Abstract
Transient receptor potential (TRP) family channels are involved in sensory pathways and respond to various environmental stimuli. Among the members of this family, TRPM7 is a unique fusion of an ion channel and a C-terminus kinase domain that is highly expressed in immune cells. TRPM7 serves as a key molecule governing cellular Mg2+ homeostasis in mammals since its channel pore is permeable to Mg2+ ions and can act as a Mg2+ influx pathway. However, mechanistic links between its kinase activity and channel function have remained uncertain. In this study, we generated kinase inactive knock-in mutant mice by mutagenesis of a key lysine residue involved in Mg2+-ATP binding. These mutant mice were normal in development and general locomotor activity. In peritoneal macrophages isolated from adult animals the basal activity of TRPM7 channels prior to cytoplasmic Mg2+ depletion was significantly potentiated, while maximal current densities measured after Mg2+ depletion were unchanged in the absence of detectable kinase function. Serum total Ca2+ and Mg2+ levels were not significantly altered in kinase-inactive mutant mice. Our findings suggest that abolishing TRPM7 kinase activity does not impair its channel activity and kinase activity is not essential for regulation of mammalian Mg2+ homeostasis.
TRPM7 is a member of the transient receptor potential (TRP) family of cation channels, displaying outward rectification and permeable to a number of divalent metal ions, including Mg2+ and Ca2+ 1,2,3,4. A closely related gene, TRPM6, was identified as being mutated in familial hypomagnesemia with secondary hypocalcemia5,6, and a key role for both TRPM6 and TRPM7 has been suggested in cellular Mg2+ homeostasis5,6,7,8,9,10. TRPM7 channel is widely expressed, with particularly high numbers of functional channels in immune cells and cardiac and smooth muscle cells1,4,7,11,12. A hallmark of TRPM7 channels is their sensitivity to cytoplasmic Mg2+ 1,2. Using patch-clamp electrophysiology, we recently demonstrated that inhibition by Mg2+ is biphasic, with ~10 μM and ~165 μM IC50 values12. Interestingly, Mg2+ sensitivity of TRPM7 channels is not constant but increases with multiple Mg2+ applications to inside-out patches13. In addition to Mg2+, TRPM7 is also inhibited by other metal cations Zn2+, Mn2+, Ca2+ and polyvalent cations such as spermine or neomycin14. Both native (endogenous) and heterologously expressed TRPM7 can be activated by cytoplasmic alkalinization and inhibited by acidification14. We determined that intracellular pH dependence of native human TRPM7 channels is monophasic with an IC50 of pH 6.312.
TRPM7 channels are expressed in mammalian cell lines commonly used for heterologous gene expression, such as HEK293 and CHO-K12,12,15. This presents a problem in studies of TRPM7 kinase function since the background kinase activity existing in these cell lines may reduce the effects of introducing inactivating kinase mutations. The TRPM7 kinase-dead knock-in mouse model, therefore, would allow us to study TRPM7 channel activity a) in its native environment at physiological expression levels, and b) in the complete absence of kinase activity.
TRPM7 is a unique protein that contains an atypical kinase domain at its C-terminus, closely similar to eukaryotic elongation factor-2 kinase (eEF2K)16,17,18,19. This kinase is reported to have several substrates in vitro, such as TRPM7 itself, annexin A1, phospholipase Cγ2 (PLCγ2) and eEF2K15,20,21,22. The TRPM7 kinase domain plays a structural role and is essential for ion channel activity8,10,15. Deletion of the kinase domain resulted in significantly reduced TRPM7 currents and increased sensitivity to Mg2+ 8,10,15. However, the role of the kinase activity and autophosphorylation in the ion channel function remains controversial8,14,15,16,23. In some reports, the kinase activity was shown to affect the channel function8,16,23, while others showed that kinase activity did not change channel current amplitudes or sensitivity to high concentrations of Mg2+ 15. This discrepancy could be caused by the use of heterologous expression of TRPM7 mutants or by deletion of the kinase domain.
In the present study, to address if TRPM7 kinase activity has any roles in channel function, we generated kinase-inactive mutant mice and analyzed their phenotype and channel activity. Our results clearly show that kinase activity of TRPM7 is not required for the native channel function. In macrophages isolated from kinase-inactive mutant animals the basal TRPM7 channel activity was increased yet maximal activity was not significantly changed. We also found that the mutant mice exhibited normal development and serum total Mg2+ and Ca2+ concentrations. Our findings suggest that TRPM7 kinase activity is dissociated from its channel activity and regulation of mammalian Mg2+ homeostasis.
Results
Generation of the TRPM7 kinase-dead mutant mouse
We generated TRPM7 kinase-inactive mutant mice by homologous recombination in embryonic stem (ES) cells to replace the wild-type (WT) Trpm7 gene with a point mutant Trpm7 allele encoding TRPM7 K1646R protein. The construct of targeting vector is shown in Figure 1A. K1646 is equivalent to the conserved lysine residue of classical kinases that is often mutated to produce a kinase-inactive protein, and its mutation to arginine results in diminished kinase activity15. After homologous recombination in ES cells, heterozygotes were confirmed by Southern blot analysis (Fig. 1B). Subsequently, heterozygote mice were generated using standard procedures. Deletion of the neo cassette was performed by microinjection of pCAGGS-Cre into pronuclei of fertilized eggs derived from heterozygous mice24, and confirmed by Southern blot analysis (Fig. 1C). Homozygous “kinase-dead” mice expressing TRPM7 K1646R were obtained by breeding between heterozygous males and females; they will hereafter be referred to as TRPM7R/R animals. The scheme of PCR genotyping is shown in Fig. 1D: using this PCR product, we confirmed the presence of the desired point mutation in the genomes of mice by DNA sequencing (Fig. 1E).
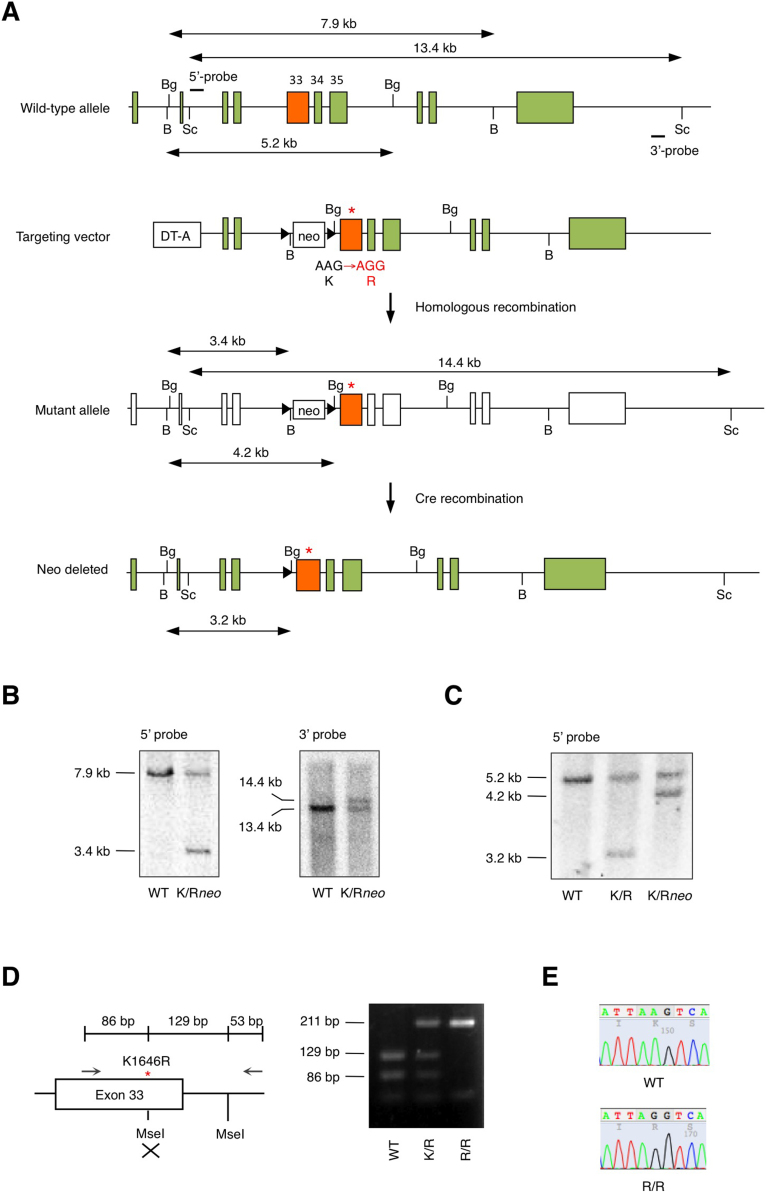
Figure 1. Generation of TRPM7 kinase-dead knock-in mice.
(A) Schematic diagram of the endogenous Trpm7 locus and the targeting vector used to engineer embryonic stem (ES) cells by homologous recombination. This vector contains a point mutation in exon 33 that changes lysine residue at position 1646 to arginine (K1646R). Restriction enzyme sites are indicated (B, BamHI; Bg, BglII; Sc, ScaI). The length of the genomic fragment is also shown when digested by the indicated enzymes. Probes 5′ and 3′ were used to screen ES cells for homologous recombination by Southern blotting. DT-A, diphtheria toxin-A; neo, neomycin-resistant gene. (B) Southern blot analysis of genomic DNA from targeted ES cells using 5′-probe or 3′-probe after digestion with BamHI or ScaI. Full length blots are shown in Fig. S1. (C) Southern blot analysis of genomic DNA from loxP-excised heterozygous mice using 5′-probe after digestion with BglII. Full length blot is shown in Fig. S2. (D) PCR strategy used to genotype the animals. The region surrounding K1646R mutation was amplified by PCR and digested with MseI. A 211 bp fragment undigested at the mutated region is produced from wild-type (WT) allele, while the amplification product from the KR allele is digested into 129 bp and 86 bp fragments at the mutated region. PCR was performed on DNA isolated from tail biopsies; results from WT, TRPM7K/R, and TRPM7R/R animals are shown. Full length gel image is shown in Fig. S3. (E) PCR amplification products from WT and TRPM7R/R animals were subcloned and sequenced to demonstrate the presence of the K1646R mutation; representative traces are shown.
TRPM7R/R mice display normal development and serum Mg2+ levels
In order to investigate key physiological characteristics of TRPM7R/R mice, we performed biochemical analysis of the serum total Mg2+ and Ca2+ levels. TRPM7R/R mice had normal serum total Mg2+ and Ca2+ levels (Fig. 2A). Under standard conditions, growth and viability was normal in TRPM7R/R mice that were followed up for ~10 months, and there were no remarkable differences in body weight, food intake or locomotor activity between WT and TRPM7R/R animals (Fig. 2B–D).
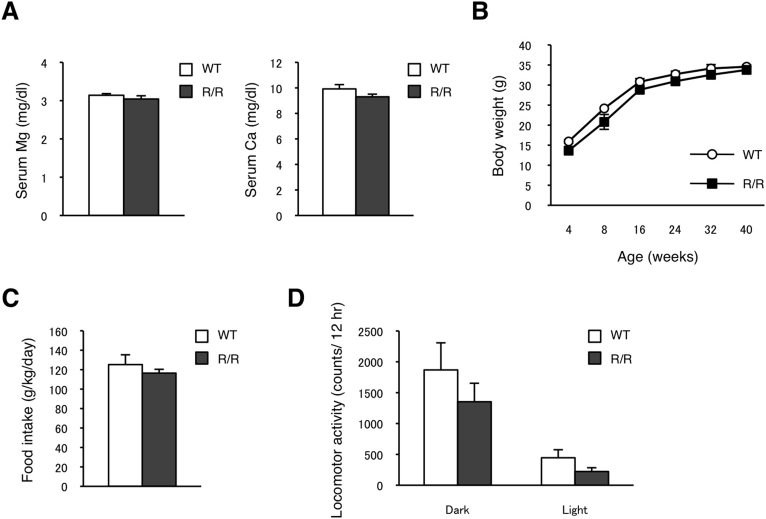
Figure 2. Serum total Mg2+ and Ca2+ levels and general behaviors in TRPM7R/R mice.
(A) Total Mg2+ and Ca2+ levels in serum obtained from WT and TRPM7R/R mice. Here and below values represent means ± s.e.m. (n = 10–14). (B) Male WT and TRPM7R/R mice were weighed at the indicated times (n = 6). (C,D) General behavior was analyzed in 16 to 18 week-old WT and TRPM7R/R mice. (C) Food intake data. (D) Locomotor activity data (n = 6).
TRPM7R/R mice have greatly diminished kinase activity
To determine if the kinase activity of TRPM7 was abolished in TRPM7R/R mice, we analyzed the phosphorylation levels of TRPM7 by protein immunoprecipitated from lysates of mouse embryonic fibroblasts (MEF) using an anti-TRPM7 antibody. Autophosphorylation was clearly absent in mutant protein derived from TRPM7R/R MEFs (Fig. 3, left panel). Phosphorylation of an exogenous substrate, myelin basic protein (MBP), was also abolished in these mice (Fig. 3, left panel). We confirmed the presence of similar levels of TRPM7 protein in immunoprecipitates from WT and TRPM7R/R MEFs (Fig. 3, right panels).
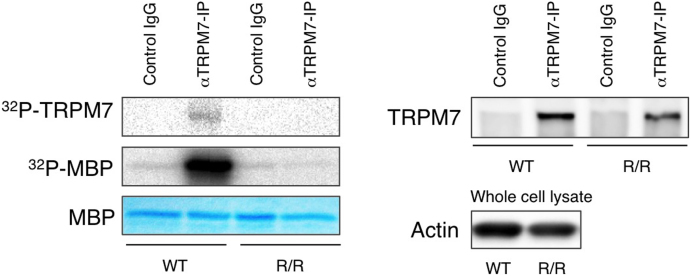
Figure 3. TRPM7 kinase activity in TRPM7R/R mice.
Kinase assay of TRPM7 proteins from WT and TRPMR/R mouse embryonic fibroblasts (MEF). Left panel shows 32P incorporation into autologous TRPM7 and exogenous myelin basic protein (MBP) by TRPM7 immunoprecipitated from WT or TRPM7R/R MEF lysates. Coomassie blue staining of MBP was used to ensure equal quantities of MBP. Right panel shows Western blot analysis of TRPM7 levels in the immunoprecipitates used in the left panel. Probing for actin in the whole cell lysates was used to ensure the presence of equal amount of protein in the lysates before immunoprecipitation. Full length blots and gel images are shown in Fig. S4.
TRPM7 channel activity in the TRPM7R/R mutant macrophages
In our earlier study, which employed a heterologous expression system, we reported that channel function is not noticeably affected by knockdown of kinase activity15. In order to address the question whether TRPM7 channel activity is altered in TRPM7R/R mice, we measured TRPM7 channel currents in peritoneal macrophages derived from WT and TRPM7R/R mice. RT-PCR experiments with specific primer sets for Trpm7 and Trpm6 showed that murine peritoneal macrophages expressed high levels of Trpm7 but very little Trpm6 (Fig. 4A, left panel). By contrast, kidney total RNA used as a positive control showed a strong TRPM6 signal as expected25 (Fig. 4A, right panel). Kinase assay showed that TRPM7 kinase activity was compromised in TRPM7R/R mutant macrophages (Fig. 4B), whereas protein levels were similar between WT and TRPM7R/R mice (Fig. 4C). TRPM6 is the only other channel-kinase in the murine genome and its biophysical properties closely resemble TRPM7 at the whole-cell level26, therefore it was important to ascertain that the cell type under investigation expresses primarily TRPM7 and not a mixture of TRPM6 and TRPM7. TRPM7 currents in macrophages have not been reported previously. Figure 4D shows the current-voltage relations obtained in WT and TRPM7R/R mutant mouse peritoneal macrophages. The currents were evoked by depleting cytoplasmic Mg2+ with HEDTA-containing internal solution (free [Mg2+] = 400 nM)12. All WT macrophages tested exhibited robust TRPM7 currents with a characteristic outwardly rectifying current-voltage (I-V) curve (Fig. 4D, black). I-V was unchanged in the TRPM7R/R mutant (Fig. 4D, red). In both WT and mutant macrophages currents developed with a similar time course (Fig. 4E) reaching a maximum after ~5 min of recording/dialysis with low-Mg2+ solutions. Current amplitudes measured at break-in (basal) were increased whereas the maximum current amplitudes were somewhat reduced in mutant macrophages (Fig. 4F, left). We therefore normalized the absolute current magnitudes to cell capacitances (Fig. 4F, right). Normalized maximum current densities were not significantly different between WT and TRPM7R/R mutant macrophages, suggesting that the difference in absolute current stemmed from size variability of cells used in the recordings. Yet, unexpectedly, basal current densities showed significant potentiation in TRPM7R/R macrophages. In order to ascertain that the currents measured in macrophages were indeed carried by TRPM7 channels, we examined the effects of internal high Mg2+ and spermine, known inhibitors of TRPM7 channels12,14, as well as blockade of monovalent currents by external spermine1. Inclusion of 303 μM free Mg2+ or 300 μM spermine in the pipette solution resulted in a drastic reduction of maximal current in both WT and kinase-dead macrophages, which was not statistically different (Fig. 4F). This concentration of Mg2+ was sufficient to inhibit basal TRPM7 currents in most cells. By contrast, in the presence of 300 μM internal spermine, TRPM7 currents still developed but also ran down quickly (Suppl. Fig. 7B). We next compared the sensitivity of monovalent TRPM7 current in the absence of external Ca2+ to external spermine. The bathing solution was switched to DVF buffer to record monovalent TRPM7 current when the current achieved steady state amplitude with 400 nM internal Mg2+ and spermine was applied to the cell. Figure 4G shows I-V relations obtained in presence and immediately before application of 10 μM spermine. In agreement with our previous report1 we found that spermine preferentially blocked the inward component of monovalent TRPM7 current, displaying strong voltage dependence. The extent of spermine block did not significantly differ between WT and kinase-dead mutant macrophages (Fig. 4H). These experiments confirmed that the currents we recorded in murine macrophages mostly represent endogenous TRPM7 channel activity. We conclude therefore that TRPM7 channel activity is not significantly impaired when the C-terminus kinase is inactivated, in agreement with our previous findings15. Moreover, basal channel activity is higher when the kinase is inactivated.
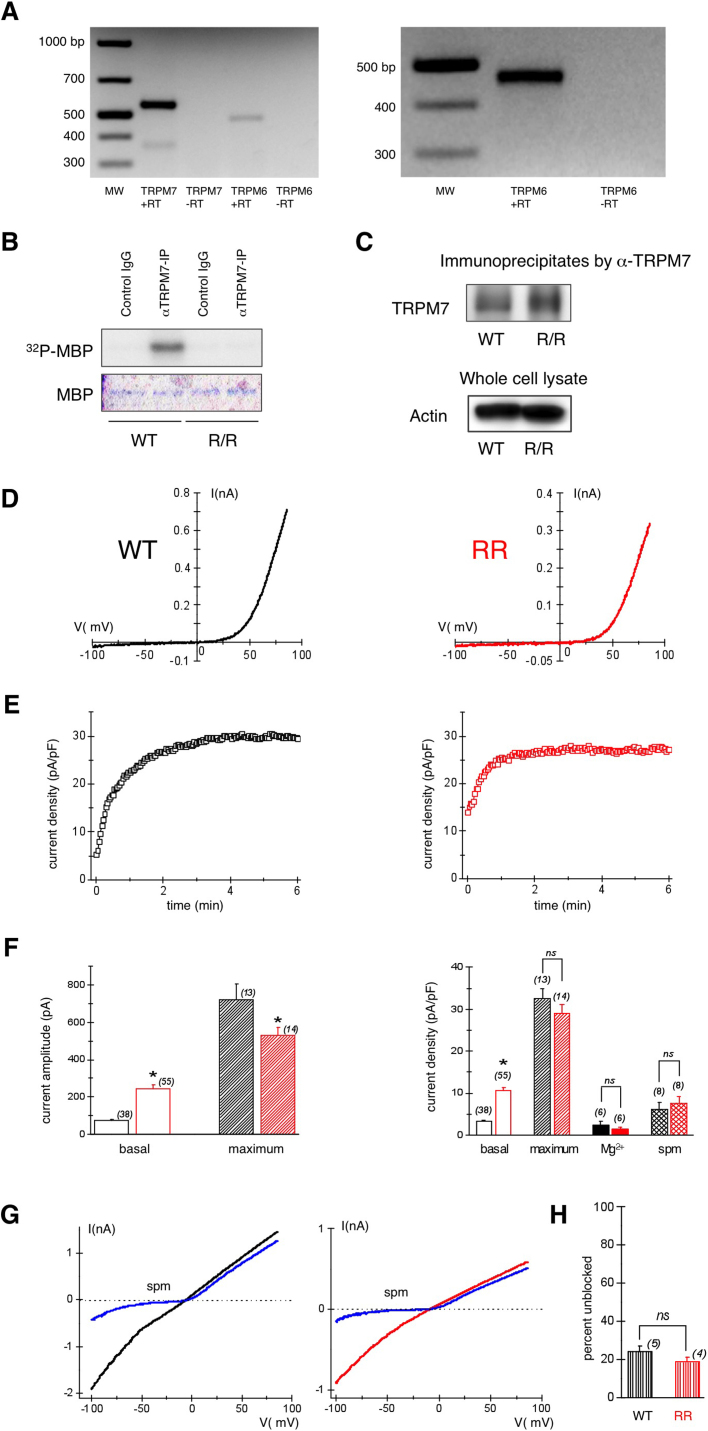
Figure 4. TRPM7 channel and kinase activities in the macrophages of WT and TRPM7R/R mice.
(A) Expression of TRPM7 and TRPM6 mRNA in peritoneal macrophages. RT-PCR analysis of TRPM7 and TRPM6 expression in mouse peritoneal macrophages (left panel) and kidney (right panel). RT, reverse transcriptase. 40 and 38 cycles PCR were used for macrophage and kidney cDNA, respecitvely. Predicted TRPM7 size = 550 bp, predicted TRPM6 size = 475 bp. Full length gel images are shown in Fig. S5. (B) Kinase assay of TRPM7 protein isolated from peritoneal macrophages. 32P incorporation into exogenous MBP by TRPM7 immunoprecipitated from lysates of WT or TRPM7R/R macrophages is shown. Coomassie staining of MBP was used to ensure equal levels of MBP. Full length images are shown in Fig. S6. (C) Western blot analysis of TRPM7 in the immunoprecipitates used in (B). Actin in whole cell lysates was used to ensure the presence of equal protein amounts before immunoprecipitation. Full length gel images are shown in Fig. S7. (D) TRPM7 current-voltage relations recorded in WT (black) and TRPM7R/R (red) peritoneal macrophages. (E) Time course of TRPM7 current development in the same cells. (F) Basal (break-in) and maximum TRPM7 current amplitudes (left) and densities (right) in WT and TRPM7R/R mice. Internal solutions for measurement of basal and maximum currents contained 400 nM free Mg2+ (left). Internal Mg2+ and spermine solutions contained 303 μM Mg2+ or 300 μM spermine with no Mg2+ (F, right). Current amplitudes were obtained from the 125th ramp after break-in, P < 0.01 for basal, P < 0.05 for maximum. Time courses of current development with 300 μM spermine are shown in Fig. S8.(G) Blockade of monovalent TRPM7 current by 10 μM external spermine in macrophages isolated from WT (left) and kinase-dead mutant mice (right). (H) Percent unblocked monovalent TRPM7 current in WT and TRPM7R/R mouse macrophages at −100 mV. Values are means ± s.e.m. Numbers of cells are shown in parentheses.
Discussion
In this study, we generated TRPM7 kinase-dead mutant mice and analyzed the relationship between its kinase activity and channel function. These mutant mice lacked any detectable kinase activity in their MEFs and macrophages. However, they exhibited normal serum Mg2+ and Ca2+ levels and developed normally. Furthermore, in macrophages isolated from TRPM7R/R mice, maximum channel activity was not affected by the absence of kinase activity. Interestingly, we find that basal TRPM7 channel activity, which represents their activity in an intact cell, was significantly potentiated.
TRPM7 is a member of the transient receptor potential (TRP) family of ion channels with a characteristic strong outward rectification and permeability to a number of divalent metal cations, including Mg2+ and Ca2+ 2,3. TRPM7, and its closely related gene TRPM6 have key roles in Mg2+ homeostasis5,6,7,8,9,10. It was reported that deletion of TRPM7 in DT-40 chicken lymphoma cells arrests cell cycle progression8. Moreover, deletion of the entire mouse TRPM7 is lethal at embryonic day 7.57,27. Deletion of the kinase domain alone results in early embryonic lethality in homozygous mice10. These results suggest that TRPM7 is essential for embryonic development and cell survival. In contrast, our present study demonstrates that the removal of TRPM7 kinase activity by point mutation does not affect cell growth and embryonic development, since TRPM7R/R mice were born normally and weight gain was similar to WT mice. Both Schmitz et al. and Ryazanova et al. reported that deletion of the entire TRPM7 protein or the kinase domain disrupts Mg2+ homeostasis8,10. In our TRPM7R/R mice, the serum Mg2+ concentration was normal. This suggests that removal of kinase activity has little effect on Mg2+ homeostasis. This is supported by our findings in peritoneal macrophages, showing that the maximum number of functional TRPM7 channels is essentially the same in TRPM7R/R mice. Ryazanova et al. reported that deletion of TRPM7 kinase domain significantly reduced TRPM7 channel activity in cells isolated from heterozygous mice10. This difference appears to be due to deletion of the kinase domain in full or in part10 vs. inactivation of phosphotransferase activity by point mutation (present study). The TRPM7 kinase domain plays a structural role and is essential for ion channel activity10,15, and the absence of kinase domain was reported to alter TRPM7 channel sensitivity to Mg2+ 8,28. Therefore, in these reports, deletion of the kinase domain seemed to lead to fatal deficits of TRPM7 channel function. Our results suggest, therefore, that embryonic lethality of kinase domain deletion10 is a consequence not of inactive kinase but rather of severely reduced ion channel function.
Runnels et al. showed that kinase activity regulates channel activity using heterologously expressed kinase-inactivated mutants in CHO-K1 cells16. Several reports also mentioned that kinase activity is related to channel function using kinase domain-deleted mutants8,10,23,28. However, kinase domain has a structural role, and its deletion leads to not only deletion of kinase activity but also structural deficits15. Therefore, it has not been clarified whether kinase activity has a role in channel function. Here we show using knock-in strategy that deletion of kinase activity does not reduce maximal channel activity achieved with Mg2+ depletion. Unexpectedly, the basal activity of TRPM7 channels was increased. Basal channel activity reflects the portion of TRPM7 channels that are functional in an intact cell, before it is infused with low Mg2+ buffers. This pre-activation phenomenon was observed in other cell types (with presumably normal TRPM7 kinase function) and its nature is not known12. In a heterologous expression system it is difficult to study channel pre-activation since overexpressed TRPM7 is always active whereas native TRPM7 is mostly inactive4,12,15,16. It was previously reported that kinase-dead TRPM7 is less sensitive to inhibition by high Mg2+ (~2–8 mM)8,28. Reduced Mg2+ sensitivity could lead to the larger pre-activated currents we observe (Fig. 4), however, 300 μM free Mg2+ was sufficient to inhibit basal TRPM7 currents in both WT and R/R macrophages equally, suggesting that cytoplasmic Mg2+, estimated to be in the range of 1–2 mM free, does not inhibit all TRPM7 channels. The reasons for this are not known and may include increased availability of PI(4,5)P2 or increased sensitivity to this phospholipid in intact cells11,13,14. The possibility that Mg2+ levels in kinase-dead mutant cells are significantly reduced causing greater channel activity cannot be ruled out although this seems unlikely in view of our finding that serum Mg2+ levels are not changed27. Finally, we found a weak presence of TRPM6 channel-kinase in our RT-PCR experiments on WT macrophages (Fig. 4A), and it might play a role in facilitating TRPM7 basal activity23. We did not detect single TRPM6 channel activity in macrophages, however (data not shown). Our future efforts will compare Mg2+ sensitivity of WT and R/R mutant TRPM7 channels in detail at the whole-cell and single-channel level, in view of the newly identified high affinity inhibitor site12,13.
Several Mg2+ transporters are known to regulate cellular Mg2+ homeostasis, CNNM2, SLC41A1/2, Mrs2 and MagT129,30,31,32,33. TRPM7 kinase-dead mice exhibited normal levels of serum magnesium, however, cellular Mg2+ levels in various tissues were not investigated. The possibility that TRPM7 kinase activity influences the function of these transporters and cellular Mg2+ levels cannot be excluded. We suggest that kinase activity is not essential for Mg2+ homeostasis and embryonic/adult mouse development, because kinase-dead mice did not show abnormal serum magneisum levels (i.e. hyper- or hypomagnesemia).
TRPM7 kinase is reported to have several substrates. Therefore, its kinase activity might have various physiological or pathophysiological roles in mammals. Our mutant mice, under standard conditions, developed normally and we did not observe any significant deficiencies. Further studies are needed for definition of the roles of TRPM7 kinase in physiology and pathophysiology.
Methods
Generation of TRPM7R/R knock-in mice
A targeting vector was constructed to replace the exon 33 in Trpm7 genomic locus with the DNA fragment containing a point mutation-inserted exon 33 (5′-AAG-3′ to 5′-AGG-3′) changing K1646 to R and loxP-flanked thymidine kinase promoter and neomycin resistance gene. This point mutation was generated by PCR and introduced into the targeting vector. A diphtheria toxin A fragment gene driven by MC-1 promoter was introduced at 5′ end of the targeting vector. The linearized targeting vector was introduced into 129/SV ES cells by electroporation, and cells selected in G418. Genomic DNA from ES clones was screened for homologous recombination from both ends by Southern blot analysis using external probes 5′-probe and 3′-probe (Fig. 1A). Identified ES clone as targeted was injected into fertilized blastocysts from C57BL/6 female mice. Chimeric mice were mated with C57BL/6 female mice for germinal transmission. To excise the loxP-flanked neomycin cassette, fertilized eggs from C57BL/6 female paired with heterozygous mutant males were collected and pCAGGS-Cre was microinjected into the pronucleus24. These eggs were then transferred into pseudopregnant mice and their offsprings were analyzed for excision of neomycin cassette by Southern blot. Heterozygous mice were backcrossed to C57BL/6 for at least seven generations. Homozygous mice (TRPM7R/R) were obtained by intercrossing of heterozygous mice.
Experiments involving animals
All procedures involving mice were performed in compliance with the National Institutes of Health guidelines and were approved by the Laboratory Animal Care and Use Committees of Mitsubishi Kagaku Institute of Life Sciences, University of the Ryukyus, Kumamoto University and Wright State University.
For measurements of locomotor activity and food intake, the mice were acclimated to the single housing environment for 2 days, and locomotor activity data was collected with ACTIMO-100 (Shinfactory), and the food intake data was collected manually. Serum total Mg and Ca levels shown in Figure 2A were measured with an autoanalyzer (FUJI DRI-CHEM 3500 V, Fuji Film) at Skylight Biotech Inc. Mg was measured with FUJI DRI-CHEM SLIDE Mg-PIII (Fuji Film), and Ca was measured with FUJI DRI-CHEM SLIDE Ca-PIII (Fuji Film). These SLIDE-s detect both ionized and bound magnesium and calcium.
TRPM7 kinase assay
Mouse embryonic fibroblasts (MEFs) were obtained from WT or TRPM7R/R mouse embryos. Peritoneal macrophages were isolated from adult mice34. Cells were lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 120 mM NaCl, 0.5 mM DTT, 1.5 mM MgCl2, 0.2 mM EDTA, 1% Triton X-100 supplemented with protease inhibitors) on ice for 20 min and insoluble materials were removed by centrifugation at 15,000 rpm for 10 min. Cell extracts were incubated with rabbit polyclonal anti-TRPM7 antibody (Millipore) overnight at 4°C, followed by incubation with protein A sepharose beads for 1 hr. Subsequently, the beads were washed three times in the lysis buffer and once in kinase buffer (50 mM HEPES, pH 7.0, 4 mM MnCl2, 5 mM DTT). Immunoprecipitates were suspended in kinase buffer with 50 μg/ml myelin basic protein (MBP). The kinase reaction was initiated by adding 0.1 mM ATP in combination with 5 μCi [γ-32P] ATP and proceeded for 30 min at 30°C. Proteins bound to the beads were eluted in Laemmli sample buffer, subjected to SDS-PAGE, and analyzed by autoradiography.
Isolation of peritoneal macrophages and RT-PCR
Peritoneal macrophages were isolated from WT or TRPM7R/R mice of both sexes according to previous reports34,35. Peritoneal cells were collected in Dulbecco's PBS containing 3% fetal bovine serum (FBS) through lavage of abdomen, and centrifuged at 1,500 r.p.m. for 8 min. The pellet was resuspended in RPMI medium and placed in cell culture dishes containing glass coverslips. After incubation for 1 hour at 37°C, the medium was replaced with RPMI containing 10% FBS, and attached cells were used for electrophysiological experiments and total RNA isolation. For RT-PCR experiments, Verso 1-step RT-PCR kit (Thermo Scientific) was used with RNA isolated from wildtype mouse macrophages and kidneys and forward and reverse primer pairs specific for murine TRPM7 GAATGGTCTGTGGAAAAGCACACG/CTTCTGCCCCATACTTTCCAAC and TRPM6 GAGAGGAGGCCACAGTCAAG/CAGGCCCTGGTCACTGTAAT.
Patch clamp electrophysiology
TRPM7 channel currents were recorded from murine peritoneal macrophages plated on glass coverslips using whole-cell patch clamp essentially as previously described1,12. The basic internal solution contained (in mM) 10 HEDTA, 106 glutamic acid, 5 CsF, 8 NaCl, 10 HEPES and pH adjusted to 7.3 with CsOH. 60 μM MgCl2 was added to this solution yielding estimated free [Mg2+] of 400 nM (Webmaxc software, Stanford University). For 303 μM free Mg2+ internal solution HEDTA and glutamic acid concentrations were reduced to 9 mM and 10 mM respectively and 7.74 mM MgCl2 added. The standard external solution contained (in mM) 2 CaCl2, 140 NaAspartate, 4.5 KCl, 3 CsCl, 10 HEPES-Na+, 0.5 dextrose, pH 7.3. The divalent cation free external solution (DVF) contained (in mM) 140 NaAspartate, 6 HEDTA, 10 HEPES-Na+, pH 7.31,13. Macrophages have been reported to express substantial voltage-gated and inwardly rectifying K+ currents35,36,37. In order to record TRPM7 in isolation, the internal solution contained Cs+ instead of K+ and the external solution contained 3 mM CsCl to reduce K+ channel contamination. Glutamate and aspartate were used as the main anions to reduce chloride conductances. For estimating external spermine block TRPM7 currents were allowed to develop to steady state with 400 nM free Mg containing internal solution, upon which the bathing solution was switched to DVF. Spermine was added to DVF1. In experiments addressing internal 300 μM spermine inhibition, spermine tetrachloride was added to the 10 mM HEDTA internal solution. Cells were held at 0 mV and instantaneous current-voltage relations were obtained by applying −100 to +85 mV voltage ramps every 2.5 seconds. Voltage ramps were preceded by a 50 ms step to −100 mV. Time course of current development was plotted by taking current magnitudes measured at +84.82 mV and plotting against time. EPC10 patch clamp amplifier (HEKA Elektronik) operated by PatchMaster software was used for command voltage generation and data acquisition. Capillary glass from Warner Instruments was used to manufacture patch pipettes on a robotic Zeitz Universal puller or a vertical Narishige puller. Pipettes were fire polished to resistances of 3–5 mOhm using a Narishige MF830 microforge. Origin v. 8 (OriginLab) was used for data analysis and graphing. In Fig. 4F and H, two sample t-test was used to determine stasitsically significant differences between means. Osmolalities were adjusted with D-mannitol. All experiments were performed at room temperature (~25°C).
Author Contributions
T.K., C.K. and M.M. designed experiments. T.K. and C.K. performed most of the experiments, P.B., S.H. and J.A.K. designed and carried out electrophysiological analysis and RT-PCR experiments. K.N. supported the generation of knock-in mice. K.T. provided critical advice. T.K., C.K., J.A.K. and M.M. wrote the paper.
Supplementary Material
Supplementary informations
Acknowledgments
We thank Rikki Chokshi, Urszula Osinska, Sai Kollipara for excellent technical assistance and Mark Rich for useful comments on the manuscript. This research was funded by Japanese Ministry of Education, Culture, Sports, Science and Technology (MM), The Promotion Project of Medical Clustering of Okinawa Prefecture (MM) and American Heart Association, National Center (JAK). SH was supported in part by GM090122 NIGMS R25 grant.
References
- Kozak J. A., Kerschbaum H. H. & Cahalan M. D. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 120, 221–235 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler M. J. et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001). [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller M. K., Hermosura M. C., Nadler M. J., Scharenberg A. M., Penner R. & Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwanyanya A. et al. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J. Physiol. 559 (Pt 3), 761–776 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann K. P. et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 31, 166–170 (2002). [DOI] [PubMed] [Google Scholar]
- Walder R. Y. et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 31, 171–174 (2002). [DOI] [PubMed] [Google Scholar]
- Brandao K., Deason-Towne F., Perraud A. L. & Schmitz C. The role of Mg2+ in immune cells. Immunol. Res. 55, 261–269 (2013). [DOI] [PubMed] [Google Scholar]
- Schmitz C. et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 (2003). [DOI] [PubMed] [Google Scholar]
- Chubanov V. et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. U. S. A. 101, 2894–2899 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanova L. V. et al. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 1, 109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwanyanya A., Sipido K. R., Vereecke J. & Mubagwa K. ATP and PIP2 dependence of the magnesium-inhibited, TRPM7-like cation channel in cardiac myocytes. Am. J. Physiol. Cell Physiol. 291, C627–635 (2006). [DOI] [PubMed] [Google Scholar]
- Chokshi R., Matsushita M. & Kozak J. A. Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am. J. Physiol. Cell Physiol. 302, C1004–1011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokshi R., Matsushita M. & Kozak J. A. Sensitivity of TRPM7 channels to Mg2+ characterized in cell-free patches of Jurkat T lymphocytes. Am. J. Physiol. Cell Physiol. 302, C1642–1651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J. A., Matsushita M., Nairn A. C. & Cahalan M. D. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J. Gen. Physiol. 126, 499–514 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M. et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J. Biol. Chem. 280, 20793–20803 (2005). [DOI] [PubMed] [Google Scholar]
- Runnels L. W., Yue L. & Clapham D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001). [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Matsushita M., Nairn A. C. & Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7, 1047–1057 (2001). [DOI] [PubMed] [Google Scholar]
- Drennan D. & Ryazanov A. G. Alpha-kinases: analysis of the family and comparison with conventional protein kinases. Prog. Biophys. Mol. Biol. 85, 1–32 (2004). [DOI] [PubMed] [Google Scholar]
- Nairn A. C. et al. Elongation factor-2 phosphorylation and the regulation of protein synthesis by calcium. Prog. Mol. Subcell. Biol. 27, 91–129 (2001). [DOI] [PubMed] [Google Scholar]
- Dorovkov M. V., Kostyukova A. S. & Ryazanov A. G. Phosphorylation of annexin A1 by TRPM7 kinase: a switch regulating the induction of an α-helix. Biochemistry 50, 2187–2193 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deason-Towne F., Perraud A. L. & Schmitz C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase Cγ2 (PLCγ2) using TRPM7-kinase. Cell Signal. 24, 2070–2075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud A. L., Zhao X., Ryazanov A. G. & Schmitz C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k. Cell Signal. 23, 586–593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. et al. The TRPM6 kinase domain determines the Mg ATP-sensitivity of TRPM7/M6 heteromeric ion channels. J. Biol. Chem. 289, 5217–5227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. et al. Humanized prion protein knock-in by Cre-induced site-specific recombination in the mouse. Biochem. Biophys. Res. Commun. 222, 742–747 (1996). [DOI] [PubMed] [Google Scholar]
- Bödding M. TRPM6: A Janus-like protein. Handb. Exp. Pharmacol. 179, 299–311 (2007). [DOI] [PubMed] [Google Scholar]
- Topala C. N. et al. Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium 41, 513–523 (2007). [DOI] [PubMed] [Google Scholar]
- Jin J., Desai B. N., Navarro B., Donovan A., Andrews N. C. & Clapham D. E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeuse P., Penner R. & Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 127, 421–434 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuiver M. et al. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am. J. Hum. Genet. 88, 333–343 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabakken T., Rian E., Kveine M. & Aasheim H. C. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem. Biophys. Res. Commun. 306, 718–724 (2003). [DOI] [PubMed] [Google Scholar]
- Goytain A. & Quamme G. A. Functional characterization of the mouse solute carrier, SLC41A2. Biochem. Biophys. Res. Commun. 330, 701–705 (2005). [DOI] [PubMed] [Google Scholar]
- Zsurka G., Gregán J. & Schweyen R. J. The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics 72, 158–168 (2001). [DOI] [PubMed] [Google Scholar]
- Goytain A. & Quamme G. A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics 6, 48 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L. & Gordon S. Murine macrophages: a technical approach. Methods Mol. Biol. 415, 255–272 (2008). [DOI] [PubMed] [Google Scholar]
- Ypey D. L. & Clapham D. E. Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc. Natl. Acad. Sci. U. S. A. 81, 3083–3087 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C. & Trautmann A. Ionic channels in murine macrophages. J. Cell Biol. 105, 761–769 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K. Ion channels in leukocytes. Physiol. Rev. 71, 775–811 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary informations