Abstract
Despite existing vaccination strategies targeting TRP-2, its function is not yet fully understood. TRP-2 is an enzyme involved in melanin biosynthesis and therefore discussed as a differentiation antigen. However, in mice Trp-2 was shown to be expressed in melanocyte stem cells of the hair follicle and therefore also considered as an indicator of stemness. A proper understanding of the TRP-2 function is crucial, considering a vaccination targeting cells with stemness properties would be highly effective in contrast to a therapy targeting differentiated melanoma cells.
Analysing over 200 melanomas including primaries, partly matched metastases and patients’ cell cultures we show that TRP-2 is correlated with Melan A expression and decreases with tumor progression. In mice it is expressed in differentiated melanocytes as well as in stem cells. Furthermore, we identify a TRP-2 negative, proliferative, hypoxia related cell subpopulation which is significantly associated with tumor thickness and diseases progression. Patients with a higher percentage of those cells have a less favourable tumor specific survival.
Our findings underline that TRP-2 is a differentiation antigen, highlighting the importance to combine TRP-2 vaccination with other strategies targeting the aggressive undifferentiated hypoxia related subpopulation.
Abbreviations: DCT, Dopachrome tautomerase; TMA, Tissue microarray; TRP-2, Tyrosinase related protein-2
Introduction
Melanoma is a highly aggressive neoplasm. Patients with distant metastases often face very poor prognosis, with a median survival rate of approximately 9 months, and with less than 10% of patients surviving beyond 5 years [1], [2]. Tumor growth and spread is known to be regulated by the crosstalk between tumor cells and stroma including immune cells. Moreover spontaneous tumor regression has been shown in melanoma and demonstrated to be regulated by the immune system. The ability of the immune system to recognize melanoma cells is based on the presence of immunogenic antigens capable of triggering a specific immune response. A continuous search for tumor antigens, which could be used to direct the human immune system against cancer lead to the discovery of several families of key-cancer-related molecules [3], [4], [5], [6], [7]. Between these tyrosinase related protein 2 (TRP-2; also known as dopachrome tautomerase; DCT) represents to date a major target of immunotherapy for melanoma.
TRP-2 is a membrane-bound melanosomal enzyme involved in melanin biosynthesis also known as a melanoma differentiation antigen expressed in normal melanocytes, melanomas, normal retinal tissue and brain [8]. TRP-2 was identified by screening a tumor cDNA library with a T cell line exhibiting an in vivo antitumor activity. This finding demonstrated the immunogenicity of TRP-2 and to date several epitopes of this protein have been described to be recognized by specific cytotoxic T lymphocytes in humans. Based on these findings, TRP-2 represents a good target for immunotherapeutic treatment of melanoma [9], [10], [11].
Although several vaccination strategies targeting TRP-2 have been developed so far [12], [13], [14], [15], its expression in melanoma tissues is not yet fundamentally investigated. It has been reported that TRP-2 is neural crest specific and only expressed in melanocytes, in the pigment epithelium of the retina and in the brain [8]. Of major interest is that TRP-2 has been described to be hypoxia related [16].
In this project we investigated the expression of TRP-2 in over 200 melanoma biopsies and cell cultures from primary melanomas and metastasis. Moreover, we characterized the subpopulation of melanoma cells expressing TRP-2. Trp-2 (Dct) is a marker of melanocytic lineage and in mice its expression in the bulge region of the hair follicle identifies stem cell population [17]. However, Trp-2 (Dct) is expressed throughout the melanocytic lineage including not only melanocyte stem cells, which are c-Kit negative but also melanoblasts and differentiated melanocytes, which express c-Kit marker.
Taken together our findings illustrate that TRP-2 is a melanoma differentiation antigen and not a stem cell marker.
Furthermore, we identified an aggressive, proliferative TRP-2-negative subpopulation in primary melanoma, which significantly increases with tumor progression. Interestingly, the presence of this subpopulation in primary melanoma is associated with Breslow tumor thickness, hypoxia and indicates a less favourable tumor specific survival. This is in contradiction with the idea that TRP-2 might label the melanocyte stem cell population, while it is believed that stem cells are associated with more aggressive behaviour and less differentiation in many tumors. Our findings suggest that a TRP-2 vaccination would target the more differentiated melanoma cells. This approach should be ideally combined with other therapies able to target the aggressive hypoxia related undifferentiated subpopulation.
Materials and Methods
Tumor Specimens
All analyses involving human melanoma tissue were performed in accordance with the ethical committee in canton Zurich. Immunohistochemistry was performed on three different tissue microarrays (TMAs) representing a total of 81 primary melanomas, 59 melanoma metastasis and 65 melanoma patients’ derived cell cultures. The TMAs partly included matched tumor samples from primary tumors, metastases and cell cultures. Totally, 9 triplets consisting of primary melanoma, metastases and cell cultures, 5 pairs including primary melanoma and metastases and 25 pairs of melanoma tissue (9 primary and 16 metastases) matched with cell cultures were analysed. One TMA consisted of primary melanomas (Breslow tumor thickness > 1 mm) with available clinical data and follow up information about the patients included. Detailed clinical information of this TMA has been reported in a previous study [18].
The melanoma cells cultures were derived from surgical specimen of melanoma patients included in a life bio bank project. Written informed consent was approved by the local IRB (EK647 and EK800). TMA containing melanoma cell cultures and melanoma tissue were constructed as previously described [19].
Approval for the use of melanoma TMAs and melanoma metastases was obtained from the official ethical authorities of the Canton Zurich (StV 16–2007).
Mice
All animal experiments were performed in accordance with Swiss law and have been approved by the veterinary authorities of Zurich.
Immunohistochemistry
For the mouse experiments: skin samples were fixed with 4% formaldehyde and frozen in OCT compound. For immunohistochemistry, sections were stained as previously described [20]. Anti-Dct (rabbit, ab74073, Abcam) was used.
Sections of 2 μm from a tissue TMA were stained with antibodies against Melan A, Hif-1α, TRP-2 and Mib-1. The immunohistochemical staining for all antigens was performed on automated staining systems Melan A, TRP-2/Mib-1 on Ventana Bench Mark, Ventana Medical Systems, Tucson, AZ, USA and Hif-1α on Bond Refine, Vision BioSystems Ltd, Newcastle Upon Tyne, UK. The following antibodies were used: Hif-1α clone mgc3 (Abcam Limited), dilution 1:400; Melan A clone A103 (DAKO A/S), dilution 1:30; Mib-1 clone 30–9 (Ventana-Roche), prediluted.
Evaluation of TRP-2, Melan A and Hif-1α Expression
To determine the expression frequencies of TRP-2, the hot spot of a tumor sample was chosen and the percentage of positive cells per 100 melanoma cells was recorded. In addition, using a co-staining for Mib-1, four different combinations of positive and negative cells for Mib-1 and TRP-2 were recorded. For the evaluation of Melan A and Hif-1α a semi quantitative scoring system was applied following the German immunohistochemical scoring (GIS) system in which the final immunoreactive score equaled the product of the percentage of positive cells times the highest staining intensity. The percentage of positive cells was graded as follows: 0: negative; 1: up to 10% positive cells; 2: 11% to 50%; 3: 51% to 90%; and 4: > 90%. Staining intensity was graded as follows: 0: negative; 1: weakly positive; 2: moderately positive and 3: strongly positive [21]. All stainings were evaluated by an experienced pathologist (D.L.).
Hypoxia Treatment
Cells were cultured in a Modular Incubator Chamber (MIC-101, Billups-Rothenberg inc.), flushed with 20 liters/minute (flow meter; RMA-23-SSV; Dwyer) with certified premixed gas composed of 1% O2 , 5% CO2 and 94% N2 (CARBAGAS, Switzerland). The O2 concentration inside the chamber was measured with an oxygen sensor (VTI-122, Disposable Polarographic Oxygen Cell; 100122, Vascular Technology). The hypoxia chamber was placed in an incubater at 37 °C for 72 hours before RNA isolation.
qRT-PCR
Total RNA was extracted from primary melanoma cell cultures using TRIzol according to manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Total RNA was used for cDNA synthesis using Promega’s Reverse Transcription System (Promega, Madison, WI, USA) according to the supplied protocols. Gene expression was quantified using the FastStart Universal SYBR Green Master (ROX; 04913914001, Roche Basel, Switzerland) and the Viia7 system from Applied Biosystems. The primers for DCT and RPL28 were purchased from Qiagen (Venlo, The Netherlands).
Statistical Analyses
Correlations between TRP-2, Melan A, Mib-1 and Hif-1α in melanoma were analyzed using Spearman’s rank correlation. TRP-2, TRP-2/Mib-1, Hif-1α and Melan A were compared between different patient groups using the Mann–Whitney test. Wilcoxon signed ranks test was used to analyse the expression of TRP-2, Melan A and Hif-1α in matched tumor samples. Survival differences between groups were calculated by a log rank test. The Cox-regression analysis was applied for analysis of the association between tumor TRP-2/Mib-1 expression and tumor-specific survival.
p-values below 0.05 were considered as significant. IBM SPSS Statistics 20 (SPSS Inc., Chicago, IL) was used for statistical analyses. GraphPad Prism 5 was used for Boxplots and Kaplan-Meier curve.
Results
TRP-2, Melan A and Mib-1 Expression in Primary Melanoma and Metastases
We found a correlation between expression of TRP-2 and the melanoma differentiation anitgen Melan A in primary melanomas (p = 0.0001; Spearman’s correlation coefficient 0,6) as well as in metastases (p = 0.0001; Spearman’s correlation coefficient 0,6).
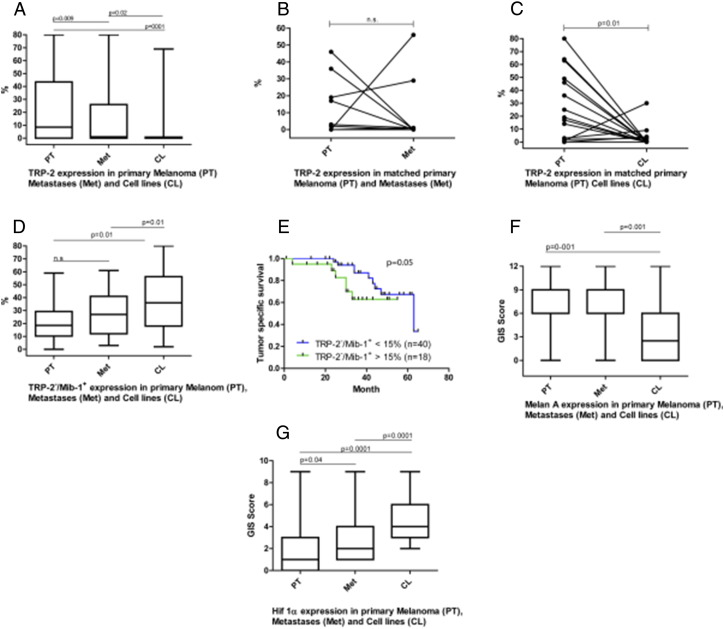
Importantly, there was a significant more frequent TRP-2 expression in primary melanomas compared to metastases (p = 0.009; Figure 1A). Thirty-six of 81 (44%) primary melanomas and 14 of 59 (24%) metastases showed TRP-2 expression in over 10% of melanoma cells. In 9 out of 12 matched samples a decrease in TRP-2 expression was detected in the metastases compared to the primaries; in 2 out of 12 samples an increase of TRP-2 in the metastases compared to the primaries was detected and in 1 out of 12 the expression of TRP-2 was absent in the primary as well as in the metastases. However these results are statistically not significant (p = 0.08) due to the low number of samples and the weak expression of TRP-2 in the metastases (Figure 1B). In addition, we found also a significant decrease of TRP-2 positive cells in cell culture compared to their matched primary tumor tissue (p = 0.01; Figure 1C). These findings indicate the survival benefit of TRP-2 negative cells in cell culture.
Figure 1.
Graphs with statistical analysis of TRP-2, TRP-2 negative/Mib-1 positive and Melan A in primary melanomas, metastases and cell cultures (mean and standard deviation).
Using our newly developed co-staining of Mib-1 and TRP2, we analyzed the proliferating (MIB-1 positive) melanoma cells depending on their TRP-2 expression in primary melanoma, and metastases (Figure 2A-D). In melanoma metastases, proliferating TRP-2 negative cells were significantly more frequent compared to the primaries (p = 0.01; Figure 1D), whereas non-proliferating TRP-2 positive cells were significantly less frequent in melanoma metastases compared to the primaries (p = 0.01). For the subgroups, which were either negative or positive for both markers, we found no significant difference between primary melanomas and metastases. Interestingly the percentage of TRP-2−/Mib-1+ cells significantly correlated with Breslow tumor thickness in the patient group with Breslow tumor thickness over 1 mm (p = 0.048; Spearman’s correlation coefficient 0,3). Furthermore, these cells were significantly correlated with Hif-1α expression (p = 0.03; Spearman’s correlation coefficient 0,3) and therefore with hypoxic condition in primary melanoma. In addition patients who had less than 15% of TRP-2−/Mib-1+ in their primary melanoma had statistically an approaching significance for a better tumor specific survival (p = 0.05; Figure 1E).
Figure 2.
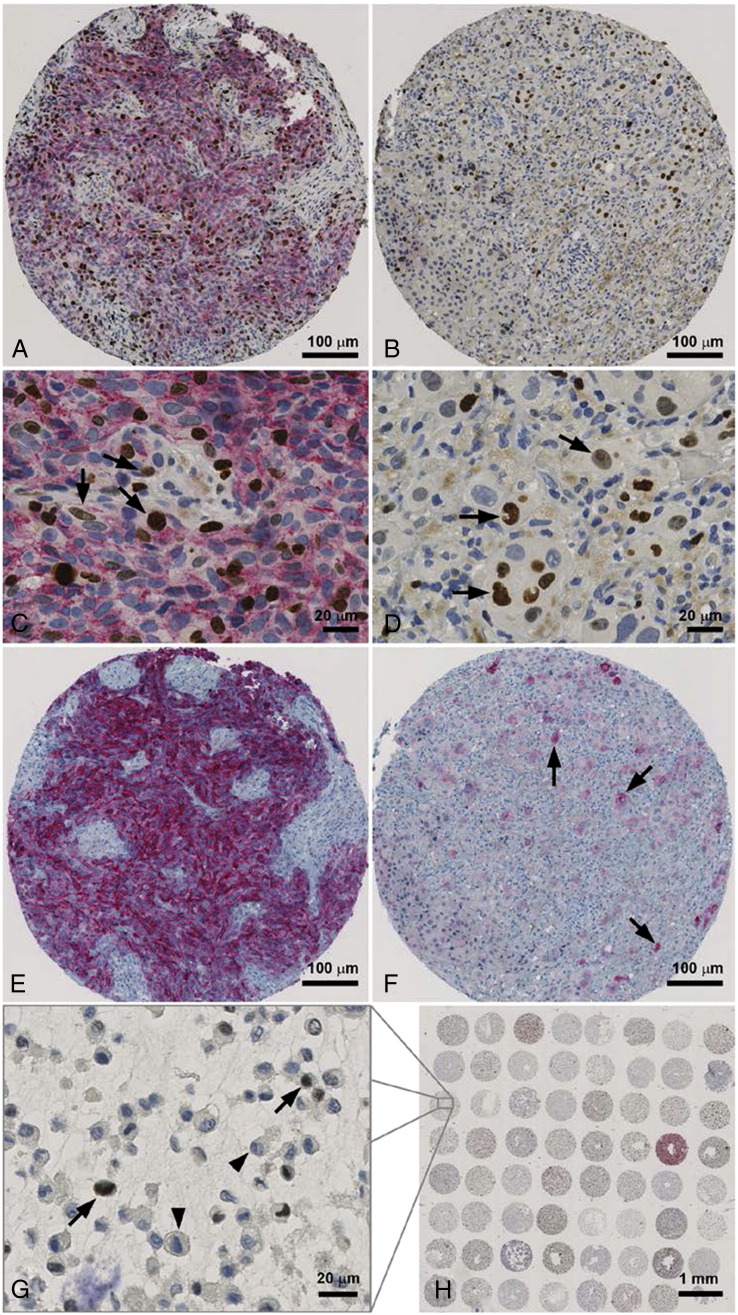
Co-staining of Mib-1/TRP-2 and Melan A staining in primary melanoma and corresponding metastasis (A-F). Moderate TRP-2 expression in over 50 percent of primary melanoma cells (A x100; C x400) and negativity in the metastasis (B x100; D x400). Greater extent of proliferating TRP-2 negative cells (arrow) in the metastasis (D x400) in comparison to the primary melanoma (C x400). Primary melanoma with strong Melan A expression in over 50% of tumor cells (E x100) and less than 10% in the metastasis (arrow; F x100). Overview of melanoma cell culture array with abcence of TRP-2 expression in most melanoma patient's cell cultures (H x10). Cell culture with TRP-2 negative proliferating cells (arrow; G x400).
TRP-2, Melan A and Mib-1 Expression in Melanoma Tissue Compared to Melanoma Cell Cultures
Melanoma patients’ cell cultures expressed significantly less Melan A than primary melanomas (p = 0.001) or metastases (p = 0.001; Figure 1 F). In addition TRP-2 was significantly less expressed in cell cultures if compared to primaries (p = 0.001) or to metastases (p = 0.02; Figure 1A).
Hypoxia Inducible Factor 1α (Hif-1α) and TRP-2 Expression
Hif-1α expression was significantly higher in melanoma metastases (p = 0.04) and cell cultures (p = 0.0001) when compared to primary melanomas (Figure 1G).
Analysing all melanoma samples primary melanomas, metastases and melanoma cell cultures we found a significant correlation between Hif-1α expression and the the presence of TRP-2−/Mib-1+ cells (p = 0.002; Spearman’s correlation coefficient 0,2) as well as with proliferation (Mib-1) alone (p = 0.01 Spearman’s correlation coefficient 0,2). However, analysing separately the different groups, only a significant correlation between Hif-1α expression and the presence of TRP-2−/Mib-1+ cells in melanoma patient’s cell cultures persisted (p = 0.01; Spearman’s correlation coefficient 0,3).
We found no significant correlation between Hif-1α, and TRP-2 expression neither in primary melanoma, melanoma metastases nor melanoma cell cultures as expected by cell line experiments.
Hypoxia is Downregulating TRP-2 in Primary Human Melanoma Cell Cultures
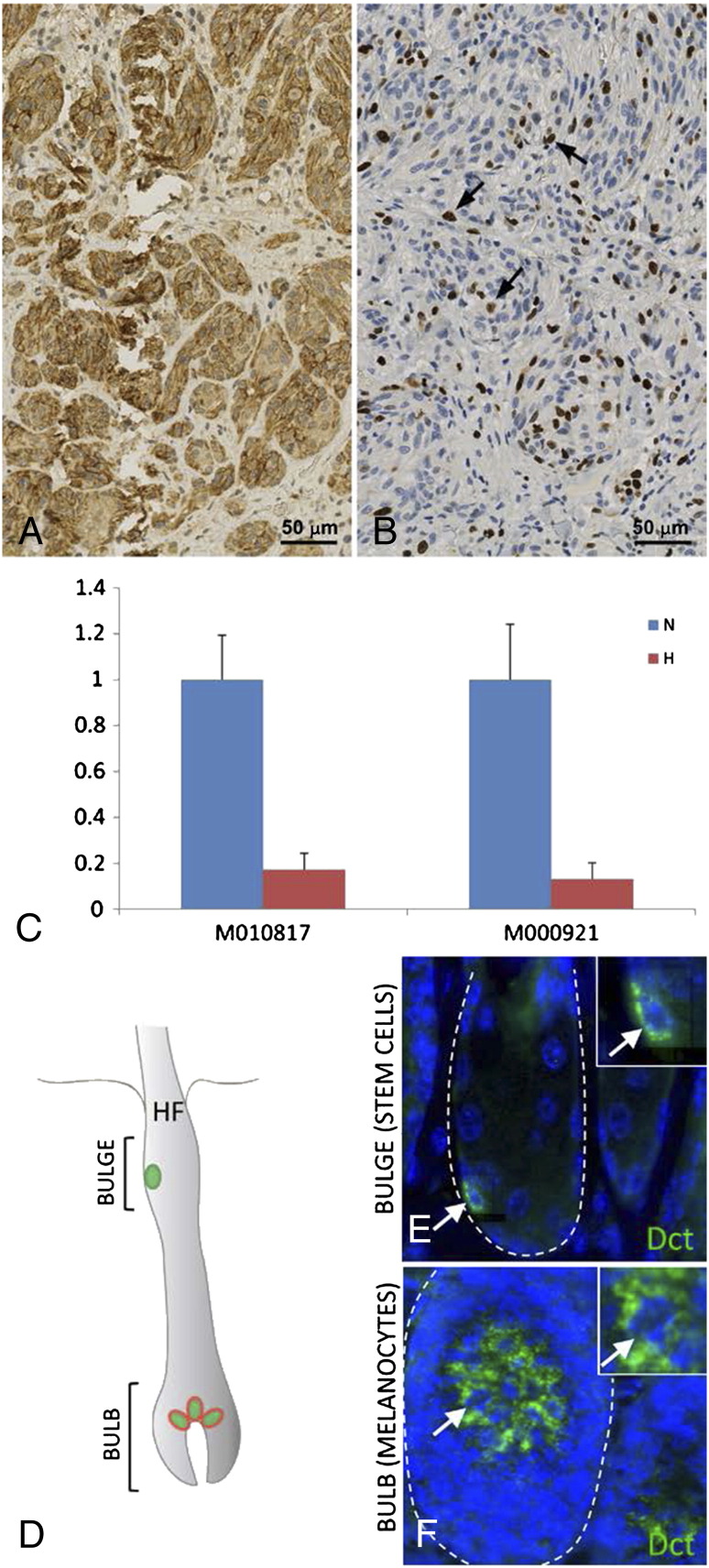
We treated primary human melanoma cell cultures with hypoxia for 72 hours and subsequently performed qRT-PCR for TRP-2 (Figure 3C). The results show a significant downregulation (5.8- resp. 7.6-fold) of TRP-2 in the hypoxia treated samples compared to untreated control cells, supporting the observed correlation of Hif-1α expression and the the presence of TRP-2−/Mib-1+ cells.
Figure 3.
Primary melanoma with strong Hif-1α expression in over 50% of tumor cells (A x200). Identical primary melanoma with co-staining for Mib-1/TRP-2 without TRP-2 expression (B x200). Proliferating TRP-2 negative tumor cells (arrow). Two different melanoma patient's cell lines (M010817 and M000921) under normoxic (blue) and hypoxic (red) conditions: Significant downregulation of TRP-2 in the hypoxia treated samples (C). Fluorescence labelling for Dct in mouse skin (D-F): Dct expression in melanocyte stem cells of the hair follicle bulge (E) as well as in melanocytes of the hair follicle bulb (F).
TRP-2 Fluorescence Labeling in the Hair Follicle in the Mouse Skin
As previously shown [17], Dct expression in the hair follicle bulge labels melanocyte stem cells. Fluorescence labelling of Trp-2 (Dct) showed positivity for Dct in the melanocytes in the hair bulb region, thereby showing that Trp-2 expression is not restricted to the stem cell compartment (Figure 3D). These findings show that TRP-2 is a melanocytic differentiation antigen and not a stem cell marker.
Discussion
In this study, we characterize TRP2 as a melanoma differentiation antigen without evidence to be a stem cell marker. Our data are consistent with a model that an aggressive proliferative TRP-2-negative subpopulation exists in primary melanoma, which significantly increases with tumor progression.
In the past years a major effort was to define new tumor targets for immunotherapeutic purposes. Ideally these targets should be stably and specifically expressed in the tumor and able to trigger an immune response.
TRP-2 is an immunogenic enzyme involved in the melanin synthesis and considered as a melanoma differentiation marker but also as a melanocyte stem cell marker. There is evidence that cancer stem cells are involved in the tumor progression and dissemination, which includes a series of distinct steps that together comprise the “invasion–metastasis cascade” [22]. Therefore, a therapy that targets cancer stem cells could be highly effective if not curative. Accordingly, the role of TRP-2 in melanoma as a stem cell or differentiation marker is a relevant issue for therapeutical purposes. In mice, we show that Trp2 (Dct) is a differentiation antigen and not a stem cell marker demonstrated by the fact that the population of melanoblasts/melanocytes express Trp-2 as well as melanocyte stem cells, located in the bulge region of the hair follicle (Figure 3D-F). In order to study the expression of TRP-2 (DCT) in humans, we analysed primary and metastatic melanomas as well as patients’ derived primary melanoma cell cultures. We could demonstrate that TRP-2 expression is significantly correlated with expression of the melanoma differentiation antigen Melan A in primary melanomas, and melanoma metastases. These data suggest that TRP-2 expression is rather correlated with the differentiation degree of melanocytes as indicated by the co-expression with Melan A. In addition, there is a significant loss of TRP-2 expression with tumor progression. These results underline that TRP-2 is a differentiation antigen and not a stem cell marker also in human melanoma.
From molecular profiling studies it is established that progression of tumors, including malignant melanomas, is associated with an accumulation of new genetic hits [23]. It is therefore reasonable that differentiation antigens are lost with tumor progression. Interestingly despite several TRP-2 vaccination studies [12], [13], [14], [15], TRP-2 expression has not been studied yet in human melanoma. Our study is the first report on TRP-2 expression in over 200 melanomas and melanoma cell cultures.
According to our data TRP-2 negative cells are considered an aggressive subpopulation, which has a survival benefit and which is highly proliferative. Interestingly, this TRP-2 negative/Mib-1 positive subpopulation is significantly associated with Breslow tumor thickness. Furthermore, patients with more than 15 percent of TRP-2 negative/Mib-1 positive cells in their primary melanoma, approached significance for a less favourable tumor specific survival. The course of their disease was more aggressive with earlier development of metastases and death (Figure 1E).
Remarkably, the presence of the TRP-2 negative/Mib-1 positive subpopulation is significantly hypoxia related. TRP-2 and other genes involved in the pigment production pathway, including Melan A, are transcriptional targets of the transcription factor microphthalmia-associated transcription factor (MITF). Hoek et al. and others have developed a model of tumor progression, in which melanoma cells are switching between two cell phenotypes of proliferation and invasion. MITF and many of its target genes, including TRP-2, were shown to be downregulated in the dedifferentiated invasive phenotype cells compared to the more melanocytic proliferative phenotype cells.
Our experiments show a clear downregulation by TRP-2 by hypoxia, supporting recent studies which show that hypoxia, through Hif-1α is leading to a downregulation of melanocytic markers like MITF and its targets and therefore causing a dedifferentiation of the melanoma cells with increased invasive potential [24], [25]. Hypoxia plays an important role in the differentiation process of cells [26], [27] as well as in tumor progression [28]. Therefore, our finding in melanoma that the TRP-2 negative/Mib-1 positive cells are hypoxia related is of relevance as this indicates that this subpopulation of cells would not be targeted by vaccination. Several chemotherapies target hypoxic cells and moreover hypoxic specific therapies have been developed (ie TH302) [29].
In the field of tumor immunology, a successful strategy implies polyvalent immunization and synergistic combination of chemotherapies and vaccination. Taken together our results demonstrate TRP-2 as a good differentiation marker highlighting the importance to combine TRP-2 vaccination with other strategies targeting the aggressive undifferentiated hypoxia related subpopulation.
Acknowledgments
We are grateful to N. Wey for photographic reproductions.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors state no conflict of interest.
Grant Support: This work was supported by the Swiss Cancer League.
References
- 1.Balch C.M. Cutaneous melanoma: prognosis and treatment results worldwide. Semin Surg Oncol. 1992;8:400–414. doi: 10.1002/ssu.2980080611. [DOI] [PubMed] [Google Scholar]
- 2.Ho R.C. Medical management of stage IV malignant melanoma. Medical issues. Cancer. 1995;75:735–741. doi: 10.1002/1097-0142(19950115)75:2+<735::aid-cncr2820751418>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S.A. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst. 1996;88:1635–1644. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- 4.van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 5.Gaugler B., Van den Eynde B., van der Bruggen P., Romero P., Gaforio J.J., De Plaen E., Lethe B., Brasseur F., Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Eynde B., Peeters O., De Backer O., Gaugler B., Lucas S., Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boel P., Wildmann C., Sensi M.L., Brasseur R., Renauld J.C., Coulie P., Boon T., van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 8.Tief K., Schmidt A., Beermann F. New evidence for presence of tyrosinase in substantia nigra, forebrain and midbrain. Brain Res Mol Brain Res. 1998;53:307–310. doi: 10.1016/s0169-328x(97)00301-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang R.F., Appella E., Kawakami Y., Kang X., Rosenberg S.A. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkhurst M.R., Fitzgerald E.B., Southwood S., Sette A., Rosenberg S.A., Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]
- 11.Bloom M.B., Perry-Lalley D., Robbins P.F., Li Y., el-Gamil M., Rosenberg S.A., Yang J.C. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonuleit H., Tuting T., Steitz J., Bruck J., Giesecke A., Steinbrink K., Knop J., Enk A.H. Efficient transduction of mature CD83 + dendritic cells using recombinant adenovirus suppressed T cell stimulatory capacity. Gene Ther. 2000;7:249–254. doi: 10.1038/sj.gt.3301077. [DOI] [PubMed] [Google Scholar]
- 13.Cho H.I., Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avogadri F., Merghoub T., Maughan M.F., Hirschhorn-Cymerman D., Morris J., Ritter E., Olmsted R., Houghton A.N., Wolchok J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triozzi P.L., Aldrich W., Ponnazhagan S. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 2010;28:7837–7843. doi: 10.1016/j.vaccine.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 16.Sendoel A., Kohler I., Fellmann C., Lowe S.W., Hengartner M.O. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465:577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura E.K., Jordan S.A., Oshima H., Yoshida H., Osawa M., Moriyama M., Jackson I.J., Barrandon Y., Miyachi Y., Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 18.Mihic-Probst D., Mnich C.D., Oberholzer P.A., Seifert B., Sasse B., Moch H., Dummer R. p16 expression in primary malignant melanoma is associated with prognosis and lymph node status. Int J Cancer. 2006;118:2262–2268. doi: 10.1002/ijc.21608. [DOI] [PubMed] [Google Scholar]
- 19.Mihic-Probst D., Kuster A., Kilgus S., Bode-Lesniewska B., Ingold-Heppner B., Leung C., Storz M., Seifert B., Marino S., Schraml P. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int J Cancer. 2007;121:1764–1770. doi: 10.1002/ijc.22891. [DOI] [PubMed] [Google Scholar]
- 20.Shakhova O., Zingg D., Schaefer S.M., Hari L., Civenni G., Blunschi J., Claudinot S., Okoniewski M., Beermann F., Mihic-Probst D. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 21.Krajewska M., Krajewski S., Epstein J.I., Shabaik A., Sauvageot J., Song K., Kitada S., Reed J.C. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger T.R., Peeper D.S. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Vogelstein B., Kinzler K.W. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 24.Widmer DS, Hoek KS, Cheng PF, Eichhoff OM, Biedermann T, Raaijmakers MI, Hemmi S, Dummer R, Levesque MP (2013). Hypoxia Contributes to Melanoma Heterogeneity by Triggering HIF1alpha-Dependent Phenotype Switching J Invest Dermatol. [DOI] [PubMed]
- 25.Cheli Y., Giuliano S., Fenouille N., Allegra M., Hofman V., Hofman P., Bahadoran P., Lacour J.P., Tartare-Deckert S., Bertolotto C. Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene. 2012;31:2461–2470. doi: 10.1038/onc.2011.425. [DOI] [PubMed] [Google Scholar]
- 26.Ezashi T., Das P., Roberts R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Q., Lee Y.J., Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 28.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 29.Moyer M.W. Targeting hypoxia brings breath of fresh air to cancer therapy. Nat Med. 2012;18:636–637. doi: 10.1038/nm0512-636b. [DOI] [PubMed] [Google Scholar]