Abstract
BACKGROUND: Efficacy of second-line chemotherapy in platinum-pretreated non–small cell lung cancer (NSCLC) is poor. This study investigated efficacy of computed tomography–guided percutaneous fine-needle 5% ethanol-cisplatin intratumoral injection (CT-PFNECII) combined with second-line chemotherapy in patients with platinum-pretreated stage IV NSCLC. PATIENTS: Between October 2011 and July 2013, 34 eligible patients were randomly assigned to receive either CT-PFNECII combined with second-line chemotherapy (combination group, n = 17) or second-line chemotherapy alone (chemotherapy group, n = 17). The primary end points were the proportions of patients who achieved an overall response rate (ORR) and disease control rate (DCR). Secondary end points were median survival and progression-free survival (PFS). RESULTS: The ORR and DCR in the combination group were significantly higher than in the chemotherapy group (23.53% vs 11.76% for ORR, P < .01; and 58.82% vs 35.29% for DCR, P < .01). Compared with patients in the chemotherapy group, patients in the combination group had significantly longer PFS (5.4 months vs 3.0 months, P < .01) and median survival (9.5 months vs 5.3 months, P < .01). CONCLUSIONS: CT-PFNECII combined with second-line chemotherapy provided a higher response rate and improved survival than second-line chemotherapy for patients with platinum-pretreated stage IV NSCLC.
Introduction
Lung cancer is the most common cancer in the world, and non–small cell lung cancer (NSCLC) accounts for approximately 80% of all cases of lung cancer. Platinum-based chemotherapy is the standard first-line care for NSCLC [1], [2]. However, platinum resistance and tumor recurrence, which are believed to be mediated by cancer stem-like cells (CSCs) or side-population cells, invariably develop [3], [4], [5]. Currently, second-line chemotherapy is the standard of care for platinum-pretreated NSCLC even though its efficiency is poor [1], [2], [5].
Docetaxel and pemetrexed are currently the standard second-line chemotherapy agents for NSCLC. Treatment with pemetrexed generally results in clinically equivalent efficacy outcomes with docetaxel in the second-line treatment of patients with advanced NSCLC [1]. However, pemetrexed and docetaxel only produced overall response rates (ORRs) of 9.1% and 8.8% with a median survival time of 8.3 and 7.9 months, respectively, in platinum-pretreated NSCLC [1].
The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib have also been used as standard second-line agents in treating NSCLC. Sensitivity to EGFR TKIs is dependent on the activation of the EGFR pathway or the presence of EGFR-interacting proteins [5]. Studies showed that no significant differences in efficacy were noted between patients treated with TKIs and those treated with docetaxel or pemetrexed in platinum-pretreated NSCLC [5], [6], [7].
Therapeutic inhibition of EGFR with TKIs has resulted in favorable response rates only in 11.14% to 15.25% of platinum-pretreated NSCLC, mostly because the EGFR mutation or gene amplification rate is only 16.6% in NSCLC [5], [6]. In addition, median survival of 7.6 months for gefitinib in platinum-pretreated NSCLC and 5.3 months for erlotinib in platinum-resistant NSCLC indicate the desperate need for novel approaches to treat the patient population [5], [7], [8], [9].
We previously found that 5% ethanol-cisplatin injected intratumorally could eradicate cisplatin-resistant lung tumors and extend survival by improved killing of lung CSCs in mice [10]. We believe that 5% ethanol improves the efficacy of CSC killing by inhibiting breast cancer resistance protein (BRCP/ABCG2) drug transporter function and by improving the penetration of cisplatin into the tumor cells [10]. On the basis of our model organism studies, it is possible that computed tomography (CT)–guided percutaneous fine-needle 5% ethanol-cisplatin intratumoral injection (CT-PFNECII) might also regress platinum-pretreated or even platinum-resistant tumors in patients with NSCLC by killing chemoresistant cancer stem cells and cancer cells. Furthermore, it is possible that the residual unkilled but damaged tumor cells after 5% ethanol-cisplatin treatment might be more fragile and sensitive to systemic second-line chemotherapy agents. Thus, combination of CT-PFNECII with systemic second-line chemotherapy might provide a new way to improve survival of this patient population.
This study is aimed to investigate the efficacy and safety of CT-PFNECII combined with second-line chemotherapy in patients with platinum-pretreated stage IV NSCLC.
Patients and Methods
Patients
The study protocol was approved by the Institutional Review Boards of the No. 309 People’s Liberation Army Hospital in Beijing, and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Adult patients with histologically documented NSCLC who received ≥ 1 platinum-based chemotherapy regimen, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, were potentially eligible for this study. Patients were excluded if they had a life expectancy of less than 1 month or had an indication for liver, renal, or heart failure. Thirty-four eligible patients were enrolled in this study and asked for written informed consent. Information collected at baseline included sex, age, ECOG performance status, tumor size, histology, disease stage, lung tumor–related chest pain or dyspnea, time since last chemotherapy (interval from last chemotherapy to inclusion), times of CT-PFNECII, and platinum resistance. Protocol design, data collection, and analysis were solely the responsibility of the authors.
Study Design
Eligible patients were randomly assigned to receive either CT-PFNECII combined with second-line chemotherapy (standard pemetrexed or docetaxel dosing schedule) (combination group, n = 17) or second-line chemotherapy (standard pemetrexed or docetaxel dosing schedule) alone (chemotherapy group, n = 17).
If a patient received prior taxane treatment, such as docetaxel or paclitaxel, pemetrexed was given as second-line chemotherapy. Otherwise, docetaxel was given as second-line chemotherapy.
Ethanol-cisplatin (5%) was freshly prepared with 10 mg (2 ml) of cisplatin (Qilu Pharmaceutical Co, Ltd, Shandong, China) dissolved into an ethanol solution of 20 to 30 ml with the final ethanol concentration of 5% (vol/vol). Next, the freshly prepared 20 to 30 ml of 5% ethanol-cisplatin solution was percutaneously injected into the lung tumor individually with a 22-gauge fine needle (Gallini Medical Devices, Via Frattini, Italy) under CT (GE Healthcare, Waukesha, WI) guidance, once a week. This procedure was performed weekly for two consecutive weeks, and a third week with no treatment completed one cycle. Single chemotherapy agent pemetrexed (Alimta; Eli Lilly and Company, Indianapolis, IN) (500 mg/m2 as a 10-minute IV infusion on day 1 of a 21-day cycle) or docetaxel (Taxotere; Aventis Pharmaceuticals, Bridgewater, NJ) (75 mg/m2 as a 1-hour IV infusion on day 1 of a 21-day cycle) was administered IV 1 day after CT-PFNECII every 3 weeks as a cycle. Each patient in the combination group received one to two cycles of CT-PFNECII and four cycles of pemetrexed/docetaxel, and each patient in the chemotherapy group received four cycles of pemetrexed/docetaxel. Patients on the pemetrexed arm were instructed to take folic acid (350-1000 μg) orally daily beginning approximately 1 to 2 weeks before the first dose of pemetrexed and continuing daily until 3 weeks after the last dose of pemetrexed. A 1000-μg vitamin B12 injection was administered intramuscularly 1 week before the first dose of pemetrexed and was repeated approximately every 9 weeks until after discontinuation. Patients on the pemetrexed arm were instructed to take dexamethasone (Guizhou Guangzheng Pharmaceuticals, Guizhou, China) (4 mg orally twice daily the day before, the day of, and the day after pemetrexed) as a prophylactic measure against skin rash. Patients on the docetaxel arm were instructed to take dexamethasone (8 mg orally twice daily the day before, the day of, and the day after docetaxel).
Follow-up and Study End Points
All patients were followed up every 2 months regularly after the treatment protocol was finished. Patients were evaluated and followed up with ORR, disease control rate (DCR), progression-free survival (PFS), median overall survival (OS), and safety profile. Responses were assessed with the use of the Response Evaluation Criteria in Solid Tumors (RECIST, set by an international collaboration including the European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group), and toxic effects were assessed according to the Common Toxicity Criteria of the National Cancer Institute (Bethesda, MD) (version 2.0). Lung tumor–related symptoms including chest pain and dyspnea before and after CT-PFNECII were observed. CT-PFNECII–related side effects including pain, cough, fever, hemoptysis, and pneumothorax and chemotherapy-related side effects including myelosuppression and gastrointestinal reaction were observed in this study.
All patients were followed up until death or until the end of the study, with a minimum of 2 months and maximum of 18 months of follow-up.
Statistical Analysis
All primary analyses were performed on an intention-to-treat principle. The RECIST analysis was calculated according to the ordered one-way data of Ridit analysis. The effect of two kinds of treatment regimens was calculated using a two-sided log-rank test. Survival analysis was calculated according to the Kaplan-Meier method with SPSS software (IBM, Armonk, NY). Ninety-five percent confidence intervals (CIs) were calculated when appropriate. Differences were considered significant at P < .05.
Results
Patients
Between October 1, 2011 and July 1, 2013, a total of 34 patients were randomly assigned to receive either CT-PFNECII combined with second-line chemotherapy or second-line chemotherapy alone. Among them, 17 patients received CT-PFNECII combined with second-line chemotherapy, and 17 patients received standard second-line chemotherapy alone. In the combination group, 7 patients received two cycles (four times) of CT-PFNECII, and 10 patients received one cycle (two times) of CT-PFNECII. The average cycle of CT-PFNECII received by patients in the combination group was 1.41. Seven patients in the combination group and six patients in the chemotherapy group had tumor-related chest pain or dyspnea. In each group, there were five (29.41%) platinum-resistant patients (disease recurred within 3 months to previous chemotherapy). The overall study population had a median age of 56 years (range = 32-76 years), and baseline characteristics were generally well balanced between the treatment groups (Table 1).
Table 1.
Demographics of the Patients and Their Cancers.
| Characteristic |
CT-PFNECII + Chemotherapy |
% |
Chemotherapy |
% |
|---|---|---|---|---|
| (n = 17) |
(n = 17) |
|||
| No. of Patients | No. of Patients | |||
| Age, yr | ||||
| Median | 55 | 57 | ||
| Range | 32-74 | 35-76 | ||
| Male sex | 13 | 12 | ||
| Weight, kg | ||||
| Median | 65 | 64 | ||
| Range | 41-105 | 38-94 | ||
| Interval from last chemotherapy to inclusion, months | ||||
| Median | 2.5 | 2.5 | ||
| Interquartile range | 2-9 | 2-10 | ||
| Patients with tumor-related chest pain or dyspnea | 7 | 6 | ||
| Times of CT-PFNECII | ||||
| 4 | 7 | 41.18 | ||
| 2 | 10 | 58.82 | ||
| ECOG status⁎ | ||||
| ≤ 1 | 5 | 29.41 | 5 | 29.41 |
| 2 | 12 | 70.59 | 12 | 70.59 |
| Cancer stage | ||||
| IV | 17 | 100 | 17 | 100 |
| Histologic type of cancer | ||||
| Adenocarcinoma | 8 | 47.06 | 8 | 47.06 |
| Squamous | 5 | 29.41 | 7 | 41.18 |
| Sarcomatoid carcinoma | 1 | 5.88 | 0 | 0 |
| Large cell | 0 | 0 | 0 | 0 |
| Other | 3 | 17.65 | 2 | 11.77 |
| Tumor size, cm | ||||
| 3-5 | 11 | 10 | 58.82 | |
| > 5 | 6 | 7 | 41.18 | |
| Platinum-resistant patients | 5 | 5 | 29.41 | |
Higher scores on the ECOG scale indicate poorer performance.
Efficacy
In the combination group, 10 of 17 (58.82%) patients benefited from our treatment in terms of disease control, and all 7 patients (100%) who had lung tumor–related chest pain and dyspnea before the treatment achieved significant symptom relief within 48 to 72 hours after CT-PFNECII treatment. By contrast, in the chemotherapy group, only 6 of 17 (35.29%) patients achieved disease control, and 1 of 6 (16.67%) patients with tumor-related chest pain or dyspnea acquired symptom control. Of the 17 patients in the combination group, tumor was completely destroyed in 1 patient, and tumors were controlled in 9 other patients with 3 patients (17.64%) judged as partial response (PR) and 6 patients (35.29%) judged as stable disease (SD) after two cycles of treatment. The CT scans of two patients before and 6 months after the combination treatment are shown in Figure 1. The ORR and DCR in the combination group were 8 of 17 (23.53%) and 10 of 17 (58.82%), respectively. Of the seven patients who received two cycles of CT-PFNECII, one complete response (CR), one PR, and three SD were achieved (ORR = 28.57%; DCR = 71.43%). And among 10 patients who received one cycle of CT-PFNECII, two PR and three SD were achieved (ORR = 20%; DCR = 50%). ORR and DCR of patients who received two cycles of CT-PFNECII tended to be higher than those of patients who received one cycle of CT-PFNECII. By comparison, 2 patients (11.76%) achieved PR, 4 patients (23.53%) achieved SD, and 11 patients (64.71%) achieved progressive disease (PD) in chemotherapy group (ORR = 11.76%; DCR = 35.29%).
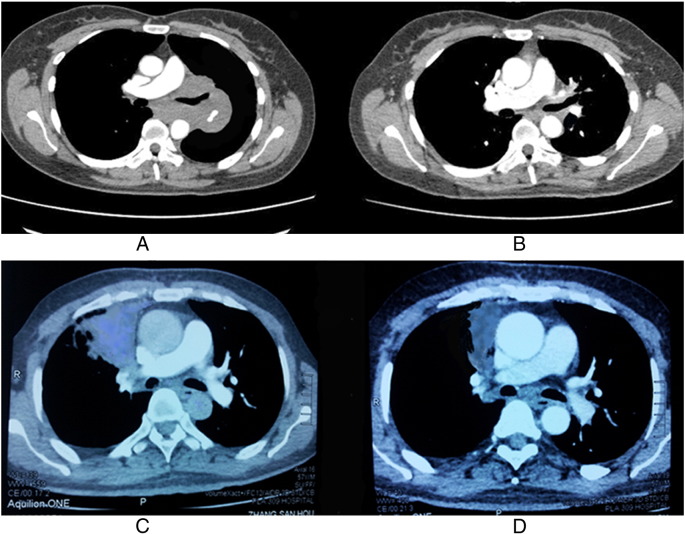
Figure 1.
Comparison of two patients' CT scan before and 6 months after the combination treatment. (A) CT scan of patient A before the treatment. This patient had progressive dyspnea before the treatment. (B) CT scan of patient A 6 months after the combination treatment. Lung tumor regressed, and symptoms were relieved 6 months after the combination treatment. (C) CT scan of patient B before the treatment. This patient had severe chest pain. (D) CT scan of patient B 6 months after the combination treatment. Lung tumor shrank significantly, and pain disappeared 6 months after the combination treatment.
Ranked data Ridit analysis for RECIST showed that the ORR and DCR in the combination group were significantly higher than ORR and DCR in the chemotherapy group, respectively (23.53% vs 11.76% for ORR, P < .01; 58.82% vs 35.29% for DCR, P < .01) (Table 2).
Table 2.
Efficacy Results.
| Efficacy End Point | CT-PFNECII + Chemotherapy (n = 17) |
Chemotherapy (n = 17) |
P | Hazard Ratio | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | ||||
| OS, mo |
|||||||||
| Median | 9.5 | 7.91-10.49 | 5.3 | 3.66-6.94 | .004 | .29 | .11-.80 | ||
| PFS, mo | |||||||||
| Median | 5.4 | 4.30-6.70 | 3.0 | 2.23-3.77 | .001 | .26 | .15-.76 | ||
| Range | 0-10 | 0-7 | |||||||
| Best overall response⁎ | .024# | ||||||||
| CR | 1 | 0 | |||||||
| PR | 3 | 2 | |||||||
| SD | 6 | 4 | |||||||
| PD | 7 | 11 | |||||||
| ORR (CR+PR) | 4 | 23.53 | 2 | 11.76 | .000⁎ | ||||
| DCR (CR+PR+SD at first tumor assessment) | 10 | 58.82 | 6 | 35.29 | .000⁎ | ||||
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
n = 17 for each group.
χ2 test.
Ridit analysis.
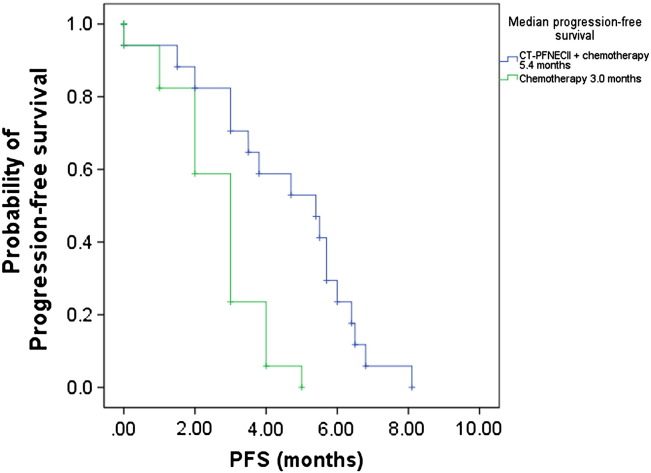
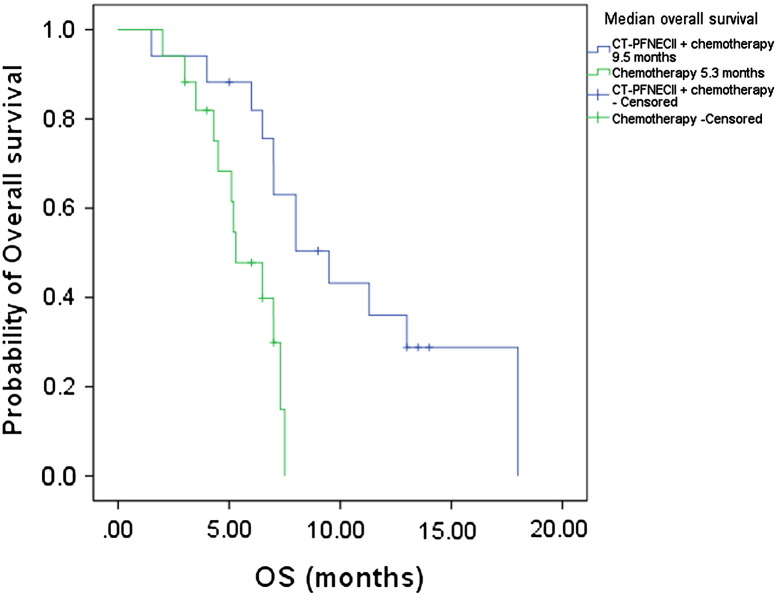
The median survival time was 9.5 months in the combination group (95% CI, 6.38-12.62 months) and 5.3 months in the chemotherapy group (95% CI, 3.66-6.94 months). The time to progression was 5.4 months (95% CI, 3.11-7.69 months) in the combination group and 3.0 months (95% CI, 2.43-3.57 months) in the chemotherapy group (Table 2). Compared with patients in the chemotherapy group, the patients in the combination group had significantly longer PFS (P < .01) and OS (P < .01) (Figure 2, Figure 3).
Figure 2.
Kaplan-Meier estimates of the probability of PFS in CT-PFNECII + chemotherapy–treated and chemotherapy-treated patients with NSCLC. PFS of CT-PFNECII + chemotherapy–group patients was significantly longer than that of chemotherapy group. The P value (P = .001) was derived from a log-rank test comparing both treatment groups. The hazard ratio of mortality was 0.48 (95% CI, 0.44-5.35) in favor of the CT-PFNECII + chemotherapy group.
Figure 3.
Kaplan-Meier estimates of the probability of OS in CT-PFNECII + chemotherapy–treated and chemotherapy-treated patients with NSCLC. Survival of CT-PFNECII + chemotherapy–group patients was significantly longer than that of chemotherapy group. The P value (P = .004) was derived from a log-rank test comparing both treatment groups. The hazard ratio of mortality was 0.29 (95% CI, 0.11-0.80) in favor of the CT-PFNECII + chemotherapy group.
Safety
Adverse events associated with CT-PFNECII and chemotherapy are summarized in Table 3. The adverse events associated with CT-PFNECII were transient mild local pain (7 of 17 patients, 41.18%), cough (8 of 17 patients, 47.06%), and mild pneumothorax (2 of 17 patients, 11.76%) during the procedure and mild hemoptysis (2 of 17 patients, 11.76%) for 3 to 5 days after the procedure. All the side effects were mild and well tolerated and did not need further medications or invasive procedures to control. Grades 3 to 5 adverse events associated with chemotherapy were observed in four patients (23.53%) of the combination group and in four patients (23.53%) of the chemotherapy group. No significant difference was found between the two groups (23.53% vs 23.53%; P > 0.05). No serious adverse events were observed (Table 3).
Table 3.
Side Effects in CT-PFNECII–Treated Patients.
| Side Effects | Patient (n = 17) | |
|---|---|---|
| Adverse events associated with intratumoral injection | All | Grade 3/4 |
| Chest pain | 7 | 0 |
| Cough | 8 | 0 |
| Pneumothorax | 2 | 0 |
| Hemoptysis | 2 | 0 |
| Adverse events associated with chemotherapy | ||
| Myelosuppression | 5 | 2 |
| Gastrointestinal reaction | 2 | 2 |
Discussion
The results of our study suggest that CT-PFNECII combined with second-line chemotherapy produced a higher response rate and improved survival than second-line chemotherapy in platinum-pretreated stage IV NSCLC. In addition, side effects of this combination therapy were generally well tolerated.
Compared with ORR of 11.76% and DCR of 35.29% in the chemotherapy group, the combination therapy provided an ORR of 23.53% and a DCR of 58.82% in platinum-pretreated stage IV NSCLC. Of note, one complete tumor regression was achieved in a patient by two cycles of combination treatment. More importantly, all patients who had lung tumor–related chest pain or dyspnea before our treatment achieved significant symptom relief even within 72 hours after CT-PFNECII treatment. Our pilot study suggests that CT-PFNECII combined with second-line chemotherapy has potent antitumor activity against platinum-pretreated NSCLC tumors.
The benefit of our combination treatment in terms of survival outcomes was also quite encouraging. Considering that 29.41% of patients in our study population were platinum resistant (five patients in each arm) and 58.82% of the patients (10 of 17) received CT-PFNECII two times, the PFS of 5.4 months and OS of 9.5 months by our combination treatment were more valuable.
The side effects of CT-PFNECII such as transient mild pain and cough in patients with lung cancer were minimal and well tolerated because only quite small amount of cisplatin and quite low concentration of ethanol were injected intratumorally. In addition, mild pneumothorax and mild hemoptysis relating to the procedure were uncommon because we used a 22-gauge fine needle under the precise guidance of CT. Furthermore, combination of CT-PFNECII with second-line chemotherapy did not worsen common side effects of chemotherapy. No significant differences in chemotherapy-related adverse events in the two groups were noted, indicating clinical safety of CT-PFNECII.
We previously found that 5% ethanol could potently inhibit ABCG2 pump, which is a major drug transporter in protecting platinum-resistant NSCLC cells from cytotoxic agents. We also found that 5% ethanol-cisplatin injected intratumorally could eradicate cisplatin-resistant lung tumors by killing chemoresistant lung CSCs and normal lung cancer cells [10]. We speculate that the residual unkilled but damaged tumor cells in the 5% ethanol-cisplatin treatment group might be more fragile and sensitive to second-line chemotherapy agents. As a result, we speculate that CT-PFNECII treatment might have synergistic effects with systemic second-line chemotherapies, such as docetaxel or pemetrexed, in controlling platinum-pretreated NSCLC.
Our study showed that second-line chemotherapy pemetrexed or docetaxel produced an ORR of 11.76% and a median survival time of 5.3 months in our patients. The ORR in our study is similar with that in a previous report, but the median survival in our study is a little shorter than in their study [1], [11]. Possible reasons for this could be that patients in our study were all with stage IV disease and almost 30% of them were platinum resistant, whereas only 74.8% of the patients with NSCLC in the previous study were stage IV [1].
However, when pemetrexed or docetaxel was combined with CT-PFNECII, the combination approach showed an ORR of 23.53% and a median survival time of 9.5 months in our patients with platinum-pretreated NSCLC. Considering that the ORRs were only 9.1% and 8.8% for pemetrexed and docetaxel, respectively, in the previous study [1], these data are quite encouraging. In addition, we found that CT-PFNECII could efficiently control lung tumor–related chest pain or dyspnea even within 72 hours in all patients who had these symptoms before. This suggests that 5% ethanol-cisplatin injected intratumorally could have potent antitumor activity against platinum-pretreated NSCLC. Our previous studies in mouse xenografts showed that 5% ethanol could inhibit the ABCG2 pump in tumor cells as well as drive the penetration of cisplatin into tumor cells [10], [12]. Our results also support the previous findings that decreased platinum accumulation in NSCLC tumor tissues might be an important mechanism of platinum resistance in patients with NSCLC [13].
Compared with a median survival of 5.2 months produced by docetaxel and 9.4 months by selumetinib plus docetaxel in patients with platinum-pretreated KRAS-mutant NSCLC, the median survival of 9.5 months by our combination treatment shows promising potential [14].
In contrast to the median survival of 7.6 months for gefitinib in platinum-pretreated NSCLC and 5.3 months for erlotinib in platinum-resistant NSCLC, the median survival of 9.5 months by our combination approach suggests that it might compare favorably to the more expensive EGFR TKIs [7], [8].
Intratumoral injection of chemotherapeutic agents in ethanol mixtures might also be effective in treating other types of cancer. Studies by Pietronigro and his colleagues showed that intratumoral injection of chemotherapeutic agent bis-chloroethylnitrosourea, dissolved in 100% ethanol could produce a 40% cure rate in rats bearing intracranial T9 tumors and 72% SD in patients with recurrent malignant glioma [15], [16], [17]. However, our previous results showed that the chemotherapeutic agent cisplatin, when dissolved in high concentrations of ethanol such as 50% ethanol, produced minimal tumor inhibition [10], [18]. However, the glioma tumors in patients in the Pietronigro studies were smaller than the tumors in our patients. We speculate that smaller tumors might be easier to be suffused by 100% ethanol, leading to complete tumor necrosis. Further studies on the efficacy of chemotherapy-ethanol mixtures are needed to determine the optimal therapies for specific types of cancers.
We found that the CT-PFNECII–related side effects were mild and well tolerated even by quite frail patients with NSCLC, and these patients did not need further medications or invasive procedures to control the side effects. Because a 22-gauge fine needle was used in our CT-PFNECII administration, the procedure is essentially “noninvasive” to the patients with NSCLC and could be safely performed in any parts of either lung lobe under CT guidance. This is less invasive than other procedures such as cryoablation that often uses two cryoprobes of 15 to 17 gauges inserted percutaneously into the lung tumor. Accordingly, the risks of pneumothorax and hemothorax by cryoablation are more than likely higher that in our procedure [19].
We also found in our pilot study that CT-PFNECII combined with second-line chemotherapy might provide a higher response rate and improved survival for patients with platinum-pretreated stage IV NSCLC. Importantly, CT-PFNECII could efficiently control lung tumor–related symptoms such as chest pain and dyspnea in patients with platinum-pretreated NSCLC even within 3 days after the procedure. Because 5% ethanol-cisplatin injected intratumorally could regress platinum-pretreated lung tumor in NSCLC and CT-guided percutaneous fine-needle intratumoral injection is a quite safe clinical procedure, application of CT-PFNECII in platinum-pretreated NSCLC warrants further study [10].
In conclusion, this study conducted in a small patient population showed that CT-PFNECII combined with second-line chemotherapy provides a higher response rate and improved survival for patients with platinum-pretreated stage IV NSCLC than second-line chemotherapy alone. As side effects of this approach were well tolerated by the patients with cancer, its further clinical applications in lung and other types of cancer deserves further study in larger cohorts.
Footnotes
The project was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, China State Education Ministry (No. 2007–1108) to Q.N.
References
- 1.Hanna N., Shepherd F.A., Fossella F.V., Pereira J.R., De Marinis F., von Pawel J., Gatzemeier U., Tsao T.C., Pless M., Muller T. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 2.Roth B.J., Krilov L., Adams S., Aghajanian C.A., Bach P., Braiteh F., Brose M.S., Ellis L.M., Erba H., George D.J. Clinical cancer advances 2012: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2013;31:131–161. doi: 10.1200/JCO.2012.47.1938. [DOI] [PubMed] [Google Scholar]
- 3.Niu Q., Wang W., Li Y., Ruden D.M., Wang F., Li Y., Wang F., Song J., Zheng K. Low molecular weight heparin ablates lung cancer cisplatin-resistance by inducing proteasome-mediated ABCG2 protein degradation. PLoS One. 2012;7:e41035. doi: 10.1371/journal.pone.0041035. [Epub 2012 Jul 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 5.Murphy M., Stordal B. Erlotinib or gefitinib for the treatment of relapsed platinum pretreated non-small cell lung cancer and ovarian cancer: a systematic review. Drug Resist Updat. 2011;14:177–190. doi: 10.1016/j.drup.2011.02.004. [Epub 2011 Mar 24] [DOI] [PubMed] [Google Scholar]
- 6.Rosell R., Moran T., Queralt C., Porta R., Cardenal F., Camps C., Majem M., Lopez-Vivanco G., Isla D., Provencio M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Kim E.S., Hirsh V., Mok T., Socinski M.A., Gervais R., Wu Y.L., Li L.Y., Watkins C.L., Sellers M.V., Lowe E.S. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 8.Ciuleanu T., Stelmakh L., Cicenas S., Miliauskas S., Grigorescu A.C., Hillenbach C., Johannsdottir H.K., Klughammer B., Gonzalez E.E. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 9.Fung-Kee-Fung M., Oliver T., Elit L., Oza A., Hirte H.W., Bryson P. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol. 2007;14:195–208. doi: 10.3747/co.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu Q., Wang W., Li Y., Ruden D.M., Li Q., Wang F. Cisplatin in 5% ethanol eradicates cisplatin-resistant lung tumor by killing lung cancer side population cells and non-SP cells. Front Genet. 2013;4:163. doi: 10.3389/fgene.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu Q., Wang W., Li Y., Qin S., Wang Y., Wan G., Guan J., Zhu W. Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies. Int Immunopharmacol. 2011;11:449–456. doi: 10.1016/j.intimp.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Hamstra D.A., Moffat B.A., Hall D.E., Young J.M., Desmond T.J., Carter J., Pietronigro D., Frey K.A., Rehemtulla A., Ross B.D. Intratumoral injection of BCNU in ethanol (DTI-015) results in enhanced delivery to tumor—a pharmacokinetic study. J Neurooncol. 2005;73:225–238. doi: 10.1007/s11060-004-5675-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim E.S., Lee J.J., He G., Chow C.W., Fujimoto J., Kalhor N., Swisher S.G., Wistuba I.I., Stewart D.J., Siddik Z.H. Tissue platinum concentration and tumor response in non–small-cell lung cancer. J Clin Oncol. 2012;30:3345–3352. doi: 10.1200/JCO.2011.40.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jänne P.A., Shaw A.T., Pereira J.R., Jeannin G., Vansteenkiste J., Barrios C., Franke F.A., Grinsted L., Zazulina V., Smith P. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 15.Pietronigro D., Drnovsky F., Cravioto H., Ransohoff J. DTI-015 produces cures in T9 gliosarcoma. Neoplasia. 2003;5:17–22. doi: 10.1016/s1476-5586(03)80013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassenbusch S.J., Nardone E.M., Levin V.A., Leeds N., Pietronigro D. Stereotactic injection of DTI-015 into recurrent malignant gliomas: phase I/II trial. Neoplasia. 2003;5:9–16. doi: 10.1016/s1476-5586(03)80012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkinson M.D., Smith T.S., Haylock B., Husband D., Shenoy A., Vinjamuri S., Walker C., Pietronigro D., Warnke P.C. Phase II trial of intratumoral BCNU injection and radiotherapy on untreated adult malignant glioma. J Neurooncol. 2010;99:103–113. doi: 10.1007/s11060-010-0113-0. [DOI] [PubMed] [Google Scholar]
- 18.Tan W., Bailey A.P., Shparago M., Busby B., Covington J., Johnson J.W., Young E., Gu J.W. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol Ther. 2007;6:1211–1217. doi: 10.4161/cbt.6.8.4406. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi Y., Izumi Y., Hashimoto K., Yashiro H., Inoue M., Nakatsuka S., Goto T., Anraku M., Ohtsuka T., Kohno M. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One. 2012;7:e33223. doi: 10.1371/journal.pone.0033223. [Epub 2012 Mar 8] [DOI] [PMC free article] [PubMed] [Google Scholar]