Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with relevant morbidity and mortality. Besides hypertension, valvular disease and cardiomyopathy, mainly ischemic and dilated, also other conditions like obesity, alcohol abusus, genetic factors and obstructive sleep apnea (OSA) are discussed to contribute to the progression from paroxysmal to persistent AF. The prevalence of OSA among patients with AF is 40-50%. OSA is characterized by periodic or complete cessation of effective breathing during sleep due to obstruction of the upper airways. Obstructive respiratory events result in acute intrathoracic pressure swings and profound changes in blood gases together leading to atrial stretch and acute sympatho-vagal dysbalance resulting in acute apnea related to electrophysiological and hemodynamic alterations. Additionally, repetitive obstructive events in patients with OSA may lead to sympathetic and neurohumoral activation and subsequent structural and functional changes in the atrium creating an arrhythmogenic substrate for AF in the long run.
This review focuses on the acute and chronic effects of negative thoracic pressure swings, changes in blood pressure and sympatho-vagal dysbalance induced by obstructive respiratory events on atrial electrophysiology and atrial structure in patients with obstructive sleep apnea.
Keywords: Animal experiment, atrial electrophysiology, atrial fibrillation, continuous positive airway pressure, obstructive sleep apnea, renal denervation.
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with relevant morbidity and mortality [1]. Different classical risk factors for AF are hypertension, valvular disease and cardiomyopathy, mainly ischemic and dilated. Interestingly, also obesity, alcohol abusus, genetic factors and inflammation have been discussed as additional risk factors for AF [2]. Another condition, which is associated with a high prevalence of AF is obstructive sleep apnea (OSA) [3-5].
In this review, we will describe the pathophysiology of obstructive apneas in OSA potentially influencing atrial structure and electrophysiology and discuss studies investigating the role of different mechanisms on the development of a substrate for AF in OSA.
2. CLINIC OF OSA
A high prevalence of sleep disordered breathing can be found in young patients with both paroxysmal and persistent AF with preserved LV function (62% vs. 38% in patients without AF) [6]. However, the prevalence of daytime symptoms like excessive daytime sleepiness in OSA patients with AF is rare [7]. Therefore, the prevalence of OSA in AF patients may be even underestimated. Gami et al. [5] showed that the severity of OSA is a strong predictor of incident AF in individuals younger than 65 years. Additionally, a prospective analysis by Kanagala et al. [4] demonstrated that patients with OSA have a higher recurrence rate of AF after initial successful cardioversion than patients without OSA. Treatment with continuous positive airway pressure (CPAP) reduced the recurrence rate in OSA patients. Patients with OSA have a higher risk of AF recurrence after catheter ablation than those without OSA and effective treatment of OSA by CPAP improved catheter ablation success rates [8]. AF patients with severe OSA show a lower response rate to antiarrhythmic drug therapy than those with milder forms of OSA [9].
3. PATHOPHYSIOLOGY OF OSA
OSA is characterized by periodic or complete cessation of effective breathing during sleep due to occlusion of the upper airways. An apnea-hypopnea index (AHI) higher than 30 apneas per hour is considered severe OSA. Obstructive apneas caused by the collapse of the upper airway during sleep result in intrathoracic pressure swings provoking myocardial stretch of the heart chambers and lead to changes in transmural pressure gradients, particularly affecting the thinwalled atria [10]. Additionally, obstructive respiratory events induce intermittent apnea-associated hypoxaemia and hypercapnia, leading to sympathetic activation and subsequent intraapneic and postapneic hemodynamic fluctuations.
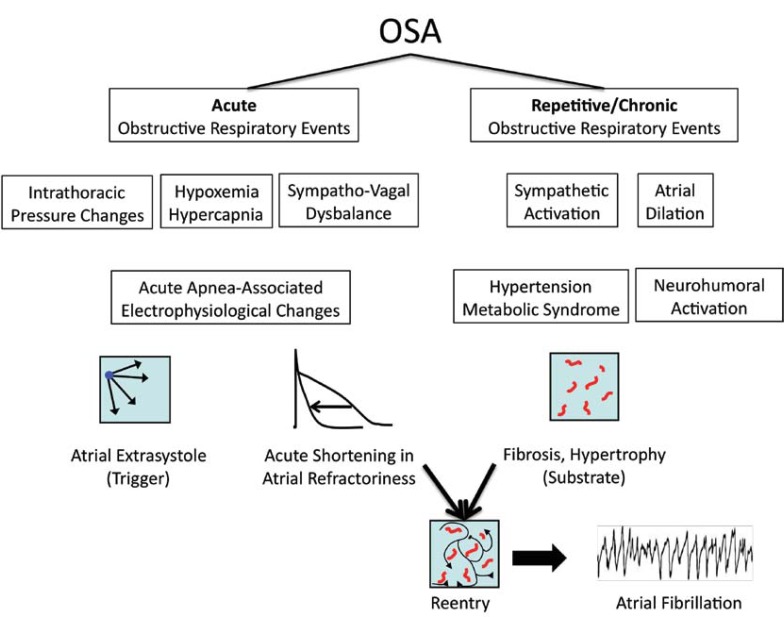
Interestingly, in OSA, paroxysms of AF were found to be nocturnal and, at least partly, temporally related to respiratory obstructive events [11, 12]. This temporal link between sleep disordered breathing and the occurrence of AF suggests that the trigger for AF may be mainly caused by acute changes during apneas and not by chronic remodeling processes in the atria alone [13]. Acute factors directly associated with obstructive respiratory events like intrathoracic pressure changes, changes in blood gases and sympatho-vagal dysbalance may contribute importantly to the occurrence of AF in OSA (Fig. 1).
Fig. (1).
Effects of different conditions during acute (see Chapter 3.1) and chronic (see Chapter 3.2) obstructive respiratory events on atrial electrophysiology, atrial substrates and triggers of atrial fibrillation.
3.1. Acute Effects of Obstructive Respiratory Events on Atrial Arrhythmogenesis
Intrathoracic pressure changes: Obstructed inspirations generate wide fluctuations in intrathoracic pressure, resulting in changes in cardiac transmural pressure, which in turn lead to atrial stretch. Negative tracheal pressures reaching down to -80 to -100 mbar were observed in OSA patients [10, 11]. Tracheal occlusion with applied negative tracheal pressure at -80 mbar resulted in a negative right atrial pressure of -16 mbar and a negative intrathoracic pressure of -65 mbar in a pig model for OSA [14]. This suggests an increase in atrial transmural pressure gradients, which results in atrial distension. Comparable intrathoracic pressure changes and alterations in atrial dimensions were observed during repetitive Mueller maneuvers in humans (attempted inspiration against the obstructed upper airways) [15].
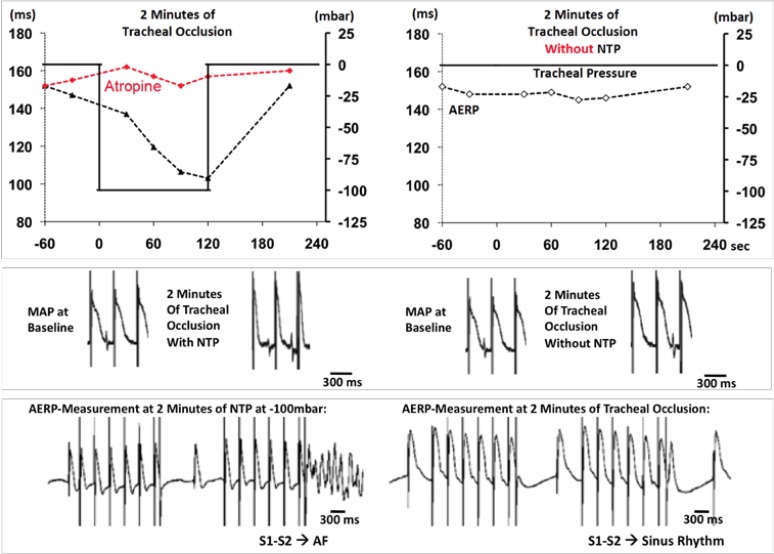
Acute atrial dilation caused by fluctuations in thoracic pressure may result in arrhythmogenic atrial electrophysiological alterations. In isolated Langendorff- perfused rabbit hearts, atrial dilation has been shown to shorten atrial refractoriness [16], slow conduction and increase the amount of intra-atrial conduction blocks [17]. Shortened atrial refractoriness together with lower conduction velocity and increased heterogeneity in local conduction increases the excitable gap, potentially resulting in increased AF-susceptibility [18]. In a pig model for OSA, application of negative tracheal pressure during tracheal occlusion -but not tracheal occlusion without applied negative tracheal pressure- reproducibly and reversibly shortened the atrial refractory period and strongly enhanced the inducibility of AF [14] (Fig. 2). These acute electrophysiological changes were reversible within 2 minutes after the release of the tracheal occlusion and were mainly mediated by sympatho-vagal imbalance, since they could be influenced by atropine, bilateral vagotomy or beta-receptor blockade [14, 19, 20]. Besides pronounced shortening in atrial refractoriness, obstructive respiratory events also resulted in increased occurrence of spontaneous premature atrial contractions, representing potent triggers for spontaneous AF-episodes in a pig model for OSA [21]. Arrhythmogenic electrophysiological changes were also observed in a rat model for obesity and OSA, which were partly prevented by combined autonomic blockade [22]. Of note, clinically used antiarrhythmic drugs like amiodarone or sotalol were not able to suppress the acute shortening in atrial refractoriness induced by applied negative thoracic pressure in the above mentioned pig model for OSA [20]. In accordance with this finding, Monahan et al. [9] demonstrated “that patients with severe OSA show a lower response rate to antiarrhythmic drug therapy for AF than those with milder forms of OSA. Interestingly, nonresponders to antiarrhythmic drugs had higher apnea-hypopnea indexes than responders” [9]. However, “minimum oxygen saturation did not differ between the 2 groups” [9]. This suggests that not hypoxia but the obstructive respiratory events, preferably negative thoracic pressure, seem to be important for AF recurrence, limiting antiarrhythmic drugs efficacy in patients with OSA.
Fig. (2).
Changes in atrial electrophysiology during 2 min of tracheal occlusion with (left) or without (right) applied negative tracheal pressure (NTP) at -100 mbar.Individual example of changes in atrial effective refractory period (AERP) (first line) and corresponding original recordings of monophasic action potentials (MAP) at baseline and at 2 minutes of tracheal occlusion (second line). Individual example of corresponding MAP signals during representative AERP-measurements at the end of 2 minutes of tracheal occlusion (bottom). Applied NTP at -100 mbar resulted in a progressive shortening of AERP and MAP and AF was inducible by a premature beat during the S1-S2 AERP measurement procedure. (Heart Rhythm 2011; 8: 1436-43.).
Changes in blood gases: Hypoxemia, hypercapnia and acidosis are invariably linked with obstructive apneas as occurring in OSA [10, 11]. “The magnitude of nocturnal oxygen desaturation, which is an important consequence of OSA, is an independent risk factor for incident AF.” [5] The electrophysiological effects of isolated hypoxia or hypercapnia were investigated in a sheep model with continuous ventilation under autonomic blockade [23]. Isolated hypercapnia resulted in atrial effective refractory period (AERP)-prolongation. AERP “rapidly returned to baseline, but recovery of conduction was delayed about 2 hours following equalization of hypercapnia. AF vulnerability was reduced during hypercapnia (with increased AERP) but increased significantly with subsequent return to eucapnia (when AERP normalized but conduction time remained disturbed), suggesting that differential recovery of AERP and conduction might be responsible for increased vulnerability to AF” [23]. In superfused rabbit atria, hypoxia caused a transient prolongation and an increase in heterogeneity of refractory periods. Additionally, hypoxia caused depressed conduction velocity and a marked increase in inhomogeneity in conduction both leading to increased vulnerability of the atrium for reentrant arrhythmias [24] . In isolated rabbit pulmonary vein preparations, hypoxia followed by reoxygenation induced pulmonary vein burst firings [25]. However, in a pig model for OSA [14] and in a rat model for obesity and OSA [22], changes in blood gases alone were insufficient to promote AF.
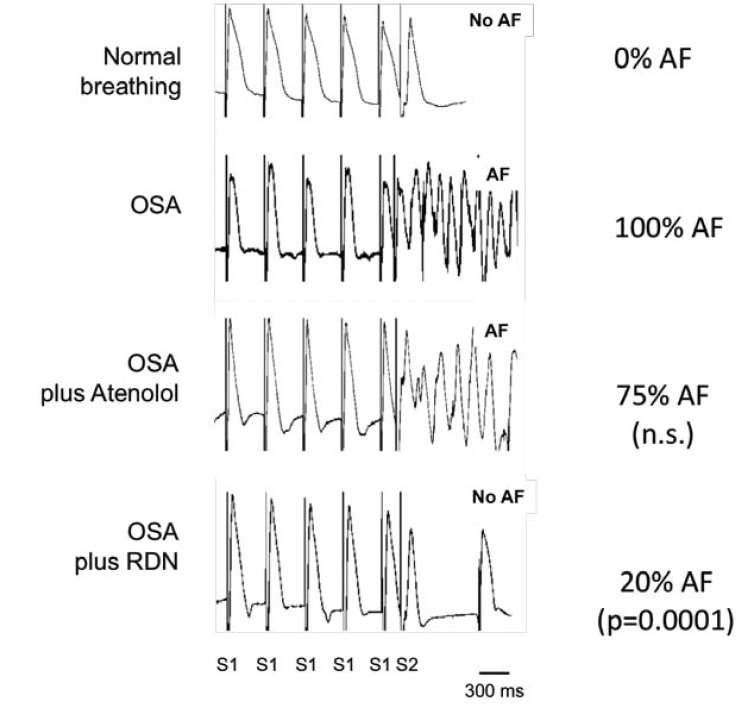
Sympatho-vagal dysbalance: Direct recordings of muscle sympathetic nerve activity showed increased sympathetic activation during apneic episodes in OSA patients [11] or in apnea divers [26]. Severe bradycardia and atrioventricular conduction disturbances together with the arousal reaction that limits the apnea duration, characterized by pronounced activation of the sympathetic system and pronounced blood pressure rises due to vasoconstriction and tachycardia, are frequently seen in OSA and suggest sympatho-vagal dysbalancecit_bfcit_af ref_bf(Kohler, 2010 ref_num874 / Kasai, 2011 #863)ref_af. The sympathetic and parasympathetic nervous system has been considered to play a role in the initiation and the maintenance of AF in humans (reviewed in [27, 28]). Forced inspiration-induced acute atrial distension causes an arrhythmogenic atrial electrical remodeling in a rat model for obesity and OSA, which was partly prevented by combined autonomic blockade [22]. Another indicator of pronounced autonomic activation during apnea has been provided by a dog model for central apnea [29]. Inducibility of AF could be attenuated by the ablation of the ganglionated plexi at the right pulmonary artery or by combined pharmacological neurohumoral blockade [29]. In a pig model, negative tracheal pressure during obstructive apneas leads to pronounced shortening of atrial refractoriness thereby perpetuating AF. These electrophysiological changes were mainly mediated by sympatho-vagal dysbalance, since they could be influenced by atropine, bilateral vagotomy or beta-receptor blockade [14, 19-21]. In this pig model, the effect of modulation of sympathetic nervous system by renal sympathetic denervation (RDN) was investigated. RDN is a strategy to modulate sympathetic nervous system and has been shown to reduce muscle sympathetic nerve activity in patients with therapy resistant hypertension [30], suggesting a reduction of central sympathetic drive. Compared to beta-blocker treatment, RDN resulted in a more pronounced attenuation of AERP-shortening induced by negative thoracic pressure, which might explain the superior antiarrhythmic effect of RDN compared to beta-blocker therapy in this animal model [19] (Fig. 3). Additionally, RDN has also been shown to reduce OSA-severity in hypertensive patients as well as it reduces blood pressure [31].
Fig. (3).
Representative atrial multiple action potential (MAP) recordings during AERP-measurements before and after RDN or atenolol in a pig model for OSA. Percent of OSA-maneuvers with inducible atrial fibrillation (AF) and the effect of either atenolol or RDN. (Hypertension 2012; 60: 172-8.).
3.2. Longterm OSA and Atrial Structural Remodeling
In patients, longterm OSA has been shown to be associated with significant atrial remodeling characterized by “atrial enlargement, local conduction disturbances and longer sinus node recovery” [32], atrial electromechanical delay and left atrial dysfunction [33].
Several mechanisms have been considered to cause OSA-related myocardial damage including systemic inflammation, neurohumoral activation, chronic atrial dilation by repetitive changes in intrathoracic pressure and multiple comorbidities like obesity and hypertension:
Inflammation and neurohumoral activation: OSA has been shown to be associated with elevations of circulating markers of inflammation [34, 35]. C-reactive protein is associated with vascular inflammation and its serum concentration correlates with AF stability, predict postoperative AF and failure of AF ablation [2].
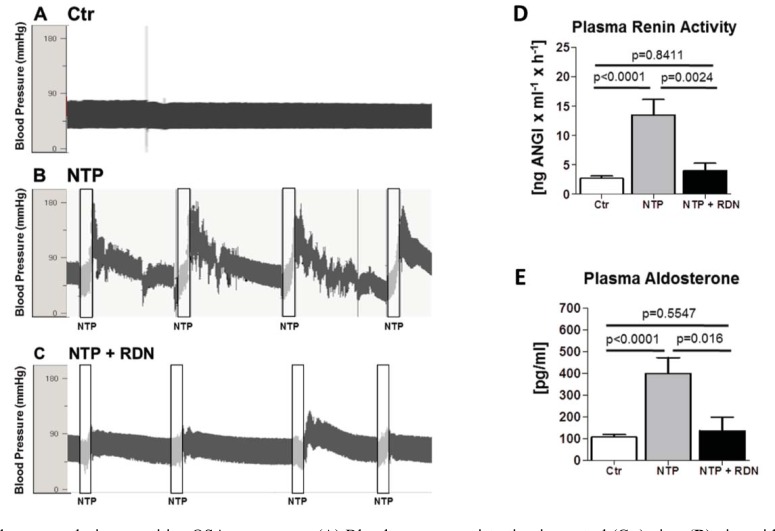
Neurohumoral activation, namely the circulating renin angiotensin aldosterone system (RAAS) together with increased oxidative stress, has been shown in OSA patients, which may underlie atrial structural and electrical remodeling in OSA [36, 37]. The RAAS has been shown to play a relevant role for the development of a structural atrial remodeling under different pathophysiological conditions [18]. In a pig model for OSA, RAAS activation was mainly driven by increased sympathetic activation, as it was almost completely attenuated by renal sympathetic denervation [21, 38] (Fig. 4).
Fig. (4).
Blood pressure during repetitive OSA-maneuvers: (A) Blood pressure registration in control (Ctr) pigs, (B) pigs with repetitive obstructive respiratory events with applied negative tracheal pressure (NTP) without and (C) with RDN (NTP + RDN). RAAS-signaling components (D and E): Effect of 4 hours of repetitive OSA-maneuvers on (D) plasma renin-activity and (E) plasma aldosterone concentrations. (Hypertension 2013; 62: 767-74.).
Effect of hypertension and obesity on structural remodeling: OSA is considered an etiological factor in the development of hypertension and in the evolution of drug resistant hypertension [39, 40]. In a sheep model, chronically elevated blood pressure was associated with significant atrial electrical and structural remodeling characterized by local conduction disturbances, shortening of atrial wavelength, and increased occurrence of AF, atrial myocyte hypertrophy and myolysis, increased atrial collagen and apoptosis [41]. In another sheep model with induced "one-kidney, one-clip" hypertension, hypertension was associated with early and progressive changes in atrial remodeling [42]. In spontaneously hypertensive rats hypertension was associated with the development of a substrate for atrial tachyarrhythmia “involving left atrial fibrosis without changes in the atrial effective refractory period” [43]. Longterm hypertension is associated with increased afterload leading to chronic atrial dilation. Longterm atrial dilation during chronic atrioventricular block in the goat caused “intra-atrial conduction defects and a higher contribution of anatomically defined re-entrant circuits” leading to increased AF-susceptibility [44].
Obesity and the metabolic syndrome, both prevalent in OSA patients, may be associated with the development of AF by systemic changes related to these conditions [45]. “There is a 3-8% higher risk of new AF -onset with each unit increase in body mass index, and this association is independent of other cardiovascular risk factors like lipid levels, blood pressure or diabetes” [45]. Pericardial fat is associated with occurrence of AF, persistence of AF, left atrial enlargement, and bad outcomes of AF ablation [46]. Additionally, obesity results in progressive atrial structural and electrical remodeling. In sheep, following a high-calorie diet, obesity was associated with atrial electrostructural remodeling. “With progressive obesity, there were changes in atrial size, conduction, histology, and expression of profibrotic mediators. These changes were associated with spontaneous and more persistent AF” [47].
4. TREATMENT OPTIONS OF ATRIAL FIBRILLATION IN PATIENTS WITH OSA
The data reviewed herein suggest that effective prevention of obstructive respiratory events may result in less AF in patients with OSA. Accordingly, in a case report, treatment with CPAP in an OSA patient with severe metabolic syndrome resulted in lower recurrence of symptomatic AF after electrical cardioversion, lowered high blood pressure and improved metabolic conditions, which were drug resistant at baseline [48]. More importantly, the initiation of a specific antiarrhythmic therapy could be prevented in this patient [48]. These antiarrhythmic effects of CPAP therapy are also confirmed in several clinical studies (see chapter 2) [8, 9, 49]. However, whether rate or rhythm control strategy should be aspired in OSA-patients despite the prevention of obstructive respiratory events by CPAP is unknown and deserved further clinical trials.
5. SUMMARY
The substrate for AF in OSA seems to be more complex compared to other conditions associated with AF. In addition to the development of a structural remodeling, acute electrophysiological changes during obstructive respiratory events caused by acute changes in blood gases, breathing associated fluctuations in intrathoracic pressure and activation of autonomic system may contribute critically to the development of an arrhythmogenic substrate. This highly arrhythmogenic substrate, characterized by the combination of permanent increased atrial size and the presence of conduction disturbances and intraapneic arrhythmogenic electrophysiological alterations likely contributes to increased recurrence rate of AF following cardioversion or catheter ablation. OSA-associated factors should be considered during the development of future antiarrhythmic pharmacologic and interventional treatment strategies for AF.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kirchhof P, Bax J, Blomstrom-Lundquist C , et al. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET-EHRA consensus conference “research perspectives in AF”. Eur Heart J. 2009;30:2969– 77. doi: 10.1093/eurheartj/ehp235. [DOI] [PubMed] [Google Scholar]
- 2.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of 'not-so-lone atrial fibrillation'. Europace. 2008;10:668–73. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KG, Johnson DC. Obstructive sleep apnea is a risk factor for stroke and atrial fibrillation. Chest. 2010;138:239. doi: 10.1378/chest.10-0513. [DOI] [PubMed] [Google Scholar]
- 4.Kanagala R, Murali NS, Friedman PA , et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Hodge DO, Herges RM , et al. Obstructive sleep apnea. obeity.and the risk of incident atrial fibrillation. J Am Coll Cardiol . 2007; 49:565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–9. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 7.Albuquerque FN, Calvin AD, Sert Kuniyoshi FH , et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–73. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel D, Mohanty P, Di Biase L , et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysio. 2010;3:445–51. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 9.Monahan K, Brewster J, Wang L , et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110:369–72. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 11.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 12.Monahan K, Storfer-Isser A, Mehra R , et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 14.Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8:1436–43. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Orban M, Bruce CJ, Pressman GS , et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea. atrial fibrillaion.and heart failure. Am J Cardiol . 2008; 102:1557–61. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96(5):1686–95. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 17.Eijsbouts SC, Majidi M, van Zandvoort M, Allessie MA. Effects of acute atrial dilation on heterogeneity in conduction in the isolated rabbit heart. J Cardiovasc Electrophysiol. 2003;14(3):269–78. doi: 10.1046/j.1540-8167.2003.02280.x. [DOI] [PubMed] [Google Scholar]
- 18.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 19.Linz D, Mahfoud F, Schotten U , et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60:172–8. doi: 10.1161/HYPERTENSIONAHA.112.191965. [DOI] [PubMed] [Google Scholar]
- 20.Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Combined blockade of early and late activated atrial potassium currents suppresses atrial fibrillation in a pig model of obstructive apnea. Heart Rhythm. 2011;8:1933–9. doi: 10.1016/j.hrthm.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Linz D, Hohl M, Nickel A , et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–74. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki YK, Shi Y, Benito B , et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;9:1409–16. doi: 10.1016/j.hrthm.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson IH, Roberts-Thomson KC, Kistler PM , et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm. 2010;7:1263–70. doi: 10.1016/j.hrthm.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Lammers WJ, Kirchhof C, Bonke FI, Allessie MA. Vulnerability of rabbit atrium to reentry by hypoxia.Role of inhomogeneity in conduction and wavelength. Am J Physiol. 1992;262:H47–55. doi: 10.1152/ajpheart.1992.262.1.H47. [DOI] [PubMed] [Google Scholar]
- 25.Lin YK, Lai MS, Chen YC , et al. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin Sci (Lond) 2012;122:121–32. doi: 10.1042/CS20110178. [DOI] [PubMed] [Google Scholar]
- 26.Heusser K, Dzamonja G, Breskovic T , et al. Sympathetic and cardiovascular responses to glossopharyngeal insufflation in trained apnea divers. J Appl Physiol. 2010;109:1728–35. doi: 10.1152/japplphysiol.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linz D, Ukena C, Mahfoud F, Neuberger HR, Böhm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy?. J Am Coll Cardiol. 2014;63:215–24. doi: 10.1016/j.jacc.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Linz D, van Hunnik A, Ukena C , et al. Renal denervation: effects on atrial electrophysiology and arrhythmias. Clin Res Cardiol Mar 29. 2014 doi: 10.1007/s00392-014-0695-1. [DOI] [PubMed] [Google Scholar]
- 29.Ghias M, Scherlag BJ, Lu Z , et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–83. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Hering D, Lambert EA, Marusic P , et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 31.Witkowski A, Prejbisz A, Florczak E , et al. Effects of renal sympathetic denervation on blood pressure. sleep apnea corse.and glycemic control in patients with resistant hypertension and sleep apnea. . Hypertension. 2011; 58:559–65. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 32.Dimitri H, Ng M, Brooks AG , et al. Atrial remodeling in obstructive sleep apnea: Implications for atrial fibrillation. Heart Rhythm. 2011;9:321–7. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Yagmur J, Yetkin O, Cansel M , et al. Assessment of atrial electromechanical delay and influential factors in patients with obstructive sleep apnea. Sleep Breath. 2012;16:83–8. doi: 10.1007/s11325-010-0477-6. [DOI] [PubMed] [Google Scholar]
- 34.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1.L-8.and MCP-1. J Appl Physiol . 2003;94:179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 35.Shamsuzzaman AS, Winnicki M, Lanfranchi P , et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 36.Møller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–80. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso M, Bonanni E, LoGerfo A , et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2012;13:632–6. doi: 10.1016/j.sleep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Linz D, Mahfoud F, Linz B , et al. Effect of Obstructive Respiratory Events on Blood Pressure and Renal Perfusion in a Pig Model for Sleep Apnea. Am J Hypertens Mar 12. 2014 doi: 10.1093/ajh/hpu036. [DOI] [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 40.Logan AG, Perlikowski SM, Mente A , et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Kistler PM, Sanders P, Dodic M , et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–56. doi: 10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 42.Lau DH, Mackenzie L, Kelly DJ , et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010;7:1282–90. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Choisy SC, Arberry LA, Hancox JC, James AF. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension. 2007;49:498–505. doi: 10.1161/01.HYP.0000257123.95372.ab. [DOI] [PubMed] [Google Scholar]
- 44.Neuberger HR, Schotten U, Blaauw Y , et al. Chronic atrial dilation. electrical remodeing.and atrial fibrillation in the goat. J Am Coll Cardiol. 2006; 47:644–53. doi: 10.1016/j.jacc.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 45.Dublin S, French B, Glazer NL , et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166:2322–8. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 46.Wong CX, Abed HS, Molaee P , et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–51. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 47.Abed HS, Samuel CS, Lau DH , et al. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 48.Linz D, Ukena C, Neuberger HR. Treatment of obstructive sleep apnoea improves metabolic conditions and prevents initiation of antiarrhythmic therapy in a patient with atrial fibrillation. Europace. 2014;16:245. doi: 10.1093/europace/eut233. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Wang ZW, Li J , et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace Apr 2. 2014 doi: 10.1093/europace/euu066. [DOI] [PubMed] [Google Scholar]