Abstract
In the stent era, in addition to restenosis, there are many important consequences deserving more attention. Firstly described in peripheral vascular interventions, it took several years for stent fracture to be known as an appreciable complication of coronary intervention. Especially with the introduction of drug eluting stents and the use of coronary stents in more complex cases, its prevalence has raised and new data have been published concerning its mechanism, predictors, diagnosis, clinical course and treatments. This review will discuss the available literature about stent fracture.
Keywords: Coronary stent, percutaneous coronary intervention, stent fracture.
INTRODUCTION
The role of stents in the coronary interventions is changing: the first generation stainless steel bare metal stents (BMS) bailed out flow-limiting dissection and recoil and inadvertently proved to decrease restenosis, the “Achilles’ heel” of PCI. After this honeymoon for intervention, the unresolved obstacle addressed through the development of the drug-eluting stent (DES). DES, by itself faced with the many new important phenomenon: Endothelial dysfunction, edge effect, malapposition, late and very late stent thrombosis.
Stent thrombosis and fracture albeit infrequent [1-5], are now recognized as important complications of stenting. These surprisingly interconnected complications seem to occur especially after the DES implantation. Despite its low prevalence, stent fracture may be one of the main causes of target vessel revascularization (TVR).
This article will review the various aspects of coronary stent fracture.
INCIDENCE
Although stent fracture is a common complication in the peripheral vascular interventions [1], it remained unrecognized in percutaneous coronary intervention (PCI) for a long period of time. The reported incidence of stent fracture ranges between 0.8 and 19% [2-4]. This firstly may be due to the asymptomatic nature of stent fracture. In addition, in reported series, coronary angiography was performed in 60- 90% of cases during follow up, thus is liable to potentially miss some instances of stent fracture [1, 2, 4]. Another explanation is that some studies have included only complete stent fractures [6-8] and neglecting partial ones [2]. Selection of different populations may also have impact on the reported incidence of stent fracture insofar this complication is more common in high-risk groups. Shaikh et al. analysed 188 patients with restenosis and found a high incidence rate of SF (18.6% of the study population) [4]. Furthermore, it seems that stent fracture does not happen equally in the different types of stents, so the incidence is likely to change with different stent types [1-4].
Diagnostic modalities have different accuracy to detect SF. Some studies have combined fluoroscopy and ultrasound [2] or fluoroscopy and computer tomography [9], influencing the rate of SF. (See the “Diagnosis” section for further detail).
With regard to these possible explanations, it is not surprising to see a much greater incidence of SF (29%) in a post-mortem DES registry [6]. Chakravarty et al. in their meta-analysis, reported a mean stent fracture incidence of 4.9% [5] and posited that the incidence correlated with procedural (stent type, deployment method, etc.) and lesional (vessel characteristics, native vs. graft, etc.) factors as well as diagnostic methods.
PREDICTORS AND MECHANISMS
Stent fracture came into recognition with the advent of the DES [4]. According to published data, the incidence of stent fracture in the BMS is inconsiderable [3, 10] and limited to the saphenous vein graft (SVG) [11, 12]. Excluding the Kawai et al. report showing a nonsignificant difference with respect to stent fracture between the BMS and the DES (4.4% for the DES and 1.3% for the BMS, p value = 0,078) [13], the bulk of the evidences have shown statistically significant differences between the two major stent types [1-5]. It has been suggested that a greater neointimal proliferation in the BMS reduces the risk of fracture [14, 15] or /and less radio opacity renders the diagnosis of fracture more difficult [4]. Besides, in routine practice, the BMS is usually not deployed for long and complex lesions, which are regarded as predictors of stent fracture [16].
Amongst the various types of the DES, the sirolimus Stent (SES) is the main convict [1-5]. Although there are few investigations reporting equal frequencies of stent fracture in the different types of the DES [13], the majority of the available studies underscore a higher frequency of SF in SES [1-4]. Chakravarty et al. [5] and Chhatriwalla et al. [17] found that more than 95% of their stent fracture cases had occurred in the Cypher Stent, and Lee et al. in their analysis 3636 of Cypher Stents and 1162 Taxus Stents in 530 patients, reported a stent fracture prevalence of 1.9%, all in the Cypher Stents [1].
Several hypotheses have been proposed for this predilection. The interaction between stent and vessel geometry during stent implantation is considered the most important factor in the pathophysiology of SF. Stents change the vessel geometry, thus creating a new vessel angulation. This geometric distortion imposes a considerable mechanical force, increasing metal fatigue and finally the likelihood of stent fracture [4, 18]. In this context, stent flexibility which influences the vessel geometry appears to be directly related to fracture resistance. In DES group, the least flexible device undergoing bench testing for flexibility (Cypher) was the most susceptible to stent fracture [19]. Also, the fact that the SES enjoys relatively high radio opacity is believed to enhance the possibility of fracture diagnosis [3]. There is currently a paucity of data in the existing literature on the rate of stent fracture in the new generation of the DES [18]; nonetheless, the incidence appears to be lower [5, 18, 20]. A recent large consecutive series of everolimus-eluting stents demonstrated stent fracture in 2.9% of lesions [21]. In this report, fracture was evaluated angiographically, with only ~50% of patients having intravascular ultrasound evaluation (IVUS) and the analysis was performed at 6-9 months following stent deployment. Both the early time course for evaluation (6-9 months) and the use of IVUS in only ~50% would tend to underestimate the true prevalence of stent fracture in this population [21].
It should be noted that the flexibility/comformability and fracture resistance has been addressed in the design of new generation of stents. The new platinum chromium everolimus-eluting stent (PROMUS Element) with “a modified scaffold design” try to create a more flexible and fracture resistant stent [22, 23].
Apart from stent type some other stent characteristics play role in stent fracture. Longer stents are thought to be more vulnerable to fracture [2, 15, 17]. Doi et al. reported that in their two groups of patients, fracture was more frequent in the group with longer stents (45.2 ± 23.0 vs. 28.5 ± 14.9 mm, p value =0.0003) [15] and Park et al. found a similar difference in terms of the correlation between the stent length and stent fracture in their two patient groups (55.25 ± 22.26 mm vs. 40.07 ± 25/51 mm, p value =0.005) [18]. Radial force is believed to be more pronounced in longer stents, (not) least in the mid-part of the stent, and it increases metal fatigue and consequently the rate of fracture [4, 5, 18].
Overlapping stents by enhancing “axial stiffness” possibly increases the risk of stent fracture [1, 9, 24-26]. A 60% rate of fracture in overlapped stents was observed in one study [3], and elsewhere, higher frequencies of restenosis along with fracture were reported in overlapped stents [27]. Excessive pressure during stent deployment, balloon post-dilation and resultant damage to the stent strut has also been blamed for a rise in the risk of stent fracture [3, 7, 15, 18, 25, 28]. This notion was borne out by the Park et al. study, which found a higher maximal inflation pressure in the stent fracture group (13.42 ± 3.86 atm vs. 11.62 ± 3.39 atm, p value =0.015) [18].
The stent and balloon diameter [4, 16, 18, 24, 28], number of the implanted stents [18], residual post-stenting stenosis [18], smaller minimal lumen diameter, and greater acute gain and late loss [18, 29] are some other procedural predictors. Park et al. demonstrated a significant relation between the number of the implanted stents and stent fracture (2.17 ± 0.19 vs. 1.61 ± 0.91, p value =0.005) [18], and Kim et al. showed a higher incidence rate of stent fracture in lesions with smaller minimal lumen diameters before the procedure (0.38 ± 0.55 vs. 0.71 ± 0.46 mm, p value =0.04) and larger acute gain (2.28 ± 0.39 vs. 1.44 ± 0.60 mm, p value =0.001) and late loss (0.81 ± 0.49 vs. 0.42 ± 050 mm, p value =0.001) [29].
Although there have been a few reports showing no specific predilection for the location of stent fracture [1], the right coronary artery (RCA) appears to be favoured [1, 5, 18, 29, 30]. In a meta-analysis on stent fracture, the incidence rates of this complication in the RCA, left anterior descending artery (LAD), left circumflex artery (LCX), SVG, and left main artery were 56.4%, 30.4%, 10.9%, 1.7%, and smaller than 0.01%, respectively [5]. In another review of eleven studies, Lee et al. noticed that 54% of all fractures were reported in the RCA [3]. The higher rate of stent fracture in the RCA may be due to its tortuosity and sharp angularity, which increases metal fatigue during a cardiac cycle [2, 3, 18]. In their review, Lee et al. reported excessive tortuosity in most cases of stent fracture [3], and Shaikh et al. explained that tortuosity increased the flexion points during a cardiac cycle and consequently led to stent fracture [4]. Ino et al. having meticulously analyzed the hinge motion angle of vessels during systole and diastole, and concluded that a greater degree of motion induces a higher degree of metal fatigue [2]. Furthermore, they have reported that the degree of the hinge motion of the RCA was greater than that of the LAD or LCX (31.0 ± 3.º vs. 22.8 ± 4.9º) and stated that stenting changes the vessel angulation, thereby creating different mechanical forces which might increase metal fatigue. Different angulations have been proposed as measures of increased mechanical forces [8, 29, 31]. The mean measured angles in the Chang et al. study was 67º [26], which differed from that in the Shaikh et al. study (≥75°) [4] and the one in the Yang et al. and Umeda et al. studies (≥ 45°) [8, 31]. Researchers believe that the higher incidence of stent fracture in the SVG stems from the same mechanism [7].
Halwani et al. in their detailed study, showed that the calcification of plaque increases the risk of stent fracture [32]. In addition, ostial [33] and bifurcation lesions [28] are reported as the predictors of stent fracture.
Whereas a number of studies have reached the conclusion that patient-related predictors and coronary artery disease risk factors do not increase the risk of stent fracture [4, 5], Park et al. demonstrated that hypertension and chronic kidney disease were correlated with stent fracture [18].
In a meta-analysis on stent fracture, overlapping stents, stent length, and stenting in the RCA were the only significant predictors of this complication [5]. Elsewhere, after a multivariate analysis, Park et al. found that stent length, use of the SES, minimum stent diameter, and maximal inflation pressure were the only significant predictors of stent fracture [30]. And finally, Kuramitsu et al. who analyzed SF in a new generation of DES (everolimus-eluting stent), found ostial lesion and lesions with hinge motion, tortuosity, or calcification as independent predictors of SF [21].
CLASSIFICATION
Diagnostic modalities have helped devise different classifications for stent fracture over the years [1, 25, 29-31]. In the fluoroscopic classification, stent fracture is graded with regard to the number of the fractured struts seen during angiography: single strut fracture as type I; ≥2 strut fracture as type II; ≥2 strut fracture with deformation as type III; fracture with transection but no gap as type IV; and fracture with a gap within as type V [6, 34].
Park et al. reported the incidence of stent fracture in the above-mentioned types as follows: 50% type I; 7.7% type II; 38.4% type III; 3.9% type IV; and 0% type V [18]. Nakazawa et al. in their thorough assessment, showed that most stent fractures were types I, II, and III [6]. Also in the report of Kuramitsu et al. 97 % of SF in the everolimus-eluting stent were classified as type II and III [21]. There are also other classifications for the grading of stent fracture, which are on the basis of intravascular ultrasound (IVUS) [35] or computed tomography (CT) scan [28].
CLINICAL PRESENTATION
Doi et al. observed equal frequencies of stent fracture at one year and a year after stent implantation [15], but some reported series, regarded stent fracture as a late stent failure phenomenon. They stated that the incidence of stent fracture has been directly correlated with time duration from stent deployment and explained that geometry distortion of the vessel wall is more pronounced with time. Surprisingly, the one year comparison of the two different stent platforms (platinum chromium vs. cobalt chromium) with regard to flexibility in PLATINUM Trial, showed no significant differences [22], but the two year analysis of the trial were in favour of the more flexible one (platinum chromium) [23]. The possible explanation could be that SF was more pronounced after one year of stent implantation.
Stent fracture is usually associated with binary restenosis, thrombosis, aneurysm, embolization, ischemic events, and target lesion revascularization (TLR) and could thereby increase morbidity and mortality [3].
Several studies have reported a rise in in-stent restenosis (ISR) in tandem with stent fracture [4, 7, 31], in a wide range of 10-90 % [16, 28]. It has been hypothesised that stent fracture renders local drug delivery at the stent site uneven and impaired [7, 8]. In contrast, Halkin et al. reported that in their series, stent fracture usually occurred long after drug delivery had been terminated and that ISR could not have been caused by the proposed mechanism [36].
Is this type of restenosis significantly harmful? Chakravarty et al. in their meta-analysis, concluded that as much as stent fracture could be asymptomatic, the probability of ISR and TVR exhibited an upward trend amongst the reviewed studies [5]. Ino et al. also reported that ISR and TVR after stent fracture were not allied to major adverse cardiac events (MACE) [2]. Lee et al. reported that their cases of ISR were focal and limited to stent fracture types III and IV, half of them asymptomatic, and that there were no cardiac deaths [37]. Park et al. also showed a greater incidence of binary restenosis in their group of patients with stent fracture (one patient with stent fracture presented with non ST-elevation myocardial infarction [NSTEMI]), but there was no difference in terms of clinical manifestation between patients with or without stent fracture [18]. In contrast, other investigations have suggested that stent fracture could be potentially harmful [17]. Chhatriwalla et al. reported a 6% incidence rate of STEMI and 42% of NSTEMI in their stent fracture population [17], and Nakasawa et al. reported that all their cases of fatal stent fracture were in stage V [6], although there was a low risk of adverse events with low-grade stent fracture.
Aneurysm is more common with complete stent fracture, and a combination of stent fracture and aneurysm is normally expected to happen one year after stenting [15]. And finally, stent thrombosis, albeit apparently uncommon [1, 6], has also been proposed as a complication of stent fracture [3, 6, 17, 38]. Thrombosis by SF can be seen at any time, and was reported as a risk factor for late stent thrombosis. It has been suggested that direct contact of free metal to luminal surface can cause platelet activation [39].
Kuramitsu et al. also reported an increased risk of TLR and MI caused by SF following everolimus- eluting stent implantation (5.1% versus 0.4%; P=0.018 and 25.6% versus 2.0%; p<0.001, in SF and non-SF group respectively) [21]. Stent thrombosis was also more common in the SF group than in the non-SF group (5.1% versus 0.4%; P=0.018) [21].
DIAGNOSIS
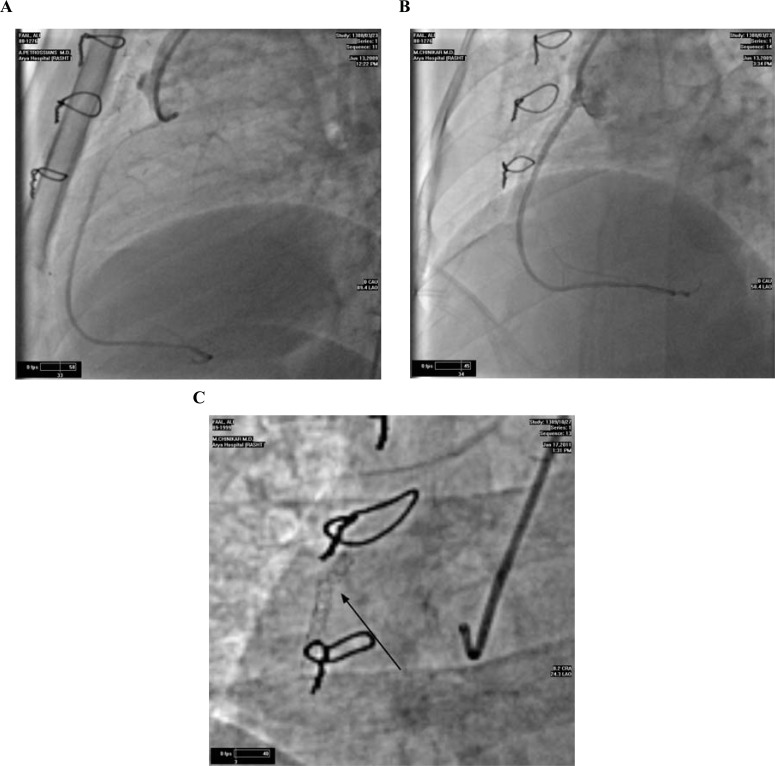
The existing literature abounds with imaging modalities suggested for optimal diagnosis of stent fracture. Most of the relevant studies have utilized fluoroscopy with or without contrast injection: while some maintain it as the best possible way to diagnose stent fracture [40] (Fig. 1), others urge that complimentary modalities be drawn upon [37, 41]. Ino et al. argued that some cases of stent fracture were only detected by plain fluoroscopy and that contrast injection might mask them [2].
Fig. (1).
Percutaneous coronary intervention was performed on a saphenous vein graft lesion (A). A Cypher stent was deployed with apparently satisfactory result (B). Nine months later, the patient hospitalized for an episode of acute coronary syndrome. Stent fracture was detected on fluoroscopy at the proximal third of the stent (C).
Some innovative designs have increased the accuracy of flouroscopic diagnosis. One of these novel methods is Stent Boost (StentBoost Substract, Phillips Healthcare, Best, Netherlands), which employs a marker balloon to detect stent fracture [42].
IVUS has enjoyed a rise in its use for the diagnosis of stent fracture in recent years. As opposed to a limited number of studies reporting a low diagnostic yield [43], the majorities have acknowledged the efficacy of IVUS as a diagnostic modality and even proposed stent fracture classification based on it [1, 5]. Yamada et al. showed that IVUS was more reliable than angiography [44] and posited that the high ability of the metallic strut for the reflection of ultrasound must be the reason for the superiority of IVUS.
Multislice CT scan is another diagnostic modality in the medical armamentarium for the detection of stent fracture [28, 43, 45-47]. All the studies using CT scan for diagnosis have underlined its high accuracy in comparison with angiography [43, 45]. Pang et al. reported an overall accuracy, sensitivity, and specificity of 84.1%, 80.7%, and 100%, respectively, for CT scan compared to 73.9%, 77.2%, and 58.3%, respectively, for conventional angiography [43].
Finally, optical coherence imaging has also been reported as a diagnostic modality for revealing stent fracture [38].
A review of the literature on a comparison between the different modalities for diagnosis of stent fracture yields the following observations. Pang et al. compared 64-slice CT scan, conventional angiography, and IVUS and the result was superiority of CT scan [43]. Hecht et al. asserted that CT scan and IVUS were both highly valuable for detecting stent gap [28]; an important determinant for ISR, stent fracture, and overlap failure, so considering the noninvasiveness of CT scan, it could be a valuable modality for diagnosis and prognosis.
TREATMENT
There is no consensus for treatment of stent fracture. Many consider stent fracture benign and usually asymptomatic, accompanied by a negligible incidence rate of cardiac events and, therefore, advocate only the continuation of antiplatelet therapy [37], while others opt for treatment and apparently 1-2% of TVR procedures are undertaken for lesions with stent fracture [18]. Lee and coworkers in their study conclude that if patients with SF continued the dual antiplatelet therapy irrespective of symptoms, a low MACE would occur [37]. Sianos et al. report an immediate symptom relief following re-stenting in lesion with SF [24]. Park et al. in their series of SF only treated patients with SF who were complicated by ISR and a decreased fractional flow reserve [18]. Other patients in their series were only followed and they had an excellent clinical course.
As far we know, stent fracture could result in ISR. There are three approaches regarding stent fracture with ISR. Some tend to leave asymptomatic ISR without treatment and reserve intervention for symptomatic ISR [25]. Others choose to treat stent fracture with ISR irrespective of symptoms [37]. Lee et al. presented an algorithm for the treatment of stent fracture [37]. They proposed the continuation of antiplatelet therapy regardless of ischemic symptoms and suggested intervention for the following patients: a) symptomatic or asymptomatic ISR with > 70% stenosis and b) symptomatic ISR with 50-70% stenosis, which shows positive results in physiological stress test with or without IVUS. Balloon-only, BMS, and DES have all been applied for the treatment of stent fracture; the usage of the DES, however, seems to be more reasonable. Of note some studies have suggested the use of Paclitaxel-eluting stents in the SES fracture [25].
CONCLUSION
Since its first case report in 2004 [24], stent fracture has gradually been recognized as an important complication of coronary stenting. Stent fracture may have a low overall clinical incidence and benign course, but under no circumstances should underestimate its potential consequences. Usage of the SES, length of the stent, and stenting of the RCA are amongst the major contributors. Stent fracture is usually diagnosed during fluoroscopy, but CT scan and IVUS seems to have more advantages in the detection of this complication.
For all the research conducted thus far, there is still no consensus about the treatment of stent fracture; nevertheless, the DES has conferred favourable results in symptomatic lesions.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007;69(3):387–94. doi: 10.1002/ccd.20942. [DOI] [PubMed] [Google Scholar]
- 2.Ino Y, Toyoda Y, Tanaka A , et al. Predictors and prognosis of stent fracture after sirolimus-eluting stent implantation. Circ J. 2009;73(11):2036–41. doi: 10.1253/circj.cj-09-0343. [DOI] [PubMed] [Google Scholar]
- 3.Canan T, Lee MS. Drug-eluting stent fracture: incidence. contributing facors.and clinical implications. Catheter Cardiovasc Interv. 2010,; 75(2):237–45. doi: 10.1002/ccd.22212. [DOI] [PubMed] [Google Scholar]
- 4.Shaikh F, Maddikunta R, Djelmami-Hani M, Solis J, Allaqaband S, Bajwa T. Stent fracture. an incidental finding or a significant marker of clinical in-stent restenosis?. Catheter Cardiovasc Interv. 2008;71(5):614–8. doi: 10.1002/ccd.21371. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty T, White AJ, Buch M , et al. Meta-analysis of incidence.clinical characteristics and implications of stent fracture. . Am J Cardiol. 2010;106(8):1075–80. doi: 10.1016/j.amjcard.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Nakazawa G, Finn AV, Vorpahl M , et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54(21):1924–31. doi: 10.1016/j.jacc.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 7.Aoki J, Nakazawa G, Tanabe K , et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(3):380–6. doi: 10.1002/ccd.20950. [DOI] [PubMed] [Google Scholar]
- 8.Yang TH, Kim DI, Park SG , et al. Clinical characteristics of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;131(2):212–6. doi: 10.1016/j.ijcard.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Lousinha A, Fiarresga A, Cardona L , et al. Stent fracture: case report and literature review. Rev Port Cardiol. 2011;30(2):213–21. [PubMed] [Google Scholar]
- 10.Kwon SU, Doh JH, Namgung J, Lee SY. Stent strut fracture-induced restenosis in the right coronary artery: detection by MDCT. Heart. 2008;94(2):221. doi: 10.1136/hrt.2006.108175. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury PS, Ramos RG. Images in clinical medicine.Coronary-stent fracture. N Engl J Med. 2002;347(8):581. doi: 10.1056/NEJMicm020259. [DOI] [PubMed] [Google Scholar]
- 12.Dorsch MF, Seidelin PH, Blackman DJ. Stent fracture and collapse in a saphenous vein graft causing occlusive restenosis. J Invasive Cardiol. 2006;18(4):E137–9. [PubMed] [Google Scholar]
- 13.Kawai T, Umeda H, Ota M , et al. Do drug elution components increase the risk of fracture of sirolimus-eluting stents?. Coron Artery Dis. 2010;21(5):298–303. doi: 10.1097/mca.0b013e32833aa6a1. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Park JS, Shin DG , et al. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am J Cardiol. 2007;100(4):627–30. doi: 10.1016/j.amjcard.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 15.Doi H, Maehara A, Mintz GS , et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009;103(6):818–23. doi: 10.1016/j.amjcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos D, Xanthopoulou I. Coronary stent fracture: how frequent it is?.Does it matter?. Hellenic J Cardiol. 2011;52(1):1–5. [PubMed] [Google Scholar]
- 17.Chhatriwalla AK, Cam A, Unzek S , et al. Drug-eluting stent fracture and acute coronary syndrome. Cardiovasc Revasc Med. 2009;10(3):166–71. doi: 10.1016/j.carrev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Park KW, Park JJ, Chae IH , et al. Clinical characteristics of coronary drug-eluting stent fracture: insights from a two-center des registry. J Korean Med Sci. 2011;26(1):53–8. doi: 10.3346/jkms.2011.26.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ormiston JA, Webber B, Webster MW. Stent longitudinal integrity bench insights into a clinical problem. JACC Cardiovasc Interv. 2011;4(12):1310–7. doi: 10.1016/j.jcin.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu MC, Cheng CC, Huang TY. Fracture of zotarolimus-eluting stent after implantation. Tex Heart Inst J. 2009;36(6):618–20. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuramitsu S, Iwabuchi M, Haraguchi T , et al. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv. 2012;5(5):663–71. doi: 10.1161/CIRCINTERVENTIONS.112.969238. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Teirstein PS, Meredith IT , et al. A prospective. randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospecive.Randomized., Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. . J Am Coll Cardiol. 2011; 57(16):1700–8. doi: 10.1016/j.jacc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Teirstein PS, Meredith IT , et al. Two-year results of the PLATINUM randomized trial comparing PLATINUM chromium promus element and cobalt chromium PROMUS/XIENCE V everolimus-eluting stents in de novo coronary artery lesions. J Am Coll Cardiol. 2012;59(13s1):E323–3. [Google Scholar]
- 24.Sianos G, Hofma S, Ligthart JM , et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004;61(1):111–6. doi: 10.1002/ccd.10709. [DOI] [PubMed] [Google Scholar]
- 25.Adlakha S, Sheikh M, Wu J , et al. Stent fracture in the coronary and peripheral arteries. J Interv Cardiol. 2010;23(4):411–9. doi: 10.1111/j.1540-8183.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- 26.Leong DP, Dundon BK, Puri R, Yeend RA. Very late stent fracture associated with a sirolimus-eluting stent. Heart Lung Circ. 2008;17(5):426–8. doi: 10.1016/j.hlc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee SE, Jeong MH, Kim IS , et al. Clinical outcomes and optimal treatment for stent fracture after drug-eluting stent implantation. J Cardiol. 2009;53(3):422–8. doi: 10.1016/j.jjcc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Hecht HS, Polena S, Jelnin V , et al. Stent gap by 64-detector computed tomographic angiography relationship to in-stent restenosis. fracure.and overlap failure. . J Am Coll Cardiol . 2009; 54(21):1949–59. doi: 10.1016/j.jacc.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Kim YH, Lee SW , et al. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133(3):354–8. doi: 10.1016/j.ijcard.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Park MW, Chang K, Her SH , et al. Incidence and clinical impact of fracture of drug-eluting stents widely used in current clinical practice: Comparison with initial platform of sirolimus-eluting stent. J Cardiol. 2012;60(3):215–21. doi: 10.1016/j.jjcc.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Umeda H, Kawai T, Misumida N , et al. Impact of sirolimus-eluting stent fracture on 4-year clinical outcomes. Circ Cardiovasc Interv. 2011;4(4):349–54. doi: 10.1161/CIRCINTERVENTIONS.110.958306. [DOI] [PubMed] [Google Scholar]
- 32.Halwani DO, Anderson PG, Brott BC, Anayiotos AS, Lemons JE. The role of vascular calcification in inducing fatigue and fracture of coronary stents. J Biomed Mater Res B Appl Biomater. 2012;100(1):292–304. doi: 10.1002/jbm.b.31911. [DOI] [PubMed] [Google Scholar]
- 33.Bradley JT, Schmoker JD, Dauerman HL. Complete Cypher stent fracture and migration in the ostium of the right coronary artery. J Invasive Cardiol. 2007;19(4):E99–101. [PubMed] [Google Scholar]
- 34.Jaff M, Dake M, Pompa J, Ansel G, Yoder T. Standardized evaluation and reporting of stent fractures in clinical trials of noncoronary devices. Catheter Cardiovasc Interv. 2007;70(3):460–2. doi: 10.1002/ccd.21240. [DOI] [PubMed] [Google Scholar]
- 35.Carter AJ. Stent strut fracture: seeing is believing. Catheter Cardiovasc Interv. 2008;71(5):619–20. doi: 10.1002/ccd.21569. [DOI] [PubMed] [Google Scholar]
- 36.Halkin A, Carlier S, Leon MB. Late incomplete lesion coverage following Cypher stent deployment for diffuse right coronary artery stenosis. Heart. 2004;90(8):e45. doi: 10.1136/hrt.2004.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SE, Jeong MH, Kim IS , et al. Clinical outcomes and optimal treatment for stent fracture after drug-eluting stent implantation. J Cardiol. 2009;53(3):422–8. doi: 10.1016/j.jjcc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Shite J, Matsumoto D, Yokoyama M. Sirolimus-eluting stent fracture with thrombus. visualization by optical coherence tomography. . Eur Heart J. 2006;27(12):1389. doi: 10.1093/eurheartj/ehi542. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Park JS, Shin DG , et al. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am J Cardiol. 2007;100(4):627–30. doi: 10.1016/j.amjcard.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 40.Choe H, Hur G, Doh JH , et al. A case of very late stent thrombosis facilitated by drug eluting stent fracture: comparative images before and after stent fracture detected by 64-multidetector computed tomography. Int J Cardiol. 2009;133(3):e125–8. doi: 10.1016/j.ijcard.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 41.Lemos PA, Saia F, Ligthart JM , et al. Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases. Circulation. 2003;108(3):257–60. doi: 10.1161/01.CIR.0000083366.33686.11. [DOI] [PubMed] [Google Scholar]
- 42.Kim MS, Eng MH, Hudson PA , et al. Coronary stent fracture: clinical use of image enhancement. JACC Cardiovasc Imaging. 2010;3(4):446–7. doi: 10.1016/j.jcmg.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Pang JH, Kim D, Beohar N, Meyers SN, Lloyd-Jones D, Yaghmai V. Detection of stent fractures: a comparison of 64-slice CT. conventional cine-angiogr. phy.;and intravascu-lar ultrasonography. Acad Radiol 2009, 16(4):412–7. doi: 10.1016/j.acra.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Yamada KP, Koizumi T, Yamaguchi H , et al. Serial angiographic and intravascular ultrasound analysis of late stent strut fracture of sirolimus-eluting stents in native coro-nary arteries. Int J Cardiol. 2008;130(2):255–9. doi: 10.1016/j.ijcard.2007.08.082. [DOI] [PubMed] [Google Scholar]
- 45.Lim HB, Hur G, Kim SY , et al. Coronary stent fracture: detection with 64-section multidetector CT angiography in patients and in vitro. Radiology. 2008;249(3):810–9. doi: 10.1148/radiol.2493088035. [DOI] [PubMed] [Google Scholar]
- 46.Mitsutake R, Miura S, Nishikawa H, Saku K. Usefulness of the evaluation of stent fracture by 64-multi-detector row computed tomography. J Cardiol. 2008;51(2):135–8. doi: 10.1016/j.jjcc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Li P, Gai L. Coronary stent fracture detected by multidetector computed tomography. Int J Cardiovasc Imaging. 2010;26(7):729–30. doi: 10.1007/s10554-010-9624-1. [DOI] [PubMed] [Google Scholar]