Abstract
Background
The global obesity epidemic has increased the prevalence of fatty liver disease. At present, 14% to 27% of the general population in the industrialized world has non-alcoholic fatty liver disease (NAFLD).
Methods
We review pertinent publications retrieved by a selective search of the PubMed database for the years 1995 to 2013.
Results
The term “non-alcoholic fatty liver disease” covers cases of a wide spectrum of severity, ranging from bland fatty liver without any inflammation and with little or no tendency to progress all the way to non-alcoholic steatohepatitis (NASH) with inflammatory reactions and hepatocyte damage, with or without fibrosis. Some 5% to 20% of patients with NAFLD develop NASH, which undergoes a further transition to higher-grade fibrosis in 10% to 20% of cases. In fewer than 5% of cases, fibrosis progresses to cirrhosis. These approximate figures lead to an estimate of 0.05% to 0.3% for the prevalence of cirrhosis in the general population. About 2% of all cirrhosis patients per year develop hepatocellular carcinoma. The diagnosis of fatty liver disease can be suspected initially on the basis of abnormally high aspartate aminotransferase (ASAT) and/or alanine aminotransferase (ALAT) levels and abnormal ultrasonographic findings. The positive predictive value of an ultrasonographic study for mild steatosis is 67% at most. The NAFLD fibrosis score, which is computed on the basis of multiple parameters (age, body-mass index, diabetes status, ASAT, ALAT, platelet count, and albumin level), has a positive predictive value of 82% to 90% and a negative predictive value of 88% to 93%. Liver biopsy is the gold standard for diagnosis but should be performed sparingly in view of its rare but sometimes life-threatening complications, such as hemorrhage. The treatment of NAFLD and NASH consists mainly of changes in lifestyle and nutrition.
Conclusion
NAFLD can, in principle, be reversed. This is only possible with weight reduction by at least 3% to 5%.
The term “non-alcoholic fatty liver disease” (NAFLD) refers to hepatic steatosis accounting for more than 5–10% of the total weight of the liver or macrosteatosis of the same extent, which is not caused by excessive consumption of alcohol (women ≤20 g/d, men ≤30 g/d). Mixed forms of NAFLD and alcoholic fatty liver disease are possible. In order to make a diagnosis, imaging or histological techniques need to be applied to confirm hepatic steatosis, in the confirmed absence of any other cause of secondary steatosis (Box). The term NAFLD refers to a spectrum of hepatic disorders, ranging from simple or bland fatty liver (NAFL, non-alcoholic fatty liver)—in which no inflammatory changes are seen except for macrovesicular or microvesicular steatosis—to non-alcoholic steatohepatitis (NASH), which is characterized by an inflammatory reaction with hepatocytic injury, such as balloonic degeneration and necroapoptosis with or without fibrosis (1).
Box. Causes of secondary hepatic steatosis (adapted from [1]).
-
Macrovesicular steatosis
Increased consumption of alcohol
Hepatitis C (especially genotype 3)
Wilson’s disease
Lipodystrophy
States of hunger
Parenteral nutrition
Abetalipoproteinemia
-
Medications
(for example, amiodarone, methotrexate, steroids)
-
Microvesicular steatosis
Reye’s syndrome
Acute fatty liver of pregnancy
HELLP syndrome
Metabolic disorders (for example, lecithin-cholesterol-acyltransferase [LCAT] deficiency)
-
Medications
(for example, valproate, antiretroviral drugs)
Epidemiology
In Europe as well as in the UK, non-alcoholic fatty liver has become the most commonly diagnosed cause of chronic liver disease. The reasons include greater general awareness of the problem (2, 3). According to data from the US National Health and Nutrition Examination Survey (NHANES), the proportion of NAFLD among chronic liver diseases rose from 47% to 75% between 1988 and 2008. The reason for this increase is most probably an increase in metabolic risk factors, also in the context of aging populations. Over the same time period, the prevalence of the five metabolic syndrome conditions also increased substantially:
Obesity from 21% to 33%
Visceral obesity from 35% to 51%
Type 2 diabetes from 5.6% to 9.1%
Insulin resistance from 23% to 35%
Arterial hypertension from 22% to 34% (2).
It is a well known fact that NAFLD is closely associated with these factors. In patients with fatty liver, the prevalence of obesity is between 30% and 100%, and that of type 2 diabetes between 10% and 75% (4). According to data from the OECD (Organization for Economic Cooperation and Development), in 2010, 14.7% of adults in Germany were obese (body mass index [BMI] >30 kg/m2)—a clear increase compared with 2000 (11.5%) (5).
In Europe, the prevalence of NAFLD in the population is estimated to be 20% to 30% (3). After adjusting for biases occurring even when the latest, state of the art ultrasound technology is used (sensitivity 88%, specificity 91% [e1]) the prevalence is 13.9% to 26.6%, which indicates that the actual prevalence may be below that observed by using ultrasound. A look at special subgroups in the population reveals a wide range of the observed prevalence rates—from 2% in unselected children to 44% in selected risk groups, such as people with type 2 diabetes (3). No reliable data are available on the prevalence of advanced NAFLD and cirrhosis.
A strong increase of NAFLD has recently been observed especially in adolescents and in older people. An Australian cohort study found a prevalence of 12.8% in adolescents, and this was found to be higher in girls than in boys (16.3% versus 10.1%) (6). Data from the Netherlands showed a prevalence of 35% in older patients (mean age 76 years) (7).
The prevalence depends not only on the population under study but also on the study method; the differences may be substantial. Two studies in potential live liver donors found histologically confirmed NAFLD in 20% and 51% of cases, respectively (8, e2). Sonographically the prevalence rate varied between 17% and 46%, depending on the population under study (9, e3). Worldwide, the assumed prevalence of NAFLD in the general population is between 6% and 33%, with a median of 20%; the estimated prevalence of NASH is notably lower, at 3% to 5% (9).
Obesity is a known risk factor for NAFLD; both a high BMI and visceral obesity increase the risk. In patients with morbid obesity (BMI>40 kg/m2) who undergo bariatric surgery, the prevalence of NAFLD may even be in excess of 90% (1). Recently, however, physical constitution and fat distribution were found to be better indicators of mortality than BMI alone (10, e4).
The proportion of NAFLD is also higher in patients with type 2 diabetes than in the general population (9). An ultrasound based study found a prevalence of 69% among patients with type 2 diabetes (11). Lipid metabolism also seems to have a substantial influence. In many type 2 diabetes patients, raised concentrations of triglycerides and lowered concentrations of HDL cholesterol are also observed. In patients with dyslipidemia who were attending an outpatient clinic, the prevalence of NAFLD was 50% (e5). Interestingly, the risk of NAFLD increases independently of the presence of diabetes with each individual metabolic risk factor (4). Factors such as age, sex and ethnicity have also been found to play a role: male sex, older age, and Hispanic origin are associated with a significantly higher risk of developing NAFLD (9, e6).
Natural clinical course
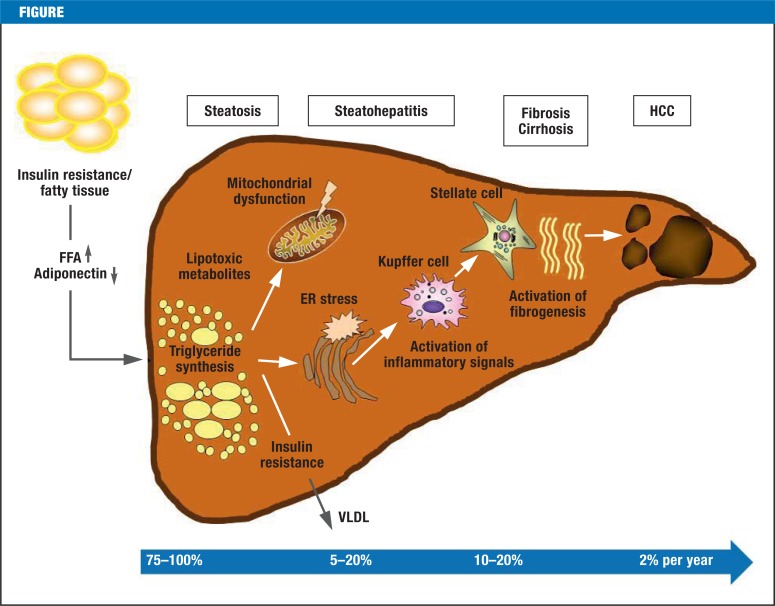
The Figure shows the pathogenesis and natural clinical course of non-alcoholic fatty liver disease. Patients with NAFLD can be prognostically categorized into two groups: patients with simple NAFL experience no progression or only a very mild progression of their disease. Liver injury after NASH is qualitatively no different to that caused by alcohol, although the progression in NASH is slower and the histological changes are less pronounced (12). However, this needs to be qualified by mentioning the fact that there are numerous studies that investigated the natural clinical course und histological changes over time in NAFL and NASH, but these studies mostly included small numbers of patients and relatively short observation periods. It is highly probably that a substantial proportion of the cases of hepatic cirrhosis that used to be classed as “cryptogenic” is due to NAFLD or NASH. This is supported by the fact that patients with cryptogenic cirrhosis disproportionally often have metabolic risk factors, such as type 2 diabetes, obesity, or metabolic syndrome, and characteristics of NASH are often detected in their liver biopsies (13, e7). Between 5% and 20% of patients with fatty liver develop NASH over the clinical course; in some 10–20% this develops into higher-grade fibrosis; in <5% this progresses to full-blown cirrhosis (14). A sequential estimate assuming the variance of these progression frequencies yields a prevalence of NAFLD cirrhosis of 0.05–0.3% of the general population. The direct development from simple NAFL to cirrhosis has also been described (15). Furthermore, patients with NAFLD have an increased risk for hepatocellular carcinoma (HCC), although the risk is mostly restricted to those with advanced fibrosis and hepatic cirrhosis (16). If cirrhosis is present, the annual risk for HCC is 2% (13). However, HCC has also been described in NAFLD patients without cirrhosis (15). According to international estimates, the incidence of HCC will double by 2020, owing to the massive increase in non-alcoholic fatty liver disease (the incidence in Germany in 2010 was 8330) (17, e8, e9). In addition to this, NAFLD is a cardiovascular risk factor that is independent of the classic risk factors (18). Furthermore, patients with NASH have been found to be subject to a higher overall mortality (survival 70% versus 80%, mean observational interval 13.7 years) compared with a control population adjusted for risk factors, in contrast to patients with bland steatosis (NAFL) (19). Similarly, in patients with NASH—but not in those with NAFL—the liver specific mortality rate is increased (1). The most common causes of death are malignancies, followed by cardiovascular disorders; liver-associated mortality is in third place (13%) (20).
Figure.
Pathogenesis and natural clinical course of non-alcoholic fatty liver disease. The figure shows the frequencies of the individual stages of disease (modified from [e18]). Pathogenesis: an important part in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) is played by insulin resistance, oxidative stress, and the inflammatory cascade. According to the “multiple hit” theory, hyperinsulinemia in the context of insulin resistance leads, as a first step, to an increased release of free fatty acids from adipocytes and myocytes, which are then absorbed by the liver, where they accumulate and result in steatosis. This initial step is then followed by a series of complex interactions between hepatocytes, Kupffer cells, adipocytes, inflammatory mediators, and oxygen radicals. The result is steatohepatitis. Free fatty acids are oxidized in mitochondria, peroxisomes, and microsomes, which leads to reactive by-products. The chronic inflammation contributes to hepatocytic injury and, in the long term, to the development of fibrosis and cirrhosis. The inflammatory mediators tumor necrosis factor alpha (TNF-alpha) and interleukin-1 beta (IL-1beta), as well as adiponectin (a hormone from adipocytes that reduces fatty acid oxidation and inhibits hepatic gluconeogenesis) seem to be of particular importance in this setting. Hepatocellular carcinoma (HCC) develops with an incidence of about 2% per year, and the cancer can also develop in a non-cirrhotic liver (e16-e18)
In principle, NAFLD is reversible; weight reduction plays a vital part in this. Two retrospective studies and one prospective study showed that hepatic steatosis reversed in a majority of morbidly obese patients who had bariatric surgery, and the proportion of patients with fibrosis also fell. Similarly, a change in different serum markers was noted, including fetuin-A expression (21, e10, e11). The association of weight gain and incidence of hepatic steatosis, as well as that of weight loss and reversal of steatosis, has been prospectively confirmed over seven years (22). Interestingly, even a moderate weight reduction of up to 4% of body weight is sufficient to yield a reduction in hepatic steatosis in 56% of patients (22). Consumption of coffee (but not espresso) and even small quantities of alcohol (<20 g/day) seemed beneficial in this setting. Coffee consumption was found to be an independent protective factor against fibrosis in NASH (odds ratio [OR] 0.75; 95% confidence interval [CI] 0.58 to 0.98); moderate consumption of alcohol reduced in NAFLD the risk for NASH (OR 0.56; 95% CI 0.39 to 0.84), fibrosis (OR 0.56; 95% CI 0.41 to 0.77), and ballooning degeneration of hepatocytes (OR 0.66; 95% CI 0.48 to 0.92) (23, 24).
Clinical presentation and diagnostic evaluation
The findings in NAFLD tend to be non-specific. Most patients have no symptoms or signs of hepatic disease at the time of diagnosis; some complain about increased tiredness or a sensation of pressure in the right upper abdomen.
In terms of laboratory chemistry, pathological values of glutamate-oxalacetate-transaminase (GOT) (aspartate-aminotransferase [ASAT]) and glutamate-pyruvate-transaminase (GPT) (alanine-aminotransferase [ALAT]) may be noted; raised GPT is mostly leading and often in isolation (4). However, even normal readings for transaminases do not rule out cirrhosis, and raised concentrations are partly re-normalized when NASH develops (25). The ferritin concentration is raised in about half of patients, and transferrin saturation increased in 6–11%. The liver’s iron content is typically within the normal range (4), in contrast to the situation in hemochromatosis. Furthermore, commercial combined tests and apoptosis markers (cytokeratine-18 fragments [e12]) are available; to date, however, these have not gained any importance in routine clinical practice.
Liver biopsy is still the gold standard in the diagnostic evaluation of NAFLD, because NASH can be formally diagnosed only by means of histological testing. However, a biopsy is an invasive procedure, which carries a risk—albeit a rare one—of potentially life threatening complications—hemorrhage, for example (e13). It needs to be borne in mind that NASH is erroneously not identified in up to one-third of patients, and that the degree of fibrosis may be subject to overestimation as well as underestimation (26).
Since patients are usually asymptomatic and the laboratory parameters often normal, the question in routine clinical practice is which patients should be investigated for NAFLD. A practical recommendation is urgently needed in this setting. The current US NAFLD guideline advises against general NAFLD screening at this time, owing to the lack of evidence of benefit and the relatively high cost, not even in high-risk groups such as obese patients or patients with diabetes (1). In Germany, the clinical practice guideline (S3) on the diagnosis and treatment of hepatocellular carcinoma does, however, consider the risk factors listed above and recommends a general ultrasound follow-up not only in cirrhosis but also in patients with NASH (27). In principle this requires a liver biopsy in all patients with a fatty liver, in order to identify high-risk patients with NASH or higher-grade fibrosis. Such an approach—with careful weighing of the risks and benefits—is of course only justified in patients with an increased primary risk for the presence of NASH or fibrosis, since these conditions are associated with the development of hepatic cirrhosis and its complications (HCC, among others). To date, no clear guidance is given in the literature with regard to defining an indication for liver biopsy in NAFLD.
The non-invasive investigative method that is certainly most suitable for detecting hepatic steatosis is ultrasound (sensitivity 60–94%, specificity 66–97%), but this is rather less precise in milder degrees of steatosis (28). According to available study data, the positive predictive value in mild steatosis is only 67% at best (28). Outside the study setting, the positive predictive value is likely to be even lower. The degree of hepatic fibrosis can now be estimated non-invasively by using several techniques of elastography (including FibroScan and acoustic radiation force impulse imaging [ARFI]) (e14). The FibroScan investigation enables distinction between fibrosis (F1–F3) and cirrhosis (29), but in morbid obesity it is not equal to the task.
For routine clinical practice, the question remains how high-risk patients can be identified with a justifiable amount of apparative diagnostics and interventional risk. Recently, a number of simple clinical risk scores has shown excellent consistency with the degree of fibrosis in patients with steatosis (30). The best result was seen for the NAFLD fibrosis score (http://nafldscore.com), which consists of the parameters age, BMI, diabetes, GOT, GPT, thrombocytes, and albumin (positive predictive value 82–90%, negative predictive value 88–93%). An increased risk of higher-degree fibrosis was described for patients with a BMI >32 kg/m2, age >45 years, diabetes, and a ratio of GOT to GPT >1 (31). New genetic markers, such as variants of PNPLA3 (adiponutrin), which indicate an increased risk of progression towards NASH, fibrosis, and HCC, have not yet become established in routine clinical practice (32, 33, e15).
In combination with findings from sonography or elastography, these clinical scores can help identify patients for diagnostic liver biopsy or close clinical and sonographic monitoring of the clinical course (six-monthly in NASH). The high coincidence of type 2 diabetes and NAFLD justifies routine diabetes screening (HbA1c and oral glucose tolerance test, if required).
Treatment
Therapeutic options in NAFLD and NASH are currently limited mainly to interventions in terms of diet and lifestyle. A medication with long-term effectiveness that would beneficially affect the course of fibrosis does currently not exist. The most effective treatment consists of weight reduction and intensive lifestyle modification with an increase in physical activity/exercise, which has been confirmed to be able to improve histological results (1). In a randomized controlled trial, an increase in physical activity of moderate intensity to some 200 minutes per week resulted in weight loss of 9% and significant improvements of steatosis and necroinflammation on hepatic histology over 48 weeks (34). In general, a reduction in weight of at least 3–5% seems required to positively affect the steatosis; with regard to necroinflammation, a weight loss of at least 9% is required (1).
In recent years, different drug based approaches have been investigated in randomized, placebo controlled studies. Metformin has not been found to have any effect of significance. A recent meta-analysis has shown that treatment with metformin for 6–12 months, combined with a lifestyle intervention, did not improve transaminases nor liver histology compared with a lifestyle intervention alone (9). Another meta-analysis and a case–control study in combination with an in-vitro study indicated, however, that metformin may have a positive effect in terms of the incidence of HCC (35, 36). Pioglitazone seems to have a positive effect, which in several studies improved the steatosis as well as the inflammation (37, 38). The effect on fibrosis is not clear. Data on the long-term effects and safety are lacking (9, 1).
As oxidative stress seems to have a central role in hepatic cell injury in the context of NASH, the influence of the antioxidant vitamin E on the disorder has also been investigated. In the randomized, placebo controlled PIVENS study in non-diabetic patients, vitamin E lowered transaminases and histologically improved steatosis and inflammation after two years of treatment, but did not affect the degree of fibrosis (38). The US guideline therefore does not recommend vitamin E in non-diabetic patients with histologically confirmed NASH, but it does recommend against its use in diabetes patients, in the absence of histological results, and in case of NASH cirrhosis or cryptogenic cirrhosis, until robust data are available (1).
Patients with NAFLD are subject to vitamin D deficiency, the extent of which depends on the degree of fibrosis and necroinflammation (39). In view of its beneficial metabolic and anti-inflammatory effects, substitution therapy in such patients seems to make sense, in principle, and is currently being investigated in studies (40).
Conclusion
With increasing prevalence rates of obesity, NAFLD has become the most common chronic liver disorder that physicians in inpatient and outpatient clinics in Europe as well as in the US will find themselves confronted with. At-risk patients can be identified by using a combination of clinical presentation, sonography (if required, in combination with elastography) and validated risk scores for close monitoring of the clinical course; doctors in private practice have an important steering function in this context.
Key Messages.
The prevalence of NAFLD in the normal population is 20–30% and that of NASH is 3%; if risk factors—such as the metabolic syndrome—are present, these rates can rise to 75% and 15–50%, respectively.
NAFLD has become the most common chronic hepatic disorder in Europe and the US.
If NASH or fibrosis are present, the affected patients’ mortality is significantly increased.
Complete reversal is possible; weight reduction has the most important role in this context.
Therapeutic methods using medications have not become established for all groups of patients; similarly, a long-term effect of drug treatment on the progression of fibrosis has not been confirmed.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Prof. Geier has received material resources from Burgerstein Vitamine (study medication SASL34) and from the Velux Foundation (third party funding SASL34 study).
The other authors declare that no conflict of interest exists.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe. A Review of available epidemiological data. European Association for the Study of the Liver 2013. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. Non alcoholic fatty liver disease. N Engl J Med. 2002;346:2121–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.OECD Health Data 2012. Eurostat Statistics Database. WHO Global Infobase. http://dx.doi.org/10.1787/9789264183896-en (last accessed on 15 April 2014)
- 6.Ayonrinde OT, Olynyk JK, Beilin LJ, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809. doi: 10.1002/hep.24097. [DOI] [PubMed] [Google Scholar]
- 7.Koehler EM, Schouten JNL, Hansen BE, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J Hepatol. 2012;57:1305–1311. doi: 10.1016/j.jhep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non alcoholic fatty liver disease and non alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS, Lazar MA. The Health risk of obesity-better metrics imperative. Science. 2013;341:856–858. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 11.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors in non alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 12.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of non alcoholic fatty liver: a follow up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 13.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and non alcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25:231–244. doi: 10.1016/j.bpg.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 16.Ertle J, Dechêne A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006 44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 20.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044–1049. doi: 10.1007/s11695-011-0559-y. [DOI] [PubMed] [Google Scholar]
- 22.Zelber-Sagi F, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012 56:1145–1151. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Anty R, Marjoux S, Ianelli A, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012;57:1090–1096. doi: 10.1016/j.jhep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57:384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease in normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 26.Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 27.Malek NP, Schmidt S, Huber P, Manns MP, Greten TF. The diagnosis and treatment of hepatocellular carcinoma. Dtsch Arztebl Int. 2014;111:101–106. doi: 10.3238/arztebl.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–1019. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Gaia S, Carenzi S, Barilli AL, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64–71. doi: 10.1016/j.jhep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 32.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trépo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: evidence from a meta-analysis of individual participant data. Hepatology. 2013 doi: 10.1002/hep.26767. doi: 10.1002/hep.26767 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.Pomrath K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR. Randomized controlled trial testing the effects of weight loss on nonalcolholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in dose-dependend manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 37.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006 355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal AJ, Chalasani L, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targher G, Bertolini L, Scala L, et al. Associations between serum 25-hydroxy vitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007 17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Geier A. Shedding new light on vitamin D and fatty liver disease. J Hepatol. 2011;55:273–275. doi: 10.1016/j.jhep.2010.12.026. [DOI] [PubMed] [Google Scholar]
- e1.Soresi M, Giannitrapani L, Florena AM, et al. Reliability of the bright liver echo pattern in diagnosing steatosis in patients with cryptogenic and HCV-related hypertransaminasaemia. Clin Radiol. 2009;64:1181–1187. doi: 10.1016/j.crad.2009.06.013. [DOI] [PubMed] [Google Scholar]
- e2.Marcos A, Fisher RA, Ham JM, et al. Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation. 2000;69:2410–2415. doi: 10.1097/00007890-200006150-00034. [DOI] [PubMed] [Google Scholar]
- e3.Williams CD, Stenger J, Asike MI, et al. Prevalence of non alcoholic fatty liver disease and non alcoholic steatohepatitis among a largely middle aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- e4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45:1929–1934. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- e6.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- e7.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- e8.Robert Koch-Institut. Krebs in Deutschland 2009/2010. 9th edition. Berlin: Robert Koch-Institut; 2013. Gesellschaft der epidemiologischen Krebsregsiter in Deutschland e.V. (eds.) 40 pp. [Google Scholar]
- e9.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Mottin CC, Moretto M, Padoin AV, et al. Histological behavior of hepatic steatosis in morbidly obese patients after weight loss induced by bariatric surgery. Obes Surg. 2005;15:788–793. doi: 10.1381/0960892054222830. [DOI] [PubMed] [Google Scholar]
- e11.Kahraman A, Sowa JP, Schlattjan M, et al. Fetuin mRNA expression is elevated in NASH compared with NAFL patients. Clin Sci (Lond) 2013;125:391–400. doi: 10.1042/CS20120542. [DOI] [PubMed] [Google Scholar]
- e12.Canbay A, Feldstein A, Kronenberger B, Schulze-Osthoff K, Bantel H. Zytokeratin-18 als Marker zur nicht invasiven Diagnostik und Prognose akuter und chronischer Lebererkrankungen. Z Gastroenterol. 2014;52:290–295. doi: 10.1055/s-0033-1356138. [DOI] [PubMed] [Google Scholar]
- e13.Tannapfel H, Dienes HP, Lohse AW. The indications for liver biopsy. Dtsch Arztebl Int. 2012;109:477–483. doi: 10.3238/arztebl.2012.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Wiegand J, Berg T. The etiology, diagnosis and prevention of liver cirrhosis—part 1 of a series of liver cirrhosis. Dtsch Arztebl Int. 2013;110:85–91. doi: 10.3238/arztebl.2013.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- e16.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- e17.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- e18.Cusi K. Role of obesity and lipotoxicity in the development of non-alcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]