Abstract
The facultative human pathogen Vibrio cholerae, the causative agent of the severe secretory diarrheal disease cholera, persists in its aquatic reservoirs in biofilms during interepidemic periods. Biofilm is a likely form in which clinically relevant V. cholerae is taken up by humans, providing an infective dose. Thus, a better understanding of biofilm formation of V. cholerae is relevant for the ecology and epidemiology of cholera as well as a target to control the disease. Most previous studies have investigated static biofilms of V. cholerae and elucidated structural prerequisites like flagella, pili and a biofilm matrix including extracellular DNA, numerous matrix proteins and exopolysaccharide, as well as the involvement of regulatory pathways like two-component systems, quorum sensing and c-di-GMP signaling. However, aquatic environments are more likely to reflect an open, dynamic system. Hence, we used a biofilm system with constant medium flow and a temporal controlled reporter-system of transcription to identify genes induced during dynamic biofilm formation. We identified genes known or predicted to be involved in c-di-GMP signaling, motility and chemotaxis, metabolism, and transport. Subsequent phenotypic characterization of mutants with independent mutations in candidate dynamic biofilm-induced genes revealed novel insights into the physiology of static and dynamic biofilm conditions. The results of this study also reinforce the hypotheses that distinct differences in regulatory mechanisms governing biofilm development are present under dynamic conditions compared to static conditions.
Keywords: Cholera, Biofilm, Dynamic, Flow cell, c-di-GMP
Introduction
Cholera remains a significant public health problem with over 250,000 cases from the Americas, Asia, Europe and Oceania as reported by the WHO in 2012 (WHO, 2013). This severe watery diarrheal disease is caused by the facultative human pathogen V. cholerae (Koch, 1884). The lifecycle of the clinically relevant V. cholerae isolates O1 and O139 is characterized by transitions between two very different habitats, the aquatic ecosystem and the human gastrointestinal tract (Nelson et al., 2009; Sack et al., 2004; Schild et al., 2008). In the aquatic environment, attachment of V. cholerae to zoo- and phytoplankton, algae, crustaceans and insects (Colwell, 1996; Huq et al., 1990; Tamplin et al., 1990) and the formation of matrix-enclosed communities called biofilms serve as survival strategies during interepidemic periods (Lutz et al., 2013; Reidl and Klose, 2002; Yildiz and Visick, 2009).
In the majority of cases, V. cholerae is transmitted to the human host by the ingestion of contaminated water or food in areas without proper water treatment or sewage disposal systems. Biofilms are a likely form in which clinically relevant V. cholerae is taken up by humans, providing a concentrated infective dose (Colwell et al., 2003; Hall-Stoodley and Stoodley, 2005; Huo et al., 1996). In addition, biofilm derived cells have been shown to be hyperinfective, which suggests the existence of factors specifically induced during biofilm formation that facilitate infection by V. cholerae (Seper et al., 2011; Tamayo et al., 2010). Taken together these findings point toward an important role of biofilm in the ecology and epidemiology of cholera.
Biofilm formation of V. cholerae requires the actions of flagella and pili and the production of a biofilm matrix including eDNA, numerous matrix proteins and the Vibrio polysaccaride (VPS) (Absalon et al., 2011; Berk et al., 2012; Chiavelli et al., 2001; Fong et al., 2006; Fong and Yildiz, 2007; Haugo and Watnick, 2002; Meibom et al., 2004; Moorthy and Watnick, 2004, 2005; Reguera and Kolter, 2005; Seper et al., 2011; Watnick and Kolter, 1999; Watnick et al., 1999, 2001). Furthermore, it has been shown that mechanisms like two-component systems, the phosphoenolpyruvate phosphotransferase system, quorum sensing (QS) and c-di-GMP signaling are involved in biofilm regulation (Hammer and Bassler, 2003; Houot et al., 2010; Houot and Watnick, 2008; Krasteva et al., 2010; Tischler and Camilli, 2004).
The structural genes for VPS production are encoded on the vps-I (vpsA to vpsK) and vps-II operons (vpsL to vpsQ), (Berk et al., 2012; Fong et al., 2010) and are involved in cell immobilization, microcolony formation, and biofilm maturation (Absalon et al., 2011; Watnick and Kolter, 1999; Watnick et al., 2001). The expression of the vps-I and vps-II operons is repressed by HapR, the master regulator of QS at high cell density in V. cholerae (Hammer and Bassler, 2003; Waters et al., 2008; Zhu and Mekalanos, 2003). HapR also controls expression of several DGC and PDE genes and decreases the intracellular levels of c-di-GMP (Hammer and Bassler, 2009; Waters et al., 2008). Consequently, c-di-GMP levels are elevated at low cell density and reduced at high cell density (Hammer and Bassler, 2009; Krasteva et al., 2010; Srivastava et al., 2011; Waters et al., 2008).
The second messenger molecule c-di-GMP positively regulates biofilm formation through induction of the vps genes (Beyhan et al., 2006b; Tischler and Camilli, 2004) and represses motility and virulence (Krasteva et al., 2010; Liu et al., 2010; Tamayo et al., 2007; Tischler and Camilli, 2005). In the current model of the V. cholerae lifecycle, c-di-GMP levels are low during infection and high in the aquatic environment where it primarily persists in biofilms (Tamayo et al., 2007). c-di-GMP is synthesized by diguanylate cyclases (DGCs) containing a GGDEF domain and is broken down by specific phosphodiesterases (PDEs) containing either an EAL or HD-GYP domain (Ryjenkov et al., 2005; Schmidt et al., 2005). The V. cholerae genome contains 61 genes encoding predicted c-di-GMP metabolic proteins, though some may be enzymatically inactive (Galperin, 2004).
To date, most studies focused on gene regulation in V. cholerae biofilms involved mutagenesis or screens in biofilms formed under static conditions (Moorthy and Watnick, 2004, 2005; Yildiz et al., 2004; Zhu and Mekalanos, 2003). Yet some findings exist which point toward a difference in gene regulation under dynamic biofilm conditions. Beyhan et al. reported that in a once-through flow cell system, the structures of RvpsT biofilms differed greatly from the ones formed under non-flow conditions (Beyhan et al., 2007). In addition, Yildiz et al. observed a drastic difference between smooth and rugose biofilms under static conditions, but well developed smooth and rugose biofilms under dynamic flow conditions (Yildiz et al., 2004). A recent study by Müller et al. reported a hydrodynamically grown V. cholerae biofilm form, independent of vpsA and luxO and a rpoS-dependent dissolution of hydrodynamic biofilms (Muller et al., 2007).
Taken together these findings suggest that in a dynamic system, where fresh nutrients are continuously provided and waste materials and extracellular signaling molecules are removed, a limited accumulation of QS signals may occur leading to different regulation or importance of genes. Furthermore, dynamic flow conditions are closer to the environmental situation than a closed static system. To our knowledge, so far no study has focused mainly on gene induction and regulation during dynamic biofilm development of V. cholerae.
Reporter gene systems are useful tools to explore gene expression in heterogeneous environments like biofilms (An and Parsek, 2007; Beloin and Ghigo, 2005; Stewart and Franklin, 2008). In this study we used a modified version of the recombination-based in vivo expression technology (RIVET) to identify genes induced during dynamic biofilm formation of V. cholerae O1 El Tor, the serotype that remains the dominant cause of cholera. RIVET resembles a temporally controlled reporter system allowing the detection of gene induction based on TnpR resolvase-mediated excision of a reporter gene cassette flanked by res sites (res-cassette) and was originally designed to identify genes induced during infection and successfully used in a variety of studies (Camilli and Mekalanos, 1995; Lee et al., 1998, 1999; Osorio et al., 2005; Schild et al., 2007). This technology is capable of detecting gene induction in sub-populations according to their spatial and temporal expression and could therefore reveal novel insights into biofilm physiology (Angelichio et al., 1999, 2004; Schild et al., 2007). Upon induction of a gene transcriptionally fused with the promotorless tnpR, the resolvase is produced, leading to excision of the res-cassette containing the two selectable marker genes neo and sacB, which confer KmR and SucS, respectively. Hence, the induction of a gene can be monitored by a change to KmS and SucR. To gain insight into biofilm formation under dynamic conditions, we applied RIVET to a dynamic biofilm setup (Fig. 1). Herein, this modified version will be consequently referred to as recombination-based in biofilm expression technology (RIBET). This dynamic biofilm screen allowed us to identify genes specifically induced under this condition and validate their induction in a static and dynamic biofilm system. We identified genes involved in c-di-GMP signaling, motility and chemotaxis, metabolism, and transport, as well as hypothetical genes. Subsequent mutagenesis of interesting candidates, their phenotypic characterization and comparison under static and dynamic biofilm conditions revealed insights into the importance of these genes in differently grown biofilms.
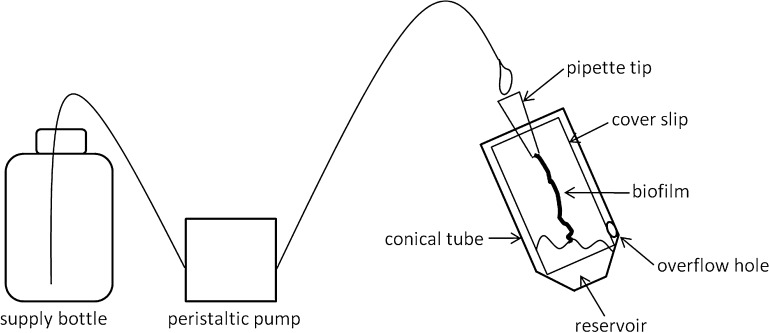
Fig. 1.
Setup of the dynamic biofilm system. The supply bottle filled with fresh LB-Sm broth is connected via silicon tubing to a 50 ml conical tube, which is fixed in a 45° angle on a metal stand. The conical tube contains a cover slip with a sterile P1000 pipette tip glued on to it. A suspension of a pIVET5n library pool is added to the conical tube and serves as reservoir for biofilm formation. By the action of a peristaltic pump, medium is pumped out of the supply bottle into the sterile P1000 pipette tip, flowing down the cover slip into the reservoir. An overflow hole at the 5 ml mark of the conical tube allows constant draining of the excess medium and keeps the reservoir volume constant. Biofilm is formed along the track of the continuous medium flow on the cover slip between the P1000 tip and the reservoir. For more details refer to section “Materials and methods”.
Materials and methods
Bacterial strains, culture conditions and supplements
V. cholerae AC53 was used as the wild type (WT) strain and is a spontaneous streptomycin-resistant (SmR) derivative of the O1 El Tor Ogawa clinical isolate E7946 (Miller et al., 1989). All V. cholerae mutant strains used in this study are derivatives of AC53. E. coli strains DH5αλpir and SM10λpir were used for genetic manipulations. Strains and plasmids used for this study are listed in Table S1. Unless stated otherwise strains were grown in LB broth with aeration at 37 °C or for biofilm formation under static or dynamic conditions at room temperature (RT). If required, antibiotics and other supplements were used in the following final concentrations: streptomycin 100 μg/ml (Sm); ampicillin (Ap) 100 μg/ml or 50 μg/ml in combination with other antibiotics; kanamycin (Km) 50 μg/ml; isopropyl-β-thiogalactopyranoside (IPTG) 0.5 mM; glucose (Gluc) 0.2%; sucrose (Suc) 10%; 5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside (X-Gal) 30 μg/ml.
Construction of in-frame deletion mutants, expression plasmids and phoA fusions
DNA manipulations including purification of chromosomal, plasmid or PCR product DNA, PCRs, and construction of in-frame deletion mutants, expression plasmids and chromosomal transcriptional phoA fusions were carried out as described previously using derivatives of pCVD442, pGP704 or pMMB67EH (Seper et al., 2011). GFP expressing strains were generated by insertion of gfp in the lacZ locus using the suized plasmid pJZ111. Oligonucleotides used for in-frame deletion mutagenesis are labeled as gene_restriction enzyme_1–4 and oligonucleotides for expression plasmid construction are labeled as gene_restriction enzyme_fw and_rv. In addition, for construction of expression plasmids for N- or C-terminally His-tagged proteins the oligonucleotides VCA0785_BamHI_fw and VCA0785_XmaI_rv as well as VC2750_NcoI_fw and VC2750_BamHI_rv were used in combination with pQE-30 or pQE-60, respectively. All oligonucleotides are listed in Table S2.
Screening for in biofilm induced (ibi) genes
A slightly adapted version of the recombination-based in vivo expression technology (RIVET) in combination with a library of 12,000 random transcriptional gene-tnpR fusions (Camilli et al., 1994; Schild et al., 2007), was used to identify genes induced during biofilm formation, which was consequently renamed recombination-based in biofilm expression technology (RIBET). An aliquot of each mutant pool of the library was grown over night at 37 °C on LB-Sm/Ap/Km agar plates, suspended in LB-Km broth, adjusted to an OD600 of 2 and used to inoculate the reservoir in the dynamic biofilm system. To ensure that the gene-tnpR fusions that we later identify as induced in the biofilm do not originate from genes induced in the planktonic phase of the reservoir, but in the process of biofilm formation, we used Km in the reservoir. Seven ml of the inoculum was added to a 50 ml conical tube, which was fixed on a metal stand with a 45° angle, see Fig. 1. As diagramed, a cover slip (borosilicate) with a sterile P1000 pipette tip glued to one end was then put in the conical tube. After a 1 h adaptation phase, LB-Sm was pumped through silicon tubing (1.5 mm × 3 mm, VWR) with a peristaltic pump (Watson-Marlow 205S) from the supply bottle into the 1 ml tip (2 rpm, equivalent to a flow rate of approximately 10 ml/h). A hole (1 cm wide and 2 cm high) punched into the conical tube at the 5 ml mark allowed constant draining of the overflow medium and consequently kept the reservoir volume constant. Visible biofilm formed on the cover slip along the continuous medium flow during a 24 h period of incubation at RT. To remove unadhered cells, the flow rate was turned to maximum for 5 s before the biofilm was taken. For each mutant pool of the library, the dynamic biofilm formation procedure was repeated three times independently. At 1–2 cm above the reservoir, approximately 1 cm length of biofilm was removed from the cover slip and suspended in LB. Serial dilutions were plated on Sm/Suc agar plates (1% tryptone; 0.5% yeast extract; 1.6% agar) to select for resolved strains. After 2 days incubation at RT, colonies were picked, patched in parallel on LB-Sm/Ap and LB-Sm/Km agar plates and incubated over night at 37 °C. ApR and KmS clones were screened for diverse gene-tnpR fusions via different PCR product sizes using the RIBET oligonucleotides IVET-1 and IVET-2 as described previously (Lombardo et al., 2007). The plasmids containing the gene-tnpR fusion (pIVETs) of five to eight different resolved strains were recovered (Qiagen Spin Miniprep Kit) and aliquots were sent to Agowa Genomics for sequencing with the RIBET oligonucleotides IVET-3 as previously described (Lombardo et al., 2007). Sequences were compared to the V. cholerae N16961 genome database TIGR with blastN (http://blast.jcvi.org/cmr-blast/). Gene annotations in Table S3 are those determined by TIGR and updated by using KEGG (Kanehisa et al., 2006). Transcriptional fusions to any annotated ORF within which tnpR had inserted in the same orientation were considered as described previously (Schild et al., 2007).
Reconstruction of fusion strains and determination of the resolution frequency
The reconstruction of unresolved V. cholerae fusion strains was carried out as described previously (Osorio et al., 2005). For all resolution frequency quantifications, strains were grown over night on LB-Sm/Ap/Km agar plates and suspended in LB broth. To determine the in vitro resolution frequency (control condition), 4 ml LB-Sm were inoculated with gene-tnpR fusion strains at an OD600 of 0.01 and incubated for 8 h at 37 °C with shaking (180 rpm). The resolution frequency under dynamic biofilm conditions was determined by growing biofilm for 24 h using the dynamic biofilm system and suspending the biofilm in LB as described above. For resolution frequency determination under static biofilm conditions, a 96 well plate was inoculated with 150 μl LB-Sm/well at an OD600 of 0.004 and statically grown for 48 h at RT. The wells were washed two times with LB-Sm broth to remove planktonic cells, and biofilm was suspended in LB broth. The resolution frequencies in vitro as well as under dynamic and static biofilm conditions were determined by plating serial dilutions on LB-Sm/Ap and LB-Sm/Km agar plates. The resolution frequency is expressed in % calculated as follows: (Sm/Ap CFU minus Sm/Km CFU) divided by Sm/Ap CFU times 100, as described previously (Schild et al., 2007).
Static biofilm assay
Static biofilms in microtiter plates were assayed by crystal violet staining essentially as previously published (Seper et al., 2011), with some modifications. Briefly, the respective strains were grown over night on LB-Sm or LB-Ap/Gluc agar plates (for plasmid containing strains), suspended in LB-Sm or LB-Ap/IPTG (for plasmid containing strains), adjusted to an OD600 0.02 and inoculated in a 96 well microtiter plate (U bottom, Sterilin) for 24, 48 or 72 h at RT. Wells were subsequently rinsed using a mircoplate washer (Anthos Mikrosysteme GmbH, Fluido2), biofilm was stained with 0.1% crystal violet, solubilized in 96% ethanol and the OD595 was measured (microplate reader: BMG Labtech SPECTROstarNano) to quantify the amount of biofilm.
Dynamic flow cell biofilm formation
For visualization and quantification of dynamically formed biofilm, the three channel flow cell system using 2% LB-Sm broth (24 h, RT) was used as described previously (Seper et al., 2011). The respective GFP expressing V. cholerae strains were used for biofilm formation to allow acquisition of fluorescent images with confocal laser scanning microscopy. Unfortunately, GFP expression was strongly reduced in presence of Ap, which was used for selection of strains containing control and expression plasmids. Thus, wild type and mutants with control or expression plasmids for complementation analysis in trans were stained with SYTO 9.
SYTO 9 from the Live/Dead BacLight Bacterial Viability kit (Invitrogen) was freshly diluted 1:1000 in 2% LB-Sm broth and approximately 250 μl were injected per flow cell channel. Biofilm was stained at RT for 20 min, and images were recorded by confocal laser scanning microscopy.
Confocal laser scanning microscopy and COMSTAT analysis
Images of biofilms were acquired using a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) with spectral detection and a Leica HCX PL APO CS 63x water objective (NA 1.2). Optical sectioning was performed in 0.13 μm steps. GFP as well as SYTO 9 was excited at 488 nm, fluorescence emission was detected between 500 and 560 nm, and images were recorded without differential interference contrast (DIC) optics. For visualization and processing of image data the Leica LAF and ImageJ 1.46 software was used. Quantification and morphological analysis of image stacks was performed using the computer program COMSTAT (http://www.comstat.dk) (Heydorn et al., 2000; Vorregaard et al.).
Alkaline phosphatase assay
Alkaline phosphatase activities (expressed in Miller Units) for chromosomal vpsA–phoA transcriptional fusions were determined as described previously (Seper et al., 2011), using cultures with a starting OD600 of 0.02 grown statically at RT for approximately 8 h (final OD600 ∼0.4).
Preparation of RNA and qRT-PCR
To elucidate HapR-dependent regulation of selected ibi genes, their gene expression in WT and a ΔhapR mutant strains was measured by qRT-PCR. Cultures were inoculated in LB broth at an OD600 of 0.02 and grown statically at RT for 24 h. Bacterial RNA extraction, cDNA synthesis and qRT-PCR were performed as previously published (Seper et al., 2013). Oligonucleotides used for qRT-PCR are listed in Table S2, labeled as following: qRTPCR_gene_fw and_rv. For each sample, the mean cycle threshold of the test transcript was normalized to the housekeeping gene 16S rRNA and to one randomly selected WT reference sample.
Purification of putative PDE enzymes
Overnight cultures of Escherichia coli BL21 containing pQE-30VCA0785, pQE-60VC2750 or empty vector were diluted 1:100 into 400 ml of LB-Ap medium with 0.5 mM IPTG. The cultures were grown at 30 °C to an OD600 of ∼0.7 for VCA0785, and ∼1.0 for VC2750. The cells were then collected by centrifugation at 6000 x g for 15 min at 4 °C. The His6-tagged proteins were affinity purified from the cells using Ni-NTA resin (Qiagen) essentially as described previously. Briefly, the cells were suspended in 5 ml of His6 lysis buffer, then lysed by freeze–thaw and sonication. Lysates were clarified by centrifugation at 10,000 × g for 30 min, then the soluble fraction was incubated with 1 ml Ni2+-nitrilotriacetic acid-agarose resin (HisPure, ThermoScientific) at 4 °C for 4 h. The samples were applied using a 5-ml polypropylene column (Qiagen). The column was washed twice with His6 buffer containing 50 mM imidazole, once with His6 buffer containing 100 mM imidazole, then proteins were eluted with His6 buffer containing 250 mM imidazole. Elution fractions were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining to assess protein purity. Protein-containing fractions were dialyzed overnight in Slide-A-Lyzer 10,000 molecular weight cut-off dialysis cassettes (Pierce) against 75 mM Tris, pH 8, 25 mM KCl, 10% glycerol. Glycerol was increased to 20% for storage at −20 °C. The VieA and WspR controls were purified as described previously (Hickman et al., 2005; Tamayo et al., 2005).
Assays for enzymatic activity
To assay for c-di-GMP phosphodiesterase activity, purified proteins were incubated with 32P-labeled c-di-GMP in PDE reaction buffer (Tamayo et al., 2005) for the indicated times at 37 °C. The [32P]-c-di-GMP substrate was synthesized using the DGC WspR as described previously (Hickman et al., 2005; Purcell et al., 2012; Tamayo et al., 2005). As negative controls, mock-purified samples from E. coli containing empty vector and buffer-only samples were tested. VieA served as the positive control. To assay for diguanylate cyclase activity, purified proteins were incubated for 0 h, 1 h, 8 h and over night (ON) with [α-32P]-GTP in buffer containing 75 mM Tris, pH 8, 250 mM NaCl, 25 mM KCl, and 10 mM MgCl2, with 2 mM CaCl2 added to inhibit PDE activity. All reaction products were analyzed by thin layer chromatography and visualized by phosphorimagery as previously described (Tamayo et al., 2005).
Detachment assay
The biofilm detachment assay was carried out as described previously (Seper et al., 2011), with the following modifications. The respective strains were grown over night on LB-Sm plates, suspended in LB-Sm broth, adjusted to an OD600 0.02 and 1.5 ml were inoculated in a 15 ml Greiner-tube. Biofilm was allowed to form for 48 h under static conditions at RT. Tubes were subsequently rinsed, and 1.6 ml spent LB broth (obtained from a 48 h biofilm culture of the respective strain, after short centrifugation and filter-sterilization) was added. For each tube, a To sample was taken to determine the original CFU in the supernatant, which results either from residual planktonic cells or mechanical disturbance of the biofilm due to addition of spent LB. The detachment rate was determined after 3 h incubation at RT. The Td sample contains the cells from To as well as cells that have detached during the 3 h incubation. The remaining biofilm was mechanically dispersed and used as sample Tb. Serial dilutions were plated on LB-Sm agar plates and incubated over night at 37 °C. The detachment rate is expressed in % of detached cells in 3 h normalized to the total cell number and calculated as follows: (CFU Td minus CFU To) divided by (CFU Td plus CFU Tb) times 100.
Motility assay
Swarm plates (1% tryptone; 0.5% NaCl; 0.5% yeast extract; and 0.3% agar) were used to assess the motility of V. cholerae strains (Moisi et al., 2009). The respective strains were grown on LB agar plates over night (ON) at 37 °C, then three to four single colonies were inoculated using a sterile P20 pipette tip into swarm plates. The plates were incubated for 24 h at RT, after which time the diameters of growth were measured.
Statistical analysis
Unless stated otherwise, the data is presented as the median with interquartile range. Data were analyzed using the Mann–Whitney U test for single comparisons or the Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons, and differences were considered significant for P values of <0.05.
Results
Identification of in biofilm induced (ibi) genes
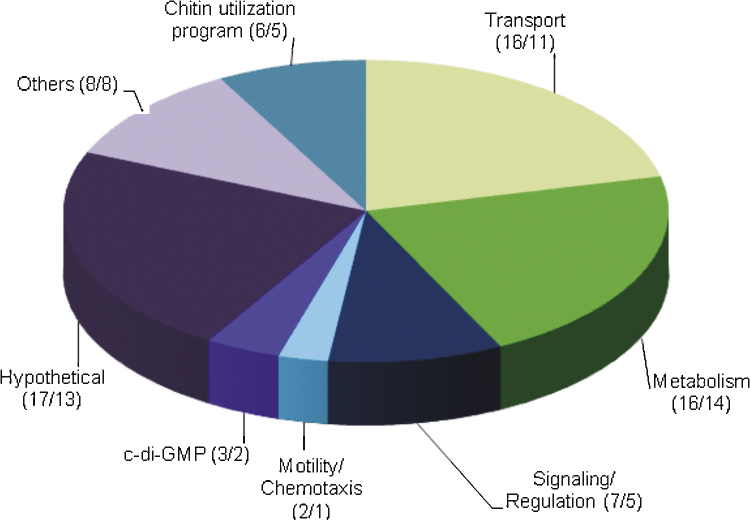
To identify genes induced during dynamic biofilm formation, we screened a library with approximately 12,000 random transcriptional gene-tnpR fusions, previously used to identify in vivo induced genes (Schild et al., 2007). We recovered and sequenced gene-tnpR fusions of ∼300 resolved strains. A total of 188 plus-stranded fusions to different genes were identified and used to reconstruct unresolved strains for the validation process. Resolution frequencies of the reconstructed strains were first determined under control conditions to eliminate false positives. Sixty-eight showed a resolution frequency of over 50% and were not tested under biofilm conditions to allow for at least a two-fold increase in resolution rate under the test conditions. The remaining 120 gave a resolution rate under 50%. To compare and validate gene induction, these 120 reconstructed strains were tested for their resolution frequencies under control, static and dynamic biofilm conditions (see section “Materials and methods” for details). A strain with a ∼2-fold or higher resolution frequency compared to control conditions was considered to be induced during biofilm formation. This was achieved for 75 fusions under dynamic biofilm conditions; 59 of these were also induced under static biofilm conditions. The median resolution frequency under control, static biofilm and dynamic biofilm conditions can be found in the supplementary material in Table S3. Fig. 2 provides an overview of the ibi genes divided in functional groups: 17 of the ibi genes are hypothetical, 6 are part of the chitin utilization program, 3 are involved in c-di-GMP metabolism, 2 are associated with motility and chemotaxis, 7 are predicted to be involved in signaling and regulatory pathways, 16 are implicated in metabolism, 16 in transport, and 8 do not fit in one of these groups and are therefore named ‘others’. To determine the role of some of these ibi genes in static and dynamic biofilm formation, we constructed in-frame deletions of 31 selected ibi genes for phenotypic characterization.
Fig. 2.
Functional distribution of in biofilm induced (ibi) genes. Shown are validated ibi genes identified with the RIBET screening technology, allocated in functional groups by their proposed function according to KEGG (Kanehisa et al., 2006). The number of ibi genes in the respective group is indicated in parenthesis as (X/Y), whereby X reflects the number of genes induced under dynamic biofilm formation and Y the number of genes also induced under static conditions. Overall, 75 genes could be validated to be induced during dynamic biofilm formation; 59 of them are also induced during static biofilm conditions.
Role of ibi genes in static biofilm formation
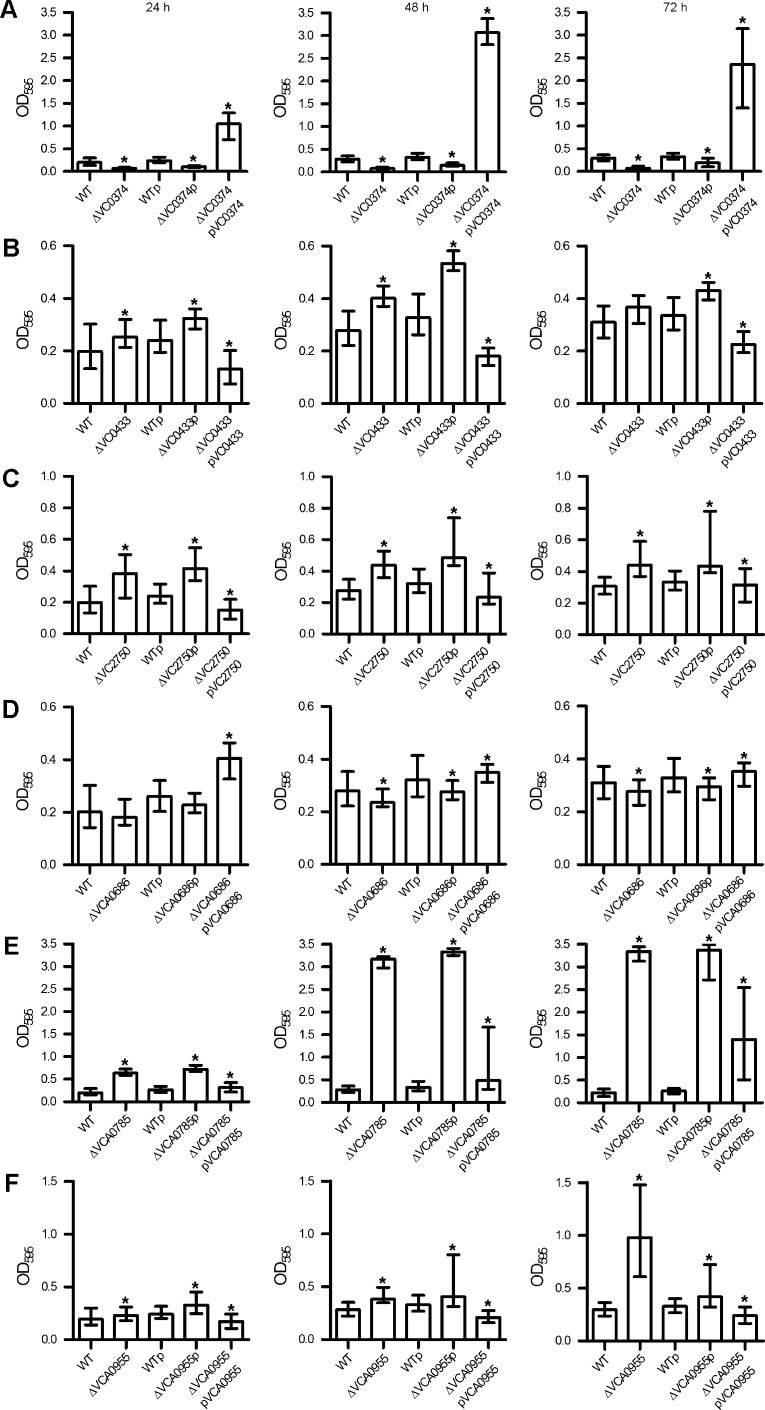
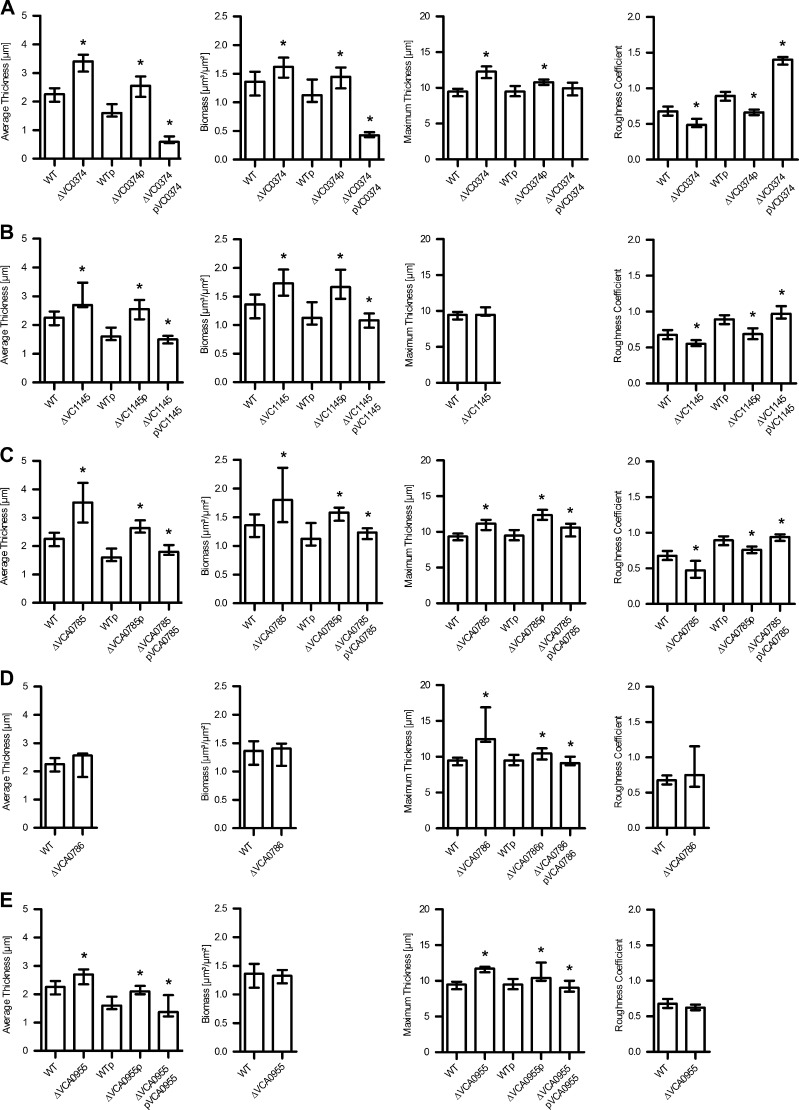
First, the constructed deletion mutants were analyzed for their biofilm formation capacity under static conditions. Therefore, a static biofilm assay in microtiter plates with crystal violet staining was used (Seper et al., 2011). The biofilm amount was quantified after 24, 48 and 72 h to allow a comprehensive phenotypic characterization. An initial screening revealed that six mutants exhibited distinct differences in biofilm formation capacity compared to wild type. These mutants were thus analyzed in independent replicates of the biofilm assay including complementation analysis (Fig. 3A–F). The ΔVC0374 and ΔVCA0686 mutants showed a decrease in biofilm production compared to wild type, which was significant at all tested time points for ΔVC0374 and at 48 h and 72 h for ΔVCA0686. VC0374 shows 77% identity to the glucose-6-phosphate isomerase of Escherichia coli K-12 and therefore may play a central role in carbon metabolism. VCA0686 is annotated as an iron(III) ABC transporter permease, but was recently characterized to be the permease of an hexose-6-phosphate uptake system of V. cholerae (Moisi et al., 2013). Deletion of ibi genes VC0433, VC2750, VCA0785 and VCA0955 resulted in increased biofilm formation, which was significantly different compared to wild type at 24 h and 48 h for ΔVC0433 and at all three time points for ΔVC2750, ΔVCA0785 and ΔVCA0955. The most pronounced phenotype was achieved by the deletion of VCA0785, which is annotated as a diguanylate cyclase. VCA0785, also known as CdgC, has been shown to modulate c-di-GMP levels, biofilm formation, motility and virulence gene expression in a rugose phase variant of V. cholerae (Lim et al., 2006, 2007; Yildiz et al., 2004). VC0433 is annotated as an arginine-ornithine antiporter; VC2750, as a GGDEF family protein; and VCA0955, as MarR family transcriptional regulator.
Fig. 3.
Deletion mutants show altered static biofilm formation compared to the wild type (WT) parental strain. Biofilms of WT, deletion mutants, and mutants and WT with control or complementation plasmids, as indicated, were quantified after 24 h, 48 h and 72 h. In detail, impact on static biofilm formation of VC0374 (A), VC0433 (B), VC2750 (C), VCA0686 (D), VCA0785 (E) and VCA0955 (E) is shown. The biofilm formation capacity was assayed under static conditions by crystal violet staining and subsequent determination of the OD595. Shown are the medians from at least eight independent measurements. The error bars indicate the interquartile range. An asterisk on top of the respective mutant data set indicates a significant difference to the WT (*P < 0.05 by Mann–Whitney U test). An asterisk on top of the mutant with control plasmid indicates a significant difference to the WT with control plasmid and an asterisk on top of the mutant with complementation plasmid indicates a significant difference to the mutant with control plasmid, respectively (*P < 0.05 by Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons).
In general, wild type biofilm levels could be restored in all tested deletion mutants by the expression of the respective gene in trans on a complementation plasmid, but not by the control plasmid alone (Fig. 3A–F).
In order to exclude that the observed differences in biofilm formation are due to altered growth phenotypes of the mutants we performed growth assays in LB broth. Growth of planktonic cells of ΔVCA0686, ΔVC0433, ΔVC2750, ΔVCA0785 and ΔVCA0955 were similar to that of wild type (data not shown). Planktonic cells of ΔVC0374 reached a final OD600 of 1.1 after 24 h, while the wild type grew to an OD600 of 1.4 (Fig. S1). Nonetheless, CFU determination of cultures grown for 24 h revealed no significant reduction in colony numbers of the mutant compared to the wild type (data not shown). Thus, the reduced biofilm formation capacity of ΔVC0374 cannot simply be explained by a growth defect.
Role of ibi genes in dynamic biofilm formation
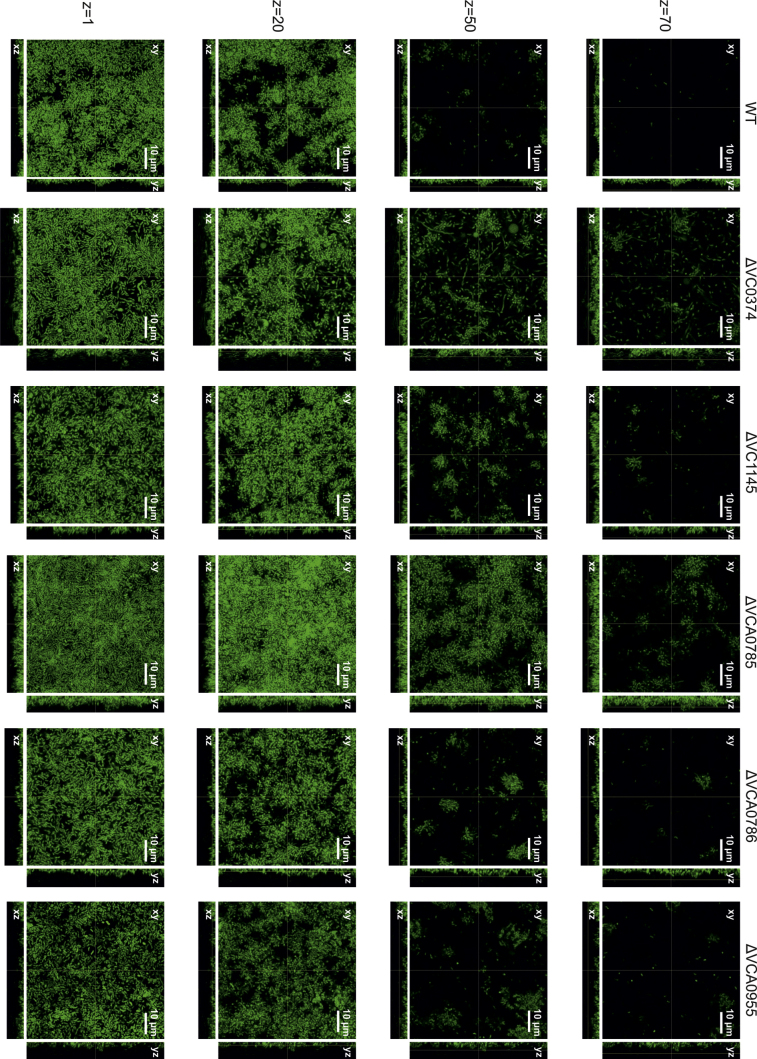
To elucidate the role of ibi genes in dynamic biofilm formation, we analyzed the biofilms of the respective deletion mutants and wild type under dynamic conditions. We used a three-channel flow cell system (Sternberg et al., 1999; Sternberg and Tolker-Nielsen, 2006) with a constant medium flow (2% LB) and quantified biofilm formation after 24 h using the COMSTAT program (http://www.comstat.dk) (Heydorn et al., 2000; Vorregaard et al.) after fluorescent image acquisition. After a first round of analysis, ibi gene mutants with distinct phenotypes as well as all mutants with significant differences in the static biofilm assay were selected for further characterization. ΔVC0433, ΔVC2750 and ΔVCA0686 showed dynamic biofilms comparable to wild type (Fig. S3), although these mutants exhibited differences in their static biofilm formation capacity (Fig. 3). A total of five ibi gene mutants showed significant alterations in their biofilm morphology under dynamic conditions compared to wild type (Figs. 4 and 5). Interestingly, ΔVC0374 showed an opposite phenotype compared to static conditions (Fig. 3A) and exhibited an increase in the average and maximum thickness as well as the biomass in the dynamically grown biofilm (Fig. 5A). The roughness coefficient, which is a measurement of the variation in thickness of a biofilm, was reduced compared to wild type, indicating a decrease in the heterogeneity of the biofilm. These changes in the structural parameters of a ΔVC0374 mutant flow cell biofilm are also visible in the fluorescent images, which in addition showed some filamentous structures that do not appear in wild type (Fig. 4). The ΔVC1145 mutant, which is a hypothetical protein also described as a putative transport protein with two predicted permease membrane regions (Kanehisa et al., 2006), showed no significant changes in static biofilm phenotype for all three time points (Fig. S2). In contrast, a change in biofilm morphology under dynamic conditions was visible and quantifiable by COMSTAT with a significant increase in average thickness and biomass as well as a decrease in the roughness coefficient (Figs. 4 and 5B). These data indicate that the ΔVC1145 mutant flow cell biofilm is denser than wild type. Deletion of VCA0785 and VCA0955 caused an increase in static (Fig. 3E and F) and dynamic biofilm formation (Figs. 4 and 5). In detail, the ΔVCA0785 mutant displayed a very dense and thick flow cell biofilm, which is consistent with a significant increase in the average and maximum thickness and biomass and a decrease in the roughness coefficient (Figs. 4 and 5C). The ΔVCA0955 mutant exhibited an increase in average and maximum thickness, but no change in biomass and roughness compared to wild type (Figs. 4 and 5E). Finally, the ibi gene mutant ΔVCA0786, annotated as a hypothetical gene, showed a significant increase in the maximum thickness of the flow cell biofilm (Figs. 4 and 5D), but had no significant phenotype under static conditions at any time point (Fig. S2). For the biofilm parameters with significant differences between wild type and respective mutant complementation analysis was performed. All phenotypes could be at least partially restored to wild type levels by the expression of the respective gene in trans, but not with the plasmid control alone.
Fig. 4.
Images of dynamically grown flow cell biofilms of mutants with altered biofilm amount or morphology compared to wild type (WT). Shown are confocal laser scanning microscopy images of GFP expressing WT and mutant biofilms as horizontal (xy) and vertical (xz and yz) projections (large and side panels, respectively). Biofilms were grown for 24 h in flow cell chambers with constant 2% LB medium flow. Large panels represent selected single optical sections through the acquired three-dimensional data sets at the indicated z position. Optical sectioning was performed in 0.13 μm steps.
Fig. 5.
COMSTAT analyses revealed significant differences in the respective parameters for dynamic mutant biofilms compared to wild type (WT). Image stacks of WT, deletion mutants, and mutants and WT with control or complementation plasmids, as indicated, were analyzed for the average thickness, the biomass, the maximum thickness and the roughness coefficient using the COMSTAT software (http://www.comstat.dk) (Heydorn et al., 2000; Vorregaard et al.). In detail, impact on dynamic biofilm formation of VC0374 (A), VC1145 (B), VCA0785 (C), VCA0786 (D) and VCA0955 (E) is shown. Shown are the medians of at least six image stacks from two independent experiments for each strain. The error bars indicate the interquartile range. An asterisk on top of the respective mutant data set indicates a significant difference to the WT (*P < 0.05 by Mann–Whitney U test). An asterisk on top of the mutant with control plasmid indicates a significant difference to the WT with control plasmid and an asterisk on top of the mutant with complementation plasmid indicates a significant difference to the mutant with control plasmid, respectively (*P < 0.05 by Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons).
Impact of ibi genes on vpsA expression and dependency on HapR regulation
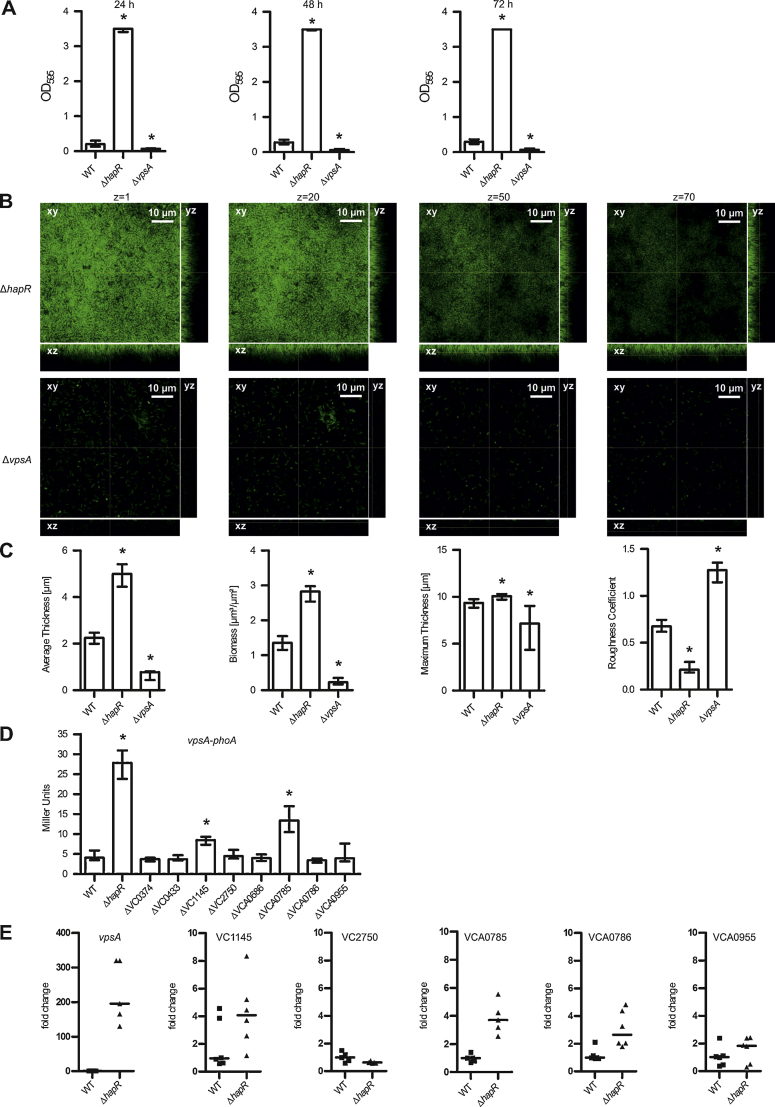
V. cholerae biofilm formation under static conditions has been shown to be dependent on vps gene expression, which synthesize the extracellular glycan-matrix. The vps genes are repressed by the QS master regulator of high-cell-density HapR, which activates and represses many target genes important for biofilm formation (Fong et al., 2010; Hammer and Bassler, 2003, 2009; Srivastava et al., 2011; Waters et al., 2008; Yildiz et al., 2004; Yildiz and Schoolnik, 1999). Recently, hydrodynamic V. cholerae biofilm formation independent of vpsA and luxO, which represent key factors for the VPS matrix and the QS cascade has been reported (Muller et al., 2007). Thus, we wanted to know whether HapR and VPS are equally important for static and dynamic biofilm formation. To elucidate the impact of HapR and VPS on biofilm formation, we used a ΔhapR and a ΔvpsA deletion mutant and assayed their biofilm formation in static and dynamic conditions as described above. The ΔvpsA mutant is VPS-deficient (Fong et al., 2010; Yildiz and Schoolnik, 1999) and was therefore chosen to test the VPS dependency of the biofilm systems. As expected and shown by others (Fong et al., 2010; Hammer and Bassler, 2003; Yildiz et al., 2004), the ΔhapR mutant exhibited a pronounced increase in biofilm amount and the ΔvpsA mutant showed essentially no biofilm formation under static conditions compared to wild type (Fig. 6A). In general, the phenotypes followed the same trends under dynamic conditions. The ΔhapR biofilm appeared dense and thick in the fluorescent images and showed a significant increase in average and maximum thickness and biomass, as well as a decrease in roughness (Fig. 6B and C). In contrast, the ΔvpsA mutant displayed no mature three-dimensional biofilm formation under dynamic conditions and only scarce events of micro-colony formation were visible (Fig. 6B). Furthermore the COMSTAT analysis revealed a decrease in average and maximum thickness as well as in biomass (Fig. 6C).
Fig. 6.
Interplay between vps gene regulation, HapR regulation and ibi genes. (A) The biofilm formation capacity of wild type (WT) and deletion mutants was quantified by crystal violet staining and subsequent determination of the OD595 after 24 h, 48 h and 72 h under static conditions. (B) Shown are confocal laser scanning microscopy images of GFP expressing ΔhapR and ΔvpsA mutant biofilms as horizontal (xy) and vertical (xz and yz) projections (large and side panels, respectively). Large panels represent selected single optical sections through the acquired three-dimensional data sets at the indicated z position. Biofilms were grown for 24 h in flow cell chambers with constant 2% LB medium flow. Images of biofilms were acquired using a Leica SP5 confocal microscope with spectral detection and a Leica HCX PL APO CS 63x water objective (NA 1.2). (C) Shown are the respective structural parameters of image stacks of WT and deletion mutants analyzed using the COMSTAT software (http://www.comstat.dk) (Heydorn et al., 2000; Vorregaard et al.). (D) Alkaline phosphatase activities (in Miller Units) were measured from 8 h statically grown cultures of indicated strains harboring a chromosomal vpsA–phoA transcriptional fusion. (A–D) Shown are the medians from at least six independent measurements. The error bars indicate the interquartile range. Significant differences between the respective data sets of the WT compared to mutants (*P < 0.05 Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons) are indicated. (E) Bacterial RNA was extracted from cultures grown statically for 24 h, reverse transcribed to cDNA and used as template for qRT-PCR analysis of the indicated genes. For each sample, the mean cycle threshold of the test transcript was normalized to the housekeeping gene 16S rRNA and to one randomly selected WT reference sample. The data are presented as medians of six independently grown samples. Significant differences compared to WT were detected for the genes vpsA, VCA0785 and VCA0786 (P < 0.05 Mann–Whitney U test).
To test whether one of the 8 ibi genes that showed a phenotypic change in static or dynamic biofilm formation affects vpsA expression, a chromosomal transcriptional vpsA–phoA fusion was introduced into the wild type and the respective mutant strains. The vpsA gene is the first gene in the vps-I locus, hence, the measured alkaline phosphatase activity reflects the transcriptional levels of vpsA. Using 8 h statically grown cultures, PhoA activity in the different strains was determined (Fig. 6D). The ΔhapR mutant served as a positive control for high vpsA expression, because it negatively regulates vps-genes (Hammer and Bassler, 2003; Yildiz et al., 2004; Zhu and Mekalanos, 2003). Consistent with these reports, vpsA–phoA expression was 6-fold increased in the ΔhapR mutant compared to wild type. Of the 8 mutants tested, the ΔVC1145 and ΔVCA0785 mutants showed a significant increase in vpsA–phoA expression compared to wild type. Thus, the increase in static and dynamic biofilm formation of ΔVCA0785 and ΔVC1145 correlates with higher vpsA expression, which consequently might lead to increased VPS production.
In addition, we analyzed whether HapR regulates the expression of selected ibi genes. To address this question, we used qRT-PCR to compare the expression of the selected genes in the wild type and ΔhapR mutant strains grown statically for 24 h (Fig. 6E). As a positive control for induction of gene expression in the absence of HapR, vpsA was used because it is repressed by HapR (Hammer and Bassler, 2003; Yildiz et al., 2004; Zhu and Mekalanos, 2003). Accordingly, vpsA expression was about 200-fold elevated in the ΔhapR mutant compared to wild type. Furthermore, VCA0785 and VCA0786 showed a significant increase in gene expression in absence of HapR. This data indicates that VCA0785 and VCA0786 are negatively regulated by HapR along with vps genes. VC1145 showed a tendency toward higher expression in the ΔhapR mutant, but also high variation of expression levels in independent wild type samples. Thus, no significant differences could be observed for VC1145 as well as for VC2750 and VCA0955 in presence or absence of HapR, indicating an independency of HapR regulation under the tested conditions.
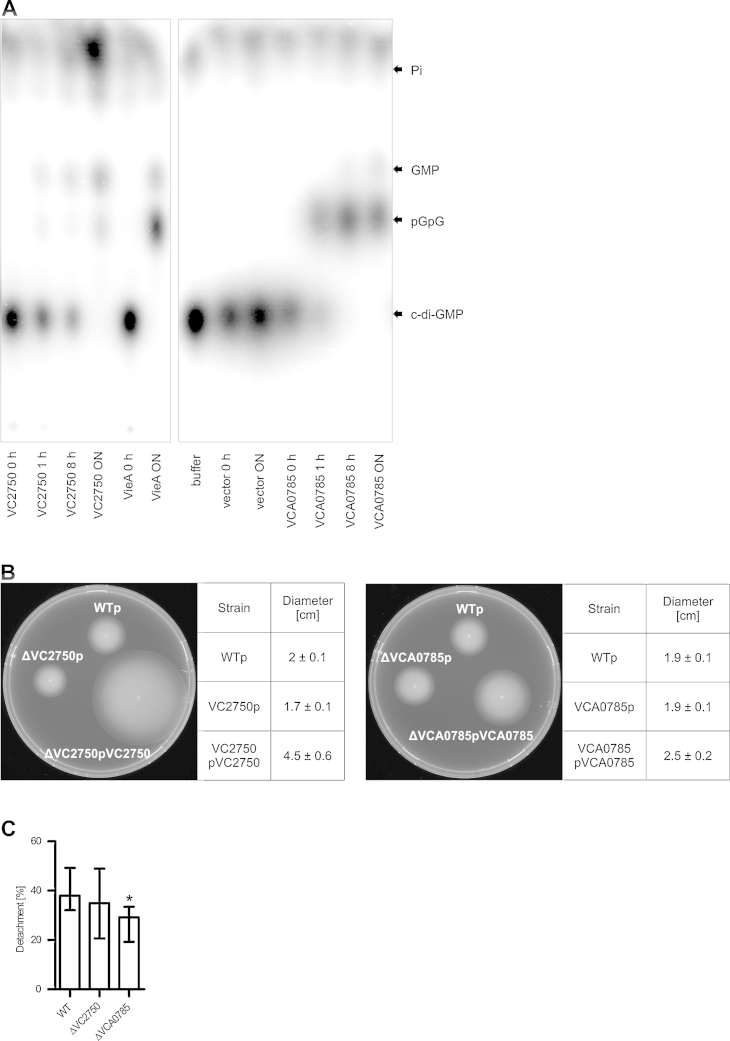
Characterization of VC2750 and VCA0785 as two c-di-GMP degrading phosphodiesterases with different impact on biofilm and motility. Both VCA0785 and VC2750 are predicted to encode proteins with tandem GGDEF and EAL domains, but seem to be differently regulated; VCA0785 is repressed by HapR, while VC2750 seems to be independent of HapR regulation. In the predicted VCA0785 gene product, the GGDEF active site residues diverge from the consensus (NSTNL in place of GG[D/E]F), while the EAL domain is well conserved, suggesting that it functions as a c-di-GMP phosphodiesterase. In the VC2750 gene product, residues that are key for enzymatic activity for both the GGDEF and EAL domains are preserved. Thus, VC2750 may function as a dual function enzyme, with the capacity to synthesize and degrade c-di-GMP. To determine whether VCA0785 or VC2750 encode a DGC, a c-di-GMP PDE, or both, the full-length gene products were tagged with hexahistidine and purified from E. coli by affinity chromatography. The purified proteins were tested for the ability to synthesize or hydrolyze c-di-GMP. Like the positive control VieA, both VCA0785 and VC2750 showed the ability to degrade c-di-GMP; the linear pGpG and GMP reaction products were apparent (Fig. 7A). The buffer only and mock-purified vector control reactions showed no hydrolysis of c-di-GMP. In contrast, no significant DGC activity was observed for either VCA0785 or VC2750, in comparison to WspR as a positive control (data not shown) (Hickman et al., 2005). Thus, full-length VCA0785 and VC2750 function as c-di-GMP PDEs in vitro.
Fig. 7.
Characterization of VC2750 and VCA0785 as c-di-GMP PDEs. (A) His-tagged purified proteins from E. coli lysates as well as buffer- and vector-only controls were tested for their ability to hydrolyze radioactive labeled c-di-GMP within 0 h, 1 h, 8 h and over night (ON). Reactions were analyzed by thin layer chromatography and visualized by phosphorimagery. The respective reactions are indicated on the bottom and the products are indicated on the right. (B) Shown are representative swarming phenotypes of wild type and mutants with control plasmid and complemented mutants incubated for 24 h at RT on swarm agar plates. The swarming diameters of six independent assays for each strain are indicated on the right as mean ± SD. (C) The detachment rate over 3 h was determined using statically grown 48 h old biofilm of the respective strain. The bars represent the percentage of detached cells normalized to the total cell number in the biofilm. Shown are the medians of at least six independent measurements. The error bars indicate the interquartile range. Significant differences between the respective data sets of the WT compared to mutants (*P < 0.05 Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons) are indicated.
As known from the current literature, c-di-GMP inversely regulates biofilm and motility of V. cholerae (Beyhan et al., 2006b; Krasteva et al., 2010; Liu et al., 2010; Tamayo et al., 2007), hence we tested the ΔVC2750 and ΔVCA0785 mutants on swarming plates containing reduced agar to allow the observation of motility phenotypes (Fig. 7B). As expected, the ΔVC2750 mutant with control plasmid showed a decrease in motility compared to wild type with control plasmid. Overexpression of VC2750 in trans on a complementation plasmid led to increased swarming compared to the wild type control. Thus, the deletion of VC2750 resulted in an increase in biofilm formation (Fig. 3C) and a decrease in motility, which is consistent with the current model of inverse regulation of biofilm and motility by c-di-GMP. Moreover, the effects of overexpression indicate that VC2750 functions predominantly as a c-di-GMP phosphodiesterase in vivo, under the conditions tested. In contrast, the deletion of VCA0785 showed no decrease in motility, but overexpression in trans also resulted in increased swarming compared to the wild type control. VCA0785 seems to have a stronger effect on biofilm formation than on motility, since the ΔVCA0785 mutant showed pronounced differences in static and dynamic biofilm formation compared to the wild type (Figs. 3E, 4 and 5C), but no effect on motility. Only after overexpression of VCA0785 was a motility effect visible, demonstrating that VCA0785 is a functional c-di-GMP phosphodiesterase in vivo and its activity can affect motility.
To further characterize the role of VC2750 and VCA0785 in biofilm formation, we performed an assay to measure detachment from a biofilm, because both mutants showed an increase in static biofilm formation. The detachment rate over 3 h of wild type, ΔVC2750 and ΔVCA0785 was determined using statically grown 48 h old biofilm (Fig. 7C). The ΔVC2750 mutant showed no significant change in the detachment rate compared to wild type. Deletion of VCA0785 resulted in a decreased detachment rate compared to wild type, which could explain the increased biofilm formation under static and dynamic biofilm conditions.
Discussion
To date, transcriptional analysis of V. cholerae during biofilm formation has been limited to static conditions, which are easy to reproduce and require only basic equipment. The data obtained by transcriptome analyses of static biofilms clearly revealed important information on the successive stages of V. cholerae biofilm development. However, a recent study suggested that biofilms grown under hydrodynamic conditions depend on a different set of biofilm-promoting factors (Muller et al., 2007), but only limited data on the transcriptional profile of V. cholerae during dynamic biofilm formation is available. Moreover, the use of global expression analysis of bacterial biofilms has been controversially discussed and remains difficult. Microarray approaches have to accommodate the consequences of averaging heterogeneity in biofilms and provide a snapshot of gene expression under a specific condition (An and Parsek, 2007; Beloin and Ghigo, 2005; Stewart and Franklin, 2008). As a consequence unique patterns of gene expression related to specific regions in a biofilm or sub-populations and transiently induced genes might be lost.
Therefore, we focused in the present study on the identification of genes induced during dynamic biofilm formation of V. cholerae using the well-established resolvase-based reporter gene system RIVET, which allows detection of spatial and temporal gene expression in sub-sets of diverse populations (An and Parsek, 2007; Angelichio et al., 1999, 2004; Beloin and Ghigo, 2005; Schild et al., 2007; Stewart and Franklin, 2008). We applied the in vivo based reporter system to a recombination-based in biofilm expression technology RIBET to identify genes induced during dynamic biofilm formation. The dynamic setup established in this study comprises a system in which V. cholerae must move out of a reservoir, against a continuous medium flow, and up an angled cover slip. This allows a direct recovery of the population within the biofilm, while detached cells, waste products or extracellular molecules are constantly removed, which may reflect a situation closer to environmental conditions. Also noteworthy, the irreversible excision of the reporter gene cassette as a consequence of induction of a gene-tnpR fusion allows the identification of genes that are only transiently induced.
An advantage of the RIBET system is the ability to recover the integrated suicide plasmids harboring the induced tnpR-fusion and to subsequently reconstruct the original unresolved strain. These reconstructed strains can be tested for their resolution frequency under control conditions as well as during biofilm formation under static and dynamic conditions a measure of gene induction. By determining the resolution frequency of each tnpR-fusion identified in the screen under these three conditions, we not only were able to validate the induction of the respective tnpR-fusion during biofilm formation per se, but also directly compare gene induction during static and dynamic biofilm formation. In total, we validated 59 genes as bona fide induced in both biofilm conditions and 16 genes that are specifically induced under dynamic biofilm conditions. Among the ibi genes were 6 genes belonging to groups previously shown to play a role during biofilm formation like extracellular nucleases, flagella, c-di-GMP related and vps genes (Absalon et al., 2011; Berk et al., 2012; Fong et al., 2010; Hammer and Bassler, 2003; Krasteva et al., 2010; Lim et al., 2007; Moorthy and Watnick, 2004; Seper et al., 2011; Tischler and Camilli, 2004; Watnick and Kolter, 1999; Watnick et al., 2001). The identification of genes with assigned roles in biofilm formation represents a valuable control to validate and strengthen the results of our screen. Interestingly, we found 6 genes of the chitin utilization program to be induced in static and dynamic biofilms, and another, the response regulator ChiS, under dynamic biofilm conditions (Meibom et al., 2004). The induction of this program under conditions where no chitin is present needs to be further investigated. Currently, we can only hypothesize that at least a subset of the chitin utilization program is part of a regulon induced during biofilm formation. One could speculate that such regulation allows induction of these genes in response to any abiotic surface or even before chitinous surfaces in the aquatic reservoirs are encountered. This pre-induction could facilitate the initial steps of biofilm formation upon the first contact with such surfaces in the environment.
Among the ibi gene mutants selected for further characterization, one mutant (ΔVC0374) revealed an inverse phenotype between static and dynamic conditions, five mutants (ΔVC0433, ΔVC1145, ΔVC2750, ΔVCA0686 and ΔVCA0786) showed a phenotype only under static or dynamic conditions and two mutants (ΔVCA0785 and ΔVCA0955) exhibited the same trend under both biofilm conditions tested.
The biofilm formed by ΔVC0374 under static conditions was comparable to a ΔvpsA mutant, which is incapable of synthesizing the VPS matrix (Fong et al., 2010; Yildiz and Schoolnik, 1999). In contrast, ΔVC0374 showed a pronounced increase in biofilm formation compared to the wild type under dynamic conditions. This increase is mainly due to morphological changes and the appearance of a filamentous phenotype, which we never observed in dynamically grown wild type biofilms. VC0374 is annotated as a glucose-6-phosphate isomerase (Kanehisa et al., 2006) and therefore may play a central role in carbon metabolism. It could be hypothesized that the accumulation of metabolic waste products, signaling molecules and other factors under static conditions may require metabolic changes and explain the impaired biofilm formation of ΔVC0374 in a closed system. In contrast, the dynamic setup provides a continuous supply of fresh medium without the need for metabolic adaptations, and could facilitate biofilm formation of ΔVC0374 even beyond wild type levels.
Additionally, we identified three ibi genes, i.e. VCA0686, VCA0955 and xds (VC2621), which have previously also shown to be induced at late stages of infection in the infant mouse model (Schild et al., 2007). Several of these in vivo late genes enhance the fitness of V. cholerae in the transition from the host to aquatic reservoirs, augmenting survival in the environment rather than contributing to colonization or virulence of the pathogen. In detail, we recently characterized the extracellular nucleases Dns and Xds as modulators of eDNA and assigned roles for them during biofilm development and in the mouse, where they facilitate escape from neutrophil extracellular traps (Seper et al., 2011, 2013). VCA0686 was recently shown to be the permease of a hexose-6-phosphate uptake system of V. cholerae. A physiological role was assigned to the hexose-6-phosphate uptake system, which correlates with increased fitness of V. cholerae after transition from the host into phosphate-limiting aquatic environments (Moisi et al., 2013). Deletion of VCA0686 decreased static biofilm formation, suggesting a role for the hexose-6-phosphate uptake system in biofilm development. Taken together, these data further strengthen the hypothesis that late during infection V. cholerae induces genes that facilitate the survival of the bacterium in the aquatic environment, where V. cholerae persists in the biofilm mode between the epidemic outbreaks (Lutz et al., 2013; Reidl and Klose, 2002; Schild et al., 2007).
We identified three deletion mutants, ΔVC0433 and ΔVC2750 and ΔVCA0686, with a phenotype under static conditions, but no dynamic biofilm phenotype. In contrast, the deletion of VC1145 or VCA0786 resulted in a dynamic, but no static phenotype. These results support that static and dynamic biofilms exhibit distinct differences. Interestingly, Müller et al. reported the formation of a hydrodynamic V. cholerae biofilm independent of vpsA and luxO, which encode key factors for the VPS matrix and the QS cascade (Muller et al., 2007). In contrast, in the present study the ΔvpsA mutant showed no significant biofilm formation under dynamic and static conditions and the ΔhapR mutant exhibited a pronounced increase in the biofilm amount compared to wild type in both conditions, which is consistent with reports by others (Fong et al., 2010; Yildiz and Schoolnik, 1999). Additionally, in this study we identified vpsE, a representative ORF of the vps-I cluster, as an ibi gene. Taken together, these data suggest that both the static and dynamic biofilms described in this study are dependent on vps genes and influenced by HapR. Thus, the different phenotypes in dynamic and static biofilms observed for some ibi mutants revealed herein cannot be simply explained by the impact of VPS or the HapR regulon. The identification of mutants with diverging phenotypes under only dynamic or static conditions is the first step toward a better understanding of the differences and common principles of biofilm formation. Future investigations are needed to comprehensively dissect the regulation under these conditions using such mutants as a platform.
Some initial characterizations have been carried out within this study. For example, ΔVC1145 demonstrated an increase in vps expression, which provides some explanation for the observed increase in biofilm formation of the deletion mutant. Furthermore VCA0785 and VCA0786 were shown to be repressed by HapR.
VCA0785, also known as CdgC, modulates intracellular c-di-GMP and it has been shown to regulate VPS production, flagellar motility, and virulence gene expression in a rugose phase variant of V. cholerae (Lim et al., 2006, 2007; Yildiz et al., 2004). Interestingly, VCA0785 was shown to regulate gene expression in a phase variant-dependent manner (Lim et al., 2007), and several studies indicate differences in gene regulation in smooth and rugose phase variants as well as different V. cholerae isolates (Beyhan et al., 2006a; Lim et al., 2007; Tischler and Camilli, 2005). Therefore, we decided to characterize the activity and phenotypes of a ΔVCA0785 mutant in the smooth variant together with the deletion mutant of the second c-di-GMP related ORF VC2750. Throughout the study, ΔVCA0785 showed the most pronounced phenotypic changes in both biofilm systems, while for ΔVC2750 solely a static biofilm phenotype was visible. Bioinformatics showed an EAL and a GGDEF domain for both VCA0785 and VC2750. It has been demonstrated previously that the EAL domain of VCA0785 is functional by overexpression of the EAL domain as well as characterization of loss-of-function point mutants (Tischler and Camilli, 2004; Yildiz and Schoolnik, 1999). Here we showed biochemically that for full-length VCA0785 and VC2750, the EAL domain of both proteins has the dominant activity and both enzymes consequently function as PDEs, consistent with the biofilm phenotypes observed.
In the current model, c-di-GMP levels are high in the aquatic environment where V. cholerae persists in biofilms (Tamayo et al., 2007). Specifically, c-di-GMP levels are elevated at low cell density and reduced at high cell density, a process controlled in part by quorum sensing and HapR (Hammer and Bassler, 2009; Krasteva et al., 2010; Srivastava et al., 2011; Waters et al., 2008). HapR controls the expression of several DGC and PDE genes and decreases overall the intracellular levels of c-di-GMP (Hammer and Bassler, 2009; Waters et al., 2008), though a QS-dependent, HapR-independent activation of a DGC was recently shown (Zhao et al., 2013). We showed that VCA0785 is repressed by HapR, as the expression level of VCA0785 increased in a ΔhapR mutant compared to wild type. Consistent with this, bioinformatic analysis predicts a HapR binding site upstream of VCA0785 (Yildiz et al., 2004). Interestingly, VCA0785 positively regulates hapR expression, as the amount of hapR message was decreased in the rugose phase variant of ΔVCA0785 (Lim et al., 2007). These findings suggest a complex regulation and feedback loop between HapR and VCA0785.
c-di-GMP inversely regulates biofilm and motility (Beyhan et al., 2006b; Krasteva et al., 2010; Liu et al., 2010; Tamayo et al., 2007; Tischler and Camilli, 2004, 2005), so we anticipated that deletion of VCA0785 or VC2750 might affect motility. Increased motility was observed for the ΔVC2750, but not the ΔVCA0785, mutant. Nevertheless both enzymes can affect motility, as demonstrated by overexpression of VCA0785 or VC2750 in trans. Although both proteins have the same enzymatic activity, these different phenotypes in biofilm and motility assays point toward some kind of specialization within the c-di-GMP regulatory cascade.
The ΔVCA0785 mutant exhibits a lower rate of detachment from a biofilm compared to the wild type, which together with the increased vps transcription provides some explanation for the pronounced increase in biofilm formation of this mutant. It has been shown previously that biofilm derived cells are hyperinfective and Tamayo et al. suggested that factors, e.g. the Pst2 system, could exist that are specifically induced during growth in a biofilm that augment infection by V. cholerae (Mudrak and Tamayo, 2012; Tamayo et al., 2010). Moreover, we recently demonstrated that biofilm clumps from the extracellular nuclease mutant ΔdnsΔxds lack this hyperinfectivity, most likely due to incomplete dissolution of biofilm-derived cells in vivo (Seper et al., 2011). Thus, it can be speculated that some of the ibi genes play a role in the hyperinfective phenotype of biofilm-derived cells, which is being investigated further.
This study provides the first comprehensive screen for genes induced during biofilm formation using a reporter gene system and a direct comparison of phenotypes under static and dynamic biofilm formation. As observed previously, phenotypes of mutants under static and dynamic biofilms do not always match (Beyhan et al., 2007; Muller et al., 2007; Yildiz et al., 2004). One might argue that dynamic conditions reflect a more natural situation with an open system and flow as in the aquatic environment. Indeed, this study revealed 16 genes to be exclusively induced under dynamic conditions, and several mutants exhibited altered phenotypes only under dynamic conditions. This strengthens the hypothesis of distinct differences in the structural features and regulatory mechanisms present under dynamic conditions compared to static conditions.
Acknowledgements
This work was supported by the Austrian FWF grants P22986 to S. S. and W901-B12 (DK Molecular Enzymology) to A. S., S. R., D. R. L., J. R. and S. S. We are grateful to S. D. Kohlwein and H. Wolinski for access to the microscope facility supported in part by the FWF (project F3005-B12 LIPOTOX). We thank Barbara Klug and Katharina Kleewein for their technical support.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Absalon C., Van Dellen K., Watnick P.I. A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 2011;7:e1002210. doi: 10.1371/journal.ppat.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D., Parsek M.R. The promise and peril of transcriptional profiling in biofilm communities. Curr. Opin. Microbiol. 2007;10:292–296. doi: 10.1016/j.mib.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Angelichio M.J., Merrell D.S., Camilli A. Spatiotemporal analysis of acid adaptation-mediated Vibrio cholerae hyperinfectivity. Infect. Immun. 2004;72:2405–2407. doi: 10.1128/IAI.72.4.2405-2407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelichio M.J., Spector J., Waldor M.K., Camilli A. Vibrio cholerae intestinal population dynamics in the suckling mouse model of infection. Infect. Immun. 1999;67:3733–3739. doi: 10.1128/iai.67.8.3733-3739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloin C., Ghigo J.M. Finding gene-expression patterns in bacterial biofilms. Trends Microbiol. 2005;13:16–19. doi: 10.1016/j.tim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Berk V., Fong J.C., Dempsey G.T., Develioglu O.N., Zhuang X., Liphardt J., Yildiz F.H., Chu S. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337:236–239. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S., Bilecen K., Salama S.R., Casper-Lindley C., Yildiz F.H. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 2007;189:388–402. doi: 10.1128/JB.00981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S., Tischler A.D., Camilli A., Yildiz F.H. Differences in gene expression between the classical and El Tor biotypes of Vibrio cholerae O1. Infect. Immun. 2006;74:3633–3642. doi: 10.1128/IAI.01750-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S., Tischler A.D., Camilli A., Yildiz F.H. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 2006;188:3600–3613. doi: 10.1128/JB.188.10.3600-3613.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomchil N., Watnick P., Kolter R. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 2003;185:1384–1390. doi: 10.1128/JB.185.4.1384-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Beattie D.T., Mekalanos J.J. Use of genetic recombination as a reporter of gene expression. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Mekalanos J.J. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol. Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavelli D.A., Marsh J.W., Taylor R.K. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 2001;67:3220–3225. doi: 10.1128/AEM.67.7.3220-3225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R.R. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Colwell R.R., Huq A., Islam M.S., Aziz K.M., Yunus M., Khan N.H., Mahmud A., Sack R.B., Nair G.B., Chakraborty J., Sack D.A., Russek-Cohen E. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M.S., Kaper J.B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J.C., Karplus K., Schoolnik G.K., Yildiz F.H. Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J. Bacteriol. 2006;188:1049–1059. doi: 10.1128/JB.188.3.1049-1059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J.C., Syed K.A., Klose K.E., Yildiz F.H. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology. 2010;156:2757–2769. doi: 10.1099/mic.0.040196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J.C., Yildiz F.H. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J. Bacteriol. 2007;189:2319–2330. doi: 10.1128/JB.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin M.Y. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 2004;6:552–567. doi: 10.1111/j.1462-2920.2004.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13:7–10. doi: 10.1016/j.tim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hammer B.K., Bassler B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Hammer B.K., Bassler B.L. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. J. Bacteriol. 2009;191:169–177. doi: 10.1128/JB.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Haugo A.J., Watnick P.I. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 2002;45:471–483. doi: 10.1046/j.1365-2958.2002.03023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A., Nielsen A.T., Hentzer M., Sternberg C., Givskov M., Ersboll B.K., Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(Pt 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Hickman J.W., Tifrea D.F., Harwood C.S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot L., Chang S., Pickering B.S., Absalon C., Watnick P.I. The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J. Bacteriol. 2010;192:3055–3067. doi: 10.1128/JB.00213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot L., Watnick P.I. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J. Bacteriol. 2008;190:311–320. doi: 10.1128/JB.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo A., Xu B., Chowdhury M.A., Islam M.S., Montilla R., Colwell R.R. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Colwell R.R., Rahman R., Ali A., Chowdhury M.A., Parveen S., Sack D.A., Russek-Cohen E. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., Hattori M., Aoki-Kinoshita K.F., Itoh M., Kawashima S., Katayama T., Araki M., Hirakawa M. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. An address on cholera and its bacillus. Br. Med. J. 1884;2:403–407. doi: 10.1136/bmj.2.1235.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva P.V., Fong J.C., Shikuma N.J., Beyhan S., Navarro M.V., Yildiz F.H., Sondermann H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327:866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Angelichio M.J., Mekalanos J.J., Camilli A. Nucleotide sequence and spatiotemporal expression of the Vibrio cholerae vieSAB genes during infection. J. Bacteriol. 1998;180:2298–2305. doi: 10.1128/jb.180.9.2298-2305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Hava D.L., Waldor M.K., Camilli A. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell. 1999;99:625–634. doi: 10.1016/s0092-8674(00)81551-2. [DOI] [PubMed] [Google Scholar]
- Lim B., Beyhan S., Meir J., Yildiz F.H. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 2006;60:331–348. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Lim B., Beyhan S., Yildiz F.H. Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J. Bacteriol. 2007;189:717–729. doi: 10.1128/JB.00834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Beyhan S., Lim B., Linington R.G., Yildiz F.H. Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. J. Bacteriol. 2010;192:4541–4552. doi: 10.1128/JB.00209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.J., Michalski J., Martinez-Wilson H., Morin C., Hilton T., Osorio C.G., Nataro J.P., Tacket C.O., Camilli A., Kaper J.B. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18229–18234. doi: 10.1073/pnas.0705636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C., Erken M., Noorian P., Sun S., McDougald D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 2013;4:375. doi: 10.3389/fmicb.2013.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom K.L., Li X.B., Nielsen A.T., Wu C.Y., Roseman S., Schoolnik G.K. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V.L., DiRita V.J., Mekalanos J.J. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 1989;171:1288–1293. doi: 10.1128/jb.171.3.1288-1293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V.L., Mekalanos J.J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisi M., Jenul C., Butler S.M., New A., Tutz S., Reidl J., Klose K.E., Camilli A., Schild S. A novel regulatory protein involved in motility of Vibrio cholerae. J. Bacteriol. 2009;191:7027–7038. doi: 10.1128/JB.00948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisi M., Lichtenegger S., Tutz S., Seper A., Schild S., Reidl J. Characterizing the hexose-6-phosphate transport system of Vibrio cholerae, a utilization system for carbon and phosphate sources. J. Bacteriol. 2013;195:1800–1808. doi: 10.1128/JB.01952-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy S., Watnick P.I. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 2004;52:573–587. doi: 10.1111/j.1365-2958.2004.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy S., Watnick P.I. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol. Microbiol. 2005;57:1623–1635. doi: 10.1111/j.1365-2958.2005.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales V.M., Backman A., Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Mudrak B., Tamayo R. The Vibrio cholerae Pst2 phosphate transport system is upregulated in biofilms and contributes to biofilm-induced hyperinfectivity. Infect. Immun. 2012;80:1794–1802. doi: 10.1128/IAI.06277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Miller M.C., Nielsen A.T., Schoolnik G.K., Spormann A.M. vpsA- and luxO-independent biofilms of Vibrio cholerae. FEMS Microbiol. Lett. 2007;275:199–206. doi: 10.1111/j.1574-6968.2007.00884.x. [DOI] [PubMed] [Google Scholar]
- Nelson E.J., Harris J.B., Morris J.G., Jr., Calderwood S.B., Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio C.G., Crawford J.A., Michalski J., Martinez-Wilson H., Kaper J.B., Camilli A. Second-generation recombination-based in vivo expression technology for large-scale screening for Vibrio cholerae genes induced during infection of the mouse small intestine. Infect. Immun. 2005;73:972–980. doi: 10.1128/IAI.73.2.972-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell E.B., McKee R.W., McBride S.M., Waters C.M., Tamayo R. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 2012;194:3307–3316. doi: 10.1128/JB.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G., Kolter R. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J. Bacteriol. 2005;187:3551–3555. doi: 10.1128/JB.187.10.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidl J., Klose K.E. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 2002;26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Ryjenkov D.A., Tarutina M., Moskvin O.V., Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D.A., Sack R.B., Nair G.B., Siddique A.K. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Schild S., Bishop A.L., Camilli A. Ins and outs of Vibrio cholerae. Microbe. 2008;3:131–136. [Google Scholar]
- Schild S., Tamayo R., Nelson E.J., Qadri F., Calderwood S.B., Camilli A. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe. 2007;2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A.J., Ryjenkov D.A., Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol. 2005;187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seper A., Fengler V.H., Roier S., Wolinski H., Kohlwein S.D., Bishop A.L., Camilli A., Reidl J., Schild S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011;82:1015–1037. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seper A., Hosseinzadeh A., Gorkiewicz G., Lichtenegger S., Roier S., Leitner D.R., Rohm M., Grutsch A., Reidl J., Urban C.F., Schild S. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9:e1003614. doi: 10.1371/journal.ppat.1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D., Harris R.C., Waters C.M. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J. Bacteriol. 2011;193:6331–6341. doi: 10.1128/JB.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg C., Christensen B.B., Johansen T., Toftgaard Nielsen A., Andersen J.B., Givskov M., Molin S. Distribution of bacterial growth activity in flow-chamber biofilms. Appl. Environ. Microbiol. 1999;65:4108–4117. doi: 10.1128/aem.65.9.4108-4117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, C., Tolker-Nielsen, T., 2006. Growing and analyzing biofilms in flow cells. Current protocols in microbiology Chapter 1, Unit 1B.2. [DOI] [PubMed]
- Stewart P.S., Franklin M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- Tamayo R., Patimalla B., Camilli A. Growth in a biofilm induces a hyperinfectious phenotype in Vibrio cholerae. Infect. Immun. 2010;78:3560–3569. doi: 10.1128/IAI.00048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo R., Pratt J.T., Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 2007;61:131–148. doi: 10.1146/annurev.micro.61.080706.093426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo R., Tischler A.D., Camilli A. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J. Biol. Chem. 2005;280:33324–33330. doi: 10.1074/jbc.M506500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M.L., Gauzens A.L., Huq A., Sack D.A., Colwell R.R. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A.D., Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A.D., Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 2005;73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance R.E., Zhu J., Mekalanos J.J. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect. Immun. 2003;71:2571–2576. doi: 10.1128/IAI.71.5.2571-2576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorregaard, M., Ersbøll, B.K., Yang, L., Haagensen, J.A.J., Molin, S., Sternberg, C. Personal communication.
- Waters C.M., Lu W., Rabinowitz J.D., Bassler B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P., Kolter R. Steps in the develompent of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P.I., Fullner K.J., Kolter R. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 1999;181:3606–3609. doi: 10.1128/jb.181.11.3606-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P.I., Lauriano C.M., Klose K.E., Croal L., Kolter R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 2001;39:223–235. doi: 10.1046/j.1365-2958.2001.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Cholera 2012. Wkly. Epidemiol. Rec. 2013;88:321–336. [PubMed] [Google Scholar]
- Yildiz F.H., Liu X.S., Heydorn A., Schoolnik G.K. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 2004;53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- Yildiz F.H., Schoolnik G.K. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F.H., Visick K.L. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009;17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Koestler B.J., Waters C.M., Hammer B.K. Post-transcriptional activation of a diguanylate cyclase by quorum sensing small RNAs promotes biofilm formation in Vibrio cholerae. Mol. Microbiol. 2013;89:989–1002. doi: 10.1111/mmi.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Mekalanos J.J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.