Abstract
BACKGROUND: This study investigated the synergistic effect of sunitinib and rapamycin on tumor growth and metastasis in murine breast cancer model. METHODS: The synergistic antitumor effect of sunitinib and rapamycin on tumor growth and metastasis was investigated. Myeloid-derived suppressor cells (MDSCs) in spleens and lungs were assessed. Tumor hypoxia, vessel density and micrometastasis were evaluated. Versican, indoleamine 2,3-dioxygenase (IDO), arginase 1, interleukin-6 (IL-6), IL-10, and transforming growth factor β (TGF-β) in the lungs and tumors were examined. IL-6 and TGF-β in the blood were evaluated. RESULTS: Synergism between sunitinib and rapamycin on tumor growth was observed. Sunitinib plus rapamycin reduced splenomegaly, MDSCs in spleens and lungs, and microvessel density in tumor microenvironment, while exacerbated hypoxia and promoted cancer lung metastasis. Sunitinib plus rapamycin markedly induced versican, IDO, arginase 1, IL-6, and TGF-β expression in the lungs, whereas it reduced IDO and IL-10 expression in the primary tumor tissues. IL-6 levels in the circulation were increased after rapamycin and combination therapies. CONCLUSIONS: The combination of sunitinib plus rapamycin reduced the tumor growth but promoted tumor metastasis. This study warrants that further mTOR inhibition treatment should be closely watched in clinical setting, especially combined with antiangiogenic therapy.
Introduction
Angiogenesis is essential for tumor growth and progression [1]. Antiangiogenic therapies have been demonstrated effective on the suppression of tumor growth [2]. Paradoxically, antiangiogenic strategies can also induce local and distant metastasis [3]. Reduced oxygen supply leads to the stabilization and activation of the transcription factor hypoxia-induced factor 1 (HIF-1) [4]. Hypoxia and the expression of HIF-1 are correlated with cancer metastasis and unfavorable prognosis [5]. Through activation of the Twist, hypoxia induces epithelial-to-mesenchymal transition [6], which was associated with cancer metastasis [7]. Sunitinib is one type of multitargeted tyrosine kinase inhibitor, which targets several receptor tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR) (VEGFR-1, VEGFR-2, and VEGFR-3), PDGFR (PDGFR-α and PDGFR-β), and stem cell factor receptor (KIT) [8]. Sunitinib monotherapy has activity in advanced breast cancers [9]. Sunitinib has also been demonstrated to be effective in combination with chemotherapy in preclinical models [10]. However, sunitinib therapy can induce intratumoral hypoxia, which enriches cancer stem cells [11].
The mammalian target of rapamycin (mTOR) promotes cell growth, proliferation, and survival in response to nutrient signals and a variety of cytokines. mTOR also plays a vital role in the regulation of cancer cell growth and progression [12]. mTOR promotes cancer cell migration and invasion [13]. mTOR has been demonstrated to impact angiogenesis. The phosphatidylinositide 3-kinases (PI3K)/Akt signaling pathway is the downstream of VEGF and promotes endothelial cell survival [14]. In the hind limb ischemia, Akt is critical for ischemia and VEGF-induced angiogenesis [15]. Endothelial cells in the tumor microenvironment have chronic Akt activation, and the sustained Akt activation induces the formation of abnormal microvessels, which mimic the effects of VEGF-A–induced angiogenesis [16]. Treatment of cultured cells with rapamycin decreased activation of Akt [17]. Rapamycin can inhibit pathologic angiogenesis through the inhibition of endothelial Akt signaling [16] and VEGF production [18]. Then, mTOR has been considered as a promising target for cancer therapy [19].
mTOR regulates the expression of HIF-1α expression [20]. We then hypothesized that rapamycin could suppress antiangiogenic therapy–induced cancer metastasis. In addition, there is no study investigating the synergism between antiangiogenic therapy and rapamycin on breast tumor model. In our present study, we demonstrate the synergistic effect of rapamycin and sunitinib on tumor regression. However, the hypothesized therapeutic effect of sunitinib combined with rapamycin on lung metastasis was not observed, and, unexpectedly, we found that the combination promoted the lung metastasis of cancer cells.
Materials and Methods
Mice
BALB/c mice (6-8 weeks old) were purchased from Beijing HFK Bioscience Co (Beijing, China) and maintained under pathogen-free conditions in the animal facility with individual ventilation. All animal experiments were carried out according to protocols approved by Sichuan University’s Institutional Animal Care and Use Committee.
Cell Lines and Reagents
Murine breast cancer cell lines (4T1) were cultured in the RPMI1640 media supplemented with 10% FBS at 37°C, 5% CO2 atmosphere. Rapamycin was obtained from Selleck Chemicals (Houston, TX). Sunitinib was purchased from Pfizer company (New York, NY).
Tumor Challenge and Treatment
Syngeneic breast cancers were established by subcutaneous inoculation of 4T1 cells. Briefly, 1 × 106 4T1 cells were injected subcutaneously in the right flank of BALB/c mice. At day 6 after tumor inoculation (tumors reached an average diameter of 5 mm), animals were randomly assigned to four groups and treated with vehicle, rapamycin (4 mg/kg), sunitinib (10 mg/kg), and rapamycin (4 mg/kg) and sunitinib (10 mg/kg), respectively. Sunitinib was administered daily by gavage, and rapamycin was intraperitoneally administered daily. Tumor diameters were measured with a caliper, and tumor volumes were calculated as previously reported [21]. Tumor burden was measured by the tumor volume and the gross wet weight of tumors. Metastatic and disseminated tumors were assessed by gross evaluation and microscopical examination.
Histopathologic Examination and Hypoxia Assessment
At 21-day posttreatment, tumor was harvested, fixed, and embedded in paraffin. Tumor sections were stained with CD31 (Abcam, Cambridge, UK) and counterstained with hematoxylin (Beyotime, Jiangsu, China). Liver and kidney metastases were evaluated on hematoxylin and eosin (H&E)-stained sections. Twenty-one days after treatment, tumor-bearing mice were injected intraperitoneally with the hypoxic cell marker Hypoxyprobe-1 (60 mg/kg; Hypoxyprobe™-1, HPI Inc., Burlington, MA) and killed 90 minutes later. Tumors were excised, and frozen tumor sections were prepared. Tumor sections were stained with fluorescein isothiocyanate–conjugated mouse anti–Hypoxyprobe-1 monoclonal antibody (1:100) at 37°C for 1 hour. The hypoxic tissues were examined under a fluorescence microscope.
Flow Cytometry
At day 21 of posttreatment, spleens were harvested, and erythrocytes were lysed. Spleen cells were centrifuged, washed with phosphate-buffered saline, and then incubated with CD11b-peridinin chlorophyll protein(PerCP)-Cy5.5 Gr-1–phycoerythrin (PE) antibodies (BD Pharmingen, San Diego, CA) for 30 minutes at 4°C. Single-cell suspension of lung cells of tumor-bearing mouse was prepared and then stained with CD11b-PerCP-Cy5.5, Gr-1–PE antibodies (BD Pharmingen) for 30 minutes at 4°C. For flow cytometry analysis, cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest software (BD Biosciences, San Jose, CA).
Reverse Transcription–Polymerase Chain Reaction
Total RNA was isolated from tumor tissues using an RNA isolation kit (Axygen, Union City, CA, AP-MN-MS-RNA-50) and reverse transcribed (Takara Bio Inc., Otsu, Japan, RR047A) following the manufacturer’s protocols. Polymerase chain reaction (PCR) was performed on a CFX 96 real-time PCR thermocycler (Bio-Rad Laboratories, Hercules, CA) using specific primers and SYBR Premix Ex Taq II (Takara Bio Inc., Otsu, Japan, RR820A). Primer pairs are as follows: mouse 18S RNA, forward—CGCCGCTAGAGGTGAAATTCT and reverse—CGAACCTCCGACTTTCGTTCT; mouse interleukin-10 (IL-10), forward—ACCTGCTCCACTGCCTTGCT and reverse—GGTTGCCAAGCCTTATCGGA; mouse IL-6, forward—GATGGATGCTACCAAACTGGAT and reverse—CCAGGTAGCTATGGTACTCCAGA; mouse arginase 1, forward—GCTGTCTTCCCAAGAGTTGGG and reverse—ATGGAAGAGACCTTCAGCTAC; mouse indoleamine 2,3-dioxygenase (IDO), forward—TGGGACATTCCTTCAGTGGC and reverse—TCTCGAAGCTGCCCGTTCT; mouse transforming growth factor β (TGF-β), forward—CTCCCGTGGCTTCTAGTGC and reverse—GCCTTAGTTTGGACAGGATCTG. Data from the real-time PCR reactions were analyzed using CFX Manager Software 2.1 (Bio-Rad Laboratories). Relative changes of mRNA expression were analyzed with the 2–△△Ct method, with 18S RNA serving as an internal reference. These standardized data were used to calculate fold changes in gene expression. All real-time PCR amplifications were performed in triplicate.
ELISA
ELISA assay was performed on serum samples taken 21 days post-therapy to determine levels of IL-6 and TGF-β protein in the circulation. Briefly, 96-well microtiter plates (MultiSciences, Hang zhou, China, Catalog No. EK2812; EK2062) were coated with serum from tumor-bearing mouse for 2 hours at 37°C. For TGF-β, serum was acidified with 1 N HCl and then neutralized with 1 N NaOH. Biotinylated secondary antibody was then added to the plates for 1 hour at 37°C. Finally, streptavidin conjugated to HRP was added for 45 minutes at 37°C. Color development was achieved using tetramethylbenzidine (TMB) (MultiSciences, Hang zhou, China) solution for 10 to 15 minutes and then stopped. Optical density was measured at 450 nm. The concentration of IL-6 and TGF-β was calculated by comparison to the standard curve.
Statistical Analysis
Comparisons between groups were analyzed by means of one-way analysis of variance. A value of P < .05 was designated as statistical significance.
Results
The Combination of Rapamycin and Sunitinib Retarded Tumor Growth
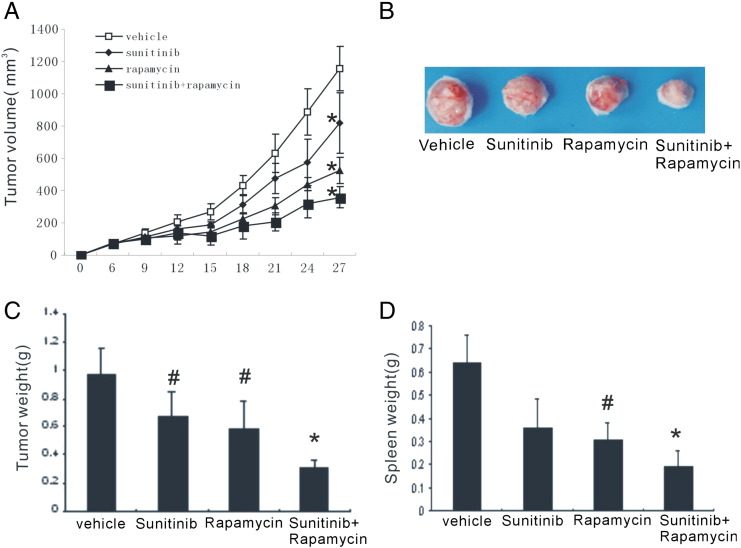
The synergistic antitumor effect of rapamycin and sunitinib on tumor growth was evaluated. Subcutaneous implantation of 4T1 breast cancer cells resulted in large tumors in the untreated group, and the mean tumor volume was 1157.02 ± 138.59 mm3 21 days after implantation. There was limited tumor growth in mice treated with sunitinib alone. Rapamycin monotherapy also significantly reduced the tumor growth. The combination treatment induced a robust delay in tumor growth, with the tumor volume only 357.81 ± 64.14 mm3 (Figure 1, A and B). As expected, the combination group had the lowest tumor weight (Figure 1C). In addition, the combinational strategy reduced splenomegaly in 4T1 breast cancer models (Figure 1D). Together, these data suggested that this combinational strategy was effective to retard tumor progression in animal breast tumor models.
Figure 1.
Combination of sunitinib and rapamycin retarded tumor growth. Tumor growth curve (A), representative images (B), and tumor weight (C) of 4T1 tumors treated with vehicle, sunitinib, rapamycin, and sunitinib and rapamycin, respectively. Combination therapy significantly reduced the splenomegaly in tumor-bearing mouse (D).*P < .01 versus vehicle and #P < .05 versus vehicle.
Combination Treatment Reduced Tumor Vessel Density
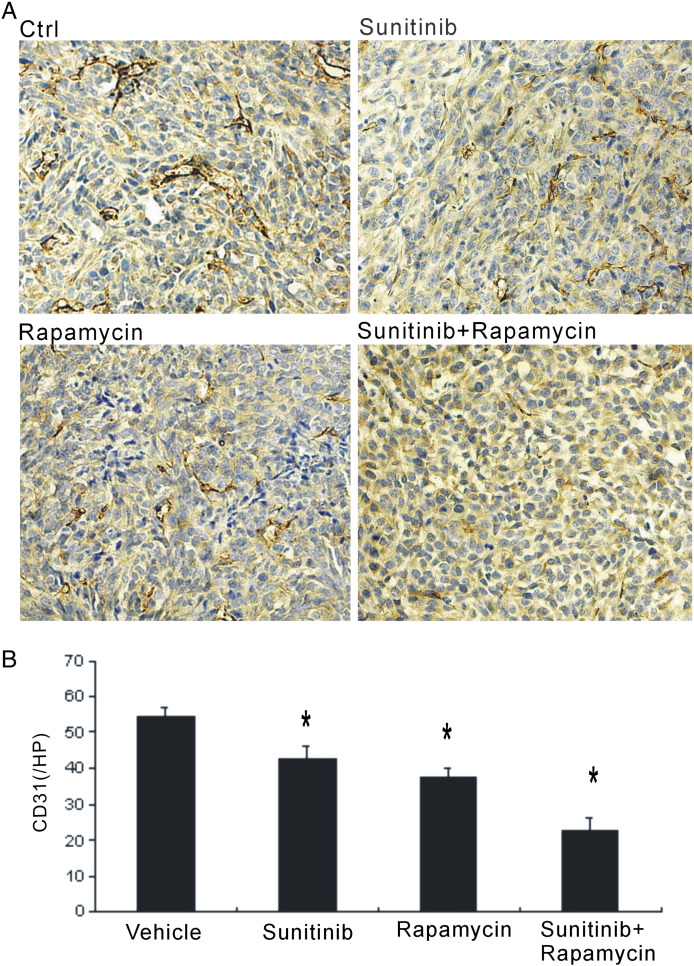
To determine the effect of combinational therapy on the tumor vessel density in tumor microenvironment, immunostaining against CD31 was performed. Compared with other groups, tumors in the vehicle group had the most vasculature, with large and tortuous morphology. The combinational strategy could robustly reduce the blood vessel density in the tumor microenvironment (Figure 2, A and B). Though rapamycin or sunitinib monotherapy could also inhibit the microvessle density, both were weaker than the combination treatment (Figure 2, A and B).
Figure 2.
Reduced microvessel density in the tumor microenvironment. (A) Immunohistochemical stain against CD31 was performed to evaluate the blood vessels in tumor sections. Control group showed large and tortuous blood vessels. (B) Combinational strategy and monothertapy with sunitinib and rapamycin robustly reduced the blood vessel density. * P < .05 versus vehicle.
Combination Therapy Reduced Myeloid-Derived Suppressor Cells in the Spleen
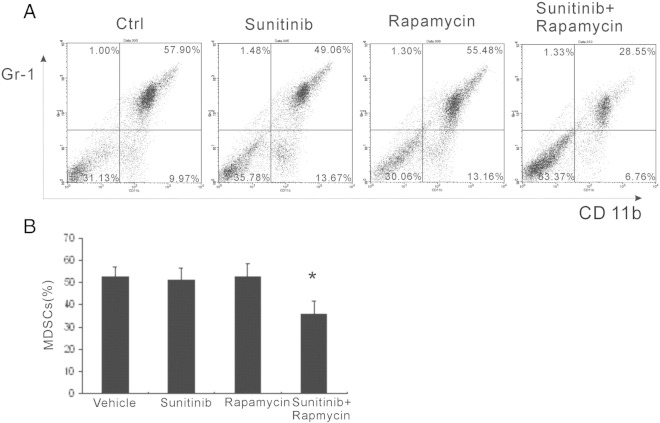
Myeloid-derived suppressor cells (MDSCs) have been shown contributing to tumor progression through immunosuppression and proangiogenesis. The quantity of MDSCs in the spleen was assessed with flow cytometry. On the 21 days of post-therapy, more than 50% of MDSCs were observed in the vehicle-treated group (Figure 3, A and B). However, there was only slightly reduction of MDSCs, but no statistical significance was observed in both sunitinib and rapamycin groups. Compared with other groups, combination treatment substantially reduced the MDSCs, and there was less than 30% MDSCs in the spleen (Figure 3, A and B). Together, the combinational strategy significantly decreased MDSC proportion in the spleen.
Figure 3.
Reduced MDSCs in spleens. (A) Twenty-one days posttreatment, the quantity of MDSCs in the spleen of tumor-bearing mice was evaluated by flow cytometry. (B) Monothertapy with sunitinib or rapamycin did not reduce the MDSCs, whereas combinational strategy significantly reduced the MDSCs in the spleen. * P < .05 versus vehicle, sunitinib, and rapamycin.
Antiangiogenic Therapy Promoted Lung Metastasis
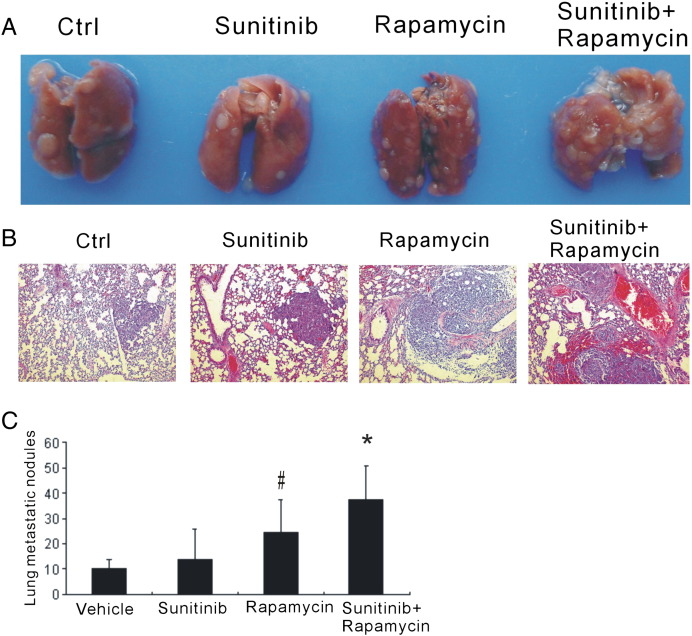
To determine whether the combined therapy reduced the cancer metastasis, we examined the metastasis macroscopically and microscopically. Unexpectedly, though the combination of sunitinib and rapamycin retarded the tumor growth, it also promoted lung metastasis. The enhanced metastasis was assessed on the day-21 of post-therapy by gross evaluation (Figure 4A) and further confirmed by the microscopical examination (Figure 4B). There was apparent lung metastasis in both rapamycin monotherapy and the combination group more lung metastasis was observed in the combination group (Figure 4C). These data indicated that rapamycin could induce metastasis in cancer therapy and make it more severe once combined with antiangiogenic therapy.
Figure 4.
Combination of sunitinib and rapamycin promoted tumor lung metastasis. (A) Lungs of tumor-bearing mice were harvested, and the metastatic nodules were grossly evaluated. (B) H&E stain showed microscopically the metastatic foci in lung sections. (C) Sunitinib combined with rapamycin promoted tumor lung metastasis. *P < .01 versus vehicle and #P < .05 versus vehicle.
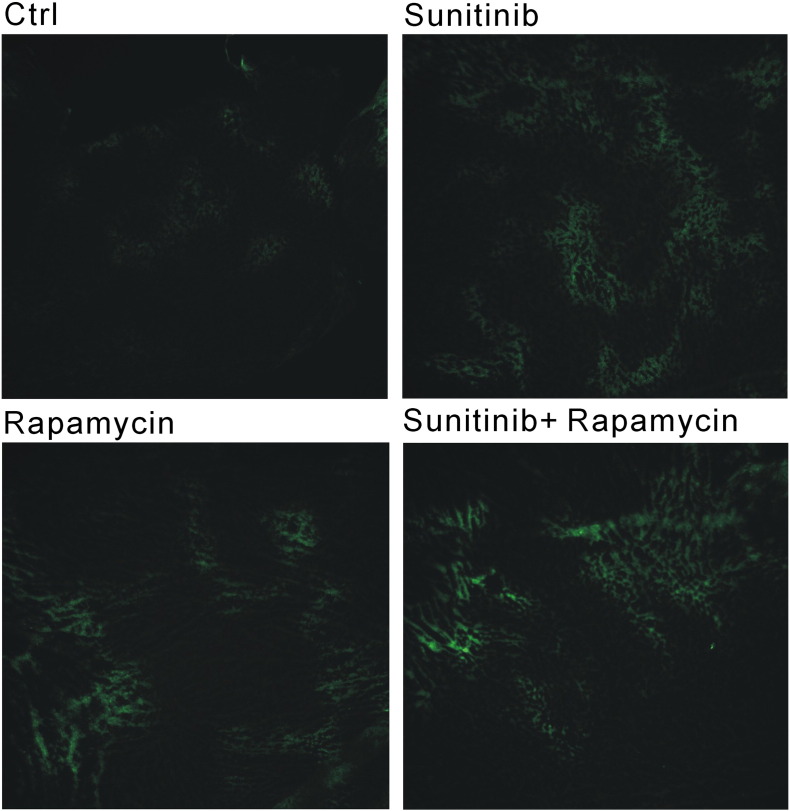
Antiangiogenic Therapy Exaggerated Hypoxia
To investigate the possible mechanism of metastasis induced by the combination therapy, immunohistochemistry of pimonidazole (Hypoxyprobe™-1, HPI Inc., Burlington, MA) adducts for hypoxic cells was evaluated in tumor sections. The results showed that significantly larger hypoxic areas exist in the tumors after antiangiogenic therapy with sunitinib or rapamycin compared with the control group (Figure 5).
Figure 5.
Antiangiogenic therapy exaggerated hypoxia. Immunohistochemistry of pimonidazole adducts for hypoxic cells was evaluated in tumor sections. Larger hypoxic areas existed in the tumor microenvironment after antiangiogenic therapy with sunitinib or/and rapamycin.
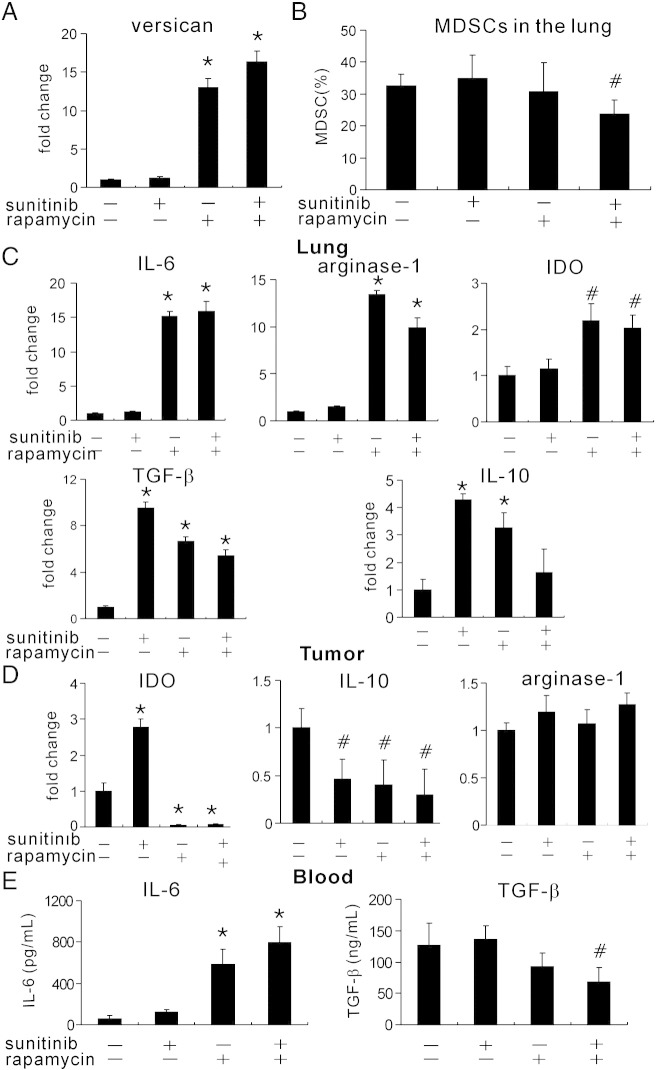
Rapamycin Induced Versican Expression and Immunosuppression in Lung Tissue
Versican secreted by MDSCs has been shown to accelerate lung metastasis. To investigate whether versican participates in rapamycin and sunitnib–induced lung metastasis, we examined the versican levels in the lungs with reverse transcription–PCR (RT-PCR) assay. Rapamycin markedly upregulated versican expression in the lungs and even more once combined with sunitinib (Figure 6A). We then assessed whether the increased versican was due to increased MDSCs in the lungs. Unexpectedly, MDSCs were decreased in the combination group (Figure 6B), which suggested other sources of versican.
Figure 6.
Rapamycin induced versican expression and immunosuppression in lung tissue. (A) Versican expression was evaluated with RT-PCR after antiangiogenic treatment. Rapamycin-based therapy increased versican expression in the metastatic lung tissue. (B) Flow cytometry was performed to detect MDSCs in the lungs. The combinational therapy reduced MDSCs in the lungs. (C) The expression of IL-6, IDO, arginase 1, TGF-β, and IL-10 in the lung tissue was evaluated with RT-PCR. (D) The expression of IDO, arginase 1, and IL-10 in the tumor tissue was evaluated with RT-PCR. (E) The circulation levels of IL-6 and TGF-β were examined with ELISA. *P < .01 versus vehicle and #P < .05 versus vehicle.
Next, we evaluated the immunosuppressive molecules and cytokines in the lungs of tumor-bearing mouse. Arginase 1, IDO, and IL-6 expression in the lungs was increased after treatment with rapamycin alone or together with sunitinib (Figure 6C). Sunitinib alone was not sufficient to induce arginase 1, IDO, and IL-6, in which it induced more TGF-β and IL-10, two other immunosuppressive cytokines. Rapamycin also significantly increased TGF-β and IL-10 expression in the lungs, whereas the combination of two drugs only induced TGF-β expression but not IL-10 (Figure 6C).
We further examined those molecules in the tumor tissues. Both sunitinib and rapamycin could decrease IL-10 in the tumor microenvironment but not arginase 1 (Figure 6D). Rapamycin significantly reduced IDO levels in the tumor (Figure 6D), which was controversial to that in lungs. IL-6 and TGF- β in the lungs were obviously increased in the lung tissues, and we then evaluated the systemic levels of those two cytokines. Antiangiogenic therapy with two drugs reduced TGF- β whereas it markedly elevated IL-6 levels in the blood (Figure 6E). Collectively, those results indicated that sunitinib and rapamycin induced an immunosuppressive microenvironment in the metastatic sites but not in the primary tumor site.
Combinational Therapy Did Not Promote Distal Metastasis in Liver and Kidney
To investigate whether rapamycin promotes cancer dissemination, important organs of tumor-bearing mice were examined. No metastatic nodule was detected macroscopically in the liver and kidney in the tumor models. In addition, we also examined the histology of livers and kidneys, and no metastasis was detected (data not shown). Those results suggested that, though rapamycin and sunitinib promoted lung metastasis, it did not cause the dissemination of cancer cells to other important organs.
Discussion
Angiogenesis is pivotal for tumor growth and metastasis [1], [22]. Antiangiogenic therapy has been designated as an effective way for cancer treatment [2]. However, a plethora of reports shows that antiangiogenic therapy can induce metastasis [3]. In our study, the combination of rapamycin and sunitinib hampered tumor growth, reduced splenomegaly and tumor microvessel density, and decreased MDSCs in the spleen. However, we found that mTOR inhibitor did not solve the problem of metastasis induced by antiangiogenic therapy. Conversely, rapamycin exaggerated the metastasis induced by antiangiogenic therapy.
mTOR regulates many basic cellular behavior, such as proliferation, survival, protein synthesis, and angiogenesis. Targeting the PI3K/Akt/mTOR pathway is a promising concept for cancer therapy. Rapamycin forms a complex with a conserved receptor FK506-binding protein 12 and then binds to mTOR, and the interaction then causes inactivation of p70S6 kinase [19]. Rapamycin inhibits albumin-induced epithelial-to-mesenchymal transition in the renal diseases [23]. Rapamycin also increases E-cadherin expression in renal cancer cells and converts the invasive to a noninvasive phenotype. Through disruption of the mTORC2 assembly and down-regulation of phosphorylated signal transducer and activator of transcription-3 (S727), rapamycin downregulates Twist1 and matrix metalloproteinase-2 expression and then suppresses the motility and the peritoneal dissemination of tumor cells [24]. Inhibitor of mTOR has achieved promising results in the clinical trials [25]. Rapamycin-based mTOR inhibition decreases angiogenesis in several tumor types [16], [18]. In our present study, we demonstrated that rapamycin and sunitinib can synergistically reduce tumor microvessel density.
In tumor-bearing hosts, MDSCs accumulate in the central and periphery lymph organ, blood, and tumor sites. MDSCs contribute to tumor progression through exerting immunosuppressive effects and promote angiogenesis in tumor microenvironment [26]. Myeloid cells treated with rapamycin could produce more IL-12 and less IL-10 [27]. Rapamycin could skew toward a proinflammatory T helper 1 pattern [27]. We found that combination can significantly reduce the splenomagly and MDSC accumulation in the spleen, which suggested that our combination strategy may inhibit the immunosuppression induced by MDSCs. However, in our study, we did not detect the decrease of MDSC in sunitinib or rapamycin therapy 21 days after treatment.
Recent report shows that mTOR promotes cancer cell migration and invasion [13]. In our present study, we did not detect the hypothesized synergistic therapeutic effect of combination of sunitinib and rapamycin on tumor metastasis. Sunitinib can slightly increase the metastasis. Rapamycin can induce robust lung metastasis of 4T1 tumors, which is assistant to the recent report, which demonstrates that mTOR inhibitor RAD001 (Novartis, Basel, Switzerland) results in the occurrence of distant metastasis [28]. Furthermore, more metastatic tumors were observed in the combination group. We also examined the liver and kidney and did not detect the metastasis in the liver and kidney in our tumor models. We observed the exaggerated hypoxia after antiangiogenic therapy. The acceleration of lung metastasis could be caused by treatment-induced hypoxia. Hypoxia has been designated as the major triggering factor for tumor metastasis. The molecular pathways regulated by hypoxia steel tumor cells to generate functionally abnormal tumor vessels through pathologic angiogenesis and recruitment of bone marrow–derived and regulatory T cells [29].
Versican is an extracellular matrix proteoglycan that stimulates mesenchymal-to-epithelial transition of metastatic cancer cells and accelerates lung metastases [30]. Elevated versican expression is also found within the metastatic lung of patients with breast cancer [30]. We evaluated versican levels in the lungs after sunitinib and rapamycin therapy. Sunitinib is not sufficient to induce versican expression. Rapamycin strongly increased expression of versican, and the combinational therapy had the highest versican levels. Versican in metastatic lungs was mainly attributed to the MDSCs [30]. We found that, however, combination therapy with sunitinib plus rapamycin reduced MDSCs in the lung tissues, which may indicate that MDSCs are not the main source of versican.
We also evaluated the immunosuppressive molecules in the lungs after antiangiogenic therapy with sunitinib and rapamycin. Arginase 1, IDO, and IL-6 expression in the lungs was increased after rapamycin or combinational therapy. Though insufficient to induce arginase 1, IDO, and IL-6, sunitinib induced more TGF-β and IL-10 in the lungs of tumor-bearing mice. Interestingly, in the tumor microenvironment, we detected less IDO and IL-10 expression after rapamycin-based therapy. Antiangiogenic therapy with those two drugs reduced TGF- β whereas it markedly induced IL-6 levels in the blood. Thus, we postulate that antiangiogenic therapy with sunitinib and rapamycin results in an immunosuppressive microenvironment in the metastatic sites and then accelerates lung metastasis.
In conclusion, though rapamycin and sunitinib could synergistically reduce tumor volume, the combination therapy exacerbated tumor metastasis. Our findings warrant that further mTOR inhibition treatment should be closely watched in clinical setting, especially when combined with other antiangiogenic therapy.
Footnotes
This work was supported by National Major Project (2011ZX09302-001-01). The authors have declared that they have no competing interests.
Contributor Information
Tao Yin, Email: yintao03073@163.com.
Sisi He, Email: chrinstina-hesisi@yahoo.cn.
Tinghong Ye, Email: yetinghong0908@163.com.
Guobo Shen, Email: shengguobo@126.com.
Yang Wan, Email: wanyang1110@gmail.com.
Yongsheng Wang, Email: wangys75@gmail.com.
References
- 1.Weis S.M., Cheresh D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 2.Fong T.A., Shawver L.K., Sun L., Tang C., App H., Powell T.J., Kim Y.H., Schreck R., Wang X., Risau W. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59(1):99–106. [PubMed] [Google Scholar]
- 3.Paez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Vinals F., Inoue M., Bergers G., Hanahan D., Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouyssegur J., Dayan F., Mazure N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 5.Birner P., Schindl M., Obermair A., Plank C., Breitenecker G., Oberhuber G. Overexpression of hypoxia-inducible factor 1α is a marker for an unfavorable prognosis in early- stage invasive cervical cancer. Cancer Res. 2000;60(17):4693–4696. [PubMed] [Google Scholar]
- 6.Yang M.H., Wu M.Z., Chiou S.H., Chen P.M., Chang S.Y., Liu C.J., Teng S.C., Wu K.J. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Mendel D.B., Laird A.D., Xin X., Louie S.G., Christensen J.G., Li G., Schreck R.E., Abrams T.J., Ngai T.J., Lee L.B. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/phar macodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 9.Burstein H.J., Elias A.D., Rugo H.S., Cobleigh M.A., Wolff A.C., Eisenberg P.D., Lehman M., Adams B.J., Bello C.L., DePrimo S.E. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;6:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 10.Cumashi A., Tinari N., Rossi C., Lattanzio R., Natoli C., Piantelli M., Iacobelli S. Sunitinib malate (SU-11248) alone or in combination with low-dose docetaxel inhibits the growth of DU-145 prostate cancer xenografts. Cancer Lett. 2008;2:229–233. doi: 10.1016/j.canlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Conley S.J., Gheordunescu E., Kakarala P., Newman B., Korkaya H., Heath A.N., Clouthier S.G., Wicha M.S. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 2012;109(8):2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw R.J., Cantley L.C. PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh A.C., Liu Y., Edlind M.P., Ingolia N.T., Janes M.R., Sher A., Shi E.Y., Stumpf C.R., Christensen C., Bonham M.J. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimmeler S., Zeiher A.M. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86(1):4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Ackah E., Yu J., Zoellner S., Iwakiri Y., Skurk C., Shibata R., Ouchi N., Easton R.M., Galasso G., Birnbaum M.J. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phung T.L., Ziv K., Dabydeen D., Eyiah-Mensah G., Riveros M., Perruzzi C., Sun J., Monahan-Earley R.A., Shiojima I., Nagy J.A. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger A.L., Linardic C.M., Chiang G.G., Thompson C.B., Abraham R.T. Differential ffects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;3(3):451–460. [PubMed] [Google Scholar]
- 18.Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., Bruns C.J., Zuelke C., Farkas S., Anthuber M. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 19.Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91(8):1420–1424. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas G.V., Tran C., Mellinghoff I.K., Welsbie D.S., Chan E., Fueger B., Czernin J., Sawyers C.L. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12(1):122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 21.Escorcia F.E., Henke E., McDevitt M.R., Villa C.H., Smith-Jones P., Blasberg R.G., Benezra R., Scheinberg D.A. Selective killing of tumor neovasculature paradoxically improves chemotherapy delivery to tumors. Cancer Res. 2010;70(22):9277–9286. doi: 10.1158/0008-5472.CAN-10-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.Y., Chang J.W., Yang W.S., Kim S.B., Park S.K., Park J.S., Lee S.K. Albumin-induced epithelial-mesenchymal transition and ER stress are regulated through a common ROS-c-Src kinase-mTOR pathway: effect of imatinib mesylate. Am J Physiol Renal Physiol. 2011;300(5):F1214–F1222. doi: 10.1152/ajprenal.00710.2010. [DOI] [PubMed] [Google Scholar]
- 24.Hong S.M., Park C.W., Cha H.J., Kwon J.H., Yun Y.S., Lee N.G., Kim D.G., Nam H.G., Choi K.Y. Rapamycin inhibits both motility through down-regulation of p-STAT3 (S727) by disrupting the mTORC2 assembly and peritoneal dissemination in sarcomatoid cholangiocarcinoma. Clin Exp Metastasis. 2013;30(2):177–187. doi: 10.1007/s10585-012-9526-9. [DOI] [PubMed] [Google Scholar]
- 25.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E., Hoosen S., StHobday T.J., Okusaka T., Capdevila J. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L., DeBusk L.M., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L.M., Carbone D.P., Lin P.C. Expansion of myeloid immune suppressor Gr+CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Ohtani M., Nagai S., Kondo S., Mizuno S., Nakamura K., Tanabe M., Takeuchi T., Matsuda S., Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112(3):635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pool S.E., Bison S., Koelewijn S.J., van der Graaf L.M., Melis M., Krenning E.P., de Jong M. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res. 2013;73(1):12–18. doi: 10.1158/0008-5472.CAN-11-2089. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch C., Giannoudis A., Lewis C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104(8):2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 30.Gao D., Joshi N., Choi H., Ryu S., Hahn M., Catena R., Sadik H., Argani P., Wagner P., Vahdat L.T. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]