Abstract
Background
Disease management programs (DMPs) are intended to improve the care of persons with chronic diseases. Despite numerous studies there is no unequivocal evidence about the effectiveness of DMPs in Germany.
Methods
We conducted a systematic literature review in the MEDLINE, EMBASE, Cochrane Library, and CCMed databases. Our analysis included all controlled studies in which patients with type 2 diabetes enrolled in a DMP were compared to type 2 diabetes patients receiving routine care with respect to process, outcome, and economic parameters.
Results
The 9 studies included in the analysis were highly divergent with respect to their characteristics and the process and outcome parameters studied in each. No study had data beyond the year 2008. In 3 publications, the DMP patients had a lower mortality than the control patients (2.3%, 11.3%, and 7.17% versus 4.7%, 14.4%, and 14.72%). In 2 publications, DMP participation was found to be associated with a mean survival time of 1044.94 (± 189.87) days, as against 985.02 (± 264.68) in the control group. No consistent effect was seen with respect to morbidity, quality of life, or economic parameters. 7 publications from 5 studies revealed positive effects on process parameters for DMP participants.
Conclusion
The observed beneficial trends with respect to mortality and survival time, as well as improvements in process parameters, indicate that DMPs can, in fact, improve the care of patients with diabetes. Further evaluation is needed, because some changes in outcome parameters (an important indicator of the quality of care) may only be observable over a longer period of time.
Chronic diseases are one of the main causes of increased morbidity and mortality risk worldwide (1). Diabetes mellitus is a chronic disease often associated with serious complications and sequelae pertaining to the eyes, kidneys, and feet; it also increases the risk of cardiovascular disease (2). According to an international analysis, the number of adults with diabetes worldwide has more than doubled in the last 30 years (3). Increasing prevalence and associated rises in spending (4, 5) are making care for chronic diseases a hot topic in health care policy in Germany and elsewhere in Europe (1). In addition to numerous prevention measures, disease management programs (DMPs) have also been introduced in many countries (6, 7).
In Germany, DMPs were rolled out nationwide in 2002 (8). Their aim was to improve the quality of health care and the treatment process (7). Although 3.86 million insured individuals took part in 1820 DMPs for type 2 diabetes in 2013 (9), it is not yet known how effective such programs are (10).
Although program evaluation is mandatory according to clause 137f, paragraph 4 in Book Five of Germany’s Social Security Code (SGB V), which is unusual among European countries (9), a comparison of data gathered on DMP participants with a control group taken from routine care is not required by law (10, 11). This means that randomized controlled trials, which are the gold standard for reporting the effects of interventions, are no longer possible for the DMPs already implemented nationwide (11). This has led research institutions and sickness funds to increase their efforts to evaluate DMPs via controlled studies in recent years, in addition to evaluation required by law.
Although systematic reviews and meta-analyses on the effectiveness of DMPs in other countries already exist (12– 18), none have been conducted for Germany. This article therefore aims to bring together the available controlled studies evaluating the effectiveness of DMPs in Germany in a systematic review.
Methods
Search strategy
A preliminary search of the literature showed that the largest number of studies available addressed the indication type 2 diabetes mellitus. This systematic review therefore examines this indication. The search was undertaken between 1 February to 15 April 2014 (most recent update). Information was processed by three independent reviewers at each stage. A comprehensive, systematic search strategy was developed to identify relevant literature. The most important components were “disease management program” as the intervention, a relation to “Germany,” and “DMP implementation period in Germany.” The final search strings contained a combination of free text and keyword searches and were suited to the specific natures of each database used (MEDLINE, EMBASE, Cochrane Library, CCMed). In addition, the reference lists cited in the included publications were screened by researchers to identify further potentially relevant studies.
Inclusion and exclusion criteria
The review included only studies published between 1 January, 2002 and 15 April 2014 that compared outcome parameters, process parameters, or economic parameters of patients in an authorized DMP for the indication type 2 diabetes mellitus within German statutory health insurance to those of patients receiving routine care. Publications that investigated the effectiveness of DMPs with no control groups, evaluated other care programs for chronic diseases or pilot projects, were not available as full text, or had not been peer-reviewed were excluded.
Study selection
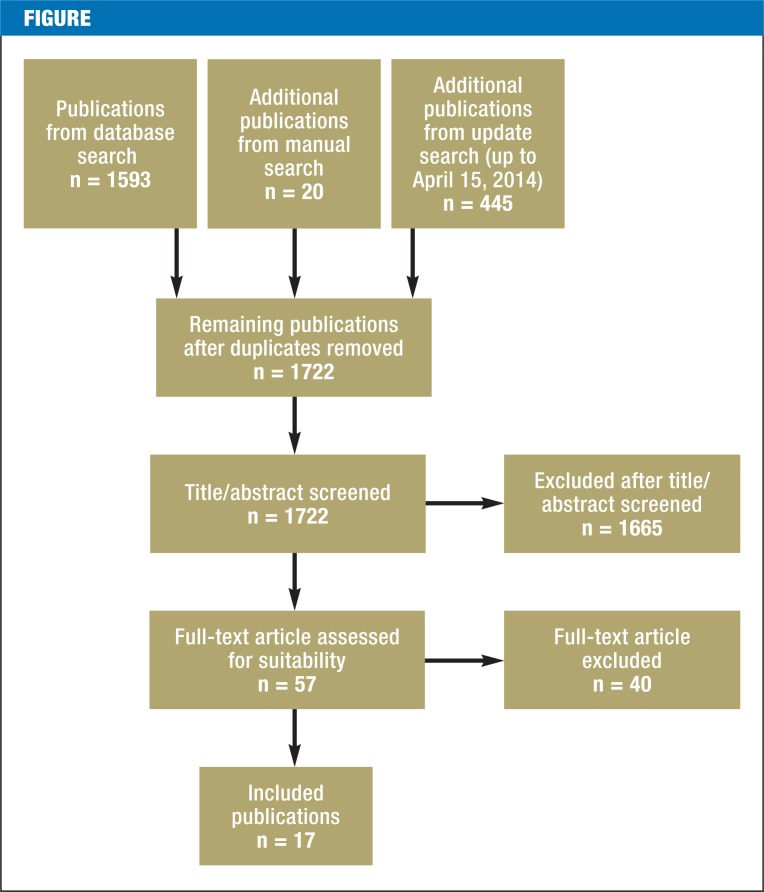
The Figure shows the study selection process and its results. Database searches yielded 1722 publications after duplicates had been removed. After these had been screened twice (first the title and abstract, then the full text) for fulfillment of the inclusion criteria, 17 publications remained.
Figure.
Study selection process
Results
Study characteristics
Table 1 shows the features of the studies included and the characteristics of their participants. Several of the 17 publications included were based on the same dataset (see Table 1), so these were treated as a single study in each case. Two publications (21 and 22) are identical other than the language in which they are written and were therefore treated as a single publication. Analysis was thus performed on a total of 16 publications based on nine studies.
Table 1a. Study characteristics.
| Study | Publication | Dataset | No. of cases | Mean age | Sex distribution (M) | Observation period | Minimum duration of continuous DMP participation before beginning of ‧analysis | Adjustment for selection bias (method: variables used) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP | CG | DMP | CG | DMP | CG | ||||||
| 1 | (19) (20) |
BARMER claims data (nationwide) |
19 888 mp | 19 888 mp | 66.3 | 66.0 | 47% | 48% | 2005 to 2007 | 36 months*3 | PSM: age, sex, zip code, insurance status, drug and hospital costs, selected hospital diagnoses, drug prescriptions |
| (21/ 22) |
19 882 mp | 19 882 mp | 67.04 | 67.02 | 46.4% | 46.4% | 2003 to 2007*2 | 36 months | |||
| 2 | (23) | BARMER claims data (nationwide) |
80 745 | 79 137 | 40 to 95 | N/A | 2006 | 12 months*4 | Evaluation: sex-specific, standardized for age | ||
| 3 | (24) | BARMER patient survey: random sample (nationwide) |
2061 | 2182 | 66.7*1 | 66.5*1 | 49.8%*1 | May to June 2007 | 1 day | Regression analysis: education, age, sex | |
| (25) | Regression analysis: active health-related ‧behavior, age, sex | ||||||||||
| 4 | (26) | TK claims data (nationwide) |
84 410 | 23 180 | 63.8 ± 10.2 | 63.6 ± 12.6 | 64.7% | 64.4% | 2007 to 2008 | 1 day*5 | PSIM: age, sex, level of care, education, ‧occupational status, drug and hospital costs, ‧scope of prescription in defined daily dose, ‧relevant comorbidities |
| 5 | (27) | KKH-Allianz claims data (nationwide) |
7131 mp | 7131 mp | 62.8 ± 10.3 | 62.8 ± 10.7 | 42.6% | July 2004 to ‧December 2006 | 18 months | Matching: age, sex, care expenditure expected ‧after adjustment for morbidity | |
| 6 | (28) | AOK claims data, part of ELSID study (Rhineland– Palatinate, Saxony–Anhalt) |
1927 mp | 1927 mp | 70.7 ± 8.6 | 70.73 ± 8.57 | 39.7% | 39.7% | 2006 to 2008 | 1 day*5 | Matching: age groups, sex, retirement status, federal state, pharmacy cost group, diagnosis cost group |
Abbreviations and notes: CG: Control group; mp: Matched pairs; M: Male; TK: Techniker Krankenkasse; AOK: Allgemeine Ortskrankenkasse; KKH: Kaufmännische Krankenkasse Halle; ELSID: Evaluation of Large Scale Implementation of Disease Management Programs for Patients with Type 2 Diabetes; PSM: Propensity score matching; PSIM: Propensity score interval matching; N/A, not available
*1In Graf et al. 2009 (25) only; N/A in Elkeles et al. 2009 (24)
*2Analysis 1: 2007; analysis 2: 2003 (baseline), 2007 (follow-up)
*3Unclear: continuous DMP participation (at least 300 days per year), 36 to 48 months
*4Continuous DMP participation from January 2006 to May 2007, data gathered in 2006
*5Unclear whether DMP participants must be enrolled throughout the observation period
All the studies are controlled observational studies. With the exception of one cohort study (33), in which data was gathered at two points in time (baseline and follow-up), they all evaluate data taken at one point only or during one observation period only. The study population of the publications (n = 16) consists of individuals insured by sickness funds BARMER and Gmünder Ersatzkasse (GEK) (n = 7), Allgemeine Ortskrankenkasse (AOK) (n = 5), Techniker Krankenkasse (TK) (n = 1), and KKH-Allianz (n = 1). In two studies, the population was based on a sample that was representative of the general population (Kooperative Gesundheitsforschung in der Region Augsburg [KORA], n = 2). The dataset of the studies consists of administrative claims data on individuals insured by these sickness funds (19– 28) and data from patient surveys (29–35). Case numbers range from 85 to 84 410 patients in DMP groups, and from 64 to 79 137 patients in control groups. The mean age in both sets of groups is between 62.8 and 70.7 years. The proportion of male members of DMP groups ranges from 39.7% to 64.7%; the corresponding figures for control groups are 39.7% to 64.4%.
With the exception of the cohort study, formal appraisal of the quality of the studies using a standardized tool such as the SIGN checklist is impossible because of study design. However, there are differences in studies’ methodological quality, and these are particularly important in the evaluation of care programs. For example, the length of the intervention being investigated before the beginning of analysis significantly affects the stability of its effects. In the studies examined here, length of intervention ranges from one day to 36 months. There may also be major differences between the structures of intervention and control groups, as study populations could not be randomized. Methods used to adjust for this selection bias also vary between the studies: for instance, four studies use matching, two of them with a propensity score. Other adjustment methods used are regression analysis (three studies) and covariance analysis (one study). In one study, evaluation was standardized for age and was sex-specific. With the exception of age or age group and sex, the variables used for adjustment are very varied (see Table 1).
Findings
The tables showing study characteristics are followed by tables showing outcome and process parameters, together with directions of effect and significance levels.
Outcome parameters
Outcome parameters are divided into the endpoints mortality, survival time, morbidity, quality of life, and satisfaction with care. Morbidity consists of diabetes-specific concomitant diseases and sequelae such as myocardial infarction. Several studies show endpoints in terms of various surrogate parameters. As a surrogate for morbidity, some studies use clinical parameters such as cholesterol or blood sugar level, while others measure attainment of therapeutic goals. Quality of life was usually ascertained using individual dimensions (e.g. satisfaction with health) on the basis of questionnaires (SF-36, EQ-5D).
In total, 15 publications (9 studies) assessed a very varied range of outcome parameters. The endpoints mortality (20, 21/22, 28) and survival time (19, 20), which were investigated in 3 and 2 publications based on 2 and 1 studies respectively, show positive effects for DMP groups. Drabik et al. and Stock et al. (21/22) find a mortality rate of 2.3% (458 deaths) in the DMP group versus 4.7% (935 deaths) in the group receiving routine care. In the study by Miksch et al. (28), the mortality rate is significantly lower in both the sample as a whole (12.8% for DMP participation versus 21.7% for routine care) and after matching (11.3% versus 14.4%). Drabik et al. (20) also find a positive effect for DMP participation. With a three-year observation period, the mortality rate was 7.17% (1425 patients) in this group versus 14.72% (2928 patients) in the control group. The same study finds a mean survival time of 1044.94 (±189.87) days in the DMP group versus 985.02 (±264.68) days in the control group. The findings for morbidity and quality of life are unclear, so only limited conclusions can be drawn regarding the effect of DMPs on these endpoints. Morbidity surrogates and quality of life dimensions show very varied results, so no general conclusions can be based on these. A clear positive effect in a DMP group can only be seen for cholesterol level (HDL cholesterol [34]), satisfaction with health (24), and satisfaction with diabetes care (25); however, like the endpoints, this information is based on only one publication. There were often only one or two publications that assess a given parameter. For example, the surrogate parameters blood sugar level and BMI were each assessed in only one publication (34); the reason for this is that the routine data used in most cases includes almost no clinical parameters. The extent to which conclusions can be drawn on the basis of the results found here is therefore limited.
Process parameters
Process parameters were investigated at both patient level (e.g. health-related behavior, attitude to health) and physician level (e.g. diagnostic measures/examinations). Process parameters such as doctor–patient relationship are also shown but cannot be attributed to either the doctor or the patient.
Eight publications (6 studies) assessed process parameters. Findings here were clearer than for outcome parameters, in terms of both trends and significance levels (see Table 3). There were clear positive effects for DMP groups regarding participation in diabetes education, for example. Statistically significant effects were found in all 5 publications (24, 25, 33– 35). Schäfer et al. (33) reported odds ratios in favor of the DMP group of 3.4 at baseline and 2.1 at follow-up.
Table 3a. Process parameters.
| Study | 2 | 3 | 4 | 5D | 8D | 9 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Publication | (23) | (24) | (25) | (26) | (27) | (33) | (34) | (35) | ||
| Subgroup analysis | L | H | B | F | ||||||
| Diagnostic measures/examinations | ||||||||||
| Blood pressure measurement (regular) | + | + | ||||||||
| Measurement of HbA1c level, creatinine level | ||||||||||
| Measurement of LDL level/cholesterol level | + | |||||||||
| Eye check (annual) | + | + | + | + | ||||||
| Foot check (annual) | + | + | + | + | + | + | ||||
| Drug therapy | ||||||||||
| Antidiabetes therapy/insulin | + | |||||||||
| Antihypertensive therapy | ||||||||||
| Guideline conform active ingredient group in hypertension treatment | + | |||||||||
| Platelet aggregation inhibitors, coronary dilators (e.g. nitrates, molsidomine) | + | |||||||||
| Lipid-reducing drugs/statins | + | |||||||||
| Statins named in guidelines | + | |||||||||
| Drug prescriptions (DDD) | ||||||||||
| Drug prescriptions (DDD) per 1000 insurance years | + | |||||||||
| Counseling on measures to change lifestyle | ||||||||||
| Healthy nutrition | + | + | ||||||||
| Physical activity | + | + | ||||||||
| Doctor contacts | ||||||||||
| Treating physician* | + | + | + | |||||||
| Coordinating physician (≥4 contacts/year) | + | |||||||||
| Ophthalmologist (annual) | + | + | ||||||||
| Diabetes specialist (≥1 contact/year) | ||||||||||
Abbreviations and notes: +: Significant positive difference for DMP groups; –: Significant positive difference for non-DMP groups; Dark blue: p<0.001, mid-blue: p<0.01, pale blue: p<0.05, pale gray: difference not significant; gray: investigated, no significance test performed or no information on statistical significance;
L: Low level of education; H: High level of education; B: Baseline; F: Follow-up; DDD: defined daily dose
*More frequent treatment by diabetes specialists in DMP groups is evaluated as a positive effect
Economic parameters
Five publications investigated economic parameters in addition to outcome and process parameters (19, 20, 21/22, 26, 27). Overall, however, the results on evaluation of direct costs, cost-effectiveness, and expenditure on care are unclear, and their scope is too limited to draw comparisons or to base conclusions on them.
Discussion
Many different outcome parameters, process parameters, and economic parameters are investigated in the 16 publications identified. However, the number of outcome parameters investigated is relatively small in comparison to the number of process parameters. Findings for outcome parameters include a positive effect on mortality identified in the 2 available studies (3 publications). The same is true of survival time. There was no clear effect on morbidity or quality of life. The overwhelming majority of German studies show improvements for DMP groups in terms of process parameters in particular. Economic effects cannot be clearly established.
Unlike Germany, in other countries the variables usually investigated are clinical parameters and morbidity surrogates. This reveals, for example, a statistically significant—albeit moderate—drop in HbA1c level (12, 13, 15, 16, 18). Results for HDL/LDL level are inconsistent; in addition, this was measured in only a small number of studies (12, 16, 18). This is also true of weight, BMI, and blood pressure (18). Turning to mortality, a systematic review (13) based on 20 randomized controlled trials (RCTs) shows no difference between groups. As quality of life and patient satisfaction are rarely investigated, there is insufficient evidence on these parameters (12, 15, 18).
The positive effects of DMPs on process parameters are in line with the results reported in international literature, which show improvements in DMP groups in terms of recommended diagnostic measures and examinations in particular (12, 15– 18). Regarding economic parameters, international studies as well fail to identify any overall findings on which conclusions can be based, due to limited scope and inconsistent results (12, 14, 15, 17, 18). Although differing designs mean that only limited comparisons can be made between DMPs used in Germany and those used in other countries, the systematic reviews and meta-analyses consulted do provide a overview of the international status of research and therefore provide a depiction of experiences in other countries.
Changes in outcome parameters become visible only if observation periods are long, so minimum duration of continuous DMP participation plays a significant role in whether conclusions can be drawn from individual studies, particularly concerning hard endpoints such as mortality and sequelae. Quality of life and economic parameters should also be subject to long-term evaluation. Although positive changes in process parameters are often needed for an improvement in outcome parameters (35), clinical parameters must undergo further evaluation.
The studies examined here do show trends in the effects of DMPs, but the evidence base is insufficient: the studies included in the review vary greatly in terms of the parameters they investigate and their design (dataset, intervention group inclusion criteria, methods, analysis). In addition, specific parameters are addressed in only one or a few studies; and the results of several studies are based on the same data. The limited extent to which conclusions can be drawn also results from the fact that findings for a study population consisting of individuals insured by a single sickness fund cannot necessarily be extrapolated to other sickness funds. Lastly, adjustment for selection effects, which is so important for controlled studies, varies in quality in these studies. The potential bias resulting from this substantially restricts the validity of the findings. Drabik et al. (36) refer to differences in adjustment for selection effects and call for minimum requirements in study design so that results can be interpreted. The final report of the DISMEVAL project (Developing and Validating Disease Management Evaluation Methods for European Health Care Systems) also compares various adjustment procedures and indicates differences between them (37). In addition, international reviews criticize the limited extent to which the results of individual studies can be compared due to substantial differences in study design, parameters measured, and DMP design (14– 16).
The discussion shows that evaluation of existing DMPs requires investigation of individuals insured by multiple sickness funds on the basis of uniform methods with specific minimum requirements. A minimum duration of continuous DMP participation must be established so that the effects of DMPs can be evaluated, in order to minimize bias in effects and rule out erroneous interpretations. Other authors have already recommended a medically sound intervention duration of three years (36). Conklin and Nolte (38) also recommend evaluating the effectiveness of such programs using cluster RCTs (group randomization) before DMPs are rolled out nationwide for any new indications.
Conclusions
The evaluations available for Germany based on controlled studies of the effectiveness of DMPs indicate positive effects on mortality and survival time. Results for the other outcome parameters and economic parameters investigated in this review are unclear. There are significant positive effects for DMP groups regarding most process parameters. As a result of weaknesses in the design of some of the included studies, selection effects are likely. This means that the effectiveness of DMPs cannot yet be considered as proven. However, the positive results for process parameters suggest that improved disease management was achieved for the individuals investigated, particularly by involving family physicians. Nevertheless, this represents an important basis for improved outcome parameters. But long-term evaluations are needed to reveal actual changes in patient relevant outcomes. This, however, should not lead to quality of life or economic parameters being overlooked.
Controlled observational studies are currently the best available option for evaluation of the potential effects of DMPs that have already been implemented. Because the ideal form of research, RCTs, is no longer possible for the DMPs already rolled out nationwide, a second-best option is needed. This should guarantee that controlled observational studies meet high quality-related criteria, are easier to compare, and evaluate the effectiveness of DMPs on the basis of important clinical parameters. Because Germany’s SHI Care Structuring Act has transferred the authority to regulate the content, design, and evaluation of DMPs to Germany’s Federal Joint Committee (G-BA, Gemeinsamer Bundesausschuss), this national body now has the option to develop suitable methodology guidelines and to implement them in statutory DMP evaluation in the future.
Table 1b. Study characteristics.
| Study | Publication | Dataset | No. of cases | Mean age | Sex distribution (M) | Observation period | Minimum duration of continuous DMP participation before beginning of analysis | Adjustment for selection bias (method: variables used) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP | CG | DMP | CG | DMP | CG | ||||||
| 7 | (29) | AOK patient survey: ELSID study random sample (Rhineland– Palatinate, Saxony–Anhalt) |
865 | 534 | 70.2 ± .3 | 70.5 ± 8.9 | 46.2% | 46.6% | November 2006 | 1 day*6 | Covariance analysis: age, BMI |
| (30) | Covariance analysis: age, sex, number of other chronic diseases, DMP participation | ||||||||||
| (31) | Covariance analysis: age, sex, number of other chronic diseases, DMP participation | ||||||||||
| (32) | None | ||||||||||
| 8 | (33) | GEK patientsurvey: random sample (nationwide) |
444 B 351 F | 494 B 345 F | 63.8 ± 8.5 B | 63 ± 10.1 B | 61.9% B | 61.1% B | B: no information F: after 10.4 months(± 0.64 months) | 6 months before survey (B)*7 | Regression analysis: age, sex, education, knowledge of German, length of time with ‧diabetes, signs of depression, duration of DMP participation |
| 9 | (34) | KORA patient questionnaire: follow-up (Augsburg region) |
89 | 77 | 67.5 ± 8.2 | 68.6 ± 9.9 | 55.1% | 55.8% | B: 1999 to 2001 F: October 2006 to May 2008 | 1 day *8,5 | Regression analysis: age, sex, education, length of time with diabetes, waist circumference, ‧clustering of care (by primary care physician) |
| (35) | 85 | 64 | 67.5 (43 to 79) | 68.5 (36 to 81) | 52.9% | 54.7% | Regression analysis: age, sex, education, length of time with diabetes, serious or moderate ‧associated diseases or complications | ||||
Abbreviations and notes: CG: Control group; B, Baseline; F: Follow-up; M: Male; AOK: Allgemeine Ortskrankenkasse; GEK: Gmünder Ersatzkasse; KORA: Kooperative Gesundheitsforschung in der Region Augsburg; ELSID: Evaluation of Large Scale ?Implementation of Disease Management Programs for Patients with Type 2 Diabetes
*5Unclear whether DMP participants must be enrolled throughout the observation period
*6Mean DMP participation according to the authors (22): male 27.2 months, female 26.6 months
*7DMP participation between the two surveys (b and f) not necessary
*8Mean DMP participation according to the authors (34): 27 months
Table 2a. Outcome parameters.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | (19) | (20) | (21, 22) | (23) | (24*1) | (25) | (26) | (27) | (28) | (29) | (30) | (31) | (32) | (33) | (34) | |||
| Subgroup analysis | F | M | F | M | ||||||||||||||
| Mortality | + | + | + | |||||||||||||||
| Survival time | + | + | ||||||||||||||||
| Morbidity | – | |||||||||||||||||
| Morbidity surrogates | Attainment of therapeutic goals: | |||||||||||||||||
|
+ | |||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
+ | |||||||||||||||||
|
||||||||||||||||||
| Hospital stays: | ||||||||||||||||||
|
+ | |||||||||||||||||
|
+ | |||||||||||||||||
|
+ | |||||||||||||||||
|
||||||||||||||||||
|
+ | + | ||||||||||||||||
|
+ | + | + | |||||||||||||||
|
||||||||||||||||||
|
+ | + | ||||||||||||||||
| Blood sugar, blood pressure | ||||||||||||||||||
| HDL cholesterol | + | |||||||||||||||||
| LDL cholesterol | ||||||||||||||||||
| Creatinine, BMI, waist circumference, physical activity | ||||||||||||||||||
| Smoker status | ||||||||||||||||||
Abbreviations and notes: +: Significant positive difference for DMP groups; –: Significant positive difference for non-DMP groups; Dark blue: p <0.001, mid-blue: p <0.01, pale blue: p <0.05, pale gray: difference not significant; gray: investigated, no significance test performed or no information on statistical significance; F: Female; M, Male
*1Subgroup analysis according to level of education. Table shows only overall finding for “satisfaction with health”
*2Based on Ullrich et al. (23): number of people with at least one hospital stay
*3Based on Nolting et al. (27): days per 1000 insurance years, cases per 1000 insurance years not significant
Table 2b. Outcome parameters.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | (19) | (20) | (21, 22) | (23) | (24*1) | (25) | (26) | (27) | (28) | (29) | (30) | (31) | (32) | (33) | (34) | |||
| Subgroup analysis | F | M | F | M | ||||||||||||||
| Quality of life | ||||||||||||||||||
| SF-36 | Physical Functioning (PF) | + | ||||||||||||||||
| Role—Physical (RP) | ||||||||||||||||||
| Bodily Pain (BP)*2 | ||||||||||||||||||
| General Health (GH) | + | |||||||||||||||||
| Vitality (VT) | + | |||||||||||||||||
| Role—Emotional (RE) | ||||||||||||||||||
| Mental Health (MH) | ||||||||||||||||||
| Physical Component Summary (PCS) | + | – | ||||||||||||||||
| Mental Component Summary (MCS) | ||||||||||||||||||
| EQ-5D | Mobility | + | ||||||||||||||||
| Self-care | + | |||||||||||||||||
| Usual activities | + | |||||||||||||||||
| Pain/discomfort | ||||||||||||||||||
| Anxiety/depression | ||||||||||||||||||
| QoL dimensions | Subjective assessment of health | + | ||||||||||||||||
| Psychological well-being | ||||||||||||||||||
| Normal life severely restricted | ||||||||||||||||||
| Normal life somewhat restricted | + | |||||||||||||||||
| Normal life not at all restricted | + | |||||||||||||||||
| Satisfaction with health | + | |||||||||||||||||
| Satisfaction with care | ||||||||||||||||||
| Satisfaction with diabetes care | + | |||||||||||||||||
| Satisfaction with medical care | + | |||||||||||||||||
Abbreviations and notes: +: Significant positive difference for DMP groups; –: Significant positive difference for non-DMP groups; Dark blue: p<0.001, mid-blue: p<0.01, pale blue: p<0.05, pale gray: difference not significant; gray: investigated, no significance test performed or no information on statistical significance; F, Female; M, Male
*1Subgroup analysis according to level of education. Table shows only overall finding for “satisfaction with health”
*2Only BP was significant in overall analysis (p <0.05)
Table 3b. Process parameters.
| Study | 2 | 3 | 4 | 5 | 8 | 9 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Publication | (23) | (24) | (25) | (26) | (27) | (33) | (34) | (35) | ||
| Subgroup analysis | L | H | B | F | ||||||
| Doctor–patient relationship | ||||||||||
| Information, communication | + | |||||||||
| Involvement in decisions | + | + | + | |||||||
| Respectful treatment | + | + | ||||||||
| Setting individual therapeutic aims | + | + | + | |||||||
| Health-related behavior/attitude to health | ||||||||||
| Treatment burden | + | + | ||||||||
| Coming to terms with disease, compliance | ||||||||||
| Awareness of prevention | + | + | + | |||||||
| Information status | ||||||||||
| Diabetes in general | + | + | + | |||||||
| HbA1c level | + | + | ||||||||
| Blood pressure | ||||||||||
| Patient education | ||||||||||
| Information on diabetes education | + | + | + | |||||||
| Information on high blood pressure education | + | + | ||||||||
| Participation in diabetes education | + | + | + | + | + | + | + | |||
| Participation in high blood pressure education | ||||||||||
| Evaluation of diabetes education | + | |||||||||
| Self-management | ||||||||||
| Blood pressure check (weekly) | + | + | ||||||||
| Blood sugar level, foot, weight check (weekly) | ||||||||||
| Diabetes diary | ||||||||||
| Program organization | ||||||||||
| Insurer-specific program structure | + | + | ||||||||
Abbreviations and notes: +: Significant positive difference for DMP groups; –: Significant positive difference for non-DMP groups; Dark blue: p<0.001, mid-blue: p<0.01, pale blue: p<0.05, pale gray: difference not significant; gray: investigated, no significance test performed or no information on statistical ?significance; L: Low level of education; H: High level of education; B: Baseline; F: Follow-up
Key Messages.
The few studies that evaluate effects on mortality and survival time show positive effects for DMP groups. However, there are varying findings for surrogate parameters for morbidity and areas within quality of life.
For process parameters, the overwhelming majority of studies show improvements in DMP groups.
Evaluation of clear positive effects for DMP groups is limited by the small number of studies, the parameters evaluated, and a great variability in study designs.
Although controlled observational studies are currently the best available option in evaluating the potential effects of DMPs that have already been implemented, uniform minimum methodological requirements must be developed for this type of studies (e.g. minimum duration of participation for participants allocated to studies’ intervention groups).
Study types such as cluster RCTs should be used to evaluate future DMPs for new indications such as depression, even if this means more time is needed to roll them out.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Busse R, Blümel M, Scheller-Kreinsen D, Zentner A. Tackling chronic disease in Europe: Strategies, interventions and challenges. Observatory Studies Series No. 20. Copenhagen. World Health Organization on behalf of the European Observatory on Health Systems and Policies. 2010 [Google Scholar]
- 2.American Diabetes Association. Position Statement. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35:64–71. [Google Scholar]
- 3.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 4.Heidmann C, Du Y, Schubert I, Rathmann W, Scheidt-Nave C. Prevalence and temporal trend of known diabetes mellitus. Results of the German Health Interview and Examination Survey for Adults (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:668–677. doi: 10.1007/s00103-012-1662-5. [DOI] [PubMed] [Google Scholar]
- 5.Koster I, Schubert I, Huppertz E. Follow up of the CoDiM-Study: cost of diabetes mellitus 2000-2009. Dtsch Med Wochenschr. 2012;137:1013–1016. doi: 10.1055/s-0032-1304891. [DOI] [PubMed] [Google Scholar]
- 6.Fullerton B, Nolte E, Erler A. Qualität der Versorgung chronisch Kranker in Deutschland. Z Evid Fortbild Qual Gesundhwes. 2011;105:554–562. doi: 10.1016/j.zefq.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Busse R, Blümel M, Ognyanova D. Akteure, Daten, Analysen. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2013. Das deutsche Gesundheitssystem. [Google Scholar]
- 8.Busse R. Disease management programs in Germany’s statutory health insurance system. Health Aff. 2004;23:56–67. doi: 10.1377/hlthaff.23.3.56. [DOI] [PubMed] [Google Scholar]
- 9.Bundesversicherungsamt (BVA) Grundlegende Informationen zu Disease Management Programmen (DMP) www.bundesversicherungsamt.de/weitere-themen/disease-management-programme/zulassung-disease-management-programme-dmp.html#c207. last accessed on 3 june 2014.
- 10.Gerlach F, Beyer M, Szecsenyi J, Raspe H. Evaluation von Disease-Management-Programmen - Aktuelle Defizite, Anforderungen, Methoden. Z Arztl Fortbild Qualitatssich. 2003;97:495–501. [PubMed] [Google Scholar]
- 11.Morfeld M, Wirtz M. Methods in health services research The example of the evaluation of the German disease management programmes. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2006;49:120–129. doi: 10.1007/s00103-005-1204-5. [DOI] [PubMed] [Google Scholar]
- 12.Egginton JS, Ridgeway JL, Shah ND, et al. Care management for Type 2 diabetes in the United States: a systematic review and meta-analysis. BMC Health Serv Res. 2012;12 doi: 10.1186/1472-6963-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimouguet C, Le Goff M, Thiébaut R, Dartiques JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ. 2011;183:E115–E127. doi: 10.1503/cmaj.091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bruin SR, Heijink R, Lemmens L, Struijs JN, Baan CA. Impact of disease management programs on healthcare expenditures for patients with diabetes, depression, heart failure or chronic obstructive pulmonary disease: a systematic review of the literature. Health Policy. 2011;101:105–121. doi: 10.1016/j.healthpol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Mattke S, Seid M, Ma S. Evidence for the effect of disease management: is $1 billion a year a good investment? Am J Manag Care. 2007;13:670–676. [PubMed] [Google Scholar]
- 16.Knight K, Badamgarav E, Henning JM, et al. A systematic review of diabetes disease management programs. Am J Manag Care. 2005;11:242–250. [PubMed] [Google Scholar]
- 17.Ofman JJ, Badamgarav E, Henning JM, et al. Does disease management improve clinical and economic outcomes in patients with chronic diseases? A systematic review. Am J Med. 2004;117:182–192. doi: 10.1016/j.amjmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22(4 Suppl1):5–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 19.Drabik A, Büscher G, Sawicki PT, et al. Life prolonging of disease management programs in patients with type 2 diabetes is cost-effective. Diabetes Res Clin Pract. 2012;95:194–200. doi: 10.1016/j.diabres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Drabik A, Büscher G, Thomas K, Graf C, Müller D, Stock S. Patients with type 2 diabetes benefit from primary care-based disease management: A propensity score matched survival time analysis. Popul Health Manag. 2012;15:241–247. doi: 10.1089/pop.2011.0063. [DOI] [PubMed] [Google Scholar]
- 21.Drabik A, Graf C, Büscher G, Stock S. Evaluation der Effektivität eines Disease Management Programms Diabetes Mellitus in der GKV - Erste Ergebnisse und methodische Überlegungen. Z Evid Fortbild Qual Gesundhwes. 2012;106:649–655. doi: 10.1016/j.zefq.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Stock S, Drabik A, Büscher G, et al. German diabetes management programs improve quality of care and curb costs. Health Aff. 2010;29:2197–2205. doi: 10.1377/hlthaff.2009.0799. [DOI] [PubMed] [Google Scholar]
- 23.Ullrich W, Marschall U, Graf C. Versorgungsmerkmal des Diabetes mellitus in Disease-Management-Programmen. Ein Vergleich von in die DMP eingeschriebenen und nicht eingeschriebenen Versicherten mit Diabetes. Diabetes, Stoffwechsel und Herz. 2007;16:407–414. [Google Scholar]
- 24.Elkeles T, Kirschner W, Graf C, Kellermann-Mühlhoff P. Health care in and outside a DMP for type 2 diabetes mellitus in Germany—results of an insurance customer survey focussing on differences in general education status. J Public Health. 2009;17:205–216. [Google Scholar]
- 25.Graf C, Elkeles T, Kirschner W. Gibt es einen Selektionsbias im DMP? Z Allg Med. 2009;85:74–81. [Google Scholar]
- 26.Linder R, Ahrens S, Koppel D, Heilmann T, Verheyen F. The benefit and efficiency of the disease management program for type 2 diabetes. Dtsch Arztebl Int. 2011;108:155–162. doi: 10.3238/arztebl.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolting HD, Gottberg A, Schiffhorst G, Buhr S, Engel J. Einfluss der Teilnahme am DMP Diabetes mellitus Typ 2 auf die Entwicklung der Leistungsausgaben - Ergebnisse einer retrospektiven kontrollierten Studie auf der Basis von GKV-Routinedaten. Gesundh ökon Qual manag. 2011;16:209–215. [Google Scholar]
- 28.Miksch A, Laux G, Ose D, et al. Is there a survival benefit within a German primary care-based disease management program? Am J Manag Care. 2010;16:49–54. [PubMed] [Google Scholar]
- 29.Miksch A, Hermann K, Trieschmann J, et al. Geschlechtsspezifische Unterschiede in der Lebensqualität von Typ-2-Diabetikern mit und ohne DMP-Einschreibung. Gesundheitswesen. 2008;70:250–255. doi: 10.1055/s-2008-1077057. [DOI] [PubMed] [Google Scholar]
- 30.Ose D, Freund T, Urban E, Kunz C, Szecsenyi J, Miksch A. Comorbidity and patient-reported quality of care: An evaluation of the primary care based German disease management program for type 2 diabetes. J Public Health. 2011;20:41–46. [Google Scholar]
- 31.Ose D, Miksch A, Urban E, et al. Health related quality of life and comorbidity. A descriptive analysis comparing EQ-5D dimensions of patients in the German disease management program for type 2 diabetes and patients in routine care. BMC Health Serv Res. 2011;11 doi: 10.1186/1472-6963-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szecsenyi J, Rosemann T, Joos S, Peters-Klimm F, Miksch A. German diabetes disease management programs are appropriate for restructuring care according to the chronic care model: an evaluation with the patient assessment of chronic illness care instrument. Diabetes Care. 2008;31:1150–1154. doi: 10.2337/dc07-2104. [DOI] [PubMed] [Google Scholar]
- 33.Schäfer I, Küver C, Gedrose B, et al. The disease management program for type 2 diabetes in Germany enhances process quality of diabetes care - a follow-up survey of patient’s experiences. BMC Health Serv Res. 2010;10 doi: 10.1186/1472-6963-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark R, Schunk M, Meisinger C, Rathmann W, Leidl R, Holle R. Medical care of type 2 diabetes in German disease management programmes: a population-based evaluation. Diabetes Metab Res Rev. 2011;27:383–391. doi: 10.1002/dmrr.1186. [DOI] [PubMed] [Google Scholar]
- 35.Stark R, Schunk M, Leidl R, Meisinger C, Holle R. Prozeßevaluation von Disease Management Programmen bei Typ 2 Diabetes auf Basis einer bevölkerungsrepräsentativen Studie in der Region Augsburg (KORA) BFuP. 2009;61:283–301. [Google Scholar]
- 36.Drabik A, Sawicki PT, Müller D, Passon A, Stock S. Die Methoden der Evaluation von Disease Management Programmen im Kontrollgruppendesign am Beispiel Diabetes mellitus - Eine systematische Übersicht. Gesundheitswesen. 2012;74:496–501. doi: 10.1055/s-0031-1301273. [DOI] [PubMed] [Google Scholar]
- 37.Conklin A, Hinrichs S. Final report. RAND Corporation: Cambridge; 2012. DISMEVAL Developing and validating disease management evaluation methods for European healthcare systems. [Google Scholar]
- 38.Conklin A, Nolte E. Cambridge: RAND Corporation; 2011. Disease management evaluation: A comprehensive review of current state of the art. [PMC free article] [PubMed] [Google Scholar]