Abstract
Objective and Importance:
The purpose of this report is to describe the clinical course of a patient referred to physiotherapy (PT) for the treatment of low back pain who was subsequently diagnosed with metastatic non-small cell carcinoma of the lung.
Clinical Presentation:
A 48-year old woman was referred to PT for the evaluation and treatment of an insidious onset of low back pain of 2 month duration. The patient did not have a history of cancer, recent weight changes, or general health concerns. The patient’s history and physical examination were consistent with a mechanical neuromusculoskeletal dysfunction and no red flag findings were present that warranted immediate medical referral.
Intervention:
Short-term symptomatic improvements were achieved using the treatment-based classification approach. However, despite five PT sessions over the course of 5 weeks, the patient did not experience long-term symptomatic improvement. On the sixth session, the patient reported a 2-day history of left hand weakness and headaches. This prompted the physiotherapist to refer the patient to the emergency department where she was diagnosed with lung cancer.
Conclusion:
Differential diagnosis is a key component of PT practice. The ability to reproduce symptoms or achieve short-term symptomatic gains is not sufficient to rule out sinister pathology. This case demonstrates how extra caution should be taken in patients who are smokers with thoracolumbar region pain of unknown origin. The need for caution is magnified when one can achieve no more than short-term improvements in the patient’s symptoms.
Keywords: Lung cancer, Medical screening, Metastatic carcinoma, Differential diagnosis, Low back pain, Orthopedic manipulative therapy
Background
Low back pain is a common condition and patients with this complaint are routinely managed by physiotherapists. While the prevalence of serious medical pathology (i.e. cancer) causing low back pain is extremely low,1,2 it is the responsibility of the physiotherapist to screen and monitor for serious medical conditions and determine if referral to another health care practitioner is indicated.
Evidence-based strategies can be utilized to screen for medical pathology that can mimic mechanical low back pain. For example, Deyo and Diehl3 reported that cancer as a cause for low back pain can be ruled out if the patient is less than 50 years old, does not exhibit unexplained weight loss, does not have a history of cancer, and is responding to conservative intervention. If a red flag is present, evidence-based screening strategies suggest that lumbar spine radiographs and laboratory testing (erythrocyte sedimentation rate) are the next appropriate steps to rule out cancer as the cause for low back pain.3,4 However, if the concern persists in the absence of abnormal laboratory and/or radiographic findings, advanced diagnostic imaging should be used to screen for cancer as a source for the pain.5,6
These screening strategies allow the physiotherapist to proceed with care of the patient and avoid unnecessary physician referrals. Some sinister pathologies may present initially with musculoskeletal symptom patterns, including the ability to reproduce or reduce symptoms through mechanical means. Therefore, it is imperative for physiotherapists to frequently reevaluate their patient’s response to intervention, since signs and symptoms of serious underlying disease can develop at any point in the course of care.7,8 This report describes the clinical course of a patient referred to physiotherapy (PT) for the treatment of low back pain, who was subsequently diagnosed with non-small cell carcinoma of the lung with metastases to the spine and brain. The purpose of this article is to enhance therapists’ understanding of when medical referral might be indicated by reporting the historical, subjective, and physical exam findings of that were indicative of sinister pathology in this case. The secondary purpose of this article is to report risk factors associated with developing lung cancer and to describe the clinical prognosis of patients with metastatic spinal carcinoma.
Patient Characteristics
The patient was a 48-year old unemployed woman (height = 165 cm; mass = 92.7 kg). She enjoyed occasional walking for physical activity. The patient smoked 15 cigarettes per day (3/4 pack per day) for the last 34 years for a total of 25.5 pack years. She also had a history of anxiety and major depression, both of which were managed by a psychiatrist and were currently under control.
She was originally evaluated in the emergency department the day after the onset of low back pain symptoms. Lumbar radiographs were significant for degenerative changes at the L5-S1 level. A urinalysis was unremarkable. She was diagnosed with a lumbar strain, prescribed naproxen, and asked to follow-up with her primary care physician if she did not improve.
There was no improvement 1 month after symptom onset when the patient was evaluated by her primary care physician. During this evaluation, the patient complained that her daily activities were being limited by fatigue. Of note, the patient had an 11-year clinical history of fatigue associated with her depression. She also noted that the pain was unchanged with activity. At this visit, she was prescribed cyclobenzaprine, provided with a general exercise handout, instructed in postural education, advised on activity modification, instructed to rest and use heat, and referred to PT.
Examination
At the PT initial evaluation (1 week after medical evaluation and 5 weeks after the emergency department evaluation), the patient’s chief complaint was upper lumbar pain centered at L1–2 and described as an intermittent, variable, and dull ache without referral into the buttock or lower extremities. Aggravating factors were sitting and lifting objects while easing factors were standing and sleeping. The patient noted that her symptoms were most intense in the evening and that the pain occasionally caused her difficulty with falling asleep. She reported that her symptoms were least intense in the afternoon and while taking medications prescribed for her pain. Her symptoms were insidious and began 8 weeks prior to the PT evaluation. There was no known cause for the pain. The patient denied any previous episodes of low back pain aside from a remote incident of pyelonephritis 19 years ago.
At the time of the initial PT evaluation, the only medications that the patient was taking were 500 mg of naproxen twice daily and 10 mg of cyclobenzaprine three times daily. Despite her report of fatigue to the primary care provider, she denied fatigue during her PT evaluation. She also denied fever/chills/sweats, shortness of breath, upper/lower extremity weakness, or changes in bladder function. There was no evidence of weight change and there was no history of cancer. The patient reported a recent onset of constipation and difficulty maintaining her balance while walking, which she felt was associated with the intake of cyclobenzaprine. The physiotherapist recommended that the patient contact her physician for medical evaluation of these potential side effects of the cyclobenzaprine.
The patient reported 3/10 pain on the numeric pain rating scale (NPRS)9 while sitting, which was alleviated to 0/10 while standing (with 0 = no pain and 10 = worst imaginable pain). Her modified Oswestry disability index (MODI) score was 32% indicating a moderate level of disability.10 She had a normal gait pattern and transitional movements were normal without signs of guarding. Balance testing was not performed at the initial evaluation. Pelvic alignment was level in standing and her thoracic kyphosis and lumbar lordosis were unremarkable.
No erythema, ecchymosis, or edema was seen in the thoracic, lumbar, or sacral regions. Lumbar active range of motion in standing was within normal limits with flexion and left lateral flexion causing a slight increase in pain (4/10 on the NPRS). Hip passive range of motion was within normal limits bilaterally and did not reproduce her symptoms. With the patient in the prone position, central posterior to anterior pressures at the L1–2 vertebral levels were hypomobile and reproduced concordant symptoms. Lumbar extension in prone (10 repetitions of prone press-ups) completely alleviated the patient’s symptoms without incurring a flexion block. Sensation testing for the L2-S1 dermatomes, strength testing for the L2-S1 myotomes, and patellar and Achilles deep tendon reflex assessment were within normal limits. Straight leg raise testing was within normal limits. Ankle clonus and Babinski reflex were not present.
Clinical Impression
Red flags for sinister pathology were present at the initial evaluation but were of minimal concern when coupled with the patient’s history and physical exam. More specifically, the patient had recently reported fatigue but also had a known 11-year history of fatigue associated with her depression. She reported recent episodes of constipation, but she associated this with a recent change in her medications. The pain was located in the upper lumbar region, which was of concern given her previous history of pyelonephritis; however, her kidney function tests had returned normal and she reported that the current symptoms did not feel similar to those of her previous bout of pyelonephritis. Despite the fact that the patient was a heavy smoker, she did not have the two most common indicators of cancer: age>50; previous history of cancer.3,4 Lastly, her symptoms were intermittent and the physical exam mimicked what one would expect of a patient with a benign musculoskeletal condition.
The most pertinent exam findings were:
pain primarily in lumbar flexion positions (sitting)
pain relieved by lumbar extension positions (standing)
active lumbar flexion and left side bending reproduced her symptoms
L1–2 was tender to palpation and hypomobile on spring testing
abolishment of her symptoms after 10 repetitions of prone press-ups in the clinic.
The initial working diagnosis was a mild L1–2 segmental dysfunction with extension bias and concurrent L1–2 hypomobility.
Intervention
The focus of intervention was to increase postural awareness in order to avoid lumbar flexed positions while increasing L1–2 mobility and thus reducing pain. A treatment-based classification (TBC) approach was used in determining treatment selection.11,12 The patient’s positive response to an extension-based exercise indicated the best treatment approach was likely specific exercise. A home exercise program was provided including continuation of her walking program and performance of 10–15 repetitions of prone press-ups 3–5 times per day.13 She was instructed in sitting ergonomics, issued a lumbar roll for sitting, and enrolled in a single-visit low back pain education class. She was offered enrollment in a smoking cessation class, but declined amidst concerns that stopping smoking would cause weight gain. Owing to the lack of referral into the lower extremities, the relatively low symptom irritability, and the patient’s ability to abolish the symptoms with one set of prone press-ups, it was determined that the patient could initially manage her symptoms through a home exercise program. For this reason, a PT follow-up was scheduled for 3 weeks based upon clinic access and what the patient could easily attend.
At the time of the PT follow-up visit 3 weeks later, the patient reported no change in her symptoms per the Global Rating of Change Scale (GROC, score = 0),14 despite reported compliance with the home exercise program. The patient reported 2/10 resting pain on the NPRS in sitting that was unchanged by standing. Her lumbar active range of motion was slightly decreased in flexion and left lateral flexion compared to the initial evaluation. Lumbar flexion increased the patient’s upper lumbar pain to 4/10 with a positive Gowers’ sign while left lateral flexion induced right-sided upper lumbar paraspinal pain. The physical examination was otherwise unchanged from the initial evaluation.
Since the patient had a lack of sustained progress using the principles of the specific exercise category, the primary author considered whether she might be re-classified in the TBC model. Owing to the relatively new onset of symptoms, the lack of pain referral into the lower extremities, and the segmental hypomobility, the patient was re-classified into the manipulation subgroup.11,12 The patient was treated with a neutral gap lumbar thrust mobilization15 targeted at L1–2. After the lumbar thrust mobilization, her lumbar flexion and left lateral flexion range of motion improved to within normal limits and her pain was reduced. She was asked to continue with her initial home exercise program with the addition of a pelvic tilt exercise; and the patient agreed to enroll in a smoking cessation class.
At her second follow-up visit with the physiotherapist 1 month following her initial evaluation, the patient reported that her symptoms were a little worse (GROC = −2). She stated that her symptoms worsened 23 days after initiating PT and that her symptoms were no longer relieved with her medications. She reported continued compliance with her home exercise program but that she no longer received pain relief from the exercises. Her resting pain had increased to 5/10 on the NPRS while sitting, which reduced with standing and with supine manual lumbar traction. Lumbar extension was normal and pain was unchanged compared to resting. Lumbar forward flexion range of motion was decreased and continued to be the most provocative active motion.
Considering that she had short-term improvement, without long-term gains, with an extension-based program and short-term improvement with long-term worsening with a mobilization program, the focus of treatment shifted back to an extension oriented treatment approach. The patient was also started on a traction based treatment program and was instructed to continue with her home exercise program. The traction treatments were performed in prone in 10–15° of lumbar extension consistent with the patient’s apparent directional preference.
Outcome
The patient was able to complete two traction treatments, which brought her total to five treatments in 5 weeks. Despite the multifaceted PT treatment approach, the patient did not experience any lasting improvements in symptoms. On arrival for her sixth appointment, she complained of a headache, left-sided upper extremity numbness and tingling, dropping items, and feeling unbalanced for the previous 2 days. She was escorted to the emergency department for immediate medical evaluation.
In the emergency department, a computed tomography scan of the brain was completed. While there was no evidence for acute hemorrhage, multiple regions of abnormal hypoattenuation were noted, which were suspicious for metastatic disease. Magnetic resonance imaging of the brain subsequently revealed multiple enhancing lesions throughout the brain approaching 1 cm in size, including the left frontal lobe and right cerebellum. These were also suspicious for metastatic disease (Fig. 1). A chest radiograph demonstrated a round to ovoid opacity in the left mid-lung measuring approximately 5 cm in diameter (Fig. 2). Chest, abdominal, and pelvic computed tomography scans demonstrated a 4.8 cm left lingular mass and osseous lesions involving L1 and L2. Computed tomography guided biopsy of the left lingular mass led to a diagnosis of non-small cell carcinoma of the lung with metastases to the spine and brain. Spinal magnetic resonance imaging showed diffuse lesions throughout the vertebral bodies consistent with metastatic disease with the largest lesion at L1 (Fig. 3). Despite radiation therapy and chemotherapy, the patient succumbed to the cancer 6 months after she was first seen in PT. The final outcome was consistent with published reports on metastatic spine tumor prognosis.16
Figure 1.
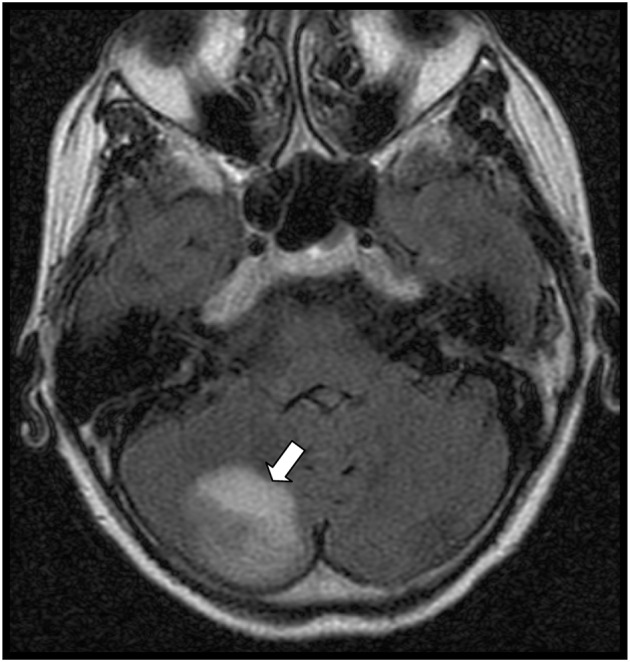
Axial view magnetic resonance image of the brain showing a metastatic lesion in the cerebellum (arrow).
Figure 2.
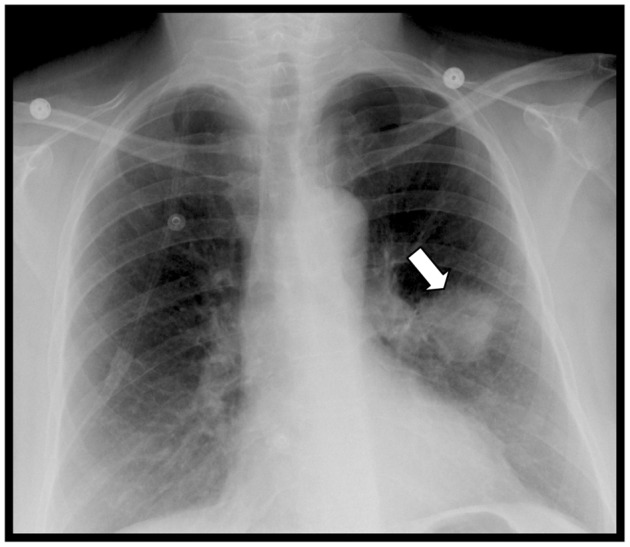
Chest radiograph revealing a radiopaque mass in the left mid-lung (arrow) consistent with a primary lung neoplasm.
Figure 3.
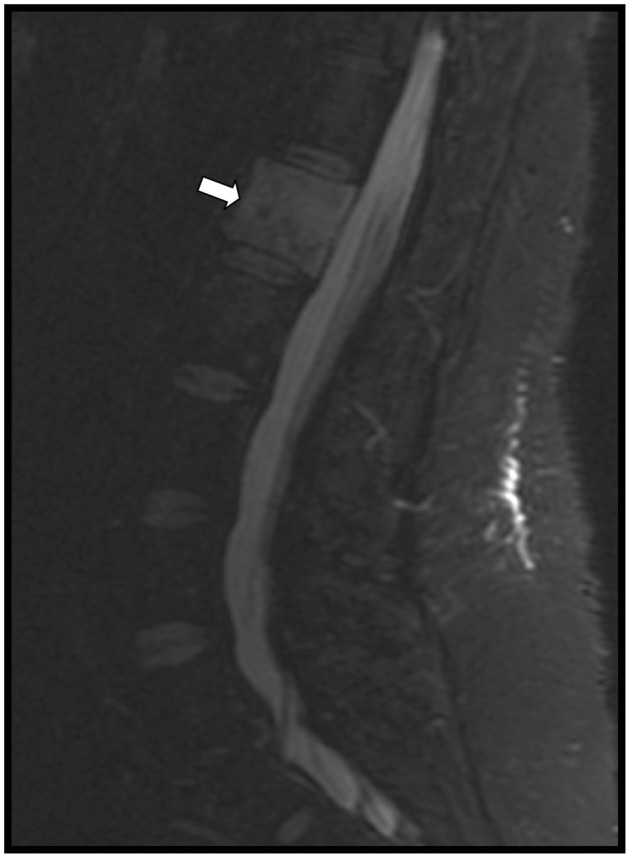
Sagittal view magnetic resonance image of the lumbar spine revealing the metastatic lesion in the L1 vertebral body (arrow).
Discussion
The spine accounts for 80% of bone metastatic lesions and is one of the most common sites for the early presentation of metastases.17,18 Cancer of the breast, lung, and prostate are the most commonly associated with bony metastases and up to 50% of patients with these types of cancer will develop bone metastases.18,19 Approximately 60% of bone metastases are found in the axial skeleton.20,21 Studies have shown that the majority of skeletal metastases can be asymptomatic with cervical and lumbar metastases tending to be more symptomatic than those in the thoracic spine.19,20 Unfortunately, metastatic disease is typically wide-spread by the time patients present symptomatically to the clinic.22
Once symptoms present, delays in diagnosis can be significant. Studies have shown symptom-to-diagnosis delay in lung cancer to be approximately 1–3 months.23–25 Overall diagnostic delay is mostly attributed to patient delay in seeking care. Delays related to the practitioner tend to be of shorter duration.25,26 Misdiagnosis and misinterpretation of tests are two factors contributing to practitioner delay.26,27 The inherently poor sensitivity of radiographs in detecting metastatic lesions can lead to misdiagnosis, adding to practitioner delay. More specifically, bony destruction of 30–50% is necessary for a lytic lesion to appear on radiographs, suggesting that some metastatic lesions may go undetected by radiographs for a considerable period of time.28 In our case, total diagnostic delay was approximately 118 days with 82 days attributed to practitioner delay. It is notable that the patient had received conventional radiographs of the lumbar spine at her initial presentation to the emergency department, which showed no signs of metastatic disease.
Evidence related to the early diagnosis and treatment tends to be paradoxical, as more rapid diagnosis and treatment has been shown to be associated with reduced survival time.25,29 These data may have a patient selection bias, however, as patients with more advanced and deadly stages of cancer tend to be diagnosed and treated more rapidly.29–31 Considering the advanced stage of our patient’s cancer, it is unlikely that the final outcome would have changed greatly with a more expedient diagnosis. However, providing a timely diagnosis in this case may have provided a psychosocial benefit to the patient and her family. Studies have shown that preparation for death is a key factor in making the dying process more favorable for patients and their families.32,33 Miyashita et al.33 identified ‘dying in a favorite place’ as a core domain in dying a ‘good death.’ Our patient survived approximately 4 months after diagnosis, allowing her to return to her native Germany for her waning days.
It is of note that our patient did not initially exhibit the typical characteristics of sinister pathology. Several factors emerged throughout the course of treatment, which indicated that her symptoms were not solely of musculoskeletal origin. While many musculoskeletal patients can present with red flag findings,1 the cascade of red flag findings in this patient was sufficient to trigger referral back to a physician. More specifically, the patient’s initial report of fatigue affecting her activities of daily living, her smoking history, her lack of response to treatment with gradually worsening symptoms, and the location of the her symptoms in the upper lumbar spine were all of concern for sinister pathology.
Papadopoulos et al.34 reported that 55% of lung cancer can be attributed to smoking. Total pack years has been found to be correlated with a higher risk of developing lung cancer.31,34 Our patient’s 25.5 pack year history placed her at a 3.5 times higher risk of developing lung cancer compared to a non-smoker.35 Overall, persons who have smoked any amount are at 2.7–8.2 times higher risk of developing lung cancer compared to those who have never smoked.31,34
Recent trends in the literature have found total pack years to be of lesser importance in cancer risk and mortality when compared to current smoking status and daily intensity.36,37 Active female smokers have a 5.2 times higher probability of contracting lung cancer compared to female non-smokers.38 Our patient was an active smoker consuming 15 cigarettes (3/4 pack) per day. The intensity of the patient’s smoking habit increased her odds of getting lung cancer by 7–13 times that of non-smokers.38,39 Similarly, this intensity increased the patient’s odds of mortality 14 times that of non-smokers.39 Research has shown that lung cancer risk in former smokers is inversely proportional to number of years since quitting.31 Despite recommendations from multiple medical providers, our patient never pursued smoking cessation.
While several components of this patient’s history and physical examination were consistent with a mechanical musculoskeletal dysfunction, the location of the patient’s symptoms (upper lumbar spine) was concerning. Upper lumbar pain has been associated with visceral referral from the kidneys.40,41 In addition, the upper lumbar spine is one of the most common sites for gastric metastases42 and vertebral compression fractures.43,44 Fornasier and Horne45 reported that 30 of 38 cases of metastatic breast cancer involved L2 and 10 of 15 cases of metastatic lung cancer involved T12, though it is important to note that other researchers have not reported similar distributions.46,47
Most non-musculoskeletal causes of back pain do not specifically target the upper lumbar spine. Sinister pathologies that propagate through hematogenous seeding (i.e. osteomyelitis or metastatic carcinoma) can be spread throughout the body with areas near the venous/lymphatic drainage being the most highly affected.48 In the case of lung cancer, the thoracic spine is the most common region for spinal metastases.46,49
The upper lumbar spine only accounts for 2–4% of lumbar disc herniations,50,51 5% of lumbar facet arthropathies,52 29% of lumbar central canal stenosis,53 and 9–11% of lumbar degenerative disc disease (DDD).54,55 Degenerative disc disease isolated to the upper lumbar spine is even more rare, accounting for only 2% of lumbar DDD patients.54,55 Iguchi et al.56 reported on 132 cases of single-level spondylolisthesis, none of which were found in the upper lumbar spine. They further reported that upper lumbar involvement was only present in cases of multi-level spondylolistheses. The infrequency of upper lumbar musculoskeletal dysfunction has led some authors to suggest that pain originating from the upper lumbar region should be viewed as a red flag.57,58
Sinister pathology is commonly thought to present as constant, unwavering pain that is unchanged by joint movement or altering body positioning and may become worse at night.59 However, metastatic lesions of the spine may mimic musculoskeletal pathology, as symptoms are often reproducible by active/passive movement and can ease with position change or rest.60,61 During the PT evaluation, the patient exhibited intermittent symptoms that were changed with position and mechanical intervention and were subjectively eased by sleep. The patient experienced short-term gains with both manual intervention and a directional preference exercise but lacked any sustained improvements. While intrasession gains with manual intervention have been shown to be an indicator of favorable prognosis,62,63 it is pertinent to note that intrasession nor short-term symptomatic improvements do not sufficiently rule out sinister pathology.
As treatment ensued, the patient began to complain of intractable pain that was unchanged by either mechanical or pharmaceutical interventions. Also of concern were her new development of headaches, left-sided upper extremity numbness and tingling, dropping items, and a decreased sense of balance. Lack of improvement with conservative therapy after a 1-month period has been shown to be an indicator of underlying sinister pathology.3,4 However, assessing long-term gains can prove challenging. For example, psychosocial factors, such as trust in one’s care provider or having care expectations met, have been shown to impact subjective reports of symptomatic improvement.64,65 Therefore, it is advisable to utilize objective measures of quality of life (i.e. GROC), function (i.e. MODI), and pain (i.e. NPRS) to accurately assess symptoms over a longer period of time. While our patient initially indicated short-term gains with treatment, her GROC and NPRS scores consistently showed a worsening trend in her symptoms.
Conclusion
While the prevalence of serious medical pathology causing low back pain is extremely low,1,2 it is the responsibility of the physiotherapist to screen for serious medical conditions and continuously monitor for the development of those conditions throughout the course of care. Our patient presented with symptoms mimicking mechanical low back pain but was later found to have spinal metastases originating from non-small cell carcinoma of the lung. The ability to reproduce symptoms with movement or palpation, or even the ability to achieve short-term symptomatic improvement through mechanical interventions does not rule out the possibility of sinister pain sources. Failure to achieve long-term symptomatic improvements with conservative management can be an indicator of sinister pathology3,4 and provides sufficient cause for medical referral. Physiotherapists should use additional caution during differential diagnosis of smokers with low back pain, especially when these symptoms are of unknown origin and are located in the upper lumbar spine.
Conflict of Interest
We affirm that we have no financial affiliation (including research funding) or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript. No other conflict of interest (i.e. personal associations or involvement as a director, officer, or expert witness) exists.
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the US Government, the Department of Defense or the Department of the Air Force.
References
- 1.Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, et al. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum. 2009;60:3072–80. doi: 10.1002/art.24853. [DOI] [PubMed] [Google Scholar]
- 2.McGuirk B, King W, Govind J, Lowry J, Bogduk N. Safety, efficacy and cost-effectiveness of evidence-based guidelines for management of acute low back pain in primary care. Spine. 2001;26:2615–22. doi: 10.1097/00007632-200112010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3:230–8. doi: 10.1007/BF02596337. [DOI] [PubMed] [Google Scholar]
- 4.Joines JD, McNutt RA, Carey TS, Deyo RA, Rouhani R. Finding cancer in primary care outpatients with low back pain: a comparison of diagnostic strategies. J Gen Intern Med. 2001;16:14–23. doi: 10.1111/j.1525-1497.2001.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feun LG, Savaraj N. Detection of occult bone metastasis by MRI scan. J Fla Med Assoc. 1990;77:881–3. [PubMed] [Google Scholar]
- 6.Grankvist J, Fisker R, Iyer V, Fründ ET, Simonsen C, Christensen T, et al. MRI and PET/CT of patients with bone metastases from breast carcinoma. Eur J Radiol. 2012;81:e13–8. doi: 10.1016/j.ejrad.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Boissonnault W, Goodman C. Physical therapists as diagnosticians: drawing the line on diagnosing pathology. J Orthop Sports Phys Ther. 2006;36:351–3. doi: 10.2519/jospt.2006.0107. [DOI] [PubMed] [Google Scholar]
- 8.Ross MD, Boissonnault WG. Red flags: to screen or not to screen? J Orthop Sports Phys Ther. 2010;40:682–4. doi: 10.2519/jospt.2010.0109. [DOI] [PubMed] [Google Scholar]
- 9.Childs JD, Piva SR, Fritz JM. Responsiveness of the Numeric Pain Rating Scale in patients with low back pain. Spine. 2005;30:1331–4. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 10.Davidson M. Rasch analysis of three versions of the Oswestry Disability Questionnaire. Man Ther. 2008;13:222–31. doi: 10.1016/j.math.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Stanton TR, Fritz JM, Hancock MJ, Latimer J, Maher CG, Wand BM, et al. Evaluation of a treatment-based classification algorithm for low back pain: a cross-sectional study. Phys Ther. 2011;91:496–509. doi: 10.2522/ptj.20100272. [DOI] [PubMed] [Google Scholar]
- 12.Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290–302. doi: 10.2519/jospt.2007.2498. [DOI] [PubMed] [Google Scholar]
- 13.Browder DA, Childs JD, Cleland JA, Fritz JM. Effectiveness of an extension-oriented treatment approach in a subgroup of subjects with low back pain: a randomized clinical trial. Phys Ther. 2007;87:1608–18. doi: 10.2522/ptj.20060297. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 15.Sutlive TG, Mabry LM, Easterling EJ, Durbin JD, Hanson SL, Wainner RS, et al. Comparison of short-term response to two spinal manipulation techniques for patients with low back pain in a military beneficiary population. Mil Med. 2009;174:750–6. doi: 10.7205/milmed-d-02-4908. [DOI] [PubMed] [Google Scholar]
- 16.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 17.Lote K, Walloe A, Bjersand A. Bone metastasis. Prognosis, diagnosis and treatment. Acta Radiol Oncol. 1986;25:227–32. doi: 10.3109/02841868609136410. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509–24. doi: 10.1200/JCO.1991.9.3.509. [DOI] [PubMed] [Google Scholar]
- 19.Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine. 1985;10:19–20. doi: 10.1097/00007632-198501000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthy GT, Tubis M, Hiss J, Blahd WH. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA. 1977;237:2504–6. [PubMed] [Google Scholar]
- 21.Qureshi A, Shams U, Akhter A, Riaz S. Metastatic bone disease as seen in our clinical practice–experience at a tertiary care cancer center in Pakistan. Asian Pac J Cancer Prev. 2012;13:4369–71. doi: 10.7314/apjcp.2012.13.9.4369. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BA.Natural history, prognosis, and staging of bone metastases. In: Stoll BA, Parbhoo S, editors. Bone metastases: monitoring and treatment. New York, NY, USA: Raven; 1983. p. 1–20 [Google Scholar]
- 23.Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56:863–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92:1959–70. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radzikowska E, Roszkowski-Śliż K, Głaz P. The impact of timeliness of care on survival in non-small cell lung cancer patients. Pneumonol Alergol Pol. 2012;80:422–9. [PubMed] [Google Scholar]
- 26.Macdonald S, Macleod U, Campbell NC, Weller D, Mitchell E. Systematic review of factors influencing patient and practitioner delay in diagnosis of upper gastrointestinal cancer. Br J Cancer. 2006;94:1272–80. doi: 10.1038/sj.bjc.6603089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98:60–70. doi: 10.1038/sj.bjc.6604096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15:1–4. [PubMed] [Google Scholar]
- 29.Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung cancer and their prognostic implications. J Thorac Oncol. 2011;6:1254–9. doi: 10.1097/JTO.0b013e318217b623. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. The symptom-to-diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J Surg Oncol. 2011;104:771–5. doi: 10.1002/jso.22006. [DOI] [PubMed] [Google Scholar]
- 31.Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86:204–6. doi: 10.1177/030089160008600305. [DOI] [PubMed] [Google Scholar]
- 32.Chunlestskul K, Carlson LE, Koopmans JP, Angen M. Lived experiences of Canadian women with metastatic breast cancer in preparation for their death: a qualitative study. Part I–preparations and consequences. J Palliat Care. 2008;24:5–15. [PubMed] [Google Scholar]
- 33.Miyashita M, Morita T, Sato K, Hirai K, Shima Y, Uchitomi Y. Good death inventory: a measure for evaluating good death from the bereaved family member's perspective. J Pain Symptom Manage. 2008;35:486–98. doi: 10.1016/j.jpainsymman.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos A, Guida F, Cénée S, Cyr D, Schmaus A, Radoï L, et al. Cigarette smoking and lung cancer in women: results of the French ICARE case-control study. Lung Cancer. 2011;74:369–77. doi: 10.1016/j.lungcan.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Xu H, Zhang C, Kong Y, Hou Y, Xu Y, et al. Combined effects of cigarette smoking, gene polymorphisms and methylations of tumor suppressor genes on non small cell lung cancer: a hospital-based case-control study in China. BMC Cancer. 2010;10:422. doi: 10.1186/1471-2407-10-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poullis M, McShane J, Shaw M, Shackcloth M, Page R, Mediratta N, et al. Smoking status at diagnosis and histology type as determinants of long-term outcomes of lung cancer patients. Eur J Cardiothorac Surg. 2013;43:919–24. doi: 10.1093/ejcts/ezs464. [DOI] [PubMed] [Google Scholar]
- 37.Peto J. That the effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer. 2012;107:406–7. doi: 10.1038/bjc.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Matteis S, Consonni D, Pesatori AC, Bergen AW, Bertazzi PA, Caporaso NE, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol. 2013;177:601–12. doi: 10.1093/aje/kws445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst. 2011;103:810–6. doi: 10.1093/jnci/djr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CL, Daniels B, Singh D, Luty S, Molinaro A. Prevalence and clinical importance of alternative causes of symptoms using a renal colic computed tomography protocol in patients with flank or back pain and absence of pyuria. Acad Emerg Med. 2013;20:470–8. doi: 10.1111/acem.12127. [DOI] [PubMed] [Google Scholar]
- 41.Shephard E, Neal R, Rose P, Walter F, Hamilton WT. Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract. 2013;63:e250–5. doi: 10.3399/bjgp13X665215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakanishi H, Araki N, Kuratsu S, Narahara H, Ishikawa O, Yoshikawa H. Skeletal metastasis in patients with gastric cancer. Clin Orthop Relat Res. 2004;423:208–12. doi: 10.1097/01.blo.0000129159.65684.b3. [DOI] [PubMed] [Google Scholar]
- 43.Patel U, Skingle S, Campbell GA, Crisp AJ, Boyle IT. Clinical profile of acute vertebral compression fractures in osteoporosis. Br J Rheumatol. 1991;30:418–21. doi: 10.1093/rheumatology/30.6.418. [DOI] [PubMed] [Google Scholar]
- 44.Burton AW, Mendoza T, Gebhardt R, Hamid B, Nouri K, Perez-Toro M, et al. Vertebral compression fracture treatment with vertebroplasty and kyphoplasty: experience in 407 patients with 1,156 fractures in a tertiary cancer center. Pain Med. 2011;12:1750–7. doi: 10.1111/j.1526-4637.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- 45.Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer. 1975;36:590–4. doi: 10.1002/1097-0142(197508)36:2<590::aid-cncr2820360240>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 46.Chen YJ, Chang GC, Chen HT, Yang TY, Kuo BI, Hsu HC, et al. Surgical results of metastatic spinal cord compression secondary to non-small cell lung cancer. Spine (Phila Pa 1976). 2007;32:E413–8. doi: 10.1097/BRS.0b013e318074d6c7. [DOI] [PubMed] [Google Scholar]
- 47.Walcott BP, Cvetanovich GL, Barnard ZR, Nahed BV, Kahle KT, Curry WT. Surgical treatment and outcomes of metastatic breast cancer to the spine. J Clin Neurosci. 2011;18:1336–9. doi: 10.1016/j.jocn.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011:107969. doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman JM, Ash CM, Souhami RL, Geddes DM, Harper PG, Spiro SG, et al. Spinal cord compression in small cell lung cancer: a retrospective study of 610 patients. Br J Cancer. 1989;59:591–3. doi: 10.1038/bjc.1989.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albert TJ, Balderston RA, Heller JG, Herkowitz HN, Garfin SR, Tomany K, et al. Upper lumbar disc herniations. J Spinal Disord. 1993;6:351–9. doi: 10.1097/00002517-199306040-00009. [DOI] [PubMed] [Google Scholar]
- 51.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–8. [PubMed] [Google Scholar]
- 52.Amundsen T, Weber H, Lilleås F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976). 1995;20:1178–86. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 53.Hall S, Bartleson JD, Onofrio BM, Baker HL, Jr, Okazaki H, O'Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103:271–5. doi: 10.7326/0003-4819-103-2-271. [DOI] [PubMed] [Google Scholar]
- 54.Hsu K, Zucherman J, Shea W, Kaiser J, White A, Schofferman J, et al. High lumbar disc degeneration. Incidence and etiology. Spine (Phila Pa 1976). 1990;15:679–82. doi: 10.1097/00007632-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Al-Saeed O, Al-Jarallah K, Raeess M, Sheikh M, Ismail M, Athyal R. Magnetic resonance imaging of the lumbar spine in young Arabs with low back pain. Asian Spine J. 2012;6:249–56. doi: 10.4184/asj.2012.6.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iguchi T, Wakami T, Kurihara A, Kasahara K, Yoshiya S, Nishida K. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech. 2002;15:93–9. doi: 10.1097/00024720-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Cyriax JH.Illustrated Manual of Orthopaedic Medicine. London: Butterworths; 1989 [Google Scholar]
- 58.Sizer PS, Jr, Brismée JM, Cook C. Medical screening for red flags in the diagnosis and management of musculoskeletal spine pain. Pain Pract. 2007;7:53–71. doi: 10.1111/j.1533-2500.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 59.Smart KM, Blake C, Staines A, Thacker M, Doody C. Mechanisms-based classifications of musculoskeletal pain: part 3 of 3: symptoms and signs of nociceptive pain in patients with low back (±leg) pain. Man Ther. 2012;17:352–7. doi: 10.1016/j.math.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Boissonnault WG, Bass C. Pathological origins of trunk and neck pain: part III – diseases of the musculoskeletal system. J Orthop Sports Phys Ther. 1990;12:216–21. doi: 10.2519/jospt.1990.12.5.216. [DOI] [PubMed] [Google Scholar]
- 61.Ross MD, Bayer E. Cancer as a cause of low back pain in a patient seen in a direct access physical therapy setting. J Orthop Sports Phys Ther. 2005;35:651–8. doi: 10.2519/jospt.2005.35.10.651. [DOI] [PubMed] [Google Scholar]
- 62.Axen I, Rosenbaum A, Robech R, Wren T, Leboeuf-Yde C. Can patient reactions to the first chiropractic treatment predict early favorable treatment outcome in persistent low back pain? J Manipulative Physiol Ther. 2002;25:450–4. doi: 10.1067/mmt.2002.126473. [DOI] [PubMed] [Google Scholar]
- 63.Hahne AJ, Keating JL, Wilson SC. Do within-session changes in pain intensity and range of motion predict between-session changes in patients with low back pain? Aust J Physiother. 2004;50:17–23. doi: 10.1016/s0004-9514(14)60244-0. [DOI] [PubMed] [Google Scholar]
- 64.Bell RA, Kravitz RL, Thom D, Krupat E, Azari R. Unmet expectations for care and the patient-physician relationship. J Gen Intern Med. 2002;17:817–24. doi: 10.1046/j.1525-1497.2002.10319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thom DH, Kravitz RL, Bell RA, Krupat E, Azari R. Patient trust in the physician: relationship to patient requests. Fam Pract. 2002;19:476–83. doi: 10.1093/fampra/19.5.476. [DOI] [PubMed] [Google Scholar]


