Abstract
Study Design:
Randomized clinical trial.
Objective:
To determine the effect of strain counterstrain (SCS) on dynamic balance and subjective sense of instability in individuals with chronic ankle instability (CAI).
Although many studies have been published on CAI, the cause for this common clinical dysfunction remains inconclusive. No studies have assessed the effectiveness of SCS on CAI.
Methods:
At baseline all participants completed a demographic questionnaire, the star excursion balance test (SEBT), and the foot and ankle ability measure (FAAM). Following the baseline evaluation, participants were randomized into the SCS experimental group (EG) (n = 13) or the sham SCS group (SG) (n = 14). All participants received the assigned treatment once a week for 4 weeks and participated in a prescribed exercise program. At week 4, all participants repeated the outcome measures and completed a global rating of change (GROC) form. The primary aim was examined with a two-way analysis of variance (ANOVA).
Results:
A significant group-by-time interaction was found for seven directions in the SEBT (P<0.031). For subjective measures, no significant group-by-time interaction was found for the FAAM (P>0.548), but the GROC revealed a significant difference (P = 0.014) in the mean score for the EG (3.92±1.66) when compared to the SG (2.43±1.66).
Discussion:
Although SCS may not have an effect on subjective ankle function in individuals with CAI, preliminary evidence suggests that SCS may lead to an improvement in dynamic ankle stability and the subjective sense of ankle instability.
Level of Evidence:
Therapy, Level 1b.
Keywords: Chronic ankle instability, Lateral ankle sprain, Manual therapy, Star excursion balance test, Strain counterstrain
Introduction
Lateral ankle sprains (LAS) represent approximately 85% of all reported ankle sprains1–3 with a recurrence rate as high as 80% leading to chronic ankle instability (CAI) in as many as 40% of cases.2,4,5 It appears that impairments present in mechanical and functional instability co-exist and are interrelated in CAI.6
There are many theories on the causes of CAI: evertor and invertor strength deficits;3,7–14 proprioceptive deficits;3,15–21 postural control deficits;15,22–26 delayed peroneal muscle reaction time;9,15,27–31 nerve deficit;15,27,32 and sensorimotor deficits.33 Other studies suggest central impairments in neuromuscular function causing an inability to prevent inversion moments,18,19,34,35 or damage to capsular and ligamentous mechanoreceptors with dysfunction in the afferent–efferent mechanism.3,11,12,18
More recently, Klykken et al.36 demonstrated a change in the motor neuron pool excitability of the tibialis anterior and the soleus muscles suggesting a less stable foot position following an acute ankle sprain.36 Muscle inhibition has been identified in the soleus and fibularis longus muscles in cases of functional instability.37 The presence of arthrogenic muscle inhibition has also been described as a possible contributor to the neuro-musculo-skeletal dysfunctions present after joint injury.38 Pietrosimone et al.39 concluded that alterations in neuromuscular function following a joint injury contribute to altered biomechanics possibly affecting long-term functional outcomes. Needle et al.40 suggest that subjective instability may be related to deficits in muscle spindle function during mechanical loading of the ankle. These studies provide support for the presence of neuromuscular dysfunction in CAI.
Strain counterstrain (SCS) is an indirect osteopathic treatment. It describes a theoretical model of neuro-musculo-skeletal dysfunction where a mechanical strain injury leads to changes in muscle spindle biasing around the involved segment known as the Proprioceptive Theory.41–47 According to this theory, as the foot turns into inversion, during a LAS, the invertor muscle spindles adapt to a newly shortened muscle length. The quick stretch and resulting contraction of the evertor muscles cause the invertor muscles to be quickly stretched from this shortened position leaving the invertor muscles in a state of increased neuromuscular hyperactivity with a facilitated spindle system. These events, of course, happen in milliseconds and micrometers. According to the Proprioceptive Theory, this scenario leaves the ankle in a state of neuro-musculo-skeletal dysfunction, leading to chronic instability and recurring ankle sprains.
In SCS, the practitioner seeks to identify the dysfunctional muscle groups through localization of significant tender points defined as small zones of tense, tender, and edematous muscle and fascial tissue about 1 cm in diameter and at least four times more tender than normal tissue.43,45–47 While Melzack and Stillwell48 stated that there may be no difference between a trigger point and a SCS point, a reduction in the degree of tenderness of a SCS tender point is associated with a modification of body position.45,46 However, no universal measurement tool is used in the assessment of SCS tender points. Wong and Schauer49 used a four-point scale for palpatory tenderness and noted poor to fair test–retest reliability (kappa = 0.228–0.327). Concurrent validity with the visual analog scale (VAS) varied from near absent to good (Spearman’s rho = 0.233–0.709).49 The reliability and validity of an appropriate pain rating scale for SCS tender points has yet to be determined.
In the SCS treatment approach, tissues containing tender points are put on slack in order to reduce excessive proprioceptive activity.43,46 This allows the muscles to return to proper length and the joint to return to proper alignment thus resolving the neuro-musculo-skeletal dysfunction.43,46,47 Despite a few initial research efforts by Lewis and Flynn,50 Wong and Schauer,51 and Collins,52 further studies on the effects of SCS are necessary. This study sought to examine the effects of SCS on dynamic stability and function in individuals with CAI.
Methods
Design overview
This study followed a prospective pre-test–post-test control group design with repeated measures, was approved by the Institutional Review Board committees of Long Island University and Nova Southeastern University, and is registered with ClinicalTrials.gov (NCT02025569).
Participants
Thirty-six volunteers with a history of LAS and a subjective sense of CAI (defined as three or more episodes of the ankle giving way in the previous year) were recruited through posting and emailing of recruitment fliers. Participants were eligible for the study if they had a history of at least one LAS at least 3 months earlier and had three or more episodes of giving way in the previous year. They were excluded if they reported a history of fracture, surgery, or other musculoskeletal injury on the side of the involved ankle. Other exclusion criteria included vestibular or balance disorders, history of central nervous system pathology, mechanical instability of the ankle, inability to speak English, inability to comply with the home exercise program (HEP), or inability to attend weekly treatment sessions for 4 weeks. Twenty-seven individuals between the ages of 18 and 55 years (mean±SD, 33.6±8.8) were deemed eligible to participate and were given an explanation of the study. All participants signed a consent form and an agreement to participate in a HEP thrice a week. See Fig. 1 for flow chart of participants.
Figure 1.
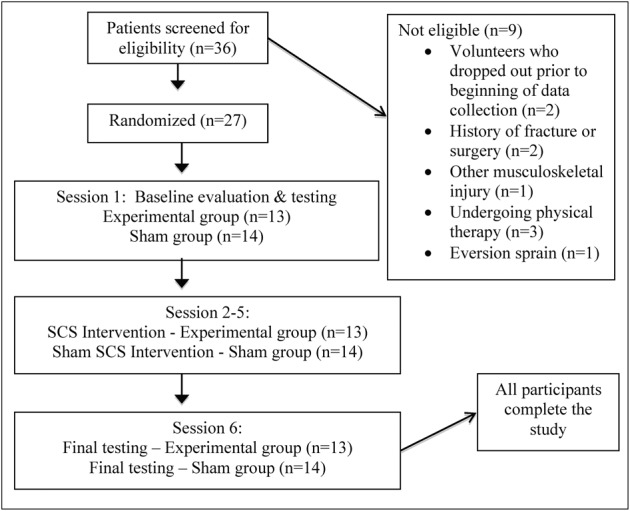
Flow chart of participants through the study. SCS: strain counterstrain.
Examination
Prior to randomization all participants were screened for inclusion and exclusion criteria and completed a demographics questionnaire including age, gender, height, weight, mechanism and date of ankle sprain, involved side, frequency of giving way, and activity level. All study participants underwent a physical examination including range of motion (ROM) and strength testing of the lower quadrant; leg length measurements for data normalization (ASIS to medial malleoli); ankle stability tests including the anterior drawer test, talar tilt test, and medial subtalar glide test to assess for mechanical instability; and an evaluation of SCS tender points. ROM, strength measures, and ankle stability tests were collected as part of a routine physical examination to ensure that participants did not present with other musculoskeletal problems. All participants presented with ROM and strength of the lower quadrant within normal limits and tested negative for ankle instability. The primary investigator, who was also the treating clinician for all participants, conducted all physical examinations. Two additional physical therapists conducted all the testing sessions.
Randomization
The first female or male participant was randomly assigned to the sham group (SG) (n = 14) or the experimental group (EG) (n = 13) by an individual not directly involved in the study. The initial assignments were done by picking a random assignment out of an envelope containing both possibilities. Other female or male participants were assigned to alternating groups, in order of recruitment.
Interventions
The intervention sessions for the EG included a re-evaluation of the SCS tender points of the lower quadrant (anterior and posterior pelvis and lower extremities). The specific tender points treated across participants varied in that each participant was treated for the tender points identified by the treating physical therapist upon examination. In each intervention session the three most tender points were treated according to the SCS treatment sequence rules (most tender point first followed by most proximal points and then the area with the greatest concentration of tender points).43 Each tender point was treated by passive positioning of the body segment in a position of comfort, as defined by Jones,43 a passive holding of the position for 90 seconds, followed by a slow passive return of the body segment to a position of rest. This protocol was followed for both the EG and the SG. In the SG, the treatment position was held for 60 seconds (less than the required 90 seconds hold) and the treatment position used did not correspond to the described SCS treatment position for that particular point, making it a sham SCS treatment intervention. To ensure that the position used in the sham treatment was incorrect, the treating clinician modified at least one component of the SCS treatment position, e.g. if the SCS treatment position called for hip flexion, internal rotation, and adduction, the clinician used hip flexion, external rotation, and adduction.
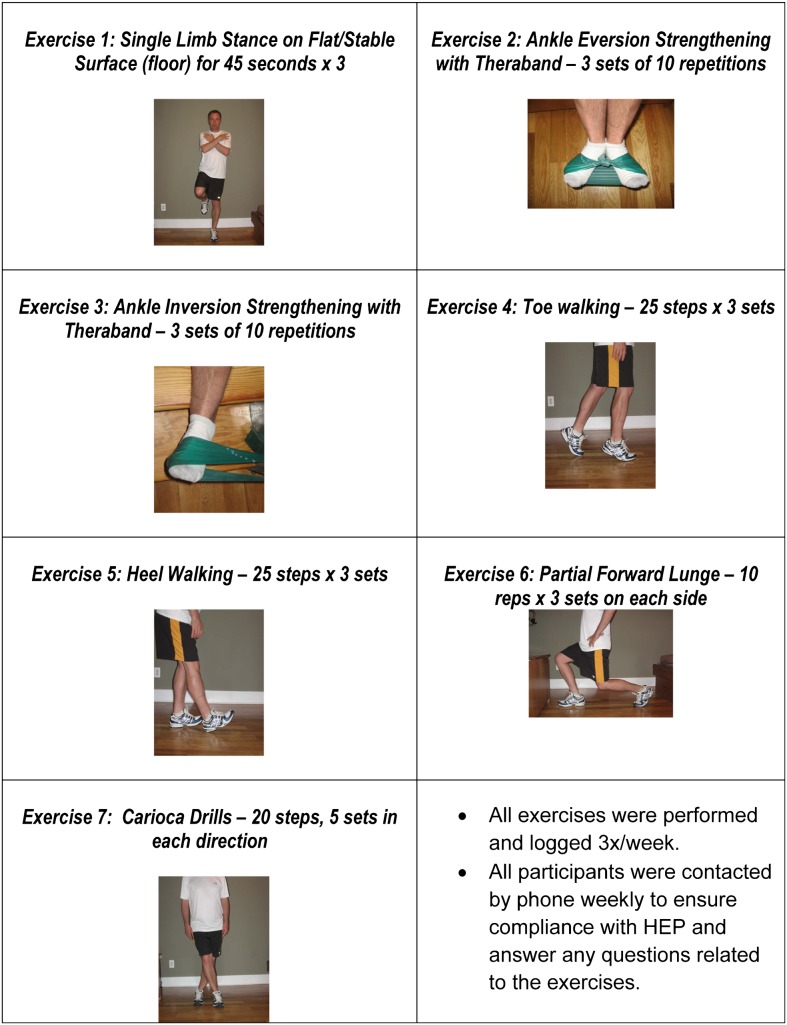
All participants were given a HEP for CAI combining basic strengthening and proprioceptive training to be performed thrice a week for the 4-week duration of the study.21,53,54 Participants were: (1) given demonstrations, explanations, and photographs of each exercise (Fig. 2); (2) asked to sign an agreement to participate in the HEP as prescribed; (3) given a check-off sheet for completion each time they completed the HEP; and (4) contacted weekly to ensure compliance. In addition, both groups received their respective interventions once-a-week for 4 weeks.
Figure 2.
Home exercise program (HEP).
The sample size and power calculations were based on detecting a difference between the two means in the SEBT of 3 cm; a within-group standard deviation of 3 cm; a two-tailed test; and an alpha level equal to 0.05. These assumptions generated a sample size of 17 participants per group. No previous studies have assessed the role of SCS on CAI, making it difficult to estimate standard deviations accurately. The estimated standard deviation of 3 cm was based on previous work by Olmsted et al.25
Outcomes measures
All participants completed a star excursion balance test (SEBT) and the functional ankle ability measure (FAAM) at baseline and at week 4. At week 4 participants also completed a global rating of change (GROC) form. The treating clinician for the sham and experimental groups was blinded to the results of the FAAM and the SEBT. Participants were blinded to their group assignment. The two clinicians who conducted the testing sessions were blinded to the group assignment of each participant.
The SEBT is a clinical test used in the assessment of dynamic balance and functional deficits associated with lower extremity injuries.55,56 The individual stands barefoot in the center of a grid and reaches with the contralateral lower extremity as far as possible, in eight directions, while maintaining balance: anterior (ANT), anteromedial (AM), medial (MD), posteromedial (PM), posterior (PO), posterolateral (PL), lateral (LAT), and anterolateral (AL). The distance reached in each direction is used as a measure of dynamic balance or dynamic stability (Figs 3 and 4). The SEBT is an effective tool for assessing deficits in individuals with CAI25,55,56 and has been found to be a valid measure of dynamic postural control with moderate to good intrarater reliability [ICC (2, 1)–0.67, 0.97] and poor to good interrater reliability [ICC (2, 1)–0.35, 0.93].57 Gribble and Hertel58 concluded that height and leg length are positively related to excursion distances on the SEBT and recommended that data should be normalized to leg length for accurate analysis. A factor analysis determined that great redundancy exists among the eight reach directions and recommended testing of only the A, PM, and PL directions.56 Schaefer and Sandrey59 have since reported a minimal detectable change (MDC) of 4.9 cm for A, 5.2 cm for PM, and 5.4 cm for P directions.
Figure 3.
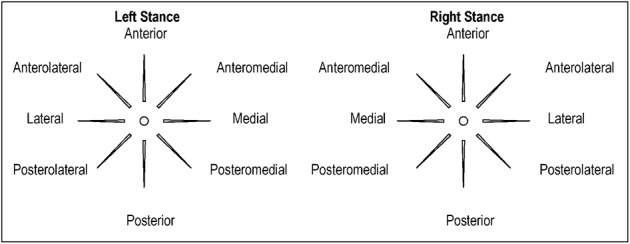
Star excursion balance test (SEBT) – eight directions referenced from the stance leg.
Figure 4.

Star excursion balance test (SEBT) in the posteromedial direction.
This study utilized the SEBT to assess dynamic postural control as follows: all participants performed a 5-minute warm-up on a stationary bicycle; five trials in each direction; a 5-minute rest followed by three trials in each of the eight directions with the mean of the three trials used for data analysis. All data were normalized for leg length by dividing the excursion distance by the leg length and multiplying by 100.
The FAAM is a self-reported instrument with a 21-item activity of daily living (ADL) subscale and an eight-item sports subscale measuring changes in physical function with possible raw score totals of 84 and 32, respectively.60 In order to account for items left unanswered, FAAM scores were transformed to percentage scores by dividing the score total by the highest potential score and multiplying by 100. It is a reliable, responsive, and valid measure of change for individuals with lower extremity musculoskeletal dysfunction undergoing physical therapy intervention with a test–retest reliability of 0.89 (ICC 2, 1) and 0.87 (ICC 2, 1) for the ADL and the sports subscales, respectively.60 Minimal clinically important difference (MCID) values for the ADL and sports subscales are scores of 8 and 9, respectively.60 In addition, individuals are asked for a subjective global rating of function (0–100%) for ADL and sports and a subjective overall categorical rating of function from 1 to 4 (severely abnormal, abnormal, nearly normal, and normal, respectively). This measurement tool was used to assess the subjective changes in ankle function before and after treatment with SCS, however, the metrics of this tool are unknown.
A GROC scale described by Jaeschke et al.61 provides individuals with a 15-point scale for subjective clinical changes and is a useful method for assessing subjective outcome in clinical studies.62,63 The scale ranges from −7 (a great deal worse) to 0 (about the same) to +7 (a great deal better), allowing individuals to classify the subjective changes experienced in a clinical parameter. The GROC has been shown to have acceptable levels of validity and reliability.61 Since functional instability is defined as a subjective sense of ‘giving way’, this study used a GROC scale to assess the participant’s perception of ankle instability at the conclusion of the 4-week intervention period through the question: ‘Please rate the overall sense of instability of your foot and ankle from the time you began treatment until now (check ONLY ONE)’.
Statistical analysis
Data analysis was performed using the SPSS Version 13.0 statistical software package (SPSS Inc., Chicago, IL, USA). A two-way analysis of variance (ANOVA), with the treatment group (SCS vs sham) as the between-subjects independent variable and time (baseline and 4 weeks) as the within-subjects independent variable, was used. Descriptive statistics included frequency counts for categorical variables and measures of central tendency and dispersion for continuous variables. Baseline demographic data and pre-test data for all outcome measures were compared across treatment groups by using independent t-tests for continuous data and chi-square tests of independence for categorical data to assess the adequacy of randomization. An intention-to-treat analysis was to be utilized to account for missing data but all participants completed the study. All data were analyzed according to the groups to which the participants were allocated. An a priori alpha level of 0.05 was used.
For analysis of the FAAM data, item score totals were transformed to percentage score by dividing the actual item score total (excluding items left unanswered) by the highest potential score. The global rating of function for the ADL and sports subscales was rated from 0 to 100% (0% indicates an inability to perform all items; 100% indicates full function). The overall categorical rating of function asked participants to rate their current overall level of function as severely abnormal (1), abnormal (2), nearly normal (3), and normal (4).
Results
Thirty-six individuals with CAI volunteered for participation in the study, Fig. 1. Twenty-nine were eligible and 27 went on to participate and complete the study after signing an informed consent (10 men, 17 women; mean±SD: age = 33.59±8.8 years; height = 167.97±9.54 cm; weight = 70.79±17.9 kg), Table 1. All participants randomly assigned to each group were treated accordingly. No significant difference in baseline data were found between the EG and SG (P = 0.237–0.713).
Table 1. Baseline participant demographics.
| Overall (n = 27) | SG (n = 14) | EG (n = 13) | P value | |
| Age, mean (SD) | 33.6 (8.8) | 30.9 (7.3) | 36.5 (9.6) | 0.113† |
| Gender (female) | 63% | 64% | 61.5% | 0.564‡ |
| Involved side | Left = 12 | Left = 6 | Left = 6 | 0.333‡ |
| Right = 15 | Right = 8 | Right = 7 | ||
| Height (cm), mean (SD) | 167.97 (9.54) | 166.19 (8.37) | 169.88 (10.65) | 0.315† |
| Weight (kg), mean (SD) | 70.79 (17.9) | 65.23 (11.8) | 76.78 (21.65) | 0.052† |
| Sprain date*, mean (SD) | 4.29 (1.96) | 4.21 (2.04) | 4.38 (1.93) | 0.880† |
SG: sham group; EG: experimental group; SD: standard deviation.
Sprain dates were classified as follows: 1 year prior = 1; 2 years prior = 2; 3 years prior = 3; 4 years prior = 4; 5 years prior = 5; more than 5 years prior = 6.
Independent samples t-test.
Chi-square test.
Significance set a priori at P<0.05.
Star excursion balance test
Means and 95% confidence intervals for normalized reach distances are presented in Table 2. In addition to a significant main effect for time in all directions (P<0.018) a significant group-by-time interaction was found in all directions (P<0.031), with the exception of the AL direction (P = 0.063) where a significant main effect for time (P = 0.013; mean difference = 2.868) was found with no main effect for group (P = 0.43). A comparison of change scores between the participants in the EG and SG demonstrated a between-group difference of 5.12 cm (1.22, 9.03) for A, 7.79 cm (2.03, 13.55) for PM, and 8.74 cm (3.19, 14.29) for P direction (Table 2).
Table 2. Pre, post and change scores for the normalized reach distances for star excursion balance test (SEBT).
| Dx | Gp | Between-group change score** | G-by-T P values | |||
| Pre* | Post* | Within-group change score** | ||||
| ANT | EG | 70.32 (9.19) | 75.67 (9.34) | 5.35 (2.20, 8.49) | 5.12 (1.22, 9.03) | 0.012 |
| SG | 76.92 (8.71) | 77.15 (9.88) | 0.22 (−2.45, 2.89) | |||
| AM | EG | 78.32 (11.83) | 84.03 (9.30) | 5.72 (0.83, 10.61) | 5.25 (0.50, 9.99) | 0.031 |
| SG | 83.65 (8.35) | 84.12 (8.59) | 0.46 (−1.44, 1.88) | |||
| MD | EG | 85.62 (10.90) | 91.63 (8.21) | 6.01 (1.33, 10.68) | 6.07 (1.25, 10.90) | 0.016 |
| SG | 92.23 (12.26) | 92.16 (11.70) | 0.07 (−2.21, 2.35) | |||
| PM | EG | 90.84 (13.26) | 100.54 (11.24) | 9.69 (3.83, 15.54) | 7.79 (2.03, 13.55) | 0.010 |
| SG | 100.45 (13.06) | 102.36 (14.05) | 1.90 (−0.31, 4.11) | |||
| PO | EG | 87.80 (13.22) | 99.53 (10.24) | 11.73 (6.53, 16.92) | 8.74 (3.19, 14.29) | 0.003 |
| SG | 98.41 (15.6) | 101.39 (16.02) | 2.98 (0.05, 5.9) | |||
| PL | EG | 79.03 (11.84) | 88.44 (10.94) | 9.41 (5.05, 13.76) | 9.12 (3.72, 14.52) | 0.002 |
| SG | 89.88 (13.53) | 90.16 (16.79) | 0.28 (−2.64, 3.20) | |||
| LAT | EG | 61.65 (15.16) | 74.09 (11.61) | 12.43 (11.77, 13.08) | 11.34 (4.06, 18.62) | 0.004 |
| SG | 73.22 (15.91) | 74.32 (16.85) | 1.09 (−1.29, 3.47) | |||
| AL | EG | 62.9 (11.62) | 67.96 (11.09) | 4.96 (1.87, 8.04) | 4.18 (−0.24, 8.62) | 0.063 |
| SG | 68.09 (8.37) | 68.87 (9.23) | 0.77 (−2.68, 4.22) |
SG: sham group; EG: experimental group; G-by-T: Group by time interactions; Dx: direction; Gp: group; ANT: anterior; AM: anteromedial; MD: medial; PM: posteromedial; PO: posterior; PL: posterolateral; LAT: lateral; AL: anterolateral.
Data are means (standard deviations).
Data are means and 95% confidence intervals.
Significance set a priori at P<0.05.
Foot and ankle ability measure
A two-way ANOVA, with treatment group (SCS vs sham) as the between-subjects independent variable, and time (baseline vs 4 weeks) as the within-subjects independent variable, was performed for the FAAM ADL and sports subscale, and the ADL and sports global rating of function subscales. Means and 95% confidence intervals for FAAM data are presented in Table 3. While no significant group-by-time interaction was found for the ADL (P = 0.854) or the sports subscale scores (P = 0.548), a significant main effect for time was found for both: ADL score (P = 0.001; mean difference = 4.57) and sports score (P = 0.001; mean difference = 11.52). No significant main effect for group was found for the ADL and sports subscale scores (P>0.46). A comparison of change scores between the participants in the EG and SG demonstrated a between-group difference of 0.44 and 3.7 in the ADL and sports scales, respectively, falling below the MCID (Table 3).
Table 3. Pre, post, and change scores for the foot and ankle ability measure (FAAM) activity of daily living (ADL) scores, sports scores, ADL global rating scale, and sports global rating scale.
| Measure | Between-group change score** | P value | ||||
| Pre* | Post* | Within-group change score** | ||||
| ADL score | EG | 90.7 (10.59) | 95.5 (5.82) | 4.8 (0.72, 8.87) | 0.44 (−5.33, 4.45) | 0.854 |
| SG | 88.6 (10.07) | 92.9 (7.29) | 4.3 (1.07, 7.52) | |||
| Sports score | EG | 73.6 (20.73) | 83.3 (17.96) | 9.68 (4.25, 15.1) | −3.7 (−8.76, 16.12) | 0.548 |
| SG | 67.2 (20.39) | 80.6 (14.48) | 13.36 (1.83, 24.88) | |||
| ADL global rating | EG | 91.3 (8.79) | 95.2 (7.63) | 3.85 (1.07, 6.62) | 0.27 (−3.88, 3.34) | 0.877 |
| SG | 81.5 (13.64) | 85.1 (13.62) | 3.57 (0.96, 6.17) | |||
| Sports global rating | EG | 81.3 (15.27) | 88.9 (14.61) | 7.62 (−1.05, 16.29) | 1.4 (−9.76, 6.96) | 0.733 |
| SG | 73.7 (13.89) | 79.9 (14.42) | 6.21 (3.39, 9.02) | |||
SG: sham group; EG: experimental group; FAAM: foot and ankle ability measure; ADL: activities of daily living.
Data are means (standard deviations).
Data are means and 95% confidence intervals.
Significance set a priori at P<0.05.
The analysis of the global rating of function for the ADL and sports subscales revealed no significant group-by-time interaction (P = 0.877 and P = 0.733, respectively) but revealed a significant main effect for time for both: ADL global rating (P<0.001; mean difference = 0.37) and sports global rating (P = 0.002; mean difference = 0.69). While a significant main effect for group was found for the ADL global rating of function (P = 0.029; mean difference = 0.99), no significant main effect for group was found for the sports global rating of function scales (P = 0.125).
Means and 95% confidence intervals for the FAAM overall categorical rating of function are presented in Table 4. In the SG, 14.3% of the participants reported an improvement (abnormal to nearly normal) in FAAM scores, while 61.6% of the EG group reported improvement (23.1% from abnormal to nearly normal and 38.5% from nearly normal to normal). A significant change in the mean categorical rating occurred in the EG (P = 0.005) while no significant change occurred in the SG (P = 0.157).
Table 4. Foot and ankle ability measure (FAAM) overall categorical rating of function.
| Pre-test | Post-test | P value* | |
| SG | 2.86 (0.663) | 3.00 (0.555) | 0.157 |
| EG | 2.77 (0.599) | 3.38 (0.650) | 0.005 |
FAAM: foot and ankle ability measure; SG: sham group; EG: experimental group.
All data are means (standard deviations).
Score ratings: severely abnormal (1), abnormal (2), nearly normal (3), and normal (4).
Wilcoxon signed ranks test.
Significance set a priori at P<0.05.
Global rating of change
At the completion of the study, all participants completed a GROC scale rating their overall subjective perception of improvement in ankle instability since the beginning of the study. A previous study reported that scores of +4 and +5 represent moderate changes and +6 and +7 represent large changes.64 Based on the GROC scores, in the current study, 28.6% of participants in the SG and 53.8% of participants in the EG had moderate to large changes (GROC score≧4). The mean GROC score was 2.43 (±1.28) for participants in the SG and 3.92 (±1.66) for participants in the EG revealing a significant difference between groups (chi-square, P = 0.014).
Discussion
The proprioceptive theory of SCS suggests that a sprain injury may lead to an imbalance in the monosynaptic stretch reflex, where the spindle mechanism of the ankle invertors is left in a state of hyperactivity, with an altered gain or hypersensitivity.41–43 This leaves the ankle joint in neuro-musculo-skeletal dysfunction, functionally unstable, and prone to repeated strains. Howell et al.65 have supported Korr’s theories that SCS leads to a reduction in the hyperactive stretch mechanism occurring after a strain injury.41,42 A shift in ankle position into slight inversion was documented in a study that used 3D joint kinematics to evaluate the function of the ankle during gait in individuals with functional instability66. The decreased ankle evertor muscles’ reflex responses may be further explained by reciprocal inhibition secondary to the proposed hyperactivity in the ankle invertors. This inhibition of the evertors could render them inefficient in their ability to protect the ankle from an inversion moment resulting in a pre-disposition for recurrent LAS. This theory is supported by Wikstrom et al.’s67 conclusion that individuals with CAI have alterations in the feedforward and feedback mechanisms involved in gait termination. A normalization of the invertor muscles stretch reflex is theorized to occur following SCS which may allow for normalization of joint and neuromuscular function.
The theory that SCS may lead to an improvement in dynamic ankle stability is supported by the results of the SEBT in this study. While the subjective portion of the FAAM asked participants to rate their functional skills at the activity limitations and participation restriction levels and found no significant changes, the GROC asked about their perception of instability at the impairment level where significant group differences were identified. The MCID found in both groups for the FAAM sports subscale, but not the FAAM ADL scale, may indicate that there is a ceiling effect for ADL function in the CAI population. These preliminary findings highlight the importance of future studies for testing this hypothesis.
Global rating of change scales have been used in clinical studies to determine the perceived amount of change, to dichotomize patients as having improved or staying stable, and to determine the MCID in quality of life measures.61,64,68 There was a significant difference in the subjective perception of change in ankle instability when comparing SG and EG (P = 0.014). These results indicate that the perceived improvement in stability was greater in the EG (53.8% reported moderate to large changes) than in the SG (28.6% reported moderate to large changes). The proposed correction in the neuro-musculo-skeletal dysfunction following SCS may lead to an improved sense of ankle stability.
Although these preliminary results are important, the final sample size was smaller than deemed necessary by a power analysis for the SEBT, increasing the risk of a Type I error. This also resulted in unequal group sizes with 13 participants in the EG and 14 participants in the SG. A sample of convenience may have led to a less than representative sample of the population with CAI, as those who volunteered may have had greater motivation to improve.
While this is an important first study on the effects of SCS on function it is important to consider the fact that one physical therapist performed all the SCS interventions and that the study did not include a long-term follow-up. In addition, the absence of a true control group makes it impossible to assess the role of a placebo effect. Future studies should consider the addition of a third group so that placebo effects may be considered in the interpretation of the results.69 A follow-up study on the effect of SCS in individuals with a shorter duration post-ankle sprain is also strongly recommended.
Conclusion
In conclusion, the preliminary results of this study support the hypotheses that SCS treatment may lead to an improvement in dynamic balance (SEBT) and the subjective sense of stability (GROC) in individuals with CAI. This study failed to support the hypotheses that SCS can lead to an improvement in subjective ankle function in individuals with CAI. Further clinical studies, including larger sample sizes and a long-term follow-up, are indicated to assess if SCS may have a clinical effect on subjective ankle function and the rate of sprain recurrence.
Disclaimer Statements
Contributors Cristiana K Collins is responsible for the study's concept and design; data acquisition, analysis, and interpretation; performing all interventions, drafting, revising, and finalizing the manuscript; approving final manuscript for submission. Joshua Cleland was responsible for providing input to study concept and design; providing input and assistance in data analysis and interpretation; providing feedback on written manuscript; approving final manuscript. Michael Masaracchio provided assistance in pre- and post-testing of study participants, data analysis and interpretation, and revisions of final manuscript. Contributors: Evangelos Pappas provided assistance in pre- and post-testing of study participants and data analysis and interpretation.
Conflicts of interest The authors declare that they have no conflict of interest.
Ethics approval This study was approved by the Institutional Review Boards of Long Island University and Nova Southeastern University.
Acknowledgments
The authors would like to acknowledge and thank Dr Evangelos Pappas for his contributions with data collection and data analysis and Dr Madeleine Hellman for her assistance in overview of the study.
References
- 1.Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ., Jr The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279–84. doi: 10.2106/JBJS.I.01537. [DOI] [PubMed] [Google Scholar]
- 2.Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73–94. doi: 10.2165/00007256-200737010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hertel J. The complex etiology of lateral ankle instability. J Orthop Sports Phys Ther. 2005;35:A9–10. [PubMed] [Google Scholar]
- 4.Hertel J, Kaminski TW. Second international ankle symposium summary statement. J Orthop Sports Phys Ther. 2005;35:A2–6. [PubMed] [Google Scholar]
- 5.Denegar CR, Miller SJ., 3rd Can chronic ankle instability be prevented? Rethinking management of lateral ankle sprains. J Athl Train. 2002;37:430–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes A, Delahunt E. Treatment of common deficits associated with chronic ankle instability. Sports Med. 2009;39:207–24. doi: 10.2165/00007256-200939030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hertel J. Overview of the etiology of chronic ankle instability. Third International Ankle Symposium: keynote lecture. J Orthop Sports Phys Ther. 2006;36:A7. [Google Scholar]
- 8.Kaminski TW, Hartsell HD. Factors contributing to chronic ankle instability: a strength perspective. J Athl Train. 2002;37:394–405. [PMC free article] [PubMed] [Google Scholar]
- 9.Hartsell HD, Spaulding SJ. Eccentric/concentric ratios at selected velocities for the invertor and evertor muscles of the chronically unstable ankle. Br J Sports Med. 1999;33:255–8. doi: 10.1136/bjsm.33.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkerson GB, Pinerola JJ, Caturano RW. Invertor vs. evertor peak torque and power deficiencies associated with lateral ankle ligament injury. J Orthop Sports Phys Ther. 1997;26:78–86. doi: 10.2519/jospt.1997.26.2.78. [DOI] [PubMed] [Google Scholar]
- 11.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47:678–85. [PubMed] [Google Scholar]
- 12.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47:669–77. [PubMed] [Google Scholar]
- 13.Lentell GL, Katzman LL, Walters MR. The relationship between muscle function and ankle instability. J Orthop Sports Phys Ther. 1990;11:605–11. doi: 10.2519/jospt.1990.11.12.605. [DOI] [PubMed] [Google Scholar]
- 14.Ryan L. Mechanical stability, muscle strength, and proprioception in the functionally unstable ankle. Aust J Physiother. 1994;40:41–7. doi: 10.1016/S0004-9514(14)60453-0. [DOI] [PubMed] [Google Scholar]
- 15.Hertel J. Functional instability following lateral ankle sprain. Sports Med. 2000;29:361–71. doi: 10.2165/00007256-200029050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Refshauge KM. Sensory deficits with ankle injuries. Second International Ankle Symposium: plenary lecture II. J Orthop Sports Phys Ther. 2005;35:A10–11. [PubMed] [Google Scholar]
- 17.Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37:487–93. [PMC free article] [PubMed] [Google Scholar]
- 18.Bullock-Saxton JE. Sensory changes associated with severe ankle sprain. Scand J Rehabil Med. 1995;27:161–7. [PubMed] [Google Scholar]
- 19.Wilkerson GB, Nitz AJ. Dynamic ankle stability: mechanical and neuromuscular interrelationships. J Sport Rehabil. 1994;3:43–57. [Google Scholar]
- 20.Riemann BL, Lephart SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37:80–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Athl Train. 2002;37:413–29. [PMC free article] [PubMed] [Google Scholar]
- 22.Hertel J, Buckley WE, Denegar CR. Serial testing of postural control after acute lateral ankle sprain. J Athl Train. 2001;36:363–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Docherty CL, Arnold BL, Gansneder BM, Hurwitz S, Gieck J. Functional-performance deficits in volunteers with functional ankle instability. J Athl Train. 2005;40:30–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Riemann BL. Is there a link between chronic ankle instability and postural instability? J Athl Train. 2002;37:386–93. [PMC free article] [PubMed] [Google Scholar]
- 25.Olmsted LC, Carcia CR, Hertel J, Shultz SJ. Efficacy of the star excursion balance test in detecting reach deficits in subjects with chronic ankle instability. J Athl Train. 2002;37:501–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Gribble PA, Hertel J, Denegar CR, Buckley WE. The effects of fatigue and chronic ankle instability on dynamic postural control. J Athl Train. 2004;39:321–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Vaes P, Duquet W, Van Gheluwe B. Peroneal reaction times and eversion motor response in healthy and unstable ankles. J Athl Train. 2002;37:475–80. [PMC free article] [PubMed] [Google Scholar]
- 28.Vaes P, Van Gheluwe B, Duquet W. Control of acceleration during sudden ankle supination in people with unstable ankles. J Orthop Sports Phys Ther. 2001;31:741–52. doi: 10.2519/jospt.2001.31.12.741. [DOI] [PubMed] [Google Scholar]
- 29.Vaes P. Efficacy of control during sudden ankle inversion. Second International Ankle Symposium: plenary lecture III. J Orthop Sports Phys Ther. 2005;35:A12. [PubMed] [Google Scholar]
- 30.van Cingel EH, Kleinrensink G, Uitterlinden EJ, Rooijens PP, Mulder PG, Aufdemkampe G, et al. Repeated ankle sprains and delayed neuromuscular response: acceleration time parameters. J Orthop Sports Phys Ther. 2006;36:72–9. doi: 10.2519/jospt.2006.36.2.72. [DOI] [PubMed] [Google Scholar]
- 31.Ebig M, Lephart SM, Burdett RG, Miller MC, Pincivero DM. The effect of sudden inversion stress on EMG activity of the peroneal and tibialis anterior muscles in the chronically unstable ankle. J Orthop Sports Phys Ther. 1997;26:73–7. doi: 10.2519/jospt.1997.26.2.73. [DOI] [PubMed] [Google Scholar]
- 32.Myers JB, Riemann BL, Hwang JH, Fu FH, Lephart SM. Effect of peripheral afferent alteration of the lateral ankle ligaments on dynamic stability. Am J Sports Med. 2003;31:498–506. doi: 10.1177/03635465030310040401. [DOI] [PubMed] [Google Scholar]
- 33.Sefton JM, Yarar C, Hicks-Little CA, Berry JW, Cordova ML. Six weeks of balance training improves sensorimotor function in individuals with chronic ankle instability. J Orthop Sports Phys Ther. 2011;41:81–9. doi: 10.2519/jospt.2011.3365. [DOI] [PubMed] [Google Scholar]
- 34.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37:364–75. [PMC free article] [PubMed] [Google Scholar]
- 35.Bullock-Saxton JE, Janda V, Bullock MI. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994;15:330–4. doi: 10.1055/s-2007-1021069. [DOI] [PubMed] [Google Scholar]
- 36.Klykken LW, Pietrosimone BG, Kim KM, Ingersoll CD, Hertel J. Motor-neuron pool excitability of the lower leg muscles after acute lateral ankle sprain. J Athl Train. 2011;46:263–9. doi: 10.4085/1062-6050-46.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005;26:1055–61. doi: 10.1177/107110070502601210. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins J, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9:135–59. [Google Scholar]
- 39.Pietrosimone BG, McLeod MM, Lepley AS. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4:31–5. doi: 10.1177/1941738111428251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Needle AR, Charles BB, Farquhar WB, Thomas SJ, Rose WC, Kaminski TW. Muscle spindle traffic in functionally unstable ankles during ligamentous stress. J Athl Train. 2013;48:192–202. doi: 10.4085/1062-6050-48.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korr IM. The neural basis of the osteopathic lesion. J Am Osteopath Assoc. 1947;47:191–8. [PubMed] [Google Scholar]
- 42.Korr IM. Proprioceptors and somatic dysfunction. J Am Osteopath Assoc. 1975;74:638–50. [PubMed] [Google Scholar]
- 43.Jones L, Kusunose R, Goering E.Jones Strain-Counterstrain. 1st edn. Boise, ID: Jones Strain-Counterstrain, Inc; 1995 [Google Scholar]
- 44.Jones LH. Spontaneous release by positioning. DO. 1964;4:109–16. [Google Scholar]
- 45.Jones LH.Strain and counterstrain. American Academy of Osteopathy; Indianapolis, IN 1981. [Google Scholar]
- 46.D'Ambrogio KJ, Roth GB.Positional release therapy: assessment and treatment of musculoskeletal dysfunction. St. Louis, MO: Mosby; 1997 [Google Scholar]
- 47.Wong CK. Strain counterstrain: current concepts and clinical evidence. Man Ther. 2012;17:2–8. doi: 10.1016/j.math.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Melzack R, Stillwell DM, Fox EJ. Trigger points and acupuncture points for pain: correlations and implications. Pain. 1977;3:3–23. doi: 10.1016/0304-3959(77)90032-X. [DOI] [PubMed] [Google Scholar]
- 49.Wong C, Schauer C. Reliability, validity and effectiveness of strain counterstrain techniques. J Man Manip Ther. 2004;12:107–12. [Google Scholar]
- 50.Lewis C, Flynn TW. The use of strain-counterstrain in the treatment of patients with low back pain. J Man Manip Ther. 2001;9:92–8. [Google Scholar]
- 51.Wong C, Schauer-Alvarez C. Effect of strain counterstrain on pain and strength in hip musculature. J Man Manip Ther. 2004;12:215–23. [Google Scholar]
- 52.Collins CK. Physical therapy management of complex regional pain syndrome I in a 14-year-old patient using strain counterstrain: a case report. J Man Manip Ther. 2007;15:25–41. doi: 10.1179/106698107791090150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattacola CG, Lloyd JW. Effects of a 6-week strength and proprioception training program on measures of dynamic balance: a single-case design. J Athl Train. 1997;32:127–35. [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkerson G. Functional rehabilitation. A protocol for management of the lateral ankle sprain. Rehabil Manag. 1996;9:54–60. [PubMed] [Google Scholar]
- 55.Gribble PA, Hertel J, Plisky P. Using the star excursion balance test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47:339–57. doi: 10.4085/1062-6050-47.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertel J, Braham RA, Hale SA, Olmsted-Kramer LC. Simplifying the star excursion balance test: analyses of subjects with and without chronic ankle instability. J Orthop Sports Phys Ther. 2006;36:131–7. doi: 10.2519/jospt.2006.36.3.131. [DOI] [PubMed] [Google Scholar]
- 57.Hertel J, Miller SJ. Intratester and intertester reliability during the star excursion balance tests. J Sport Rehabil. 2000;9:104–16. [Google Scholar]
- 58.Gribble PA, Hertel J. Considerations for normalizing measures of the star excursion balance test. Meas Phys Educ Exerc Sci. 2003;7:89–100. [Google Scholar]
- 59.Schaefer JL, Sandrey MA. Effects of a 4-week dynamic-balance-training program supplemented with Graston instrument-assisted soft-tissue mobilization for chronic ankle instability. J Sport Rehabil. 2012;21:313–26. doi: 10.1123/jsr.21.4.313. [DOI] [PubMed] [Google Scholar]
- 60.Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the foot and ankle ability measure (FAAM). Foot Ankle Int. 2005;26:968–83. doi: 10.1177/107110070502601113. [DOI] [PubMed] [Google Scholar]
- 61.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 62.Struijs PA, Damen PJ, Bakker EW, Blankevoort L, Assendelft WJ, van Dijk CN. Manipulation of the wrist for management of lateral epicondylitis: a randomized pilot study. Phys Ther. 2003;83:608–16. [PubMed] [Google Scholar]
- 63.Smidt N, van der Windt DA, Assendelft WJ, Deville WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359:657–62. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 64.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89:69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- 65.Howell JN, Cabell KS, Chila AG, Eland DC. Stretch reflex and Hoffmann reflex responses to osteopathic manipulative treatment in subjects with Achilles tendinitis. J Am Osteopath Assoc. 2006;106:537–45. [PubMed] [Google Scholar]
- 66.Delahunt E, Monaghan K, Caulfield B. Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am J Sports Med. 2006;34:1970. doi: 10.1177/0363546506290989. [DOI] [PubMed] [Google Scholar]
- 67.Wikstrom EA, Bishop MD, Inamdar AD, Hass CJ. Gait termination control strategies are altered in chronic ankle instability subjects. Med Sci Sports Exerc. 2010;42:197–205. doi: 10.1249/MSS.0b013e3181ad1e2f. [DOI] [PubMed] [Google Scholar]
- 68.Cleland JA, Abbott JH, Kidd MO, Stockwell S, Cheney S, Gerrard DF, et al. Manual physical therapy and exercise versus electrophysical agents and exercise in the management of plantar heel pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther. 2009;39:573–85. doi: 10.2519/jospt.2009.3036. [DOI] [PubMed] [Google Scholar]
- 69.George SZ, Robinson ME. Dynamic nature of the placebo response. J Orthop Sports Phys Ther. 2010;40:452–4. doi: 10.2519/jospt.2010.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]