Abstract
Although obesity is more prevalent in Hispanics than non-Hispanic whites in the United States, little is known about the genetic etiology of the related traits in this population. To identify genetic loci influencing obesity in non-Mexican Hispanics, we performed a genome-wide linkage scan in 1,390 subjects from 100 Caribbean Hispanic families on six obesity-related quantitative traits: body mass index (BMI), body weight, waist circumference, waist-to-hip ratio, abdominal and average triceps skinfold thickness after adjusting for significant demographic and lifestyle factors. We then carried out an association analysis of the linkage peaks and the FTO gene in an independent community-based Hispanic subcohort (N = 652, 64% Caribbean Hispanics) from the Northern Manhattan Study. Evidence of linkage was strongest on 1q43 with multipoint LOD score of 2.45 (p = 0.0004) for body weight. Suggestive linkage evidence of LOD > 2.0 was also identified on 1q43 for BMI (LOD = 2.03), 14q32 for abdominal skinfold thickness (LOD = 2.17), 16p12 for BMI (LOD = 2.27) and weight (LOD = 2.26), and 16q23–24 for average triceps skinfold thickness (LOD = 2.32). In the association analysis of 6,440 single nucleotide polymorphisms (SNPs) under 1-LOD unit down regions of our linkage peaks on chromosome 1q43 and 16p12 as well as in the FTO gene, we found that two SNPs (rs6665519 and rs669231) on 1q43 and one FTO SNP (rs12447427) were significantly associated with BMI or body weight after adjustment for multiple testing. Our results suggest that in addition to FTO, multiple genetic loci, particularly those on 1q43 region, may contribute to the variations in obesity-related quantitative traits in Caribbean Hispanics.
Introduction
Obesity poses a challenging global public health problem because of its increasing prevalence and strong association with elevated health-care costs, reduced quality of life, and increased risk for premature death. Significant differences in the prevalence of obesity have been observed among different racial/ethnic groups. Recent data from the Behavioral Risk Factor Surveillance System surveys showed that Hispanics had 21% greater prevalence of obesity when compared with non-Hispanic whites (CDC 2009). While socioeconomic, lifestyle, and cultural factors account for some of these observed disparities, family, twin and adoption studies have demonstrated that genetic factors are very important in body weight regulation and adiposity, with heritability estimates ranging from 16 to 85% (Yang et al. 2007). Over the past decade, much effort has been made to identify the susceptibility loci, with over 60 genome-wide linkage scans of obesity. However, no locus has been consistently implicated in the majority of these scans (Rankinen et al. 2006; Saunders et al. 2007), including region 16q12 which harbors the FTO gene and has shown the most convincing replication in genetic association studies of obesity to date (Frayling et al. 2007). The lack of replication may be partially due to varying sample size, population heterogeneity, and different biological pathways and, therefore, suggests a complex inheritance pattern with many genes having a small effect on common obesity traits.
Hispanics are the fastest growing and largest minority group in the United States and have a greater prevalence of obesity and related diseases; however, genetic studies of obesity have been sparse in non-Mexican Hispanics. To minimize genetic heterogeneity and fill the gap of knowledge in this understudied population, we performed an autosomal genome-wide linkage analysis of obesity-related quantitative traits in 100 multi-generation Caribbean Hispanic families from the Family Study of Stroke Risk and Carotid Atherosclerosis (Sacco et al. 2009a), followed by a peak-wide association analysis in an independent prospective community-based Hispanic subcohort from the Northern Manhattan Study (NOMAS) (Sacco et al. 2004).
Materials and methods
Subjects
All subjects provided informed consent to participate, and the study was approved by the Institutional Review Boards of Columbia University, University of Miami, the National Bioethics Committee and the Independent Ethics Committee of Instituto Oncologico Regional del Cibao in the Dominican Republic.
Caribbean families
The present genome-wide linkage analysis consisted of 1,390 participants from 100 Caribbean Hispanic families, with an average family size of 14, in the Family Study of Stroke Risk and Carotid Atherosclerosis. We have described the research design and detailed ascertainment scheme for the family study (Sacco et al. 2007, 2009a). Briefly, we selected probands from the Caribbean Hispanic participants in NOMAS using the following criteria: (1) reporting a sibling with a history of myocardial infarction or stroke; or (2) having 2 of 3 quantitative risk phenotypes (maximal carotid plaque thickness, left ventricular mass, or homocysteine level above the 75th percentiles in the NOMAS cohort). Eighty percent of the families were recruited based on the first criteria.
NOMAS subcohort
In the association analysis, we utilized the approach of linkage followed by finer mapping in regions of interest using a convenience and independent sample of Hispanics (n = 652, 64% Caribbean Hispanics) who had genotypes and passed quality control in genotyping from the NOMAS study. We have described the research design and subject recruitment for the NOMAS (Sacco et al. 2004, 2009b). Briefly, to be eligible, NOMAS participants had never been diagnosed with a stroke, were at least 40 years of age, and resided for at least 3 months in a household with a telephone in Northern Manhattan. A total of 3,298 community subjects were enrolled in 1993–2001 and 199 unrelated household members were recruited in 2003–2008.
Obesity-related quantitative phenotypes
We measured several anthropometric variables in the family study at baseline assessment and in the NOMAS subcohort: body weight, height, waist circumference, and hip circumference. In addition, we had triceps and abdominal skinfold thickness measures for the subjects in the family study. We calculated body mass index (BMI) as body weight (in kilograms) divided by the square of height (in meters) and waist-to-hip ratio (WHR) as waist circumference divided by hip circumference.
Genotyping and quality control
For the linkage study, we had a total of 383 autosomal microsatellite markers genotyped by the Center for Inherited Disease Research at an average interval of 10 cM. To verify and adjust family structure, we compared the putative relationship between pairs of individuals to those constructed based on the autosomal genotypes by performing maximized log-likelihood ratio test using PREST (Sun et al. 2002). Relationships with a p value <1.0 × 10−6 in a consistent manner across the family were considered an error. We checked for Mendelian errors in the final family structure using PEDCHECK (O’Connell and Weeks 1998). The average heterozygosity over 383 autosomal microsatellite markers was 78%.
For the NOMAS association study, we processed and genotyped DNA samples using the Genome-Wide Human SNP Array 6.0 chips (AffyMetrix) according to the Affymetrix procedures at the Genotyping Core of the John P Hussman Institute for Human Genomics at the University of Miami. We scanned the arrays on the GeneChip Scanner 3000 7G and analyzed image data using the Genotyping Console™. For quality control, we removed the samples from further analysis if they had call rates below 95%, relatedness or gender discrepancies, or were outliers beyond 6 SD from the mean based on EIGENSTRAT analysis (Price et al. 2006). We also excluded the SNPs if they were not in Hardy–Weinberg equilibrium (HWE) (p < 1.0 × 10−6), or had either a genotyping call rate less than 95% or an MAF less than 5% as identified by PLINK (Purcell et al. 2007).
Statistical analysis
Genome-wide linkage analysis of Caribbean families
For the autosomal genome-wide linkage analysis, we first examined all six traits for skewness, kurtosis and outliers, and their correlations using SAS software (SAS Institute Inc, Cary, NC). After removing the outliers beyond mean ± 3SD, only BMI still deviated from normality. After log transformation, the skewness and kurtosis for BMI were 0.03 and 0.02, respectively. We performed a polygenic covariate screening using sequential oligogenic linkage analysis routines (SOLAR) for a set of covariates: age, sex, age by sex, age2, education, physical activity, smoking pack years and alcohol drinking based on a threshold of p < 0.1 for inclusion of any potentially significant covariates. For all traits, we further checked the skewness and kurtosis for the residuals in the polygenic models and did not observe departure from normality after adjustment for covariates. We then employed SOLAR to calculate heritability, proportion of alleles shared identical-by-descent (IBD), and multipoint LOD scores through a variance component (VC) approach (Almasy and Blangero 1998; Amos et al. 1997). To evaluate the robustness of the results, we conducted simulation analysis to compute empirical p values for LOD scores based on 15,000 replicates in which a fully informative marker, unlinked to the specific trait, was simulated and used to compute possible LOD scores.
Follow-up association analysis of NOMAS community-based subcohort
As the NOMAS cohort included samples from a broader population, we employed two approaches to minimize the potential bias due to the population stratification. First, we limited the association analysis to Hispanics (n = 652) or Caribbean Hispanics (n = 416) to reduce the probable underlying genetic heterogeneity. Second, from our recent genetic study completed in the NOMAS subcohort, we assessed the population stratification based on the principal component approach using EIGENSTRAT (Price et al. 2006). The top two principal components from all Hispanics (PCA1, PCA2) and the top component from Carribean Hispanics (PCA1) were then used as covariates for genomic control in the respective association analyses. The strongest suggestive linkage evidence was found for body weight, BMI, and skinfold thickness; however, skinfold thickness was not available in the NOMAS subcohort, so we focused our follow-up association study on BMI (log-transformed) and body weight. We investigated the association of BMI and weight with genotypes of all available 6,640 SNPs that were located under 1-LOD unit down regions of the two linkage peaks (3,488 SNPs from 233.4 to 244.1 megabase on 1q43 and 2,791 SNPs from 21.2 to 49.4 Mb on 16p12) and within FTO (161 SNPs), with a fine mapping density of 3.3 SNP per 10 kb on 1q43 and 1 SNP per 10 kb on 16p12. Assuming an additive genetic model, we performed multiple linear regression analysis using PLINK to investigate the association of the selected SNPs with body weight and BMI after adjusting for genomic control variables, age, gender and other significant covariates identified by a stepwise selection procedure in SAS with inclusion(stay) criteria of p < 0.1 in the model. We explored the potential function of the associated SNPs using FuncPred, one of a set of web-based tools for SNP information (SNPinfo Web Server at http://snpinfo.niehs.nih.gov/index.html).
To correct for multiple testing of SNPs in the peak-wide association mapping, we applied the SimpleM approach developed by Gao et al. (2008) to take into account the dependence among the SNPs that are in linkage disequilibrium (LD). This method is particularly useful for the follow-up association analysis of SNPs within linkage peak regions or specific genes. Briefly, SimpleM first computes the eigenvalues from the pair-wise SNP correlation matrix created with composite LD from the SNP dataset and then infers the effective number of independent tests (Meff_G) through principle component analysis. Once Meff_G is estimated, a standard Bonferroni correction is applied to control for the multiple testing. We employed this approach to adjust for multiple tests performed in each follow-up region.
Power estimation
For the linkage study, we used SOLAR to calculate power through simulation. Given that the total genetic heritability for each trait is the same as that estimated from our data (Table 3), with our final data set we have at least 80% power to detect a QTL with heritability of 0.19, 0.18, 0.19, 0.20, 0.20, and 0.22 at a LOD score of 2 for BMI, body weight, waist circumference, WHR, average triceps and abdominal skinfold thickness, respectively. For the follow-up association study, assuming a minor allele frequency of 0.20, we have at least 80% power to detect an additive genetic effect (beta) of 0.046 for log-transformed BMI and 8.59 for weight in all Hispanics at a two-sided alpha of 0.001 using QUANTO (http://hydra.usc.edu/gxe/).
Table 3.
Adjusted heritability estimates for obesity-related quantitative traits based on Caribbean Hispanic families
| Traits | Heritability | Covariatesa | Trait residual in polygenic model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h2 ± SE | p | Sex | Age | Age × Sex | Age2 | Education | Physical activity |
Pack years |
Alcohol drinking |
Skewness | Kurtosis | |
| BMIb | 0.62 ± 0.06 | 2.7 × 10−37 | X | X | 0.19 | 0.26 | ||||||
| Weight | 0.66 ± 0.05 | 3.2 × 10−45 | X | X | X | X | 0.58 | 0.34 | ||||
| WC | 0.52 ± 0.06 | 5.6 × 10−29 | X | X | X | X | X | 0.58 | 0.75 | |||
| WHR | 0.25 ± 0.06 | 2.8 × 10−8 | X | X | X | X | 0.21 | 0.70 | ||||
| Abdominal skinfold thickness |
0.36 ± 0.06 | 3.7 × 10−12 | X | X | X | X | 0.03 | −0.57 | ||||
| Average triceps skinfold thickness |
0.41 ± 0.06 | 2.4 × 10−17 | X | X | X | 0.54 | 0.22 | |||||
X denotes the covariate with a nominal p≤ 0.1 for the trait
BMI was log-transformed
Results
Sample characteristics
The mean age of subjects was 46 and 69 years, respectively, for the Caribbean Hispanic family sample and community-based NOMAS Hispanic subcohort. The majority of both samples were female (60% in family sample and 62% in NOMAS subcohort). The demographic, lifestyle, and phenotypic characteristics of the study samples are summarized in Table 1.
Table 1.
Characteristics of the study samples
| Characteristicsa | Caribbean Hispanic family sample (n = 1,390) |
NOMAS Hispanic population sample (n = 652) |
|
|---|---|---|---|
| All Hispanics (n = 652) | Caribbean Hispanics (n = 416) | ||
| Age, years | 46.2 ± 17.4 | 68.9 ± 8.5 | 68.3 ± 8.3 |
| Female, % | 60 | 62 | 65 |
| Not completed high school, % | 51 | 72 | 82 |
| Smoking, pack years | 3.9 ± 10.3 | 10.2 ± 22.4 | 8.8 ± 16.9 |
| More than 1 alcohol drink/month, % | 53 | 43 | 41 |
| No leisure-time physical activity, % | 55 | 48 | 45 |
| Body mass index (BMI), kg/m2 | 28.7 ± 5.8 | 28.7 ± 4.8 | 28.8 ± 4.8 |
| Weight, lb | 166.7 ± 38.3 | 161.1 ± 30.2 | 162.0 ± 30.0 |
| Waist circumference (WC), inch | 36.5 ± 5.6 | 37.9 ± 4.6 | 38.0 ± 4.8 |
| Waist-to-hip ratio (WHR) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Abdominal skinfold thickness, mm | 36.9 ± 15.8 | NA | NA |
| Average triceps skinfold thickness, mm | 26.9 ± 11.7 | NA | NA |
NA data not available in NOMAS cohort
Values are presented as means ± SD or percentage. Physical inactivity was defined as no moderate or vigorous exercises
Trait correlation, familial correlation and heritability
Table 2 presents the correlations between obesity-related traits and the correlation of those traits between family members among the Caribbean Hispanic family sample. For trait pairwise correlation, BMI, weight and waist circumference were highly correlated with each other (≥0.79), whereas WHR showed lower correlation with other traits with correlation scores ranging from −0.06 to 0.35 except waist circumference (0.61). Intermediate correlation scores, which ranged from 0.36 to 0.59, existed between skinfold thickness and BMI, weight and waist circumference. For pairwise familial correlation, the scores between parent-offspring and between sib-sib were also higher for BMI, weight and waist circumference than those for skinfold thickness and WHR. After adjustment for significant covariates, SOLAR polygenic analyses detected a highly significant genetic component for all six traits (all p values ≤2.8 × 10−8) and calculated a higher heritability for body weight (0.66), BMI (0.62), and waist circumference (0.52), followed by average triceps skinfold thickness (0.41), abdominal skinfold thickness (0.36) and WHR (0.25) (Table 3).
Table 2.
Pair-wise correlation between obesity-related traits or between family members in Caribbean Hispanic families
| Trait/family membership | Correlation score (No. of pairs) |
|||||
|---|---|---|---|---|---|---|
| BMIa | Weight | WC | Abdominal skinfold thickness |
Average triceps skinfold thickness |
WHR | |
| Trait | ||||||
| BMIa | 0.81 (1,371) | 0.79 (1,327) | 0.59 (1,166) | 0.57 (1,325) | 0.26 (1,359) | |
| Weight | 0.79 (1,321) | 0.45 (1,166) | 0.36 (1,320) | 0.35 (1,347) | ||
| WC | 0.57 (1,164) | 0.40 (1,324) | 0.61 (1,317) | |||
| Abdominal skinfold thickness | 0.61 (1,170) | 0.21 (1,149) | ||||
| Average triceps skinfold thickness | −0.06 (1,307) | |||||
| Family Membership | ||||||
| Parent-Offspring | 0.30 (1,045) | 0.29 (1,039) | 0.25 (1,026) | 0.17 (792) | 0.16 (1,023) | 0.10 (1,016) |
| Sib-Sib | 0.30 (1,224) | 0.30 (1,245) | 0.28 (1,224) | 0.22 (904) | 0.14 (1,211) | 0.14 (1,195) |
| Half-Sibs | 0.07 (370) | 0.13 (373) | 0.17 (368) | 0.12 (272) | 0.05 (365) | 0.04 (361) |
| Cousins | 0.12 (1,351) | 0.10 (1,371) | 0.10 (1,355) | −0.01 (1,074) | 0.03 (1,347) | 0.04 (1,300) |
| Grandparent-Grandchild | 0.08 (235) | 0.12 (234) | 0.08 (229) | 0.11 (189) | 0.09 (229) | 0.00 (228) |
| Avuncular | 0.05 (1,772) | 0.07 (1,804) | 0.04 (1,778) | 0.01 (1,368) | 0.04 (1,754) | 0.03 (1,726) |
BMI was log-transformed
Autosomal genome-wide linkage scan
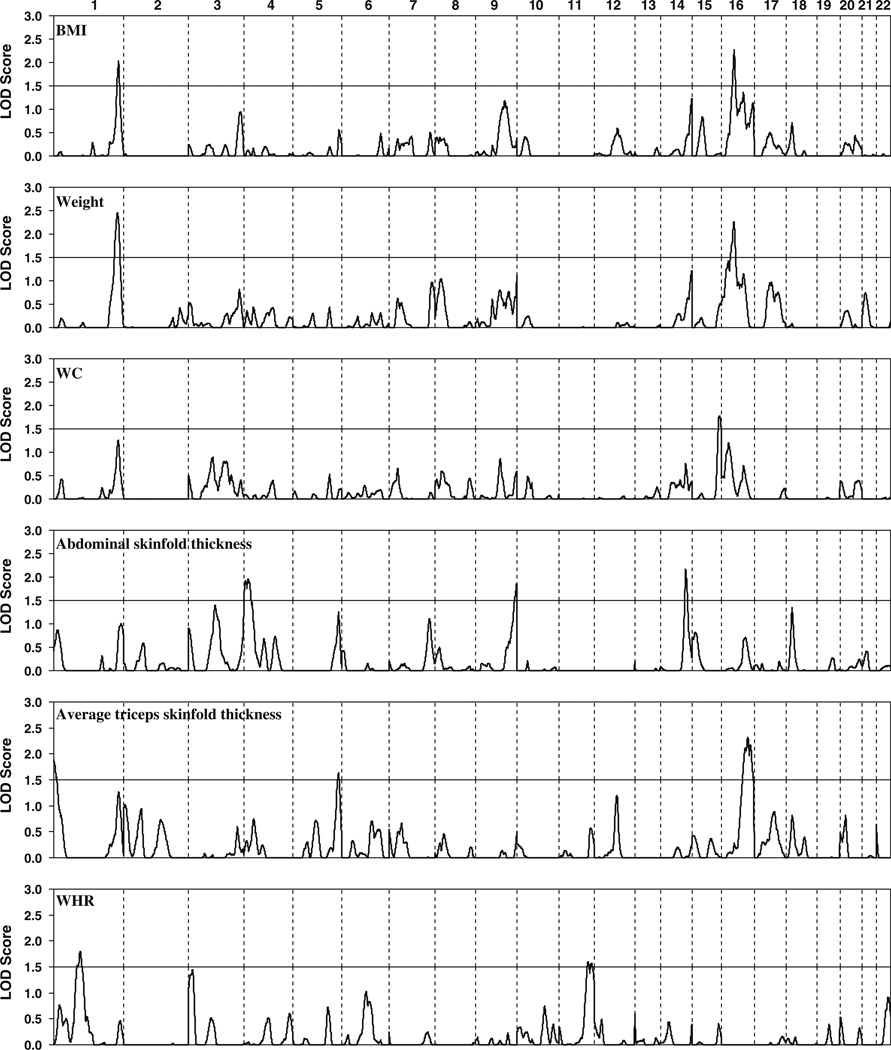
Figure 1 shows a plot of LOD scores for each phenotype based on variance component multipoint linkage analysis and Table 4 lists the chromosomal regions with a maximum multipoint LOD score over 1.5. The strongest linkage was identified on chromosome 1q43 for weight (LOD = 2.45 at 263 cM, nominal and empirical p = 0.0004). This region also showed suggestive linkage for BMI with a LOD score > 2.0 (Lander and Kruglyak 1995). In addition, there were 3 regions for which the LOD score exceeded 2 (nominal p < 0.001) for an obesity-related trait: near D16S769 on 16p12 for BMI (LOD = 2.27 at 51 cM) and body weight (LOD = 2.26 at 50 cM), near D14S1434 on 14q32 for abdominal skinfold thickness (LOD = 2.17 at 113 cM), and between D16S3091 and D16S539 on 16q23–24 for average triceps skinfold thickness (LOD = 2.32 at 105 cM) (Table 4).
Fig. 1.
Results of autosomal genome scan for obesity-related quantitative traits in Caribbean Hispanic families. Multipoint LOD scores are shown in lines for SOLAR variance component analysis. BMI was log-transformed
Table 4.
Chromosome regions with multipoint LOD score > 1.5 for obesity-related quantitative traits
| Trait | Chromosome | Marker | cM | LOD | Nominal p | Empirical pa |
|---|---|---|---|---|---|---|
| BMIb | 1q43 | D1S547 | 268 | 2.03 | 0.001 | 0.002 |
| 16p12 | D16S769 | 51 | 2.27 | 0.0006 | 0.002 | |
| Weight | 1q43 | D1S547 | 263 | 2.45 | 0.0004 | 0.0004 |
| 16p12 | D16S769 | 50 | 2.26 | 0.0006 | 0.0007 | |
| WC | 15q26 | D15S966 | 115 | 1.77 | 0.002 | 0.003 |
| Abdominal skinfold thickness | 4p15 | D4S2639 | 31 | 1.96 | 0.001 | 0.002 |
| 9q34 | D9S1838 | 164 | 1.85 | 0.002 | 0.003 | |
| 14q32 | D14S1434 | 113 | 2.17 | 0.0008 | 0.001 | |
| Average triceps skinfold thickness | 1p36 | D1S2845 | 9 | 1.88 | 0.002 | 0.002 |
| 5q35 | D5S211 | 183 | 1.64 | 0.003 | 0.004 | |
| 16q23–24 | D16S3091/D16S539 | 105 | 2.32 | 0.0005 | 0.0008 | |
| WHR | 1p31 | D1S551 | 115 | 1.80 | 0.002 | 0.002 |
| 11q24 | D11S4464 | 124 | 1.59 | 0.003 | 0.003 |
Empirical p was based on 15,000 replicates for the specific trait
BMI was log-transformed
Follow-up association analysis
Limited by phenotype availability, we focused our follow-up association analysis on the 1-LOD unit down regions of the two linkage peaks: 1q43 and 16p12, where suggestive linkage (LOD ≥ 2.0) was found for both BMI and body weight. We also investigated the association of BMI and body weight with FTO polymorphisms given that FTO has shown the most convincing replication in genetic association studies of obesity to date and is within 3 MB of our 1-LOD unit region on 16p12. Among these three regions, SimpleM analysis yielded an effective number of independent tests of 2,480 for the 1-LOD unit down region of peak 1q43, 2001 for the 1-LOD unit down region of peak 16p12, and 121 for FTO.
Table 5 reports the SNPs on 1q43 that showed an additive allelic effect on BMI or body weight with a nominal p < 0.001 in all Hispanics. The most significant association with BMI was found for SNP rs6665519 (nominal p = 1.3 × 10−5, SimpleM multiple testing corrected p = 0.03) in a highly conserved region near the lysosomal trafficking regulator gene (LYST) in Caribbean Hispanics, whereas the most significant association with body weight was found for SNP rs6692131 (nominal p = 1.8 × 10−5, SimpleM multiple testing corrected p = 0.04) with regulation potential and located in the flanking region of hypothetical gene LOC391183 in all Hispanics. Six SNPs associated with BMI were found near or in the 3′ untranslated region (3′-UTR) of GNG4 (guanine nucleotide binding protein, gamma 4 gene); three of these were at microRNA-binding sites. Several SNPs associated with both BMI and body weight were also found in or near 3 known genes, including M3 muscarinic receptor (CHRM3), ryanodine receptor 2 (RYR2), and zona pellucida glycoprotein 4 (ZP4). The remaining associated SNPs are located in the flanking region of known or hypothetical genes (Table 5).
Table 5.
SNPs on chromosome 1q43 with a p ≤ 0.001 in the follow-up association analysis of obesity-related traits in all Hispanics
| Trait | SNP | BP | Minor allele (frequencyb) |
All Hispanics |
Caribbean Hispanics |
SNP type |
Nearby gene |
||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI)c | p | β (95% CI)c | p | ||||||
| BMIa | rs6664219g | 233777195 | G (0.44) | 0.03 (0.01, 0.05) | 0.0003 | 0.05 (0.02, 0.07) | 0.00007 | Flanking | GNG4 |
| rs6676353 | 233777399 | G (0.45) | 0.03 (0.01, 0.05) | 0.0004 | 0.04 (0.02, 0.06) | 0.0002 | Flanking | GNG4 | |
| rs10802805e | 233779249 | G (0.45) | 0.03 (0.01, 0.05) | 0.0003 | 0.04 (0.02, 0.07) | 0.00009 | UTR-3 | GNG4 | |
| rs10754679 | 233779410 | G (0.45) | 0.03 (0.02, 0.05) | 0.0,003 | 0.04 (0.02, 0.07) | 0.00008 | UTR-3 | GNG4 | |
| rs10754680e | 233779701 | C (0.44) | 0.03 (0.02, 0.05) | 0.0003 | 0.04 (0.02, 0.07) | 0.0001 | UTR-3 | GNG4 | |
| rs10925976e | 233780076 | T (0.43) | 0.03 (0.02, 0.05) | 0.0003 | 0.04 (0.02, 0.07) | 0.0001 | UTR-3 | GNG4 | |
| rs6665519f | 234133095 | T (0.09) | −0.05 (−0.08, −0.02) | 0.0009 | −0.08 (−0.12, −0.05) | 0.00001d | Flanking | LYST | |
| rs6429432 | 234173864 | T (0.07) | −0.06 (−0.09, −0.03) | 0.0006 | −0.08 (−0.13, −0.04) | 0.00008 | Flanking | NID1 | |
| rs10925567 | 236351134 | A (0.05) | −0.07 (−0.11, −0.03) | 0.0006 | −0.06 (−0.11, −0.01) | 0.01 | Flanking | ZP4||KRT18P32 | |
| rs12070116 | 236510452 | T (0.06) | −0.07 (−0.10, −0.03) | 0.0002 | −0.06 (−0.11, −0.02) | 0.004 | Flanking | ZP4||KRT18P32 | |
| rs7555000g | 238017668 | C (0.07) | −0.06 (−0.10, −0.03) | 0.0003 | −0.05 (−0.10, −0.02) | 0.002 | Intron | CHRM3 | |
| rs6692131g | 240921054 | A (0.29) | −0.04 (−0.06, −0.02) | 0.00006 | −0.04 (−0.07, −0.02) | 0.0003 | Flanking | LOC391183 | |
| Weight | rs1252149 | 235225797 | G (0.28) | −5.52 (−8.77, −2.28) | 0.0009 | −5.04 (−8.92, −1.16) | 0.01 | Flanking | RYR2 |
| rs10925567 | 236351134 | A (0.05) | −11.7 (−18.30, −5.00) | 0.0007 | −10.95 (−19.21, −2.70) | 0.01 | Flanking | ZP4||KRT18P32 | |
| rs7555000g | 238017668 | C (0.07) | −10.86 (−16.73, −5.00) | 0.0003 | −10.59 (−16.92, −4.27) | 0.001 | Intron | CHRM3 | |
| rs914947 | 240916441 | A (0.30) | −5.97 (−9.31, −2.62) | 0.0005 | −5.10 (−9.29, −0.92) | 0.02 | Flanking | LOC391183 | |
| rs6692131g | 240921054 | A (0.29) | −7.36 (−10.70, −4.02) | 0.00002d | −7.57 (−11.71, −3.44) | 0.0004 | Flanking | LOC391183 | |
CI confidence interval, UTR-3 3′ untranslated region
BMI was log-transformed
Minor allele frequency in all Hispanics
β regression coefficient (change in trait per minor allele number increase, BMI was log-transformed)
Bonferroni-corrected p = 0.03 for rs6665519 and 0.04 for rs6692131 based on SimpleM
Putative microRNA-binding site based on FuncPred
In highly conserved region based on FuncPred
Regulation potential based on FuncPred
Similarly, Table 6 reports the SNPs on 16p12 and in FTO that showed an additive allelic effect on BMI or body weight with a nominal p ≤ 0.001 in all Hispanics. For the SNPs within the 1-LOD unit down region of 16p12, the most significant association with the BMI was found for intronic SNP rs7199357 (nominal p = 0.0002) in GSG1-like gene (GSG1L), whereas the most significant association with the body weight was found for the SNP rs1610 (nominal p = 0.0003) at a microRNA binding site and in a highly conserved 3′ untranslated region of the ubiquitin family domain containing 1 gene (UBFD1). The remaining associated SNPs are located in several known genes, including GGA2 (golgi-associated, gamma adaptin ear containing, ARF binding protein 2), COG7 (component of oligomeric golgi complex 7), UBFD1 (ubiquitin family domain containing 1) and SLC5A11 (solute carrier family 5, member 11). For the SNPs in FTO, 3 intronic SNPs (rs12447427, rs2540784 and rs16952951) were found to be associated with BMI and/or body weight. Among the three SNPs, rs12447427, a regulatory SNP, had the strongest association with a nominal p = 1.9 × 10−4 and SimpleM multiple testing corrected p = 0.02.
Table 6.
SNPs on Chromosome 16p12 or in FTO with a p ≤ 0.001 in the follow-up association analysis of obesity-related traits in all Hispanics
| Trait | SNP | BP | Minor allele (Frequencyb) |
All Hispanics |
Caribbean Hispanics |
SNP Type |
Nearby gene |
||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI)c | p | β (95% CI)c | p | ||||||
| BMIa | rs739498g | 23424175 | T (0.06) | 0.06 (0.02, 0.10) | 0.0009 | 0.05 (0.01, 0.09) | 0.02 | intron | GGA2 |
| rs7199357 | 27896729 | T (0.05) | −0.08 (−0.12, −0.04) | 0.0002 | −0.07 (−0.11, −0.02) | 0.004 | intron | GSG1L | |
| rs12447427 | 52648090 | G (0.07) | 0.06 (0.02, 0.09) | 0.0006 | 0.05 (0.01, 0.10) | 0.01 | intron | FTO | |
| rs2540784 | 52654835 | G (0.08) | 0.06 (0.02, 0.09) | 0.0007 | 0.05 (0.01, 0.10) | 0.01 | intron | FTO | |
| rs16952951 | 52656928 | A (0.07) | 0.06 (0.02, 0.09) | 0.0008 | 0.06 (0.02, 0.11) | 0.005 | intron | FTO | |
| Weight | rs31966f | 22627867 | T (0.36) | 5.46 (2.26, 8.67) | 0.0009 | 7.59 (3.53, 11.65) | 0.0003 | Flanking | HS3ST2 |
| rs250567 | 23301900 | A (0.09) | 8.76 (3.59, 13.92) | 0.0009 | 8.79 (2.38, 15.21) | 0.008 | Flanking |
SCNN1B// COG7 |
|
| rs394908 | 23370806 | C (0.15) | 6.95 (2.88, 11.02) | 0.0009 | 5.81 (0.81, 10.82) | 0.02 | intron | COG7 | |
| rs7204714 | 23390854 | C (0.14) | 7.40 (3.23, 11.57) | 0.0005 | 6.65 (1.56, 11.75) | 0.01 | intron | GGA2 | |
| rs1610ef | 23489654 | A (0.12) | 8.15 (3.73, 12.57) | 0.0003 | 6.78 (1.17, 12.38) | 0.02 | UTR-3 | UBFD1 | |
| rs152454eg | 23490612 | C (0.12) | 7.93 (3.48, 12.39) | 0.0005 | 6.74 (1.14, 12.34) | 0.02 | UTR-3 | UBFD1 | |
| rs890845 | 24414640 | A (0.30) | −5.93 (−9.45, −2.42) | 0.0010 | −7.15 (−11.58, −2.72) | 0.002 | intron | SLC5A11 | |
| rs12447427g | 52648090 | G (0.07) | 10.89 (5.20, 16.57) | 0.0002d | 10.80 (3.61, 18.00) | 0.003 | intron | FTO | |
| rs2540784 | 52654835 | G (0.08) | 9.87 (4.35, 15.39) | 0.0005 | 9.80 (2.91, 16.68) | 0.006 | intron | FTO | |
CI confidence interval
BMI was log-transformed
Minor allele frequency in all Hispanics
β regression coefficient (change in trait per minor allele number increase)
Bonferroni-corrected p = 0.02 for rs12447427 based on SimpleM
Putative microRNA-binding site based on FuncPred
In highly conserved region based on FuncPred
Regulation potential based on FuncPred
Discussion
To our knowledge, the present study represents the first detailed genetic analyses of obesity-related quantitative traits in Caribbean Hispanics. This is further complemented by the use of two independent samples, well-characterized Caribbean Hispanic multi-generation families and a community-based prospective Hispanic cohort, i.e., mainly composed of Caribbean Hispanics, and thus, allows us to investigate the link between genetic loci and obesity-related traits in a systematic manner.
The principal findings in this report were that both body weight and BMI mapped to one region on chromosome 1q43 by attaining the suggestive linkage threshold proposed by Lander and Kruglyak (1995) and showing significant association with the genetic variants in this region in an independent population-based sample after adjustment for multiple testing. The connection between 1q43 and obesity-related traits has not been reported in the most previous linkage scans that were mainly carried out in non-Hispanic populations; however, one recent study of French Canadians has revealed that a quantitative trait locus in this region may specifically affect total adiposity (Aissani et al. 2006). Previously, this group also found linkage for LDL-C (LOD score = 2.5) and sucrose intake at the same locus (Bosse et al. 2004; Collaku et al. 2004). In addition, the Framing-ham Offspring study detected a peak LOD score of 2.59 at the same locus for systolic blood pressure during the recovery phase of exercise testing (Ingelsson et al. 2007), and a study of Mexican Americans mapped an intermediate quantitative trait of diabetic nephropathy to this region (LOD = 3.78 at peak marker D1S547) (Schelling et al. 2008). A large genome-wide association study of 14,000 common disease cases and 3,000 shared controls has also identified several genetic variants in this region associated with cardiovascular disease, hypertension and diabetes (WTCCC 2007).
While the function of many of the associated 1q43 genes is largely unknown, some of them may be candidates of particular interest given their potential biological effects. For example, CHRM3, the muscarinic acetylcholine receptor subtype M3, is one of muscarinic receptors that may play a central role in glucose and energy homeostasis (Gautam et al. 2006, 2007, 2008). Homozygous M3 receptor deficient mice (M3R−/−) display an increase in metabolic rate, elevated body temperature and hyperactivity, leading to an increase in energy expenditure and pronounced decrease in body weight and reduced levels of serum leptin and insulin (Wess 1996; Wess et al. 2007; Yamada et al. 2001). In humans, CHRM3 gene variants may be associated with decreased acute insulin secretion and increased risk for early-onset Type 2 diabetes (Guo et al. 2006). These findings suggest that the M3 receptor may represent a potential pharmacologic target for the treatment of obesity and associated metabolic disorders (Maresca and Supuran 2008). In rats, cardiac mRNA expression of RyR2 was enhanced in the obese group, and exercise training for those with diabetes improved cardiac function by minimizing dysregulation of RyR2 (Lima-Leopoldo et al. 2008; Shao et al. 2009).
In addition to 1q43, our analysis also found three regions (14q32, 16p12, and 16q23–24) with suggestive linkage evidence and several regions with a LOD score > 1.5 on 1p36, 1p31, 4p15, 5q35, 9q34, and 15q26. In fact, all these regions have previously been linked to obesity-related traits by scans from different studies, although the trait may not be exactly the same. Among the three regions with suggestive linkage evidence, 14q32 was reported linked with BMI or leptin by at least two studies (Hsueh et al. 2001; Wu et al. 2002), whereas 16p12 and 16q22–24 were located in the bins achieving a nominally significant summed rank in a meta-analysis of 37 genome-wide linkage scans for BMI performed in families with European ancestry (16p12 in bin 16.2 from 32.1 to 67.6 cM; 16q22–q24 overlapping in bin 16.3 from 67.6 to 100.4 cM) (Saunders et al. 2007). For the remaining regions with a LOD > 1.5, some studies have found linkage of BMI to 1p36 (Deng et al. 2002; Liu et al. 2004; Stone et al. 2002), BMI change to 1p31 (Chen et al. 2004), BMI and abdominal fat to 4p15 (Perusse et al. 2001; Stone et al. 2002), BMI or central obesity to 5q35 (Feitosa et al. 2002; Kraja et al. 2005), %fat or obesity to 9q34 (Chagnon et al. 2001, 2004), BMI to 11q24.1 (Arya et al. 2004; Lindsay et al. 2001; Moslehi et al. 2003; Stone et al. 2002), and fat-free body mass to 15q26 (Chagnon et al. 2000; 2001). Our association analysis in an independent sample also found moderate association between BMI and weight and the genetic variants in multiple genes under 1-LOD unit down region of 16p12 and in FTO. Besides FTO, SCNN1B has been implicated in blood pressure or obesity (Marteau et al. 2009; Tobin et al. 2008). However, for the remaining associated genes, little is known about their function and association with obesity. They may also be potential candidates for further investigation.
We did not confirm many promising regions previously reported in the literature such as 2q14, 3q26, 12q24, 13q12–13, and 20q12 (Yang et al. 2007). This is not surprising and could be for numerous reasons. Many previous studies were carried out in families ascertained through probands with extreme obesity phenotypes and multiple affected siblings, or in samples having different ethnic or cultural backgrounds. Thus, there is both population and etiological heterogeneity across studies. Correlated phenotypes do not necessarily have common genetic causes or the same pathways because environmental factors (as well as their interactions with susceptibility genes) may also influence obesity phenotypes. In addition, factors such as varying sample size, different family structure and choice of statistical analytical approaches can also contribute to inconsistency in the findings across different studies.
It is noteworthy that our study has both several strengths and weaknesses. The strengths include: (1) well-characterized and extended Caribbean Hispanic families with relatively large family size to minimize genetic heterogeneity and achieve substantial statistical power in linkage analysis, (2) well-measured multiple related quantitative traits by the same research team to assure the consistency in phenotype assessments, (3) adjustment for lifestyle factors which were seldom controlled for in previous studies and (4) follow-up fine mapping in an independent Hispanic(primarily Caribbean) cohort with genomic control. The weaknesses may include: (1) lack of more accurate obesity phenotypes such as body fat percentage or intra-abdominal adipose tissue, (2) unavailability of skinfold thickness measures in the population-based cohort for the follow-up association analysis, (3) the estimates of polygenic and single locus effects based on the variance component approach may be subject to bias (Shugart et al. 2002) and our findings in this specific population may not be directly generalized to other populations.
In summary, the present study of multi-generation Caribbean Hispanic families reports confirmatory evidence for linkage on multiple chromosomal regions that were previously identified in other populations. It also replicates the association of FTO with obesity-related quantitative traits. The region on chromosome 1q43 is of particular interest is because it shows both linkage and association with multiple obesity traits in Caribbean Hispanics. Further large studies are needed to replicate the observed associations and to perform in depth investigations of the functional variants of the genes in these regions.
Acknowledgments
This work was supported by the National Institute of Neurologic Disorders and Stroke [R01NS40807 to R.L.S., R01NS047655 and K24 NS 062737 to T.R., R37NS29993 to R.L.S]; and Evelyn F. McKnight Center for Age-related Memory Loss. We are thankful to the study participants for their collaboration and to all staff of the Northern Manhattan Study and Family Study for their energetic efforts to this study, and in particular Edison Sabala and Janet DeRosa.
Contributor Information
Chuanhui Dong, Department of Neurology, Miller School of Medicine, University of Miami, Miami1120 NW 14th Street, FL 33136, USA.
Ashley Beecham, Department of Human Genetics, John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Susan Slifer, Department of Human Genetics, John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Liyong Wang, Department of Human Genetics, John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Mark S. McClendon, Department of Neurology, Miller School of Medicine, University of Miami, Miami1120 NW 14th Street, FL 33136, USA
Susan H. Blanton, Department of Human Genetics, John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA
Tatjana Rundek, Department of Neurology, Miller School of Medicine, University of Miami, Miami1120 NW 14th Street, FL 33136, USA.
Ralph L. Sacco, Email: rsacco@med.miami.edu, Department of Neurology, Miller School of Medicine, University of Miami, Miami1120 NW 14th Street, FL 33136, USA; Department of Human Genetics, John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; Department of Epidemiology and Public Health, Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
References
- Aissani B, Perusse L, Lapointe G, Chagnon YC, Bouchard L, Walts B, Bouchard C. A quantitative trait locus for body fat on chromosome 1q43 in French Canadians: linkage and association studies. Obesity (Silver Spring) 2006;14:1605–1615. doi: 10.1038/oby.2006.185. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos CI, Krushkal J, Thiel TJ, Young A, Zhu DK, Boerwinkle E, de Andrade M. Comparison of model-free linkage mapping strategies for the study of a complex trait. Genet Epidemiol. 1997;14:743–748. doi: 10.1002/(SICI)1098-2272(1997)14:6<743::AID-GEPI30>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Arya R, Duggirala R, Jenkinson CP, Almasy L, Blangero J, O’Connell P, Stern MP. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. Am J Hum Genet. 2004;74:272–282. doi: 10.1086/381717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse Y, Chagnon YC, Despres JP, Rice T, Rao DC, Bouchard C, Perusse L, Vohl MC. Genome-wide linkage scan reveals multiple susceptibility loci influencing lipid and lipoprotein levels in the Quebec Family Study. J Lipid Res. 2004;45:419–426. doi: 10.1194/jlr.M300401-JLR200. [DOI] [PubMed] [Google Scholar]
- CDC. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–744. [PubMed] [Google Scholar]
- Chagnon YC, Borecki IB, Perusse L, Roy S, Lacaille M, Chagnon M, Ho-Kim MA, Rice T, Province MA, Rao DC, Bouchard C. Genome-wide search for genes related to the fat-free body mass in the Quebec family study. Metabolism. 2000;49:203–207. doi: 10.1016/s0026-0495(00)91299-x. [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Rice T, Perusse L, Borecki IB, Ho-Kim MA, Lacaille M, Pare C, Bouchard L, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Genomic scan for genes affecting body composition before and after training in Caucasians from HERITAGE. J Appl Physiol. 2001;90:1777–1787. doi: 10.1152/jappl.2001.90.5.1777. [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Merette C, Bouchard RH, Emond C, Roy MA, Maziade M. A genome wide linkage study of obesity as secondary effect of antipsychotics in multigenerational families of eastern Quebec affected by psychoses. Mol Psychiatry. 2004;9:1067–1074. doi: 10.1038/sj.mp.4001537. [DOI] [PubMed] [Google Scholar]
- Chen W, Li S, Cook NR, Rosner BA, Srinivasan SR, Boerwinkle E, Berenson GS. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:462–469. doi: 10.1038/sj.ijo.0802610. [DOI] [PubMed] [Google Scholar]
- Collaku A, Rankinen T, Rice T, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. A genome-wide linkage scan for dietary energy and nutrient intakes: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study. Am J Clin Nutr. 2004;79:881–886. doi: 10.1093/ajcn/79.5.881. [DOI] [PubMed] [Google Scholar]
- Deng HW, Deng H, Liu YJ, Liu YZ, Xu FH, Shen H, Conway T, Li JL, Huang QY, Davies KM, Recker RR. A genomewide linkage scan for quantitative-trait loci for obesity phenotypes. Am J Hum Genet. 2002;70:1138–1151. doi: 10.1086/339934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitosa MF, Borecki IB, Rich SS, Arnett DK, Sholinsky P, Myers RH, Leppert M, Province MA. Quantitative-trait loci influencing body-mass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2002;70:72–82. doi: 10.1086/338144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32:361–369. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, Li JH, Wess J. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006;4:363–375. doi: 10.1016/j.cmet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Duttaroy A, Mears D, Hamdan FF, Li JH, Cui Y, Jeon J, Wess J. Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab. 2007;9(Suppl 2):158–169. doi: 10.1111/j.1463-1326.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- Gautam D, Jeon J, Li JH, Han SJ, Hamdan FF, Cui Y, Lu H, Deng C, Gavrilova O, Wess J. Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review. J Recept Signal Transduct Res. 2008;28:93–108. doi: 10.1080/10799890801942002. [DOI] [PubMed] [Google Scholar]
- Guo Y, Traurig M, Ma L, Kobes S, Harper I, Infante AM, Bogardus C, Baier LJ, Prochazka M. CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes. 2006;55:3625–3629. doi: 10.2337/db06-0379. [DOI] [PubMed] [Google Scholar]
- Hsueh WC, Mitchell BD, Schneider JL, St Jean PL, Pollin TI, Ehm MG, Wagner MJ, Burns DK, Sakul H, Bell CJ, Shuldiner AR. Genome-wide scan of obesity in the Old Order Amish. J Clin Endocrinol Metab. 2001;86:1199–1205. doi: 10.1210/jcem.86.3.7358. [DOI] [PubMed] [Google Scholar]
- Ingelsson E, Larson MG, Vasan RS, O’Donnell CJ, Yin X, Hirschhorn JN, Newton-Cheh C, Drake JA, Musone SL, Heard-Costa NL, Benjamin EJ, Levy D, Atwood LD, Wang TJ, Kathiresan S. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115:2917–2924. doi: 10.1161/CIRCULATIONAHA.106.683821. [DOI] [PubMed] [Google Scholar]
- Kraja AT, Rao DC, Weder AB, Cooper R, Curb JD, Hanis CL, Turner ST, de Andrade M, Hsiung CA, Quertermous T, Zhu X, Province MA. Two major QTLs and several others relate to factors of metabolic syndrome in the family blood pressure program. Hypertension. 2005;46:751–757. doi: 10.1161/01.HYP.0000184249.20016.bb. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lima-Leopoldo AP, Sugizaki MM, Leopoldo AS, Carvalho RF, Nogueira CR, Nascimento AF, Martinez PF, Luvizotto RA, Padovani CR, Cicogna AC. Obesity induces upregulation of genes involved in myocardial Ca2+ handling. Braz J Med Biol Res. 2008;41:615–620. doi: 10.1590/s0100-879x2008000700011. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Kobes S, Knowler WC, Bennett PH, Hanson RL. Genome-wide linkage analysis assessing parent-of-origin effects in the inheritance of type 2 diabetes and BMI in Pima Indians. Diabetes. 2001;50:2850–2857. doi: 10.2337/diabetes.50.12.2850. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Xu FH, Shen H, Liu YZ, Deng HY, Zhao LJ, Huang QY, Dvornyk V, Conway T, Davies KM, Li JL, Recker RR, Deng HW. A follow-up linkage study for quantitative trait loci contributing to obesity-related phenotypes. J Clin Endocrinol Metab. 2004;89:875–882. doi: 10.1210/jc.2003-030774. [DOI] [PubMed] [Google Scholar]
- Maresca A, Supuran CT. Muscarinic acetylcholine receptors as therapeutic targets for obesity. Expert Opin Ther Targets. 2008;12:1167–1175. doi: 10.1517/14728222.12.9.1167. [DOI] [PubMed] [Google Scholar]
- Marteau JB, Samara A, Dedoussis G, Pfister M, Visvikis-Siest S. Candidate gene microarray analysis in peripheral blood cells for studying hypertension/obesity. Per Med. 2009;6:22. doi: 10.2217/pme.09.6. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Goldstein AM, Beerman M, Goldin L, Bergen AW. A genome-wide linkage scan for body mass index on Framing-ham Heart Study families. BMC Genet. 2003;4(Suppl 1):S9. doi: 10.1186/1471-2156-4-S1-S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusse L, Rice T, Chagnon YC, Despres JP, Lemieux S, Roy S, Lacaille M, Ho-Kim MA, Chagnon M, Province MA, Rao DC, Bouchard C. A genome-wide scan for abdominal fat assessed by computed tomography in the Quebec Family Study. Diabetes. 2001;50:614–621. doi: 10.2337/diabetes.50.3.614. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Sabala EA, Rundek T, Juo SH, Huang JS, DiTullio M, Homma S, Almonte K, Lithgow CG, Boden-Albala B. Design of a family study among high-risk Caribbean Hispanics: the Northern Manhattan Family Study. Ethn Dis. 2007;17:351–357. [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Blanton SH, Slifer S, Beecham A, Glover K, Gardener H, Wang L, Sabala E, Juo SH, Rundek T. Heritability and linkage analysis for carotid intima-media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke. 2009a;40:2307–2312. doi: 10.1161/STROKEAHA.109.554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009b;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CL, Chiodini BD, Sham P, Lewis CM, Abkevich V, Adeyemo AA, de Andrade M, Arya R, Berenson GS, Blangero J, Boehnke M, Borecki IB, Chagnon YC, Chen W, Comuzzie AG, Deng HW, Duggirala R, Feitosa MF, Froguel P, Hanson RL, Hebebrand J, Huezo-Dias P, Kissebah AH, Li W, Luke A, Martin LJ, Nash M, Ohman M, Palmer LJ, Peltonen L, Perola M, Price RA, Redline S, Srinivasan SR, Stern MP, Stone S, Stringham H, Turner S, Wijmenga C, Collier DA. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity (Silver Spring) 2007;15:2263–2275. doi: 10.1038/oby.2007.269. [DOI] [PubMed] [Google Scholar]
- Schelling JR, Abboud HE, Nicholas SB, Pahl MV, Sedor JR, Adler SG, Arar NH, Bowden DW, Elston RC, Freedman BI, Goddard KA, Guo X, Hanson RL, Ipp E, Iyengar SK, Jun G, Kao WH, Kasinath BS, Kimmel PL, Klag MJ, Knowler WC, Nelson RG, Parekh RS, Quade SR, Rich SS, Saad MF, Scavini M, Smith MW, Taylor K, Winkler CA, Zager PG, Shah VO. Genome-wide scan for estimated glomerular filtration rate in multi-ethnic diabetic populations: the family investigation of nephropathy and diabetes (FIND) Diabetes. 2008;57:235–243. doi: 10.2337/db07-0313. [DOI] [PubMed] [Google Scholar]
- Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol. 2009;106:1280–1292. doi: 10.1152/japplphysiol.91280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart YY, O’Connell JR, Wilson AF. An evaluation of the variance components approach: type I error, power and size of the estimated effect. Eur J Hum Genet. 2002;10:133–136. doi: 10.1038/sj.ejhg.5200772. [DOI] [PubMed] [Google Scholar]
- Stone S, Abkevich V, Hunt SC, Gutin A, Russell DL, Neff CD, Riley R, Frech GC, Hensel CH, Jammulapati S, Potter J, Sexton D, Tran T, Gibbs D, Iliev D, Gress R, Bloomquist B, Amatruda J, Rae PM, Adams TD, Skolnick MH, Shattuck D. A major predispo-sition locus for severe obesity, at 4p15–p14. Am J Hum Genet. 2002;70:1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wilder K, McPeek MS. Enhanced pedigree error detection. Hum Hered. 2002;54:99–110. doi: 10.1159/000067666. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Tomaszewski M, Braund PS, Hajat C, Raleigh SM, Palmer TM, Caulfield M, Burton PR, Samani NJ. Common variants in genes underlying monogenic hypertension and hypotension and blood pressure in the general population. Hypertension. 2008;51:1658–1664. doi: 10.1161/HYPERTENSIONAHA.108.112664. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- WTCCC. Genome-wide association study of 14, 000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cooper RS, Borecki I, Hanis C, Bray M, Lewis CE, Zhu X, Kan D, Luke A, Curb D. A combined analysis of genomewide linkage scans for body mass index from the National Heart, Lung, and Blood Institute Family Blood Pressure Program. Am J Hum Genet. 2002;70:1247–1256. doi: 10.1086/340362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]