scholar]
- 145.Kevelaitis E, Peynet J, Mouas C , et al. Opening of potassium channels.The common cardioprotective link between preconditioning and natural hibernation?. Circulation. 1999;99:3079–85. doi: 10.1161/01.cir.99.23.3079. [DOI] [PubMed] [Google Scholar]
- 146.Koning MMG, Gho BCG, van Klaarwater E , et al. Rapid ventricular pacing produces myocardial protection by non ischemic activation of ATP potassium channels. Circulation. 1996;93:178–86. doi: 10.1161/01.cir.93.1.178. [DOI] [PubMed] [Google Scholar]
- 147.Domenech RJ, Macho P, Velez JD , et al. Tachycardia preconditions the infarct size in dogs.Role of adenosine and protein kinase C. Circulation. 1998;97:786–94. doi: 10.1161/01.cir.97.8.786. [DOI] [PubMed] [Google Scholar]
- 148.Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol. 1994;76:1608–14. doi: 10.1152/jappl.1994.76.4.1608. [DOI] [PubMed] [Google Scholar]
- 149.Powers SK, Demirel HA, Vincent HK , et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol (Reg Int Comp Physiol) 1998;44:R1468–77. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 150.Demirel HA, Powers SK, Zergeroglu MA , et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–12. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 151.Rinaldi CA, Masani MD, Linka AZ, Hall RJ. Effect of repetitive episodes of exercise induced myocardial ischemia on left ventricular function in patients with chronic stable angina: evidence for cumulative stunning or ischemic preconditioning. Heart. 1999;81:404–11. doi: 10.1136/hrt.81.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Okazaki Y, Kodama K, Sato H , et al. Attenuation of increased regional myocardial oxygen consumption during exercise as a major cause of warm-up. J Am Coll Cardiol. 1993;21:1597–604. doi: 10.1016/0735-1097(93)90374-a. [DOI] [PubMed] [Google Scholar]
- 153.Wayne EJ, Laplace LB. Observations on angina of effort. Clin Sci. 1993;1:103–29. [Google Scholar]
- 154.Paraskevaidis IA, Iliodromitis EK, Mavrogeni S , et al. Repeated exercise stress testing identifies early and late preconditioning. Int J Cardiol. 2005;98:221–6. doi: 10.1016/j.ijcard.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 155.Tomai F, Perino M, Ghini AS , et al. Exercise-induced myocardial ischemia triggers the early phase of preconditioning but not the late phase. Am J Cardiol. 1999;83:586–8. doi: 10.1016/s0002-9149(98)00918-7. [DOI] [PubMed] [Google Scholar]
- 156.Crisafulli A, Melis F, Tocco F , et al. Delayed preconditioning-mimetic action of exercise or nitroglycerin do not affect hemodynamics and exercise performance in trained or sedentary individuals. J Sport Sci. 2007;25:1393–401. doi: 10.1080/02640410601128914. [DOI] [PubMed] [Google Scholar]
- 157.McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation. 1978;57:958–62. doi: 10.1161/01.cir.57.5.958. [DOI] [PubMed] [Google Scholar]
- 158.Bowles DK, Farra RP, Starnes JW. Exercise training improves cardiac function following ischemia in the isolated working rat heart. Am J Physiol. 1992;263:H804–9. doi: 10.1152/ajpheart.1992.263.3.H804. [DOI] [PubMed] [Google Scholar]
- 159.Locke M, Tanguay RM, Klabundei RE , et al. Enhanced postischemic myocardial recovery following exercise-induction of HSP 72. Am J Physiol. 1995;269:H320–5. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- 160.Hamilton KL, Staib JL, Philips T, Hess A, Lennon SL, Powers SK. Exercise. antioxidnts.and HSP72 protection against myocardial ischemia/reperfusion. Free Radic Biol Med . 2003;34: 800–9. doi: 10.1016/s0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- 161.Yamashita N, Baxter GF, Yellon M. Exercise directly enhances myocardial tolerance to ischaemia-reperfusion injury in the rat through a protein kinase C mechanism. Heart. 2001;85:331–6. doi: 10.1136/heart.85.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–58. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 163.Gomez-Cabrera MC, Domenech E, Romagnoli M , et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–9. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 164.Ristow M, Zarse K, Oberbach A , et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acc Sci USA. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 166.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–92. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 167.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese super-oxide dismutase activation. J Exp Med. 1999;189:1699–706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suhr F, Gehlert S, Grau M, Bloch W. Skeletal muscle functions during exercise – Fine- tuning of diverse subsystems of nitric oxide. Jnt J Mol Sci. 2013;14:7109–39. doi: 10.3390/ijms14047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Welch WJ. Mammalian stress response: cell physiology. structure/function of stress protins.and implication for medicine and disease. Physiol Rev . 1992;72:1063–81. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 170.Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–36. [PubMed] [Google Scholar]
- 171.Lennon SL, Quindry J, Hamilton KL e al. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96:1299–305. doi: 10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- 172.Gho BCG, Schoemaker RG, van den Doel M, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 173.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance.Reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–6. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 174.Takaoka A, Nakae I, Mitsunami K , et al. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning”. J Am Coll Cardiol. 1999;33:556–64. doi: 10.1016/s0735-1097(98)00559-2. [DOI] [PubMed] [Google Scholar]
- 175.Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol (Heart Circ Physiol) 2002;283:H29–37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- 176.Wang YP, Xu H, Mizoguchi K, Oe M, Maeta H. Intestinal ischemia induces late preconditioning against myocardial infarction: a role for inducible nitric oxide synthase. Cardiovasc Res. 2001;49:391–8. doi: 10.1016/s0008-6363(00)00266-2. [DOI] [PubMed] [Google Scholar]
- 177.Heusch G, Schultz R. Editorial: remote preconditioning. J Moll Cell Cardiol. 2002;34:1279–81. doi: 10.1006/jmcc.2002.2093. [DOI] [PubMed] [Google Scholar]
- 178.Gross GJ. Remote preconditioning and delayed cardioprotection in skeletal muscle. Am J Physiol (Regul Integr Comp Physiol) 2005;289:R1562–3. doi: 10.1152/ajpregu.00627.2005. [DOI] [PubMed] [Google Scholar]
- 179.Dickson EW, Lorbar M, Porcaro WA , et al. Rabbit heart can be preconditioned via transfer of coronary effluent. Am J Physiol (Heart Circ Physiol) 1999;277:H2451–7. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- 180.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol (Heart Circ Physiol) 2000;278:H1571–6. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 181.Green HJ, Hughson RL, Orr GW, Ranney DA. Anaerobic threshold. blood lacate.and muscle metabolites in progressive exercise. J Appl Physiol . 1983;54:1032–8. doi: 10.1152/jappl.1983.54.4.1032. [DOI] [PubMed] [Google Scholar]
- 182.Ivy JL, Chi MMY, Hintz CS, Sherman WM, Hellendall RP, Lowry OH. Progressive metabolite changes in individual human muscle fibers with increasing work rates. Am J Physiol (Cell Physiol) 1987;252:C630–9. doi: 10.1152/ajpcell.1987.252.6.C630. [DOI] [PubMed] [Google Scholar]
- 183.Michelsen MM, Støttruo NB, Schmidt MR , et al. Exercise-induced cardioprotection is mediated by a bloodborne. transferable factor. . Basic Res Cardiol. 2012;107:260–9. doi: 10.1007/s00395-012-0260-x. [DOI] [PubMed] [Google Scholar]
- 184.Jean-St-Michel E, Manlhiot C, Li J , et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43:1280–6. doi: 10.1249/MSS.0b013e318206845d. [DOI] [PubMed] [Google Scholar]
- 185.de Groot PCE, Thijssen DHJ, Sanchez M, Ellenkamp R, Hopman MTE. Ischemic preconditioning improves maximal performance in humans. Eur J Appl Physiol. 2010;108:141–6. doi: 10.1007/s00421-009-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Crisafulli A, Tangianu F, Tocco F , et al. Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. J Appl Physiol. 2011;111:530–6. doi: 10.1152/japplphysiol.00266.2011. [DOI] [PubMed] [Google Scholar]
- 187.Bailey TG, Jones H, Gregson W, Atkinson G, Cable NT, Thijssen DHJ. Effect of ischemic preconditioning on lactate accumulation and running performance. Med Sci Sport Exerc. 2012;44:2084–9. doi: 10.1249/MSS.0b013e318262cb17. [DOI] [PubMed] [Google Scholar]
- 188.Bailey TG, Birk GK, Cable NT , et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am J Physiol (Heart Circ Physiol) 2012;303:H533–8. doi: 10.1152/ajpheart.00272.2012. [DOI] [PubMed] [Google Scholar]
- 189.Loukogeorgakis SP, Panagiotidou AT, Broadhead MV, Donald A, Deanfield JE, McAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–6. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 190.Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channnel dependent mechanism. Circulation. 2007;116:1386–95. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 191.Abu-Amara M, Yang SY, Quaglia A , et al. Nitric oxide is an essential mediator of the protective effects of remote ischemic preconditioning in a mouse model of liver is-chemia/reperfusion injury. Cli Sci (Lond) 2011;121:257–66. doi: 10.1042/CS20100598. [DOI] [PubMed] [Google Scholar]
- 192.Bøtker HE, Kharbanda R, Schmidt MR , et al. Remote ischemic conditioning before hospital admission. as a complement to angioplsty.and effect on myocardial salvage in patients with acute myocardial infarction a randomised trial. Lancet. 2010;375: 727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 193.Peart JN, Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol (Heart Circ Physiol) 2009;296:H1705–20. doi: 10.1152/ajpheart.00162.2009. [DOI] [PubMed] [Google Scholar]
- 194.Noël M, Jobin J, Marcoux A, Poirier P, Dagenais GR, Bogaty P. Can prolonged exercise-induced myocardial ischemia be innocuous?. Eur Heart J. 2007;28:1559–65. doi: 10.1093/eurheartj/ehm152. [DOI] [PubMed] [Google Scholar]
- 195.Domenech RJ. Preconditioning A new concept about the benefit of exercise. . Circulation. 2006;113:e1–3. doi: 10.1161/CIRCULATIONAHA.105.569863. [DOI] [PubMed] [Google Scholar]
- 196.Crisafulli A. Exercise and ischemic preconditioning. Curr Cardiol Rev. 2006;2:153–62. doi: 10.2174/1573403X10666140404110229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kavazis AN. Exercise preconditioning of the myocardium. Sports Med. 2009;39:923–35. doi: 10.2165/11317870-000000000-00000. [DOI] [PubMed] [Google Scholar]