Abstract
This review highlights the concepts, recent applications and limitations of High Throughput Screening (HTS) flow cytometry-based efflux inhibitory assays. This platform has been employed in mammalian and yeast efflux systems leading to the identification of small molecules with transporter inhibitory capabilities. This technology offers the possibility of substrate multiplexing and may promote novel strategies targeting microbial efflux systems. This platform can generate a comprehensive data set that may support efforts to map the interface between chemistry and transporter biology in a variety of pathogenic systems.
Keywords: transporter, multidrug resistance, high throughput flow cytometry, multiplexing, transporter-ligand interactome
Introduction
Inhibition of multidrug efflux systems has been a fundamental therapeutic challenge both in cancer and infectious diseases. There are three generations of inhibitors in mammalian systems with modest success in the clinic[1] and virtually no evidence for successful clinical validation of efflux inhibitors in prokaryotes[2].
One major contributor to resistance for many classes of chemotherapeutic and antimicrobial agents is multidrug efflux. Efflux occurs due to the activity of membrane transport systems which also perform essential roles in cellular metabolism and exhibit activity in a wide range of organisms. They differ in membrane topology, energy coupling mechanisms, and most importantly in substrate specificities [3-5]. Based on their sequence similarity and structural homology, efflux systems are classified into six super-families [3]: ATP-binding cassettes (ABC), major facilitators, resistance-nodulation cell division, small multidrug resistance family, multi-antimicrobial extrusion protein family, and multidrug endosomal transporters (MET). The first five families are found in microorganisms while the MET family appears restricted to higher eukaryotes. Representatives of all groups are expressed in mammalian cells [6]. The ABC superfamily is the largest, comprising seven subfamilies designated A to G [3,4]. ABC transporters play important roles in normal biology and therapeutic response to medications in mammalian cells. The highly conserved ATP-binding domains of ABC transporters provide the nucleotide-dependent engine that drives transport [3,5]. 48 proteins, members of the ABC super-family have been identified corresponding to approximately 42-44 transporters whereas 4 ABC proteins (subfamilies E and F) are translation factors. The ABCB, ABCC, and ABCG subfamilies are linked to human multidrug resistance (MDR) and are expressed in many human tumors. Specifically, ABCB1 (MDR1/Pgp), ABCC1 (MRP1), and ABCG2 (Breast Cancer Resistance Protein, BCRP) are known to influence the efficacy of drugs and have unambiguously been shown to contribute to cancer multidrug resistance [7,8]. ABCB1, ABCC1, and ABCG2 are highly expressed in the gut, liver, and kidneys and may restrict the oral bioavailability of administered drugs. ABCB1 and ABCG2 are also expressed in the epithelia of the brain and placenta and also in stem cells, where they perform a barrier function [9,10].
The discovery efforts for efflux inhibitors have raised key questions for drug development, including the most suitable in vitro methods for studying transporter-drug interactions, the clinical importance of transporters in drug absorption and disposition, the selection criteria, and the appropriate follow-up clinical studies required for transporter inhibition [11]. These are active fields of investigation and have triggered the discussion for different approaches to study transporter mediated drug-drug interactions [12], but most importantly have generated the need for sophisticated and current inhibition strategies.
The Transporter Inhibition Strategy
Dual treatment with efflux pump inhibitors (EPIs) in conjunction with chemotherapeutics is a common but disputed treatment strategy to circumvent MDR in cancer [13]. The concept of enhancing the utility of antimicrobials by employing EPIs appears appealing although there are a number of conceptual and methodological challenges in translating the information for generating EPIs into clinical implementation [14-16]. A number of assays to identify efflux substrates and inhibitors have been developed in the last 10 years, but their polyspecificity and overlapping roles in cell physiology makes the majority of the EPI-discovery efforts an evolving and ongoing “work in progress”.
Identifying natural substrates and inhibitors of efflux systems is an active and expanding topic. A number of structurally and functionally diverse compounds act as substrates or modulators of efflux systems. However, only a few are appropriate candidates for clinical use as MDR reversing agents [17,18].
The major focus of ABC efflux transporter inhibition has been on ABCB1. First generation chemosensitizers were formulated from existing drug compounds to include the calcium channel blocker verapamil, the immunosuppressant cyclosporin A, and the steroid progesterone. Challenges with dose-related toxicity and solubility have prevented progress into the clinic. Second and third generation inhibitors were predominantly sourced from derivatizing first-generation molecules and ABCB1 directed combinatorial chemistry efforts. Examples include: the cyclosporin A derivative valspodar; Vertex Pharmaceuticals’ biricodar; anthranilamide-based modulators tariquidar, XR9051, XR9577 and WK-X-34; the acridone carboxamide derivative elacridar; the heteroaryloxypropanolamines zosuquidar, dofequidar, and laniquidar; and the diarylimidazole ontogeny [13,19]. The fourth generation inhibitors, for example, CBT-1 [20], tend to be more potent and less toxic than the first generation compounds; however, clinical trials to demonstrate efficacy, are still in progress.
Tools for Inhibitor Discovery
The use of classic and non-functional methods (Northern blotting, RNase protection, RNA in situ hybridization, RT-PCR and immunostaining) to detect a transporter’s activity is not trivial and accuracy can be questionable. Transporter protein expression is not always correlated with mRNA levels, as transcripts are often present below the detection threshold, since relatively few active transporter molecules can cause major alterations in drug transport. Both functional and cell viability assay activities, that may employ strains that lack or overexpress efflux systems, are usually robust with reproducible results. These have been used extensively in low and middle throughput screening campaigns, and are amenable to miniaturization. Computational approaches have also been used, but those efforts were not entirely independent of experiments as an assay from either of the first two categories was typically coupled to provide proof of principle experimental information.
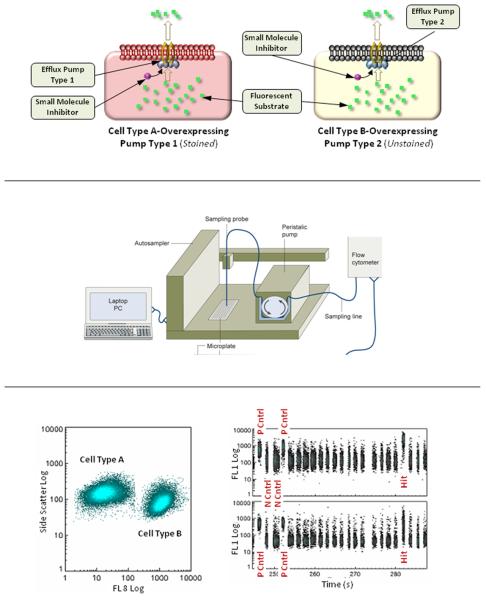
A functional efflux assay is a measure of the transporter’s ability to move compounds against the concentration gradient and across the cell membrane. Upon loading cells with a lipophilic dye capable of diffusing across cell membranes, the resulting fluorescence intensity of the cell reflects the activity of the ABC transporter. Cells with highly active transporters will exhibit lower fluorescence intensity values because of the increased efflux of the dye/substrate. In the presence of an active EPI, these substrates accumulate in the cell so they will exhibit higher fluorescence intensity values (Fig 1b). A transporter’s function can be measured by cellular uptake or efflux of fluorescent substrates over time.
Figure 1.
Schematic illustration for: the duplex assay concept and format for identification of ABCB1 and ABCG2 Inhibitors upper panel (1); the elements of the HyperCyt® HT flow cytometry platform middle (2); Flow cytometric resolution for stained ABCB1 and ABCG2 cells (lower left) and visualization of HT results (lower right)
When evaluating cells for efflux phenotypes, it is necessary to distinguish between accumulation and retention. Two subcategories of functional transporter are commonly employed: 1) the accumulation assay which measures dye uptake with or without model or under-investigation inhibitors; and 2) the retention assay where the cells are pre-loaded with the substrate in the absence of any modulator and washed, then incubated again but without dye, but in the presence of reversing agents. Both assay types potentially supply high throughput, output the increase in fluorescence intensity, and are readily automated. As the variety of efflux based campaigns has increased, assay development and screening for inhibitors has been transferred from conventional fluorometers and plate readers to fluorescence microscopes and high resolution multiparametric flow cytometers.
Rhodamines (6G, and 123), calcein, 2′,7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF), CellTrace and Red Orange [21,22] have been extensively used to report mammalian ABC-efflux activity. Most fluorophores in ABC transporter activity assays have been associated with limitations arising from protein binding, dye sequestration, or changes in dye fluorescence intensity due to intracellular parameters such as pH or free calcium levels. To combat such problems it is common to use hydrophobic ester derivatives, such as acetoxymethyl (AM) esters of the fluorescent dyes such as calcein acetoxymethyl ester (calcein-AM) [23]. The cell permeable derivatives of fluorescent dyes may be actively exported from cells by efflux proteins. However, when these ester derivatives reach the cytosol, intracellular esterases cleave the ester groups and allow the resulting free dye to be transported as well. Beyond the use of fluorescently labeled, known substrates (i.e. BODIPY® FL Verapamil etc.) the need for a new generation of dyes for transporter explorations prompted ENZO Life Sciences, Inc. to evaluate eFluxx-ID® Green or Gold probes. These xanthene-based small molecules are sensitive and specific fluorescent dyes developed for the detection of MDR activity in living cells for the three major types of mammalian ABC transporters [24].
A fundamental problem in efflux assays stems from interference with other constitutively expressed proteins and transporter background signals. Although short hairpin RNA (shRNA) and other technologies have been routinely used to silence background signals, the following example is indicative of an alternative pathway to bypass this obstacle. A permeability assay was developed that employs a modified low efflux Madin–Darby canine kidney cell line (MDCKII-LE) and an iterative fluorescence-activated cell sorting technique with calcein-AM was used as an efflux substrate. MDCKII-LE cells are a subpopulation of MDCKII cells with over 200-fold lower canine P-glycoprotein (ABCB1) mRNA level and fivefold lower protein level than MDCKII-WT. Canine MDCKII-LE cells offered clear advantages over the MDCKII-WT by providing lower efflux transporter background signals and minimizing interference from canine ABCB1 [25].
Efflux assays are also potentially useful to distinguish cell populations with special features. For example the “Side Population” discrimination assay is a flow cytometric method used to detect stem cells based on the dye efflux properties of ABC transporters [26]. An image-based high-content screening system was used to specifically identify and analyze the high drug efflux cancer cell (HDECC) population in lung cancer cells. The assay was based on a combination of fluorescent imaging and flow cytometry using Hoechst 33342 as a substrate. This system was used to screen 1,280 pharmacologically active compounds and identified 12 potent HDECC inhibitors. These inhibitors were able to overcome multidrug resistance and sensitize HDECCs to chemotherapeutic drugs, or directly reduce the tumorigenicity of lung cancer cells possibly by affecting stem-like cancer cells [27]. An alternate approach involved the synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna (nilotinib). The efflux of tyrosine kinase inhibitors by ABC transporters has been linked to the development of drug resistance in chronic myelogenous leukemia patients. The fluorescent derivative of Tasigna, (BODIPY FL Tasigna), inhibited BCR-ABL kinase activity in K562 cells and was also effluxed by ABCB1- and ABCG2-expressing cells in both cultured cells and rat brain capillaries expressing ABCB1 and ABCG2 [28].
Utilizing computational methodologies to identify efflux substrates and inhibitors remains a substantial challenge in part because the crystal structure for most integral membrane proteins has not been described. Another obstacle is polyspecificity, where the same compound can be a substrate for multiple transporters from the same family and a single transporter is able to recognize and pump different substrates. We describe some current computational tools that address these issues. The first example is a computational model of human ABCC2 that was able to distinguish inhibitors from non-inhibitors based on molecular structure. The success rate was 86% for the training set and 72% for the test set which consisted of 191 registered oral drugs and drug-like compounds [29].
In a second computational example, a support vector machine (SVM) method was developed to predict ABCB1 substrates based on data collected from the literature. This SVM model exhibited a prediction accuracy of 80% in an independent external validation dataset. Bikadi et al. built a homology model of human ABCB1 based on the mouse ABCB1 X-ray crystal structure and provided a predictive model for the transporter-substrate binding [30]. The models have not yet been experimentally validated and predictions may be subject to the high error rate usually associated with non-validated models. Virtual ligand screening was employed in the search for novel inhibitors of ABCC5. Eleven compounds were identified and tested in vitro against the known inhibitor sildenafil. Seven of the eleven predicted compounds were more potent than the control [31]. An induced fit docking model of mouse ABCB1a was developed which takes into account flexibility and non-specificity of transporters for different substrates [32]. A set of known substrates along with non-substrate drugs was used to validate the model with good results. However, blind testing on a set of known ABCB1 substrates showed very weak correlation between efflux ratios and docking score. Therefore, the predictions of this model may only be used in qualitative terms.
A method for discriminating ABCB1 substrates from non-substrates was based on calculated molecular descriptors and multivariate analysis. Fifty-three compounds were previously described as ABCB1 substrates or non-substrates. The molecular descriptors, calculated, were correlated to experimental classes using partial least squares discriminant analysis. The model correctly predicted the behavior of 72% of 272 compounds. Thirty of the 53 previously mentioned drugs were also evaluated for ABCB1 inhibition. The model was able to discriminate between substrates and inhibitors with an average accuracy of 82% [33]. Experimental studies show that the distinction between inhibitor and substrate is often artificial as an inhibitor can also be a substrate. For example, the interaction of tariquidar in vivo with ABCG2 is concentration dependent: it behaves at low concentration as an ABCG2 substrate whereas at high concentration it behaves as an ABCG2 inhibitor [34]. This example demonstrates that compound quantity is experimentally crucial to define the relation with the transporter while unfortunately these models can predict only a qualitative role.
Classification of substrates vs non-substrates and inhibitors vs non-inhibitors using several machine learning approaches and molecular descriptors reveals the most common pitfall encountered by research in the field: scarcity of public data and incomplete datasets [35]. The authors make the case for low predictive ability of the proposed models due to the biased training sets available. However, simple association rules albeit too general in some cases provide clues to functional moieties correlated with substrates and inhibitors, which along with molecular properties like solubility and logP can indicate with a degree a certainty the ability of a compound to be a substrate or an inhibitor. Given the concentration dependent nature of the interaction of drugs with ABC efflux systems, concentration dependent models could potentially provide more accurate future predictions.
The Biopharmaceutics Drug Disposition Classification System (BDDCS) was successfully employed to predict drug-drug interactions (DDIs) with respect to drug metabolizing enzymes, drug transporters and their interplay in a collection of over 900 marketed drugs. A model was proposed that, based on a computational procedure for predicting BDDCS class, could anticipate disposition and potential DDIs of new molecular entities. The model was trained on a set of 300 oral drugs and validated on an external set of 379 oral drugs. The BDDCS prediction model was also applied to a set 30,000+ medicinal chemistry compounds. Based on this application, it was suggested that solubility, and not permeability, is the major difference between new molecular entities (NMEs) and drugs [36].
HTS flow cytometry for efflux inhibition
The National Institutes of Health Molecular Libraries Probe Production Centers Network is tasked with finding small molecule probe compounds for academic investigators. The University Of New Mexico Center for Molecular Discovery (UNMCMD) has pioneered the development of cell suspension HTS transporter inhibitor assays for discovery utilizing a sensitive multiplex flow cytometry platform. The HyperCyt® HT flow cytometry platform, developed at UNMCMD [37], interfaces a flow cytometer and autosampler in which a peristaltic pump sequentially aspirates sample particle suspensions from each well. Between wells, the pump draws a bubble of air into the sample line generating a tandem series of bubbles separated samples for delivery to the flow cytometer (Fig. 1). Accurate measurements have been demonstrated in endpoint assays at rates of 20 to 40 samples/min over a 4-decade range of fluorescence intensity. Multi-parameter fluorescence based analysis is common in flow cytometric experimentation. When coupled with a high throughput front end sampling technology such as HyperCyt®, it becomes possible to analyze mixed cell populations, rapidly comparing multiple efflux pump systems. Multiplexing is typically accomplished for transporter targets by displaying highly expressed transporters in individual cell lines, then color coding those cell lines with fluorescence markers that distinguish the cells in the presence of fluorescent substrates. Fig. 1 shows an example of such a protocol in the form of a duplex.
The approach incorporates profiling libraries such as the Prestwick Chemical Library (PCL) which consists of 1200 off-patent drugs and known biologically active compounds along with the diverse Molecular Libraries Small Molecule Repository (MLSMR, http://mlsmr.glpg.com/MLSMR_HomePage/) library of greater than 350,000 compounds. We set out to develop new small molecule scaffolds with distinct efflux inhibition selectivity profiles based on multiplex transporter target screening. This approach paved the way for a series of innovations in chemical genetics including novel flow cytometry efflux assays in both mammalian (ABCB1, ABCB6, ABCG2, ABCC1) and yeast transporters including ABC (CDR1, CDR2 in Candida albicans, V-ATPase in Saccharomyces cerevisiae) as well as MFS (MDR1 in C. albicans) [38-41].
Using the fluorescent probe JC-1, and cells overexpressing ABCB1 an efflux assay based on HTS flow cytometry was developed at UNMCMD [39]. Testing the PCL revealed mometasone furoate as an ABCB1 modulator of daunorubicin DNR-mediated drug resistance. The screening campaign was expanded to include ABCG2 and ABCC1 using the MLSMR and a duplex approach in which the cells expressing individual pumps were color-coded for flow cytometric detection. This pilot study was extended into profiling the entire MLSMR library against an ABCB1 and ABCG2 duplex. Along with data uploaded to PubChem (http://pubchem.ncbi.nlm.nih.gov/), an ABCG2 efflux inhibition probe (ML230) was found with 36 fold selectivity over ABCB1 [42]. The probe reversed chemoresistance in a mouse model of ovarian cancer [43].
An HTS campaign was employed with Nile red as the fluorescent reporter substrate in a phenotypic (meaning non-differentially stained) triplex of heterologously expressed C. albicans transporters in a S. cerevisiae system where endogenous efflux systems have been disabled [40]. In this case, individual yeast strains overexpressed individual Candida transporters. The high level of transporter overexpression had two effects: 1) although the transporters interfered with dyes used for color-coding the cells, 2) each strain exhibited hundred fold fluorescence differences between the presence of the fluorescence substrate and the level when the substrate was pumped out. This made it possible to mix the three strains as a phenotypic multiplex and detect weak inhibition of pump activities of one or more strains by test inhibitors. The PCL HTS campaign revealed that the monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS efflux pump activities which reverses the azole resistance of C. albicans and Candida glabrata.[41] The MLSMR primary screening and dose response data are available in Pubchem (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=485335). One of the lessons learned from studies with new fluorescent probes involves the potential of multiple sites and/or modes of binding of probes, inhibitors and substrates with transporters. Thus the correspondence between inhibition and probe efflux and transporter activity needs to be validated in secondary assays such as viability or potentiation assays as indicated in Table 2.
Table 2. Representative Summary of Efflux Assays.
| Monolayer Effluxa | Cytometry | ATPase | Potentiation | |
|---|---|---|---|---|
| Activity measured | Transport | Efflux | ATPase stimulation | Viability |
| Analytical endpoint | LC/MS/MS | fluorescence | absorbance | Variableb |
| Throughput, cmpds/week | 12 | 100,000 | 150 | 15-40 |
| Criteria for (+) activity | BA/AB ratio >2.0 | ER >0.5 | ATPase ratio >2.0 | Viability reduction |
| Nonconfident zone | BA/AB ratio 1.5-2.0 | ER <0.5 | ATPase ratio 1.5-2.0 | Variable |
Cell monolayers (Caco-2, LLC-PK1-MDR1, MDCK-MDR1) grown on filters and placed in cluster plates, filters are typically PET or PC membranes with 0.4-1 μm pores, transport is measured in two directions: i) Apical (A) to Basolateral (B), i.e. test compound added to apical side ii) Basolateral (B) to Apical (A), i.e. test compound added to basolateral side
absorbance, luminescence
In a complementary approach, a target-based functional flow cytometry assay that measures vacuolar pH-dependent fluorescence changes in response to V-ATPase function was developed. The experimental strategy uses the pH-sensitive fluorophore BCECF-AM specific for the yeast vacuole and a potent and highly specific V-ATPase inhibitor, concanamycin A. The assay approach was also extended by using pHluorin, a ratiometric pH-sensitive GFP derivative that is used to monitor cytosolic pH [44].
This approach has been developed into a Center Driven Initiative with the ultimate goal of establishing the Transporter-Ligand Interactome as a UNMCMD predictive tool. This tool is comprised of a database and a visualization component that incorporates data from HTS flow cytometry campaigns with genomics, proteomics, structural informatics and knowledge mining using yeast and mammalian model systems for chemical probe discovery (Fig. 2).
Figure 2.
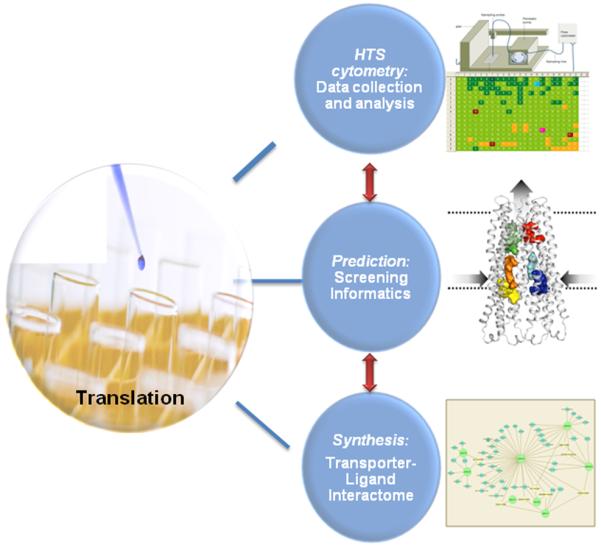
Discovery flow chart for the transporter inhibition platform.
Conclusions
From the features and analysis described it is apparent that an efflux system assay has promise but cannot be employed independently to report transporter substrates and inhibitors. While HTS flow cytometry is a powerful discovery tool, the capabilities of color-coded chemical or genetic multiplexing strategies are far from being fully exploited. For ABC transporters there remains a need for combinations of general and sensitive non-toxic fluorescent probes that allow detection of multiple modes of binding and accurate reporting of inhibitors at the primary screening stage. Furthermore, flow cytometric assays and screening campaigns other than the mammalian and yeast efflux systems are in their early stages. It remains challenging to develop and deploy similar explorations targeting the efflux systems of multidrug-resistant and pandrug-resistant Gram-negative bacteria. These pathogens pose a grave threat of truly untreatable infections. [45,46]. Finally, it should be noted that although HTS flow cytometry for transporter inhibition provides an unparalleled throughput when compared with other conventional efflux assays (Table 2) it remains largely a unique primary screening tool. Thus, secondary transporter assays that measure, for example, ATPase stimulation or chemotherapeutic potentiation with a significantly lower throughput can provide additional insight as the correspondence between these primary and secondary assays is imprecise. Exploring the alignment between primary transporter screens with secondary validation as well as remains a top priority and unmet challenge.
Highlights.
-
□
The description of a high throughput flow cytometric discovery platform for multidrug efflux inhibition.
-
□
The combination of high throughput flow cytometry campaigns for chemical probe and lead discovery with genomics, proteomics, structural informatics and knowledge mining comprises a powerful tool for discovery.
-
□
This discovery platform may enhance therapeutic countermeasures in challenging clinical conditions both in cancer and infectious diseases
Table 1. Transporter Targets and HTS flow cytometry.
Acknowledgments
Funding for George P. Tegos and Larry A. Sklar was provided by the National Institutes of Health (NIH, 5U54MH084690-02, Center Driven Initiative 1). The authors would like to thank the following: 1) Transporter target assay providers Richard Larson, Office of Research, University of New Mexico Health Sciences Center (R03MH081228); Richard Cannon, John Walsh Research Institute, University of Otago, Dunedin, New Zealand (R03 MH087406); Partha C. Krishnamurthy, Department of Pharmacology, Toxicology, and Therapeutics, University of Kansas Medical Center (R03 MH093193); Karlett Parra, Department of Biochemistry and Molecular Biology, University of New Mexico (R03): 2) The UNMCMD target team leaders and personnel for data collection and analysis; I. Ivnitski Steele, Mohuiddin Md. Taimur Khan, Susan M. Young, Anna Waller, Dominique Perez, Stephanie Chavez: 3) The director of the flow cytometry facility in the Health Sciences Center, Bruce S. Edwards and the director of Biocomputing at University of New Mexico, Tudor I. Oprea; and 4) Kristine Gouveia for a critical review of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. LAS is a co-founder of IntelliCyt which commercializes the HyperCyt® platform.
Glossary
- ATP-Binding Cassette transporters
A large family of transmembrane proteins that use ATP to move substrates across the cell membrane.
- Efflux Substrate
A molecule that is translocated by a transporter across a membrane.
- Efflux Inhibitor
A blocker (modulator) of the efflux transporter action through direct protein inhibition, competition for substrate binding or indirect interaction.
- High Throughput Screening (HTS)
a drug discovery process by which large numbers of compounds (currently 100,000+) can be rapidly tested to identify modulatory activity with a defined assay for specific cell targets. Hit compounds undergo extensive biological characterization.
- Multiplex Assay (in chemical biology)
a method that allows analysis of individual binding ligands against multiple targets in the same analytical sample. The quality and quantity of data, combined with significant reductions in analysis time and reagent consumption, provide notable advantages over other standard screening methods.
- Multidrug Resistance (MDR)
Resistance to many structurally unrelated chemotherapy agents in cells that have developed natural resistance to a single cytotoxic compound. Resistance can be attributed to the efflux action of transporter proteins that lower the concentration of multiple therapeutic drugs in the cell.
- Transporter-Ligand Interactome Tool
is comprised of a database and a visualization component that incorporates data from HTS flow cytometry campaigns with genomics, proteomics, structural informatics and knowledge mining using yeast and mammalian model systems for chemical probe discovery.
Abbreviations
- cmpds
compounds
- LC/MS/MS
liquid chromatography with tandem mass spectrometry
- BA
Basolateral to Apical
- AB
Apical to Basolateral
- ER
Efflux Ratio
- Caco-2
Human colon carcinoma cell line
- LLC-PK1-MDR1
Transfected porcine kidney cell line with low transporter background
- MDCK-MDR1
Transfected canine kidney cell line
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 2.Kourtesi C, Ball AR, Huang YY, Jachak SM, Vera DM, Khondkar P, et al. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol J. 2013;7:34–52. doi: 10.2174/1874285801307010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 5.Rees D, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Q, Paulsen IT. Comparative analyses of fundamental differences in membrane transport capabilities in prokaryotes and eukaryotes. PLoS Comput Biol. 2005:3e27. doi: 10.1371/journal.pcbi.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman B. Multidrug resistance: can new drugs help chemotherapy score against cancer? Journal of the National Cancer Institute. 2003;95:255–257. doi: 10.1093/jnci/95.4.255. [DOI] [PubMed] [Google Scholar]
- 8.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. European Journal of Pharmaceutical Sciences. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiological Reviews. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 10.Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci U S A. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Transporter Consortium GK. Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai Y, Hsiao P. Beyond the ITC White Paper: Emerging Sciences in Drug Transporters and Opportunities for Drug Development. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990467. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011;50:209–232. doi: 10.1042/bse0500209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tegos G, Haynes M, Strouse JJ, Khan MMT, Bologa CG, Oprea TI, et al. Microbial Efflux Inhibition; tactics & strategies. Current Pharmaceutical Design. 2011;17:1291–1302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikaido H, Pagès JM. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2012;36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falasca M, Linton KJ. Investigational ABC transporter inhibitors. Expert Opin Investig Drugs. 2012;21:657–666. doi: 10.1517/13543784.2012.679339. [DOI] [PubMed] [Google Scholar]
- 17.Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discovery Today. 2008;13:379–393. doi: 10.1016/j.drudis.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Eckford P, Sharom FJ. ABC Efflux Pump-Based Resistance to Chemotherapy Drugs. Chemical Reviews. 2009;109:2989–3011. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 19.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 20.Robey R, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, et al. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1((R)) Biochem Pharmacol. 2008;75:1302–1312. doi: 10.1016/j.bcp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Revalde JL, Reid G, Paxton JW. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol. 2011;68:603–610. doi: 10.1007/s00280-010-1515-6. [DOI] [PubMed] [Google Scholar]
- 22.Campanale J, Hamdoun A. Programmed reduction of ABC transporter activity in sea urchin germline progenitors. doi: 10.1242/dev.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamidipudi V, Shi T, Brady H, Surapaneni S, Chopra R, Aukerman SL, et al. Increased cellular accumulation and distribution of amrubicin contribute to its activity in anthracycline-resistant cancer cells. Cancer Chemother Pharmacol. 2012;69:965–976. doi: 10.1007/s00280-011-1782-x. [DOI] [PubMed] [Google Scholar]
- 24.Lebedeva I, Pande P, Patton WF. Sensitive and specific fluorescent probes for functional analysis of the three major types of mammalian ABC transporters. PLoS One. 2011;6:e22429. doi: 10.1371/journal.pone.0022429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di L, Whitney-Pickett C, Umland JP, Zhang H, Zhang X, Gebhard DF, et al. Development of a new permeability assay using low-efflux MDCKII cells. J Pharm Sci. 2011;100:4974–4985. doi: 10.1002/jps.22674. [DOI] [PubMed] [Google Scholar]
- 26.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Xia X, Yang J, Li F, Li Y, Zhou X, Dai Y, et al. Image-based chemical screening identifies drug efflux inhibitors in lung cancer cells. Cancer Res. 2010;70:7723–7733. doi: 10.1158/0008-5472.CAN-09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla S, Skoumbourdis AP, Walsh MJ, Hartz AM, Fung KL, Wu CP, et al. Synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna (nilotinib): evidence for transport of Tasigna and its fluorescent derivative by ABC drug transporters. Mol Pharm. 2011;8:1292–1302. doi: 10.1021/mp2001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen J, Matsson P, Bergström CA, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2) J Med Chem. 2008;51:3275–3287. doi: 10.1021/jm7015683. [DOI] [PubMed] [Google Scholar]
- 30.Bikadi Z, Hazai I, Malik D, Jemnitz K, Veres Z, Hari P, et al. Predicting P-glycoprotein-mediated drug transport based on support vector machine and three-dimensional crystal structure of P-glycoprotein. PLoS One. 2011;6:e25815. doi: 10.1371/journal.pone.0025815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sager G, Orvoll E, Lysaa R, Kufareva I, Abagyan R, Ravna AW. Novel cGMP efflux inhibitors - Identified by virtual ligand screening (VLS) and confirmed by experimental studies. J Med Chem. 2012;55:3049–57. doi: 10.1021/jm2014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolghih E, Bryant C, Renslo AR, Jacobson MP. Predicting binding to p-glycoprotein by flexible receptor docking. PLoS Comput Biol. 2011;7:e1002083. doi: 10.1371/journal.pcbi.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crivori P, Reinach B, Pezzetta D, Poggesi I. Computational models for identifying potential P-glycoprotein substrates and inhibitors. Mol Pharm. 2006;3:33–44. doi: 10.1021/mp050071a. [DOI] [PubMed] [Google Scholar]
- 34.Kannan P, Telu S, Shukla S, Ambudkar SV, Pike VW, Halldin C, et al. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2) ACS Chem Neurosci. 2011;2:82–89. doi: 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poongavanam V, Haider N, Ecker GF. Fingerprint-based in silico models for the prediction of P-glycoprotein substrates and inhibitors. Bioorganic & Medicinal Chemistry. 2012;20:5388–5395. doi: 10.1016/j.bmc.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broccatelli F, Cruciani G, Benet LZ, Oprea TI. BDDCS Class Prediction for New Molecular Entities. Mol Pharm. 2012;9:570–580. doi: 10.1021/mp2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards B, Kuckuck FW, Prossnitz ER, Ransom JT, Sklar LA. HTPS flow cytometry: a novel platform for automated high throughput drug discovery and characterization. J Biomol Screening. 2001;6:83–90. doi: 10.1177/108705710100600204. [DOI] [PubMed] [Google Scholar]
- 38.Winter S, Lovato DM, Khawaja HM, Edwards BS, Steele ID, Young SM, et al. High-throughput screening for daunorubicin-mediated drug resistance identifies mometasone furoate as a novel ABCB1-reversal agent. J Biomol Screen. 2008;13:185–193. doi: 10.1177/1087057108314610. [DOI] [PubMed] [Google Scholar]
- 39.Ivnitski-Steele I, Larson RS, Lovato DM, Khawaja HM, Winter SS, Oprea TI, et al. High-throughput flow cytometry to detect selective inhibitors of ABCB1, ABCC1, and ABCG2 transporters. Assay Drug Dev Technol. 2008;2:263–276. doi: 10.1089/adt.2007.107. [DOI] [PubMed] [Google Scholar]
- 40.Ivnitski-Steele I, Holmes AR, Lamping E, Monk BC, Cannon RD, Sklar LA. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal Biochem. 2009;394:87–91. doi: 10.1016/j.ab.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes A, Keniya MV, Ivnitski-Steele I, Monk BC, Lamping E, Sklar LA, et al. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob Agents Chemother. 2012;56:1508–1515. doi: 10.1128/AAC.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NIH Molecular Libraries Selective Efflux Inhibition of ATP-binding Cassette Sub-family G Member 2. Chapter ML230 IN Probe Reports from the Molecular Libraries Program [Internet] NLo. [Google Scholar]
- 43.Strouse JJ, Ivnitski-Steele I, Khawaja HM, Perez D, Ricci J, Yao T, et al. A Selective ATP-Binding Cassette Subfamily G Member 2 Efflux Inhibitor Revealed via High-Throughput Flow Cytometry. J Biomol Screen. 2013;18:26–38. doi: 10.1177/1087057112456875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan C, Prudom C, Raines SM, Charkhzarrin S, Melman SD, De Haro LP, et al. Inhibitors of V-ATPase Proton Transport Reveal Uncoupling Functions of Tether Linking Cytosolic and Membrane Domains of V0 Subunit a (Vph1p) J Biol Chem. 2012;287:10236–10250. doi: 10.1074/jbc.M111.321133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–440. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nature Reviews Microbiology. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 47.Polireddy P, Khan MMd T, Chavan H, Young S, Ma X, Waller A, et al. Krishnamurthy, P. A novel HTS flow cytometric assay reveals functional modulators of the ATP binding cassette transporter ABCB6. PLoS One. 2012;7:e40005. doi: 10.1371/journal.pone.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson R, Allen C, Melman SD, Waller A, Young SM, Sklar LA, et al. Identification of inhibitors of vacuolar proton-translocating ATPase pumps in yeast by high-throughput screening flow cytometry. Anal Biochem. 2010;398:203–211. doi: 10.1016/j.ab.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]