Abstract
In cardiology, optical coherence tomography (OCT) is an invasive imaging technique based on the principle of light coherence. This system was developed to obtain three-dimensional high resolution images to examine coronary artery normal and/or pathological structure. This technique replaces the ultrasound used by its main alternative procedure, intravascular ultrasound, by a near-infrared light source. Acute coronary syndromes due to atherosclerotic vascular disease are the leading cause of mortality in developed and developing countries. As a consequence, intravascular imaging systems became an important area of research and 1991 marks the first use of OCT in coronary artery observations. Since its first appearance in invasive cardiology, OCT maintains a strong presence in the research environments for the identification of vulnerable plaques, as it is able to overcome difficulties presented by other techniques such as virtual intravascular ultrasound, near-infrared spectroscopy, and histology. Moreover, OCT is increasingly being used in the clinical practice as a guide during coronary interventions and in the assessment of vascular response after coronary stent implantation. This review focuses on the relevance of OCT in research and clinical applications in the field of invasive cardiology and discusses the future directions of the field.
Keywords: Optical coherence tomography, intravascular imaging, coronary artery lesions.
INTRODUCTION
For many, the first symptom as a consequence of their atherosclerotic plaque is an acute coronary syndrome (ACS), which can have devastating clinical consequences. Unfortunately, the main problem is that there is no standard method to accurately identify the presence of vulnerable coronary atheromas in time to facilitate treatment and offer preventative options. Early detection of this condition is difficult as coronary arteries are extremely small and too deep to be examined accurately from the surface. Furthermore, most preventative procedures for asymptomatic patients are geared towards non-invasive and inexpensive procedures, which severely limit early detection. In the last decades, cardiologists have searched for new approaches and methods to define location, composition and consequences different types of plaque (stable vs. unstable). Furthermore, the introduction of percutaneous coronary intervention (PCI) created the need for the development of techniques to evaluate coronary conditions before the treatment, as well as ways to assess the results, both short and long term.
Optical coherence tomography (OCT) opened the door to a new way to assess coronary lesions, allowing the progression from indirect and less accurate methods used previously to a direct way to visualise vessel (Fig. 1), plaque morphology, coronary thrombosis (Fig. 2) as well as vascular response after stent implantation. Growing evidence indicates that OCT may hold the answer to reliable and effective intracoronary imaging and potentially make significant contributions. Although not new, this system went through several years of improvements to remove original limitations. The major question at this stage is what can OCT offer that other systems cannot? While examining OCT images, it is easy to be struck by all the detail and resolution, but ultimately the future of this technique lies on its ability to accurately and effectively detect atherosclerotic plaque and identify its composition. OCT already has a place in research and very specific clinical applications in invasive cardiology; however, defining its optimal use and clinical impact requires further large, longitudinal, and well-designed investigations. This review is structured in three parts. In light of the increasing use of OCT in invasive cardiology, we will initially focus on the basic technical aspects of OCT. Second, we will discuss the relevance of OCT in research settings, with a special focus on the detection of vulnerable plaques. Finally, we will consider the main clinical applications of OCT in invasive cardiology and discuss the future directions of the field.
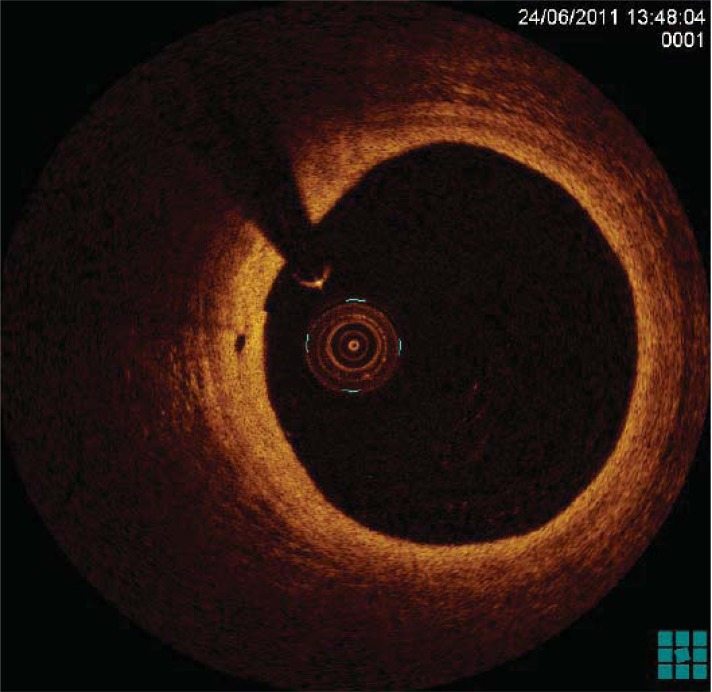
Fig. (1).
OCT imaging of a coronary artery characterised by a layered architecture.
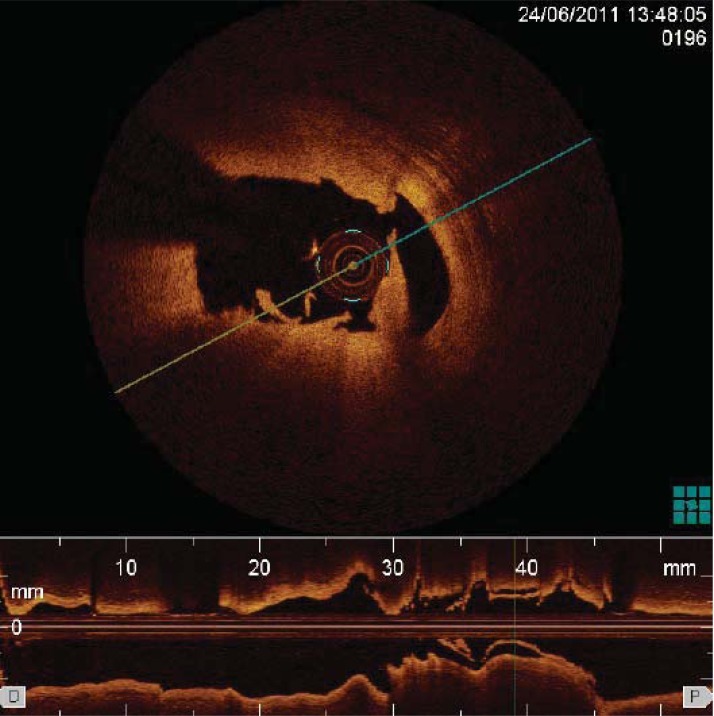
Fig. (2).
Thrombus identified by OCT as an abnormal mass protruding into the coronary lumen, with signal backscattering and various degree of attenuation.
TECHNICAL ASPECTS OF TYPES OF OCT IN CARDIOLOGY: COMPARATIVE ANALYSIS
An in-depth analysis of the principles behind OCT is outside the scope of this paper and has been extensively reviewed elsewhere [1-3]. Briefly, using an interferometer, infrared light is emitted from the OCT and split between the sample and the reference arm, creating an interference pattern analysed as a function of the signal intensity and time delay after reflection from a reference mirror or back-scattering from the tissue sample. Reflected light depends on the different refractive indices in the sample created by specific components. Multiple scans are obtained by moving the sample or pulling back the light source along the coronary artery and a three-dimensional reconstruction is possible by combining multiple scans [1-3].
FIRST GENERATION OCTs
Time domain OCTs (TD-OCTs) were the first systems to be developed, but there were severe limitations and its use never became widespread, apart from specific research applications. Its main practical limitation resulted from the need to use of an occlusion balloon to remove all blood from the vessel to be examined, potentially resulting in ischemia, as red blood cells interfere with the propagation of infrared light and introduce inaccuracies on results obtained [4].
SECOND GENERATION OCTs
Replacing the broadband light source used in TD-OCT for an adjustable laser light source with a wavelength between 1250 and 1370 nm created a much simpler and more effective system. This method is called frequency domain OCT (FD-OCT) and the reference mirror is fixed, with multiple scans obtained by altering the wavelength [4, 5]. Recently, Yoon et al. [6] demonstrated that FD-OCT is a safe technique even when covering extended areas and no serious complications, including death or myocardial infarction, were observed. This way, imaging speeds have increased to 100 fps2 and the need for an occlusion balloon was eliminated [5]. This procedure now relies on flushing the artery with contrast media to displace the blood [4, 5]. Furthermore, studies have shown that images obtained with an FD-OCT have higher resolution [5] and better reproducibility [7] than those obtained with a TD-OCT, thus providing interventional cardiologists detailed intracoronary images. This improvement means vital vessel parameters can be detected, that would otherwise be missed with earlier OCT models.
RESEARCH APPLICATIONS: OCT CAN DETECT DIFFERENT TYPES OF ATHEROSCLEROTIC PLAQUES
One of the main research applications of OCT in research settings has been in the detection of vulnerable plaques. OCT offers clear luminal images of the coronary vessels, allowing reference measurements for minimal lumen area [8] as well as identification of lipid pools and internal and external lamina [9]. In contrast to intravascular ultrasound (IVUS) where significant shadowing effect may difficult identification, calcium deposits can also be recognised with this technique. Its main limitation is a poorer axial penetration compared to IVUS which may limit observation of remodelling or an accurate measurement of stenosis [10]. This information is vital to decide whether most appropriate therapies to follow, including selection of balloon and stent dimensions and determine the optimal location for stent implantation. For example, Secco et al. [11] utilized OCT to determine the appropriate size of balloons for patients undergoing cutting balloon angioplasty for the treatment of in-stent restenosis. Moreover, Viceconte et al. [12]used OCT to guide selection of treatments, including rotablation, thrombectomy and cutting balloon angioplasty.
DETECTION OF THIN-CAP FIBROATHEROMA
It is currently accepted that thin-cap fibroatheroma (TCFA) is the most likely culprit behind serious or fatal ACS. Autopsy and clinical studies have identified that an inflamed thin fibrous cap covering a lipid-rich core is the most likely rupture point in patients suffering from ACS [13-16].Rupture can occur when an increased macrophage concentration accumulates at the shoulders of the affected plaque and starts releasing metalloproteinases, thus creating a weak point and potential thrombus release [17, 18]. Another factor that has also been linked with vulnerable plaque is remodelling, which involve physical changes to TCFAs [19] such as intraplaque neovascularisation [16].
Although some early reports suggested that OCT showed a poor TCFA detection caused by the inability to detect lipid cores located behind a fibrous covering due to low penetration [20], more recent studies have successfully used OCT to detect these fragile and vulnerable regions with a high degree of sensitivity [13, 21] and comparatively better than IVUS and angiography [22]. Van Soest et al. [21] suggested that a high attenuation OCT coefficient, µt >10 mm-1, is linked to the presence of necrotic core and macrophage infiltration, both markers for vulnerable plaque. In contrast, measurements for other less dangerous forms of atherosclerotic plaque received a lower attenuation coefficient ≈2-5 mm-1. To determine the presence of TCFAs, the currently accepted method is to use OCT to assess the thinnest part of the cap. Multiple images are generated and the one containing the field of view with the thinnest part of the TCFA is identified manually, which means this technique is highly subjective. To overcome this problem, Wang et al. [15] recently developed a computer algorithm allowing volumetric evaluation of TCFA. The authors suggest this new method is a fast and reliable way to achieve a better judgement of vulnerable plaque using OCT measurements.
OCT VERSUS OTHER TECHNIQUES IN THE DETECTION OF VULNERABLE PLAQUES
There are some limitations associated with the use of OCT to evaluate the presence of vulnerable plaques. The main issue is a limited ability to recognize necrotic cores, and due to the restricted penetration (1–2 mm), these regions simply appear with diffuse borders. Unfortunately, other non-necrotic structures are also visualized in a similar way and false-positives can occur [23]. In this case, IVUS is the better option, as it is possible to visualize the entire coronary structure and offer a more accurate distinction between necrotic cores and other structures. Differently from IVUS, OCT is obstructed by blood and may require additional contrast use, which may pose safety issues in patients with kidney impairment [24]. OCT has also limitations in the detection of aortic ostial lesions, and IVUS is superior to OCT in the detection of such abnormalities [25]. In general, however, OCT offers clear, photographic quality three-dimensional images and detects details on a microscopic level, as opposed to lower-resolution IVUS images. Recently, Yonetsu et al. [26] have compared the usefulness of intracoronary near-infrared spectroscopy (NIRS) – a system approved by the Food and Drug Administration (FDA) for the detection of coronary lipid [27] – and OCT for the detection of lipids in vulnerable plaques. The results showed modest linear correlations in the measurement of lipid. However, the accurate depth of lipid in the vessel wall could not be identified by quantitative NIRS parameters, suggesting that larger lipid and thinner fibrous cap (i.e., features consistent with plaque vulnerability), were more easily detected by OCT [26]. Notably, OCT allows to obtain high resolutions that approach that of histology. In this regard, OCT has been proposed an adjunct to histological tissue observation when it is not practical to take specimens for histological processing or when large areas need investigation [28]. Notwithstanding the potential usefulness of OCT in the detection of vulnerable plaques in a research setting, it should be emphasized that such lesions are clinically defined as plaques that would cause a cardiac event in the future. Therefore, an invasive imaging modality may be of limited use in human populations. Furthermore, it should be acknowledged that there are currently no prospective studies demonstrating the value of OCT to identify such lesions prior to events.
CLINICAL APPLICATIONS: OCT AS A GUIDE DURING CORONARY INTERVENTIONS
Due to its technical advantages, OCT is increasingly being used for in clinical practice environment. In this regard, OCT has been successfully utilized during PCI as a guide during the intervention (Fig. 3) (Table 1). This procedure is particularly useful during challenging procedures, such as chronic total occlusions [29] or bifurcation lesion stenting [30], and may lower mortality rate following the procedure [31]. During chronic total occlusions, OCT is used to confirm the guidewire is located inside the correct vessel and has not entered a false lumen [29]. In terms of bifurcation lesion stenting, with 3D reconstructions of OCT images it is possible to determine the position of the main vessel and the relative position of the side vessel, which is essential to determine a suitable strategy to conduct the procedure [30]. Also, these models help determine where a guidewire crosses a side branch through the stent implanted in the main vessel to assess strut coverage of the side branches [32].
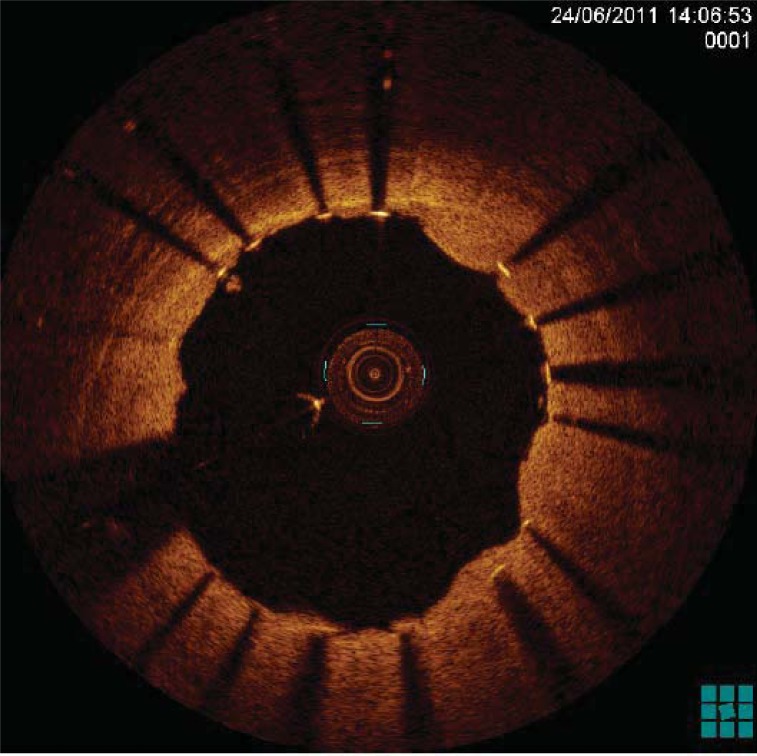
Fig. (3).
OCT imaging of a drug-eluting stent immediately after implantation in a patient with ST-segment elevation acute myocardial infarction. Note the protrusion of thrombotic material throughout stent struts.
Table 1.
Research and clinical applications of OCT in invasive cardiology.
| OCT Application | References | |
|---|---|---|
| Assessment of coronary lesions before PCI | Detection of atherosclerotic plaque | [42] |
| Detection of internal and external laminae | [9] | |
| Assessment of vulnerable plaque, including presence of thin fibrous cap, inflammation, lipid core, intraplaque neovascularisation and calcified nodule | [13, 15, 16, 21, 23] | |
| Assessment of remodelling | [42, 19] | |
| Assessment of intraplaque neovascularisation | [16] | |
| Assessment of luminal area | [8] | |
| Identification of presence and type of thrombus | [17] | |
| Determination of most suitable therapies | [11, 12] | |
| Guidance during chronic total occlusion | [29] | |
| During PCI | Guidance during bifurcation lesion stenting | [30] |
| Guidance to track exact location of stent | [31] | |
| Post-PCI evaluation | Detection of stent edge dissection, tissue protrusion, and incomplete stent apposition | [35] |
| Evaluation of stent strut coverage | [51] | |
| Mid and long-term assessments for safety and efficacy | [41] | |
| Assessment of stent coverage | [38-40] | |
| Assessment of restenosis | [56, 57] | |
| Detection of microvessels and increased neointimal hyperplasia | [58] | |
| Assessment of in-stent thrombosis | [55] | |
| Assessment of neoartherosclerosis | [60, 61] | |
CLINICAL APPLICATIONS: ASSESSMENT OF VASCULAR RESPONSE AFTER CORONARY STENT IMPLANTATION
Another important clinical application of OCT concerns evaluating possible complications following stent implantation, including detection of incomplete stent strut coverage, in stent restenosis, stent thrombosis and neoatherosclerosis. Vital information can be obtained with OCT following stent implantation, including progression and healing over the stented area [8, 33]. Therefore, OCT can be clinically used for prediction of restenosis or late thrombosis following stent implantation. Comparison studies between IVUS and OCT give greater merit to OCT in detecting any complications after PCI. Reports have shown that OCT can find more cases of malapposition [34], stent edge dissections, plaque protrusion and incomplete stent apposition than IVUS [35].
When conducting OCT after stent implantation is important to realise that infrared light cannot cross metal, and the stent is visualised as a long linear structure [36, 37] and any measurements taken need to take the thickness of the strut in consideration. Terashima et al. [36] demonstrated a high correlation and reproducibility between OCT strut measurements and actual commercial stent measurements.
STENT STRUTS COVERAGE
Various studies have been performed to assess the safety of stent procedures by using OCT to determine the extent of stent apposition and neointimal coverage after stent implantation (Fig. 4) [38-40]. This information is vital to understand how healing progresses and to establish the best treatment course for patients, especially to detect those that may be at risk of restenosis [41] and/or thrombosis, which is linked with incomplete strut coverage. In comparison with IVUS, OCT is the clear winner, capable of detecting even a layer of neointima coverage of only 10 μm, missed by other techniques [38, 42], but it may not be able to differentiate between endothelization coverage or fibrin deposits [43]. Over the last few years, there has been intense research in terms of assessing how strut coverage is influenced by the type of stent implanted. OCT detected a delay in the establishment of strut coverage between bare metal stents (BMS) and drug-eluting stents (DES), particularly first generation DESs including sirolimus-eluting (SES) or paclitaxel-eluting stents (PES) [38, 44-46]. Uncovered DES were still visible 9-12 months after the procedure [47, 48], and it was only after 5 years that complete coverage was established [49]. In contrast, Chen et al. [50] noticed that it only took 5 months for OCT images from patients that received a BMS to show complete apposition and almost all strut surfaces covered by neointima. In fact, some were starting to get covered by hyperproliferative neotintima, which could potentially cause restenosis.
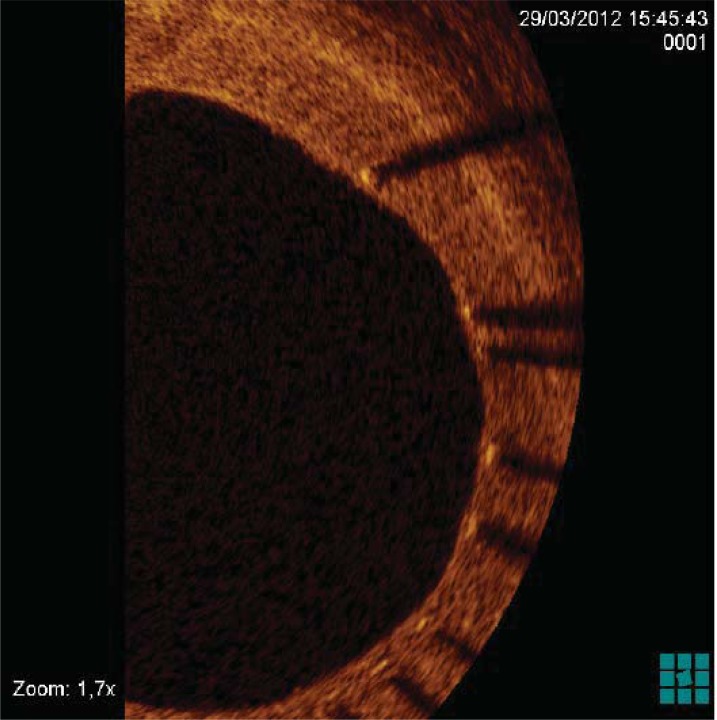
Fig. (4).
Evaluation by OCT of re-endothelization extent at follow-up in a patient who underwent drug-eluting stent implantation.
Even between different types of drug-eluting stents, Sim et al. [48] detected differences, with more uncovered struts in SES vs. PES, which means PES is associated with a faster healing response, but the mechanism is not fully understood. Second generation stents, such as zotarolimus-eluting stent or everolimus-euting stent seem to have an identical coverage rate46and show a pattern closer to BMS [51].
ASSESSMENT OF STENT THROMBOSIS
The development of drug-eluting stents has provided improvements in terms of significant reduction in restenosis after the procedure. However, other issues have arisen, as late stent thrombosis and restenosis become more common due to malapposition [41, 52, 53]. This is why a long-term treatment of dual anti-platelet therapy (DAT), sometimes lasting over a year, is currently recommended for DES-implanted patients [47, 48]. Two mechanisms have been suggested to explain stent thrombosis [54]. One mechanism involves morphological changes during vessel healing after stent implantation, as a consequence of malapposition and incomplete coverage; while the second hypothesis is associated with rupture of neoatherosclerotic tissue. Different types of thrombus collected seem to support this theory [54], however, further studies are required to fully explain the mechanisms involved in late thrombosis and their clinical implications. Alfonso et al. [55] recently conducted a study to compare between use of OCT and IVUS before and after intervention due to stent thrombosis. The authors demonstrated that OCT was able to identify all thrombus present and, despite the fact that their presence casted a shadow over the stent, OCT still recognised malapposition, uncovered struts and in-stent restenosis. OCT can detect the presence and differentiate between red and white thrombi [18], by analysing differences in the signal attenuation curve [17]. In contrast, IVUS showed severe limitations in visualising the thrombus-lumen connection and strut malaposition and failed completely to identify uncovered struts and neoatherosclerosis.
EVALUATION OF IN-STENT RESTENOSIS
In-stent restenosis (ISR) is defined as excess neointimal proliferation occurring after stent implantation [56]. This condition can progress to neoatherosclerosis and heterogenous restenosis accompanied by calcification and lipid accumulation, potentially causing luminal disruption [57]. Recently, Kim et al. [58] also identified the presence of microvessels and increased neointimal hyperplasia in some advanced ISR lesions. It is usually considered that restenosis is a common condition in patients fitted with a bare metal stent and rare with a drug-eluting stent [59], but recent studies are starting to show other important morphological and temporal differences between restenosis developed with BMS or DES [60]. BMS restenosis is characterised mostly by a rapid accumulation of smooth muscle cells, appearing in OCT images as predominantly homogeneous with high signal echogenicity, in contrast to restenosis developing with DES, which involves a slow accumulation of proteoglycan-rich tissue showing up as a layered pattern or heterogeneous tissue composition [60].
NEOATHEROSCLEROSIS
The development of in-stent neoatherosclerosis several years after implantation [60], including microvascular proliferation, lipid-rich neointima, intimal rupture, thrombi and calcification, has been identified using OCT [60, 61] and is most commonly associated with DES [62] and late stent failure [40]. Even in patients suffering from stable angina, lesions containing lipid-rich neointima were found [63]. Yonetsu et al. [61] identified several factors linked to this condition, including not only stent type and age, but also smoking, chronic kidney disease, and angiotensin-converting enzyme inhibitors/angiotensin II receptor blockade. The positive results obtained with OCT to assess vascular response to stent implantation, from stent coverage to damage caused by neoatherosclerosis, mean this technique will soon become an indispensable tool for clinical and research use (Table 1).
SPECIALS CONSIDERATIONS ON BIOABSORBABLE STENTS
New-generation bioabsorbable stents are innovative devices that have the capacity of being dissolved and assimilated into the coronary artery [64, 65]. Because they did not leave any residual foreign material in the vessel wall, their use may significantly reduce the risk of restenosis or late-stent thrombosis as compared with traditional bare-metal and drug-eluting stents [66]. The study of OCT for investigating the fate of bioabsorbable stents – including the modifications of such devices over time and the manner in which the artery returns to its normal structure – has recently gained momentum [67-69]. In the ABSORB study [70], the use of OCT has allowed demonstrating that the preserved box of a bioabsorbable stents remains in situ approximately 6 months. Subsequently, the struts predominantly become either indiscernible or integrated into the artery wall [70]. Mattesini et al. [71] have recently shown that OCT may be useful in a research setting for investigating scaffold integrity, apposition to the underlying wall, presence of thrombus and hyperplasia, and changes in the strut characteristics over time after implantation of bioabsorbable stents. Although histology remains the gold standard in the research setting for characterization of tissue maturation following the placement of bioabsorbable stents, OCT enables an in vivo assessment of stent-vessel interactions at a micron-scale level without requiring tissue preparation [72, 73]. Consequently, the complementary role of OCT as compared to histology to assess the impact of novel endovascular technologies is now well-established.
CONCLUSIONS
New advances are constantly being presented to improve and maximize benefits obtained from OCT. For example, recently developed algorithms used in automatic tissue characterisation software [63] as well as a real-time 3D and 4D phase-resolved Doppler OCT (PRDOCT) [74] allow a more objective interpretation of results. The versatility of this procedure establishes OCT as a potential imaging technique with a significant impact not only in our understanding of the vascular morphology and pathophysiology of atherosclerotic plaque, but also in aiding during coronary interventions and assessing its long-term vascular outcomes. It is currently well-established as a valuable research tool, allowing the evaluation of many conditions, including detection of thin-cap fibroatheroma. In clinical research and clinical practice, OCT is increasingly being used in the assessment of vascular response to stenting. For example, OTC has been successfully utilized for the examination of the in vivo mechanisms of late drug-eluting stent thrombosis [75] or for serial assessments of coronary artery response to DES [76, 77]. Importantly, OTC is currently being used as a clinical research tool for investigating gender disparity regarding outcomes after revascularization for STEMI. Indeed, significant sex differences still exist with higher rate of in-hospital complications, in-hospital mortality, and cardiac death at short follow-up in women undergoing PCI [78-80]. In this regard, the OCTAVIA (Optical Coherence Tomography Assessment of gender diVersity In primary Angioplasty) study (ClinicalTrials.gov Identifier: NCT01377207) will combine quantitative coronary angiography, OCT, and histopathologic analyses performed by international core labs to compare the disease’s biological mechanisms and responses to treatment in men and women. One of the greatest promises of OCT comes from the fact that it could also be used during PCI in complex procedures or even to customise long-term dual anti-platelet therapy to specific needs for each patient following stent implantation. In this case, large-scale studies are required to evaluate repeatability and reliability for specific conditions, such as ACS, thrombosis and others, to determine the most suitable applications for this technique. Following successful validation of the technology, the transition from research tool to routine procedure would need further safety evaluation and the establishment of standard measurements to be able to compare between patients. Furthermore, strict guidelines need to be developed to define under what conditions it would be beneficial to use, or when it would be more advantageous to select an alternative method [81, 82]. Ultimately, OCT can only be defined as a relevant procedure in interventional cardiology when a link between this technique and improved clinical outcomes in patients with coronary conditions can be established. In addition, the potential still present in its use in research studies is immense, from understanding plaque morphology to being able to predict vascular responses to stent implantation. The potential future applications of this technique in specific niches are also beginning to be explored. One of these areas is the assessment of cardiac allograft vasculopathy (CAV) late after heart transplantation. In this regard, it has been recently shown that OCT can provide a detailed assessment of the coronary artery wall and early morphologic changes that occur after cardiac transplantation [83]. Although the diagnosis of CAV remains challenging because of the diffuse nature of the disease and the presence of cardiac denervation, preliminary evidence seems to suggest that OCT may be more sensitive for its early detection as compared with IVUS [84]. Finally, it should be emphasized that several technological advances are currently under active investigation for intravascular OCT [85]. Such future modifications are expected to yield improved imaging times and image quality, ultimately facilitating the practical translation of OCT from research protocols to everyday clinical practice.
ACKNOWLEDGEMENTS
All images reported in this paper were obtained using a LightLab's C7-XR FD-OCT imaging system (LightLab Imaging/St. Jude Medical Italia S.p.A., Agrate Brianza, Italy).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Prati F, Regar E, Mintz GS , et al. Expert review on methodology. terminoogy.and clinical applications of optical coherence tomography physical principles., methodology of immune acquisition., and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31: 401–15. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 2.Barlis P, Schmitt JM. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention. 2009;4:529–34. doi: 10.4244/eijv4i4a89. [DOI] [PubMed] [Google Scholar]
- 3.Tearney GJ, Regar E, Akasaka T , et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT).Consensus standards for acquisiion.measurement., and reporting of intravascular optical coherence tomography studies a report from the International Working Group for Intravascular Optical Co herence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki Y, Kitabata H, Tsujioka H , et al. Comparison of contrast media and low-molecular-weight dextran for frequency-domain optical coherence tomography. Circ J. 2012;76:922–7. doi: 10.1253/circj.cj-11-1122. [DOI] [PubMed] [Google Scholar]
- 5.Takarada S, Imanishi T, Liu Y , et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–6. doi: 10.1002/ccd.22273. [DOI] [PubMed] [Google Scholar]
- 6.Yoon JH, Di Vito L, Moses JW , et al. Feasibility and safety of the second-generation. frequency domain optical coherence tomography (FD-OCT): a multicenter study. J Invasive Cardiol. 2012;24:206–9. [PubMed] [Google Scholar]
- 7.Fedele S, Biondi-Zoccai G, Kwiatkowski P , et al. Reproducibility of Coronary Optical Coherence Tomography for Lumen and Length Measurements in Humans (The CLI-VAR [Centro per la Lotta contro l'Infarto-VARiability] Study). Am J Cardiol. 2012;110:1106–12. doi: 10.1016/j.amjcard.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalo N, Serruys PW, Okamura T , et al. Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart. 2009;95:1913–9. doi: 10.1136/hrt.2009.172072. [DOI] [PubMed] [Google Scholar]
- 9.Yabushita H, Bouma BE, Houser SL , et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 10.Sawada T, Shite J, Garcia-Garcia HM , et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–46. doi: 10.1093/eurheartj/ehn132. [DOI] [PubMed] [Google Scholar]
- 11.Secco GG, Foin N, Viceconte N , et al. Optical coherence tomography for guidance of treatment of instent restenosis with cutting balloons. EuroIntervention. 2011;7:828–34. doi: 10.4244/EIJV7I7A130. [DOI] [PubMed] [Google Scholar]
- 12.Viceconte N, Chan PH, Barrero EA , et al. Frequency domain optical coherence tomography for guidance of coronary stenting. Int J Cardiol. 2013;166:722–8. doi: 10.1016/j.ijcard.2011.11.090. [DOI] [PubMed] [Google Scholar]
- 13.Barlis P, Serruys PW, Gonzalo N , et al. Assessment of culprit and remote coronary narrowings using optical coherence tomography with long-term outcomes. Am J Car-diol. 2008;102:391–5. doi: 10.1016/j.amjcard.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 14.Kume T, Okura H, Yamada R , et al. Frequency and Spatial Distribution of Thin-Cap Fibroatheroma Assessed by 3-Vessel Intravascular Ultrasound and Optical Coherence Tomography An Ex Vivo Validation and an Initial In vivo Feasibility Study. Circ J. 2009;73:1086–91. doi: 10.1253/circj.cj-08-0733. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Chamie D, Bezerra HG , et al. Volumetric quantification of fibrous caps using intravascular optical coherence tomography. Biomed Opt Express. 2012;3:1413–26. doi: 10.1364/BOE.3.001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian J, Hou J, Xing L , et al. Significance of intraplaque neovascularisation for vulnerability: optical coherence tomography study. Heart. 2012;98:1504–9. doi: 10.1136/heartjnl-2012-302445. [DOI] [PubMed] [Google Scholar]
- 17.Kume T, Akasaka T, Kawamoto T , et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–7. doi: 10.1016/j.amjcard.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Hong C, Wang W, Nan-Shan Z , et al. Using optical coherence tomography to detect peripheral pulmonary thrombi. Chin Med J. 2012;125:3171–4. [PubMed] [Google Scholar]
- 19.Yamada R, Okura H, Kume T , et al. Relationship Between Arterial and Fibrous Cap Remodeling A Serial Three-Vessel Intravascular Ultrasound and Optical Coherence Tomography Study. Circ Cardiovasc Interv. 2010;3:484–90. doi: 10.1161/CIRCINTERVENTIONS.109.928911. [DOI] [PubMed] [Google Scholar]
- 20.Manfrini O, Mont E, Leone O , et al. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2006;98:156–9. doi: 10.1016/j.amjcard.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 21.van Soest G, Goderie TP, Gonzalo N , et al. Imaging atherosclerotic plaque composition with intracoronary optical coherence tomography. Neth Heart J. 2009;17:448–50. doi: 10.1007/BF03086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo T, Imanishi T, Takarada S , et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with in-travascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–9. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 23.Kume T, Okura H, Yamada R , et al. Frequency and spatial distribution of thin-cap fibroatheroma assessed by 3-vessel intravascular ultrasound and optical coherence tomography: an ex vivo validation and an initial in vivo feasibility study. Circ J. 2009;73:1086–91. doi: 10.1253/circj.cj-08-0733. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T, Ino Y, Tanimoto T , et al. Optical coherence tomography imaging in acute coronary syndromes. Cardiol Res Pract. 2011;2011:312978. doi: 10.4061/2011/312978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terashima M, Kaneda H, Suzuki T. The role of optical coherence tomography in coronary intervention. Korean J Intern Med. 2012;27:1–12. doi: 10.3904/kjim.2012.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yonetsu T, Suh W, Abtahian F , et al. Comparison of near-infrared spectroscopy and optical coherence tomography for detection of lipid. Catheter Cardiovasc Interv. 2013 doi: 10.1002/ccd.25084. [DOI] [PubMed] [Google Scholar]
- 27.Jaguszewski M, Klingenberg R, Landmesser U. Intracoronary near-infrared spectroscopy (NIRS) imaging for detection of lipid content of coronary plaques: current experi-ence and future perspectives. Curr Cardiovasc Imaging Rep. 2013;6:426–30. doi: 10.1007/s12410-013-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung W, Boppart SA. Optical coherence tomography for rapid tissue screening and directed histological sectioning. Stud Health Technol Inform. 2013;185:109–28. [PubMed] [Google Scholar]
- 29.Schultz C, van der Ent M, Serruys PW, Regar E. Optical coherence tomography to guide treatment of chronic occlusions?. JACC Cardiovasc Interv. 2009;2:366–7. doi: 10.1016/j.jcin.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Farooq V, Serruys PW, Heo JH , et al. New insights into the coronary artery bifurcation hypothesis generating concepts utilizing 3-dimensional optical frequency domain imaging. JACC Cardiovasc Interv. 2011;4:921–31. doi: 10.1016/j.jcin.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Prati F, Di Vito L, Biondi-Zoccai G , et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823–9. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 32.Okamura T, Yamada J, Nao T , et al. Three-dimensional optical coherence tomography assessment of coronary wire re-crossing position during bifurcation stenting. EuroInter-vention. 2011;7:886–7. doi: 10.4244/EIJV7I7A138. [DOI] [PubMed] [Google Scholar]
- 33.Kawamori H, Shite J, Shinke T , et al. The ability of optical coherence tomography to monitor percutaneous coronary intervention: detailed comparison with intravascular ul-trasound. J Invasive Cardiol. 2010;22:541–5. [PubMed] [Google Scholar]
- 34.Tanigawa J, Barlis P, Dimopoulos K, Di Mario C. Optical coherence tomography to assess malapposition in overlapping drug-eluting stents. EuroIntervention. 2008;3:580–3. doi: 10.4244/eijv3i5a104. [DOI] [PubMed] [Google Scholar]
- 35.Kume T, Okura H, Miyamoto Y , et al. Natural History of Stent Edge Dissection. Tissue Protrusion and Incomplete Stent Apposition Detectable Only on Optical Coherence Tomography After Stent Implantation Preliminary Observation. Circ J. 2012;76:698–703. doi: 10.1253/circj.cj-11-0845. [DOI] [PubMed] [Google Scholar]
- 36.Terashima M, Rathore S, Suzuki Y , et al. Accuracy and reproducibility of stent-strut thickness determined by optical coherence tomography. J Invasive Cardiol. 2009;21:602–5. [PubMed] [Google Scholar]
- 37.Sawada T, Shite J, Negi N , et al. Factors that influence measurements and accurate evaluation of stent apposition by optical coherence tomography: assessment using a phantom model. Circ J. 2009;73:1841–7. doi: 10.1253/circj.cj-09-0113. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto D, Shite J, Shinke T , et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–7. doi: 10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 39.Tamburino C, La Manna A, Di Salvo ME , et al. First-in-man 1-year clinical outcomes of the Catania Coronary Stent System with Nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The LAtest Non-Thrombogenic Angioplasty stent) trial. JACC Cardiovasc Interv. 2009;2:197–204. doi: 10.1016/j.jcin.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Kubo T, Tanaka A, Kitabata H , et al. Application of optical coherence tomography in percutaneous coronary intervention. Circ J. 2012;76:2076–83. doi: 10.1253/circj.cj-12-0828. [DOI] [PubMed] [Google Scholar]
- 41.Goto I, Itoh T, Kimura T , et al. Morphological and Quantitative Analysis of Vascular Wall and Neointimal Hyperplasia After Coronary Stenting – Comparison of Bare-Metal and Sirolimus-Eluting Stents Using Optical Coherence Tomography. Circ J. 2011;75:1633–40. doi: 10.1253/circj.cj-10-1237. [DOI] [PubMed] [Google Scholar]
- 42.Capodanno D, Prati F, Pawlowsky T , et al. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in-stent tissue coverage after stent implantation. EuroIntervention. 2009;5:538–43. doi: 10.4244/eijv5i5a88. [DOI] [PubMed] [Google Scholar]
- 43.Templin C, Meyer M, Muller MF , et al. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur Heart J. 2010;31:1792–801. doi: 10.1093/eurheartj/ehq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JS, Jang IK, Fan C , et al. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc Interv. 2009;2:1240–7. doi: 10.1016/j.jcin.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Choi HH, Kim JS, Yoon DH , et al. Favorable neointimal coverage in everolimus-eluting stent at 9 months after stent implantation: comparison with sirolimus-eluting stent using optical coherence tomography. Int J Cardiovasc Imaging. 2012;28:491–7. doi: 10.1007/s10554-011-9849-7. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Chico JL, Regar E, Nuesch E , et al. Delayed coverage in malapposed and side-branch struts with respect to wellapposed struts in drug-eluting stents: in vivo assessment with optical coherence tomography. Circulation. 2011;124:612–23. doi: 10.1161/CIRCULATIONAHA.110.014514. [DOI] [PubMed] [Google Scholar]
- 47.Zhu-hua Y, Matsubara T, Inada T , et al. Neointimal coverage of sirolimus-eluting stents 6 months and 12 months after implantation: evaluation by optical coherence tomogra-phy. Chin Med J. 2008;121:503–7. [PubMed] [Google Scholar]
- 48.Sim DS, Jeong MH, Ahn Y , et al. Effectiveness of drug-eluting stents versus bare-metal stents in large coronary arteries in patients with acute myocardial infarction. J Korean Med Sci. 2011;26:521–7. doi: 10.3346/jkms.2011.26.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Räber L, Baumgartner S, Garcia HM , et al. Long-term vascular healing in response to sirolimus- and Paclitaxel-eluting stents: an optical coherence tomography study. JACC Cardiovasc Interv. 2012;5:946–57. doi: 10.1016/j.jcin.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Chen SL, Xu B, Mintz G , et al. Clinical outcome after management of unprotected left main in-stent restenosis after bare metal or drug-eluting stents. Chin Med J. 2010;123:794–9. [PubMed] [Google Scholar]
- 51.Guagliumi G, Sirbu V, Bezerra H , et al. Strut coverage and vessel wall response to zotarolimus-eluting and bare-metal stents implanted in patients with ST-segment elevation myocardial infarction: the OCTAMI (Optical Coherence Tomography in Acute Myocardial Infarction) Study. JACC Cardiovasc Interv. 2010;3:680–7. doi: 10.1016/j.jcin.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 52.La Manna A, Prati F, Capodanno D , et al. Head-to-head comparison of early vessel healing by optical coherence tomography after implantation of different stents in the same patient. J Cardiovasc Med (Hagerstown) 2011;12:328–33. doi: 10.2459/JCM.0b013e3283406428. [DOI] [PubMed] [Google Scholar]
- 53.Finn AV, Joner M, Nakazawa G , et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–41. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 54.Guagliumi G, Sirbu V, Musumeci G , et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012;5:12–20. doi: 10.1016/j.jcin.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Alfonso F, Dutary J, Paulo M , et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart. 2012;98:1213–20. doi: 10.1136/heartjnl-2012-302183. [DOI] [PubMed] [Google Scholar]
- 56.Habara M, Terashima M, Nasu K , et al. Morphological differences of tissue characteristics between early.ate.and very late restenosis lesions after first generation drug-eluting stent implantation an optical coherence tomography study. Eur Heart J Cardiovasc Imaging . 2013;14: 276–84. doi: 10.1093/ehjci/jes183. [DOI] [PubMed] [Google Scholar]
- 57.Habara M, Terashima M, Nasu K , et al. Difference of tissue characteristics between early and very late restenosis lesions after bare-metal stent implantation: an optical co-herence tomography study. Circ Cardiovasc Interv. 2011;4:232–8. doi: 10.1161/CIRCINTERVENTIONS.110.959999. [DOI] [PubMed] [Google Scholar]
- 58.Kim BK, Kim JS, Shin DH , et al. Optical coherence tomography evaluation of in-stent restenotic lesions with visible microvessels. J Invasive Cardiol. 2012;24:116–20. [PubMed] [Google Scholar]
- 59.Byrne RA, Joner M, Tada T , et al. Restenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopathology and optical intravascular imaging. Minerva Cardioangiol. 2012;60:473–89. [PubMed] [Google Scholar]
- 60.Nakazawa G, Otsuka F, Nakano M , et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–22. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yonetsu T, Kato K, Kim SJ , et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. 2012;5:660–6. doi: 10.1161/CIRCIMAGING.112.976167. [DOI] [PubMed] [Google Scholar]
- 62.Kang SJ, Mintz GS, Akasaka T , et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954–63. doi: 10.1161/CIRCULATIONAHA.110.988436. [DOI] [PubMed] [Google Scholar]
- 63.van Soest G, Goderie T, Regar E , et al. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt. 2010;15:011105. doi: 10.1117/1.3280271. [DOI] [PubMed] [Google Scholar]
- 64.Waksman R, Pakala R. Biodegradable and bioabsorbable stents. Curr Pharm Des. 2010;16:4041–51. doi: 10.2174/138161210794454905. [DOI] [PubMed] [Google Scholar]
- 65.Di Mario C, Borgia F. Assimilating the current clinical data of fully bioabsorbable stents. EuroIntervention. 2009;5(Suppl F ):F103–8. doi: 10.4244/EIJV5IFA18. [DOI] [PubMed] [Google Scholar]
- 66.Tearney GJ, Bouma BE. Shedding light on bioabsorbable stent struts seen by optical coherence tomography in the ABSORB trial. Circulation. 2010;122:2234–5. doi: 10.1161/CIRCULATIONAHA.110.980730. [DOI] [PubMed] [Google Scholar]
- 67.Slottow TL, Pakala R, Okabe T , et al. Optical coherence tomography and intravascular ultrasound imaging of bioabsorbable magnesium stent degradation in porcine coronary arteries. Cardiovasc Revasc Med. 2008;9:248–54. doi: 10.1016/j.carrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Serruys PW, Ormiston JA, Onuma Y , et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 69.Attizzani GF, Bezerra HG, Ormiston J , et al. Serial assessment by optical coherence tomography of early and late vascular responses after implantation of an absorbable-coating Sirolimus-Eluting stent (from the first-in-human DESSOLVE I trial). Am J Cardiol. 2013;112:1557–64. doi: 10.1016/j.amjcard.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 70.Onuma Y, Serruys PW, Perkins LEetal. Intracoronary optical coherence tomography and histology at 1 month and 2 3 and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. 2010;122:2288–300. doi: 10.1161/CIRCULATIONAHA.109.921528. [DOI] [PubMed] [Google Scholar]
- 71.Mattesini A, Pighi M, Konstantinidis N , et al. Optical coherence tomography in bioabsorbable stents: mechanism of vascular response and guidance of stent implantation. Minerva Cardioangiol. 2014;62:71–82. [PubMed] [Google Scholar]
- 72.Attizzani GF, Bezerra HG, Chamié D , et al. Serial evaluation of vascular response after implantation of a new sirolimus-eluting stent with bioabsorbable polymer (MISTENT): an optical coherence tomography and histopathological study. J Invasive Cardiol. 2012;24:560–8. [PubMed] [Google Scholar]
- 73.Okamura T, Onuma Y, García-García HM , et al. 3-Dimensional optical coherence tomography assessment of jailed side branches by bioresorbable vascular scaffolds: a proposal for classification. JACC Cardiovasc Interv. 2010;3:836–44. doi: 10.1016/j.jcin.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y, Liu X, Kang JU. Real-time 3D and 4D Fourier domain Doppler optical coherence tomography based on dual graphics processing units. Biomed Opt Express. 2012;3:2162–74. doi: 10.1364/BOE.3.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guagliumi G, Sirbu V, Musumeci G , et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012;5:12–20. doi: 10.1016/j.jcin.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 76.Guagliumi G, Bezerra HG, Sirbu V , et al. Serial assessment of coronary artery response to paclitaxel-eluting stents using optical coherence tomography. Circ Cardiovasc Interv. 2012;5:30–8. doi: 10.1161/CIRCINTERVENTIONS.111.965582. [DOI] [PubMed] [Google Scholar]
- 77.Guagliumi G, Sirbu V, Musumeci G , et al. Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI). Circ Cardiovasc Interv. 2010;3:367–75. doi: 10.1161/CIRCINTERVENTIONS.110.950154. [DOI] [PubMed] [Google Scholar]
- 78.Akhter N, Milford-Beland S, Roe MT , et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J. 2009;157:141–8. doi: 10.1016/j.ahj.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Roncalli J, Elbaz M, Dumonteil N , et al. Gender disparity in 48-hour mortality is limited to emergency percutaneous coronary intervention for ST-elevation myocardial infarction. Arch Cardiovasc Dis. 2010;103:293–301. doi: 10.1016/j.acvd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Benamer H, Tafflet M, Bataille S , et al. Female gender is an independent predictor of in-hospital mortality after STEMI in the era of primary PCI: insights from the greater Paris area PCI Registry. EuroIntervention. 2011;6:1073–9. doi: 10.4244/EIJV6I9A187. [DOI] [PubMed] [Google Scholar]
- 81.Tearney GJ, Regar E, Akasaka T , et al. Consensus standards for acquisition. measureent.and reporting of intravascular optical coherence tomography studies a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012; 59: 1058–72. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 82.Prati F, Guagliumi G, Mintz GS , et al. Expert review document part 2: methodology. terminology and clinical applications of optical coherence tomography for the assess-ment of interventional procedures. . Eur Heart J. 2012;33:2513–20. doi: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khandhar SJ, Yamamoto H, Teuteberg JJ , et al. Optical coherence tomography for characterization of cardiac allograft vasculopathy after heart transplantation (OCTCAV study). J Heart Lung Transplant. 2013;32:596–602. doi: 10.1016/j.healun.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Hou J, Lv H, Jia H , et al. OCT assessment of allograft vasculopathy in heart transplant recipients. JACC Cardiovasc Imaging. 2012;5:662–3. doi: 10.1016/j.jcmg.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 85.Yonetsu T, Bouma BE, Kato K , et al. Optical coherence tomography– 15 years in cardiology. Circ J. 2013;77:1933–40. doi: 10.1253/circj.cj-13-0643.1. [DOI] [PubMed] [Google Scholar]