Abstract
Brain parenchymal arterioles (PAs) are high-resistance vessels that branch off pial arteries and perfuse the brain parenchyma. PAs are the target of cerebral small vessel disease and have been shown to have greater pressure-induced tone at lower pressures than pial arteries. We investigated mechanisms by which brain PAs have increased myogenic tone compared with middle cerebral arteries (MCAs), focusing on differences in vascular smooth muscle (VSM) calcium and ion channel function. The amount of myogenic tone and VSM calcium was measured using Fura 2 in isolated and pressurized PAs and MCAs. Increases in intraluminal pressure caused larger increases in tone and cytosolic calcium in PAs compared with MCAs. At 50 mmHg, myogenic tone was 37 ± 5% for PAs vs. 6.5 ± 4% for MCAs (P < 0.01), and VSM calcium was 200 ± 20 nmol/l in PAs vs. 104 ± 15 nmol/l in MCAs (P < 0.01). In vessels permeabilized with Staphylococcus aureus α-toxin, PAs were not more sensitive to calcium, suggesting calcium sensitization was not at the level of the contractile apparatus. PAs were 30-fold more sensitive to the voltage-dependent calcium channel (VDCC) inhibitor nifedipine than MCAs (EC50 for PAs was 3.5 ± 0.4 vs. 82.1 ± 2.1 nmol/l for MCAs;P < 0.01); however, electrophysiological properties of the VDCC were not different in VSM. PAs had little to no response to the calcium-activated potassium channel inhibitor iberiotoxin, whereas MCAs constricted ∼15%. Thus increased myogenic tone in PAs appears related to differences in ion channel activity that promotes VSM membrane depolarization but not to a direct sensitization of the contractile apparatus to calcium.
Keywords: brain arterioles, calcium sensitivity, myogenic tone, smooth muscle
the myogenic response, described over a century ago by Bayliss (1), is the intrinsic property of vascular smooth muscle (VSM) to contract in response to increased pressure or stretch and relax in response to decreased pressure or stretch, thus allowing for relatively constant blood flow in the face of changing perfusion pressures (15). In the brain, the myogenic response is uniquely pronounced in both large cerebral arteries and small arterioles and is vital for autoregulation of cerebral blood flow (CBF) (10, 25). The resulting pressure-induced myogenic tone—or the amount of tone at a given pressure—importantly contributes to cerebrovascular resistance (CVR) that protects downstream capillaries from high arterial pressure (1, 10, 15, 25). In addition, because of the high metabolic demand of neuronal tissue that requires tight regulation of CBF and the need to limit hydrostatic pressure on the microcirculation to protect against vasogenic edema, myogenic tone is an important protective mechanism in the cerebral circulation that contributes to brain homeostasis (23).
Unlike most organs, in which only small arterioles comprising the microcirculation contribute to vascular resistance, the cerebral circulation has segmental vascular resistance in which large pial arteries possess significant myogenic tone and contribute ∼50% to total CVR (10). This unique arrangement allows for local changes in CBF while importantly maintaining CVR. Pial arteries on the surface of the brain give rise to smaller arteries that penetrate into the brain tissue to eventually become parenchymal arterioles (PAs) (14). PAs are long and relatively unbranched vessels in the brain that supply the cortex and striatum and directly connect the pial arteries to the capillaries. Although both are important for CBF regulation, there are significant differences between pial arteries and PAs. For example, extrinsic innervation of pial arteries is progressively lost upon entering the brain tissue where the PAs become intrinsically innervated from within the neuropil (4, 14). Likely because of this, PAs are unresponsive to some neurotransmitters (e.g., serotonin and norepinephrine) (4). In addition, PAs have greater myogenic tone at lower pressures compared with pial arteries (4, 5) due to smooth muscle that is more depolarized (16, 21) and large-conductance calcium-activated potassium channels (BKCa) that are uncoupled from ryanodine receptors and calcium sparks (7). Furthermore, under pathologic conditions such as ischemic stroke, pial arteries including the middle cerebral artery (MCA) have progressive loss of myogenic tone, whereas PAs have increased tone (6). The pronounced and persistent myogenic tone of PAs during pathologic conditions when other vessels dilate, makes them the “bottleneck” to flow in the cerebral cortex (20) and an important therapeutic target for conditions such as ischemic stroke, hypertension, and subarachnoid hemorrhage (3, 5, 6, 21).
VSM calcium has a central role in the myogenic response (16). Membrane depolarization in response to mechanosensitive channels [e.g., transient receptor potential (TRP) channels] activates L-type voltage-dependent calcium channels (VDCC), causing influx of calcium and vasoconstriction (9, 16). In cerebral pial arteries, there is a concomitant negative feedback through opening of BKCa channels in VSM that leads to cell hyperpolarization and dilation that counteracts the myogenic vasoconstriction (2). Although transmembrane calcium influx is essential for VSM contraction in response to pressure or stretch, changes in calcium sensitivity of the VSM contractile apparatus are also involved in the myogenic response (22, 27). However, the majority of studies on the role of calcium sensitivity in the myogenic response have been done on cerebral pial arteries and relatively little is known about the contribution of VDCCs vs. calcium sensitization to myogenic tone in PAs. In the present study, we investigated the relationship between myogenic tone and calcium in MCAs compared with PAs at different intravascular pressures. We used intact pressurized vessels to measure the relationship between VSM calcium and tone using Fura 2 as well as S. aureus α-toxin permeabilized vessels to measure direct calcium sensitization of the contractile apparatus. In addition, we investigated the difference in VDCC and BKCa channel activity between PAs and MCAs as the activity of these channels directly affects VSM calcium and the level of myogenic tone.
MATERIALS AND METHODS
Animals and preparation of arteries and arterioles.
Male Wistar rats weighing 350–380 g were used for all experiments. All animal procedures were approved by the University of Vermont Institutional Animal Care and Use Committee and complied with the National Institutes of Health guidelines for the care and use of laboratory animals. Rats were housed in the Animal Care Facility at the University of Vermont, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility, and were allowed food and water ad libitum. Animals were quickly decapitated under isoflurane anesthesia, and the brains were removed and placed in cold, oxygenated physiological saline solution (PSS). PAs that branched off the MCA between the M1 and M2 region within the brain parenchyma, or MCAs, were carefully dissected and mounted on glass cannulas within an arteriograph chamber, as previously described (5). Because of their different anatomic location, blinding was not possible. All vessels had intact endothelium. PAs were studied within the pressure range of 10 to 80 mmHg, whereas MCAs were studied within the pressure range of 10 to 100 mmHg. The different pressures at which PAs and MCAs were studied were chosen based on the pressure at which they operate in vivo being ∼40 mmHg for PAs and ∼75 mmHg for MCAs (10).
Preparation of isolated arteries and arterioles and measurement of VSM calcium using Fura 2.
To investigate the relationship between myogenic tone and calcium in the two different vascular segments, the calcium-sensitive dye, Fura 2-AM was used. Fura 2-AM (1 mmol/l stock dissolved in anhydrous dimethylsulfoxide, DMSO) was premixed with an equal volume of 25% pluronic acid dissolved in DMSO and then diluted in aerated PSS to yield a final concentration of 5 μmol/l. Each arterial segment (MCA and PA) was cannulated in an arteriograph, pressurized to 10 mmHg, and equilibrated at 37°C for 10–15 min. The arterial segment was then incubated in the Fura 2-AM/PSS loading solution at room temperature in the dark for 60 min. This solution was changed for freshly aerated loading solution after 30 min into the incubation period to maintain pH = 7.4. Fura 2-loaded arteries were washed two to three times with PSS and then continuously superfused at 3 ml/min with oxygenated PSS (10% O2-5% CO2-balanced N2) at 37°C. All experimental protocols were started after an additional 15-min equilibration period at 10 mmHg to allow intracellular de-esterification of Fura 2-AM. Fura 2 fluorescence was measured using a photomultiplier system (IonOptix) in which background-corrected ratios of 510-nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm.
Arterial diameter and calcium were simultaneously recorded using IonWizard software (IonOptix). Briefly, myogenic activity was evaluated by stepwise increases in pressure from 10 to 80 mmHg for PAs (n = 5) or 10 to 100 mmHg for MCAs (n = 5), and Fura 2 fluorescence was measured. At the conclusion of the experiment, diltiazem (10 μmol/l) in calcium-free PSS was added to obtain fully relaxed diameters.
Measurement of calcium sensitivity in permeabilized vessels using Staphylococcus aureus α-toxin.
In separate sets of PAs (n = 6) and MCAs (n = 5) the sensitivity of the contractile apparatus to calcium was compared between MCAs and PAs by permeabilizing the myocyte membrane with S. aureus α-toxin and measuring the contractile response to addition of calcium, as previously described (12). Briefly, blood vessel segments were carefully dissected and mounted in an arteriograph filled with HEPES-buffered PSS, pressurized to 40 mmHg for PAs or 75 mmHg for MCAs and equilibrated for 30 min. Vessel segments were permeabilized with S. aureus α-toxin (800 U/ml) in relaxing solution at room temperature for 20 min. The S. aureus α-toxin was then washed from the bath, and vessels were equilibrated in relaxing solution at 37°C for 30 min. The vasoactive response to calcium was determined by replacing relaxing solution with activating solution containing known concentrations of free ionic calcium (pCa or −log [Ca]: 7.0–6.0). For each concentration of calcium, the inner diameters were recorded once stable (5–7 min).
Measurement of electrophysiological properties of PA and MCA myocytes.
Single VDCC activity was measured as described previously (19). PAs (n = 4) and MCAs (n = 5) were isolated as described above and placed in a cell isolation solution (CIS) containing (in mmol/l): 60 NaCl, 80 Na-glutamate, 5 KCl, 2 MgCl2, 10 glucose, and 10 HEPES (pH 7.2). The arterial segments were then incubated in warmed (37°C) CIS containing 1 mg/ml papain (Worthington, Lakewood, NJ), 1 mg/ml dithioerythritol, and 0.1 mM CaCl2 for 6–8 min, followed by incubation in CIS (37°C) containing 0.7 mg/ml type F collagenase (Sigma-Aldrich, St. Louis, MO), 0.3 mg/ml type H collagenase (Sigma-Aldrich), and 0.1 mmol/l CaCl2 for 8–10 min. The digested segments were then washed three times in ice-cold CIS and triturated to release smooth muscle cells. Cells were stored on ice in CIS for use the same day. Single-channel currents were recorded in the on-cell patch clamp configuration using an Axopatch 200B amplifier equipped with a CV203BU headstage (Axon Instruments, Sunnyvale, CA). Recording electrodes (resistance, 6–10 MΩ) were pulled from borosilicate glass (1.5 mm OD, 1.17 mm ID; Sutter Instrument, Novato, CA) and coated with wax to reduce capacitance. Currents were filtered at 0.1 kHz, digitized at 1 kHz, and stored for subsequent analysis. pCLAMP version 9.2 and Clampfit version 9.2 (Axon Instruments) were used for data acquisition and analysis. Patches were initially held at a membrane potential of −70 mV, and single channel currents were recorded during 500-ms steps to membrane potentials between −40 and +40 mV. All recordings were performed at room temperature (22°C). For channel recording, the bath solution contained (in mmol/l): 145 KCl, 10 HEPES (pH 7.3), 10 TEA-Cl, 12.5 glucose, 5 EGTA, and 0.0005 Bay K8644 (Sigma-Aldrich). The pipette solution contained (in mmol/l): 40 BaCl2, 100 TEA-Cl, 10 HEPES (pH 7.3), 5 4-amino pyridine, and 12.5 glucose.
Sensitivity of intact MCAs and PAs to nifedipine and iberiotoxin.
In a separate set of PAs (n = 6) and MCAs (n = 6) that were intact (not permeabilized) and not loaded with Fura 2, the sensitivity to the VDCC inhibitor nifedipine was determined. Briefly, PAs and MCAs were equilibrated for 1 h at 40 and 75 mmHg, respectively. Because of the difference in basal tone between these vessel types, MCAs were precontracted with serotonin (∼10−7 mol/l) to the same level of constriction as PAs. Nifedipine was cumulatively added to the bath (0.001 to 1.0 μmol/l), and the diameters were recorded at each concentration. In a separate group of PAs (n = 6) and MCAs (n = 8), a single concentration of the BKCa channel inhibitor iberiotoxin (0.1 μmol/l) was given and the change in diameter recorded.
Drugs and solutions.
All isolated vessel experiments, except calcium sensitivity measurements in S. aureus α-toxin, were performed using a bicarbonate-based Ringer PSS, the ionic composition of which was (in mmol/l): 119.0 NaCl, 24.0 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4 × 7 H2O, 1.6 CaCl2, 0.26 EDTA, and 5.5 glucose. PSS was made each week and stored without glucose at 4°C. Glucose was added to the PSS prior to each experiment. PSS was aerated with 5% CO2, 10% O2, and 85% N2 to maintain pH.
For the Fura 2 calibration procedure, the following solutions were used. Calcium-free calibration solution was composed of (in mmol/l): 140.0 KCl, 20.0 NaCl, 5.0 HEPES, 5.0 EGTA, 1.0 MgCl2, 5 μmol/l nigericin, and 10 μmol/l ionomycin. Calcium-containing calibration solution was composed of (mmol/l): 140.0 KCl, 20.0 NaCl, 5.0 HEPES, 1.0 MgCl2, and 10.0 CaCl2. Both solutions were adjusted to pH 7.15 at 37°C with KOH.
For studying calcium sensitization in permeabilized vessels, the following solutions were used. HEPES-buffered PSS contained (in mmol/l): 142.0 NaCl, 4.7 KCl, 1.17 MgSO4, 0.5 EDTA, 2.79 CaCl2, 10.0 HEPES, 1.2 KH2PO4, 5.5 glucose. pH was adjusted by 10 N NaOH to 7.4 at 37°C. Relaxing solution contained (in mmol/l): 63.6 potassium methanesulfonate, 2.0 MgCl2, 4.5 Mg-ATP, 2.0 EGTA, 10.0 phosphocreatine, and 30.0 piperazine-N,N′-bis(2-ethanesulfonic acid). Relaxing solution also contained 1.0 μmol/l carbonylcyanide p-trifluromethoxyphenyl-hydrazone, a mitochondrial blocker, and 1.0 μmol/l leupeptin, a protease inhibitor. pH was adjusted to 7.1 with 8 N KOH. The composition of the activating solution was similar to that of the relaxing solution, except it contained 10 mmol/l EGTA and 10 μmol/l GTP. The amount of CaCl2 needed to yield the desired free ionic concentration of calcium in the activating solution was calculated by a web-based program Webmaxc Standard (http://www.stanford.edu/∼cpatton/webmaxcS.htm). Ionic strength was kept at 200 mmol/l by adjusting the concentration of potassium methanesulfonate.
For reagents, ionomycin and nigericin were purchased from A.G. Scientific (San Diego, CA). Fura 2-AM and pluronic acid were purchased from Life Technologies (Grand Island, NY). Fura 2-AM was dissolved in dehydrated DMSO as a 1 mmol/l stock solution and frozen at −20°C until use. S. aureus α-toxin was purchased from Calbiochem (La Jolla, CA) and aliquoted in relaxing buffer and stored at −20°C until use. All other chemicals were purchased from Sigma (St Louis, MO).
Data calculations.
Myogenic tone was calculated as a percent decrease in diameter from the fully relaxed diameter in calcium-free PSS with diltiazem by the equation: [1 − (φtone/φzero)]*100%, where φtone is inner diameter of vessel with tone and φzero is inner diameter in calcium-free PSS with diltiazem. Percent change in diameter was calculated from baseline by the equation: [1 − (φdrug/φbaseline)]*100%, where φdrug is inner diameter of vessel in iberiotoxin and φbaseline in inner diameter prior to giving the drug. Percent dilation to nifedipine was calculated from the equation: [(φnifed − φstart)/(φmaximum − φstart)]*100%, where φnifed is the inner diameter at a specific concentration of nifedipine, φstart is diameter prior to giving the first concentration of nifedipine, and φmaximum is the inner diameter at the highest concentration of nifedipine. Percent constriction to calcium in permeabilized vessels was calculated from the equation: [(φstart − φcalcium)/(φmaximum − φstart)]*100%, where φcalcium is the inner diameter at a specific concentration of calcium, φstart is diameter prior to giving the first concentration of calcium, and φmaximum is the inner diameter at the highest concentration of calcium. Wall tension for each vessel was calculated as pressure*radius after converting diameter in micrometer to radius in centimeter and pressure in millimeters Hg to dynes centimeter.
Smooth muscle concentration of intracellular calcium ([Ca2+]i) in intact vessels was calculated using the following equation: [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where Kd is the dissociation constant of Fura 2, R is experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is the ratio in the absence of [Ca2+]i, Rmax is the ratio at Ca2+ saturation, and β is the ratio of the fluorescence intensities at 380-nm excitation wavelength at Rmin and Rmax. Rmin, Rmax, and β were determined by an in situ calibration procedure using PAs and MCAs treated with nigericin (5 μmol/l) and ionomycin (10 μmol/l). These values were then used to convert the ratio values into a [Ca2+]i. This calibration procedure using nigericin and ionomycin resulted in β values for PAs and MCAs of 4.43 ± 0.28 and 4.41 ± 0.45, respectively. The Rmin and Rmax values for PAs was 0.55 ± 0.02 and 4.12 ± 0.26 and for MCAs was 0.47 ± 0.02 and 4.57 ± 0.54. The Kd was 282 nmol/l, as determined by using in situ titration of Ca2+ in Fura 2-loaded posterior cerebral arteries (16). Arterial diameter, pressure, and ratio values were recorded using an Ion Wizard data acquisition program.
Statistical analysis.
All data are presented as mean ± SE. To compare responses to pressure between PAs and MCAs, individual curves of % tone and calcium vs. pressure were fit with an exponential and values extrapolated and averaged at 50 mmHg. Student's t-test was used to compare differences between MCAs and PAs.
RESULTS
Relationship between myogenic tone, intravascular pressure, and VSM calcium in PAs and MCAs.
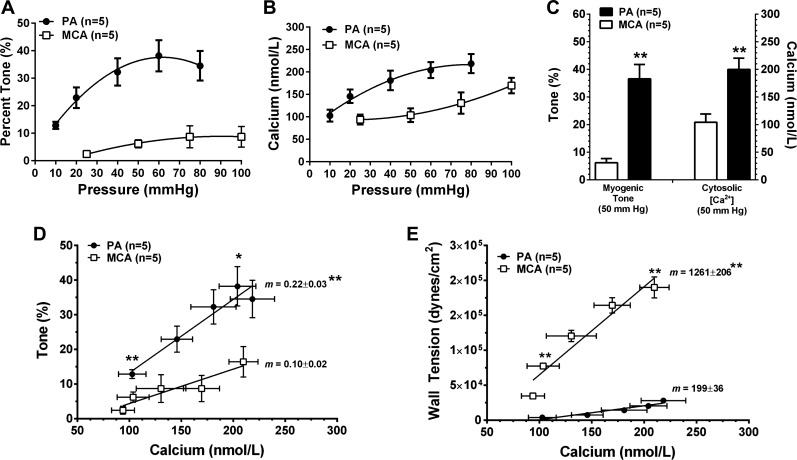
We previously showed that PAs have greater pressure-induced tone compared with upstream MCAs (4, 5); however, the underlying mechanisms by which this occurs are not completely understood. To investigate the relationship between tone and VSM calcium, Fura 2 was used. Figure 1A shows that the percent of pressure-induced tone was increased compared with MCAs. Figure 1B shows that the increase in myogenic tone in PAs was associated with increased VSM calcium. When percent tone and calcium were directly compared at 50 mmHg between both vessel types (Fig. 1C), PAs were significantly more sensitive to pressure than MCAs and responded to the same pressure with increased tone and calcium. Figure 1D compares the relationships between % tone and calcium in PAs and MCAs. Tone was significantly increased in PAs for the same level of calcium as MCAs, suggesting PAs are more sensitive to calcium as well as pressure. Because of the large difference in size between these vessels, we also considered the relationship between circumferential wall tension and calcium in these two segments. Figure 1E shows that wall tension was significantly increased in MCAs for the same level of calcium compared with PAs. Thus the increased tone in PAs may be partially explained by the considerably lower wall tension that the smooth muscle needs to constrict against to change diameter.
Fig. 1.
Relationship between myogenic tone, intravascular pressure and vascular smooth muscle (VSM) calcium in parenchymal arterioles (PAs) and middle cerebral arteries (MCAs). Graphs showing the amount of pressure-induced myogenic tone (A) and calcium (B) in PAs and MCAs, respectively. Amount of myogenic tone and calcium were increased in PAs compared with MCAs at all pressures studied. When % tone and calcium were compared at 50 mmHg, PAs were significantly more sensitive to pressure such that both tone and calcium were increased (C). When % tone was graphed as a function of calcium, PAs had greater tone at the same level of calcium compared with MCAs (D). In addition, the slope of these curves was significantly greater in PAs, suggesting an enhanced relationship between pressure, calcium, and tone in PAs compared with MCAs. E: relationship between calcium and wall tension in both vessel types. At similar levels of calcium, the wall tension of PAs was considerably less than MCAs due to their smaller size. Thus the increased tone in PAs could be partially due to their lower wall tension that would make it easier to constrict. *P < 0.05 vs. MCAs; **P < 0.01 vs. MCAs by Student's t-test.
Calcium sensitivity in S. aureus α-toxin permeabilized PAs and MCAs.
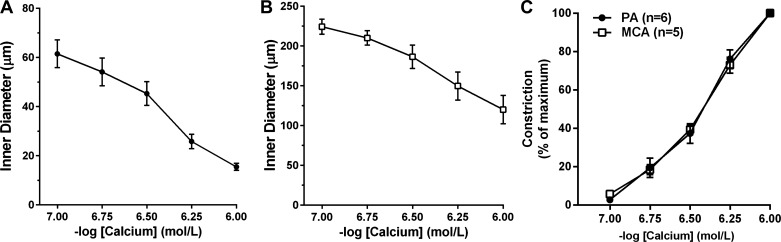
The results above suggest that increased tone of PAs may partly be due to an increase in calcium sensitivity compared with MCAs. However, because calcium was measured in vessels with intact plasma membranes in which activity of ion channels (e.g., VDCC and BKCa) could influence the level of tone, we also determined whether there was an increase in the sensitivity of the contractile apparatus to calcium in PAs. To do this, vessels were permeabilized using S. aureus α-toxin to eliminate the influence of plasma membrane ion channels and differences in membrane potential. Figure 2, A and B, shows that both PAs and MCAs constricted in response to the addition of calcium, as expected in permeabilized preparations, causing a decrease in diameter from 62 ± 6 to 16 ± 1 μm in PAs and from 224 ± 10 to 120 ± 10 μm in MCAs. One MCA was excluded because it did not respond to calcium and thus was not likely permeabilized. Figure 2C shows the sensitivity to calcium in permeabilized PAs and MCAs. Percent sensitivity is a normalized parameter that takes into account the change in diameter in response to the maximum level of calcium for each vessel. There was no difference in sensitivity to calcium between PAs and MCAs under these conditions.
Fig. 2.
Sensitivity of PA and MCA VSM to calcium in S. aureus α-toxin permeabilized vessels. A and B: graphs showing constriction of permeabilized PAs (●) and MCAs (◻), respectively, to increasing concentrations of calcium. Both vessel types constricted in response to increased extracellular calcium. C: graph showing the sensitivity to calcium of PAs and MCAs. There was no difference in the sensitivity of the contractile apparatus to calcium.
Sensitivity to nifedipine and electrophysiological properties of VDCC from PAs and MCAs.
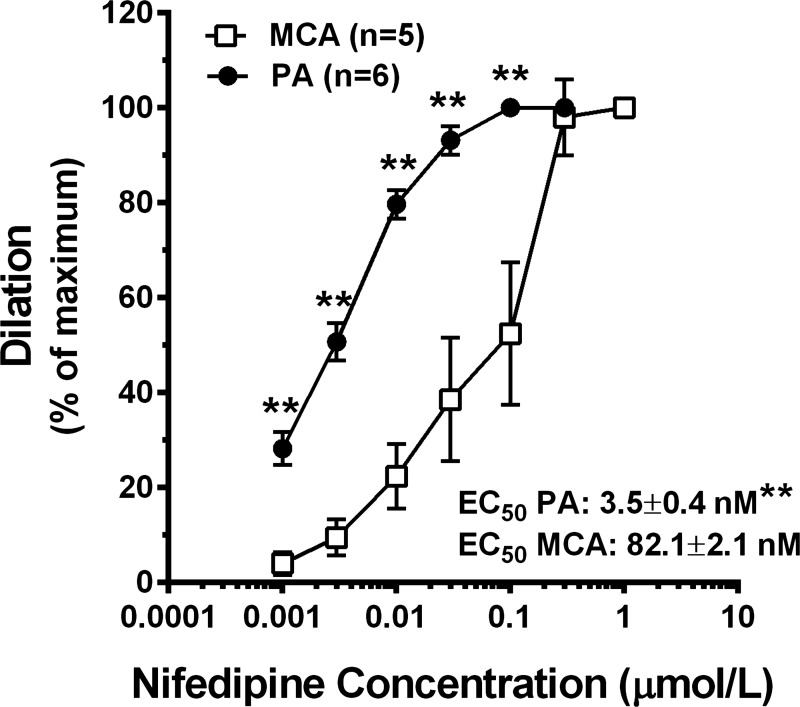
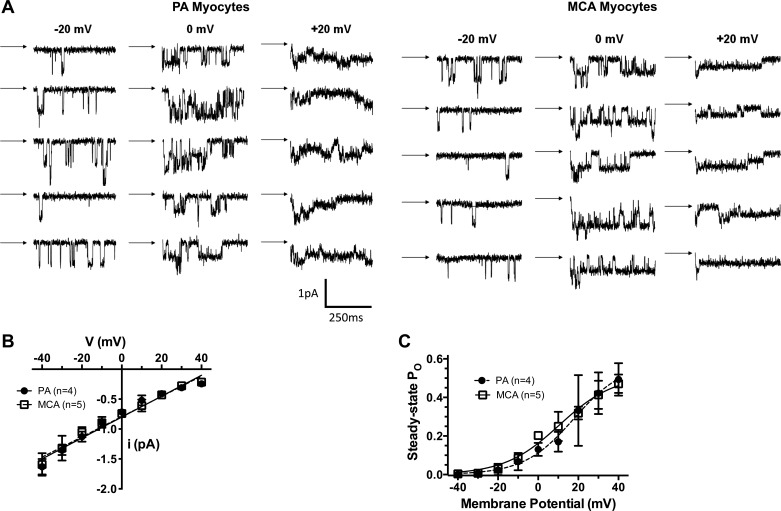
It is well-established that pressure-induced vasoconstriction involves VSM membrane depolarization that opens VDCC (6). It is therefore possible that differences in VDCC activity contribute to the increase in tone in PAs compared with MCAs. We therefore compared sensitivity of PAs and MCAs to nifedipine, a selective VDCC blocker. Figure 3 shows the concentration-response of PAs and MCAs to nifedipine at 40 and 75 mmHg, respectively. PAs were significantly more sensitive to VDCC inhibition than MCAs. The EC50 values for PAs demonstrated an ∼30-fold increase in sensitivity of PAs vs. MCAs at these pressures. To determine whether the increased sensitivity of PAs to nifedipine was due to myocytes that contained VDCC that were more active at a given membrane potential, the single channel properties of VDCC were determined in myocytes isolated from PAs and MCAs. Figure 4 shows that there were no differences in single channel properties or open state probability between myocytes from the two types of vessels. In fact, the electrophysiological properties of myocytes from the different vessels were remarkably similar.
Fig. 3.
Graph showing sensitivity of PAs and MCAs to nifedipine. Both vessel types dilated significantly to nifedipine, however, PAs were significantly more sensitive to VDCC inhibition. The concentration-response curve was performed at 40 mmHg for PAs and 75 mmHg for MCAs. **P < 0.05 vs. MCAs by Student's t-test.
Fig. 4.
Electrophysiological properties of the VDCC in PA and MCA myocytes. A: representative tracings of single channel recordings from PAs and MCAs at different voltages. B: graph showing current-voltage (I-V) relationships of single voltage-dependent calcium channels in myocytes from PAs and MCAs. There was no difference in the single channel conductances between myocytes from PAs (slope conductance: 17.5 ± 0.7 pS) and MCAs (slope conductance: 16.9 ± 0.7 pS). C: graph showing the relationships between membrane potential and steady-state open-state probably (Po) in myoctyes from PAs and MCAs. Data were fitted using the equation Po = Pmax/[1 + exp (V0.5 − Vm)/k], where Pmax is the maximal Po at positive potentials, V0.5 is the membrane potential where Po is one-half Pmax, and k is the steepness factor, which describes the voltage-sensitivity of the calcium channels. There were no differences in these parameters between the two vessel types (PA Pmax: 0.58 ± 0.13, MCA Pmax: 0.52 ± 0.05; PA V0.5: 16 ± 4 mV, MCA V0.5: 11 ± 2 mV; PA k: 6.8 ± 1.4, MCA k: 12.9 ± 1.1).
Response of PAs and MCAs to BKCa channel inhibition with iberiotoxin.
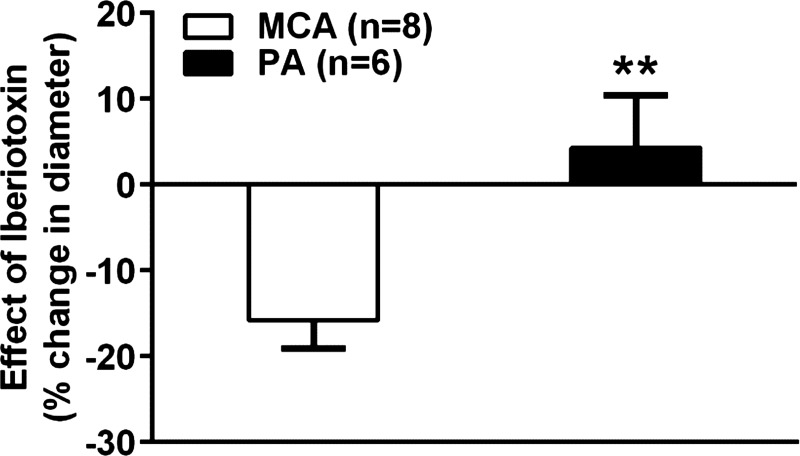
Activity of the BKCa channel has been shown to hyperpolarize VSM and counterbalance the myogenic response by promoting vasodilation (2). A previous study showed that under normal conditions, BKCa channel activity in PAs is low, thus eliminating its hyperpolarizing influence (7) that may contribute to the increased tone in these vessels. We therefore investigated the contribution of BKCa activity to myogenic tone in PAs and MCAs at 40 and 75 mmHg, respectively, by measuring the change in diameter in response to iberiotoxin, a selective inhibitor of BKCa channels. Figure 5 shows that MCAs constricted to ∼15% to iberiotoxin, demonstrating that BKCa channel activity in intact MCAs is present and inhibiting tone. In contrast, PAs had little to no response to iberiotoxin, suggesting this feedback hyperpolarization pathway is limited or absent in PAs, a condition that likely contributes to the increase in myogenic tone in PAs vs. MCAs.
Fig. 5.
Reactivity of PAs and MCAs to iberiotoxin. Graph showing the percent change in diameter to addition of a single concentration of iberiotoxin (0.1 μmol/l) in PAs and MCAs. MCAs constricted to iberiotoxin ∼ 15%, whereas PAs had little to no response. **P < 0.01 vs. MCAs by Student's t-test.
DISCUSSION
In the present study, we show that PAs had increased myogenic tone compared with MCAs that did not appear to involve direct calcium sensitization of the contractile apparatus but was associated with altered ion channel activity in response to pressure. We and others (5, 7, 21) have shown that PAs have altered ion channel function compared with pial arteries such that VSM membrane is more depolarized at lower pressures. In the present study, we found PAs were more sensitive to the dilatory effect of nifedipine, a VDCC blocker. This result is consistent with PAs having VSM with more depolarized membrane potential that causes the VDCC to be more active. It is also possible that VDCCs are more active at a given membrane potential in PAs compared with MCAs. However, we found no difference in the electrophysiological properties of the VDCC in isolated VSM from PAs and MCAs, suggesting this was not the case. This is in contrast to a previous study by Michelakis et al. (18) that found increased VDCC activity in VSM isolated from parenchymal arterioles compared with basilar arteries. The difference between the previous study and the present one may be the different pial arteries that were used—MCA in the present study and basilar in the previous study—as these perfuse different brain regions and may have different smooth muscle properties.
BKCa channel activation, through adjacent calcium spark activity, is an important negative feedback that limits myogenic vasoconstriction through VSM hyperpolarization (2, 17). Our previous study found that although BKCa channels are present in VSM from parenchymal arterioles at similar levels as cerebral pial arteries, few calcium sparks are present in PAs to activate the channel (7). In contrast, the predominant calcium signals under basal conditions in PA VSM are calcium waves that do not activate BKCa channels (7). In the present study, we confirmed this finding using the BKCa channel inhibitor iberiotoxin and showed that MCAs constricted to iberiotoxin ∼15%, demonstrating BKCa channels are active and inhibit tone in those vessels. In contrast, PAs did not constrict to iberiotoxin, demonstrating little to no influence of this channel in inhibiting tone. Thus it is likely that the lack of BKCa coupling to calcium sparks causes greater VSM depolarization in PAs due to the lack of hyperpolarizing influence of this potassium channel. VSM from PAs have been shown to be more depolarized than pial arteries (21) and this may underlie the increased myogenic tone as well as the increased sensitivity to nifedipine in PAs, given the well-recognized voltage-dependence of calcium channel block by dihydropyridines (26).
In addition to a lack of BKCa channel influence that promotes VSM depolarization in PAs, there could also be a difference in the depolarizing stimulus in response to pressure between PAs and MCAs. For example, TRP channels are nonselective cation channels that are thought to contribute to pressure-induced tone through direct calcium influx and depolarization of VSM (8, 9, 13, 24, 28). In particular, our own studies have also shown an important role for TRPM4 in the myogenic response in cerebral pial arteries (8, 9). Activation of TRPM4 currents in arterial myocytes causes membrane depolarization, activation of VDCCs and vasoconstriction (8, 9). The results shown in Fig. 1 suggest that pressure induced a greater increase in calcium and tone in PAs compared with MCAs. It is possible that a combination of decreased BKCa channel influence that promotes VSM depolarization and increased TRP channel activity in response to pressure promotes this pressure sensitization in PAs.
Differences in ion channel activation may not be the only mechanism involved in increased pressure-induced tone in PAs. In the present study, we also measured calcium sensitivity using a permeabilized vessel preparation. We demonstrate that PA VSM had similar calcium sensitivity as MCAs (Fig. 2D). In nonpermeabilized vessels in which the membrane was intact, there does appear to be an increase in calcium sensitivity in PAs. This may be due to differences in wall tension between the two vessel types. According to the law of Laplace, circumferential wall tension is related to the intravascular pressure and the radius of the vessel (11). Because PAs are considerably smaller in diameter, and were studied at lower pressures, they had significantly less wall tension compared with MCAs. Thus force production of VSM required to change diameter would be less in PAs than MCAs. This difference in wall tension between the two vessel types could influence the myogenic vasoconstriction because it would take less force to constrict the smaller PAs. The difference in wall tension could also explain why there appears to be an increase in calcium sensitivity in intact PAs (Fig. 1D), i.e., it required less force production to cause constriction of PAs at the same level of calcium as MCAs, but that the true calcium sensitivity shown in permeabilized vessels was not different. This effect, in combination with an actual increase in cytosolic calcium shown in Fig. 1C could account for the large increase in arterial tone in PAs compared with MCAs at a given pressure. An important experiment that is beyond the scope of this study would be to measure force production in isolated VSM from these different vessels in response to stretch and calcium.
Lastly, it is worth noting that we studied vessels with intact endothelium because we were attempting to understand the cellular mechanisms that regulate the differences in tone under physiological conditions between PAs and MCAs. In the cerebral circulation, the endothelium has considerable influence on tone that appears to be different between PAs and MCAs (5). It is possible that differences in endothelial nitric oxide or endothelium-derived hyperpolarization (EDH) between the two vessel types influence their basal tone. We previously showed that PAs constrict in response to SKCa and IKCa channel inhibition, suggesting that basal EDH, in addition to nitric oxide, inhibits tone in these vessels (5). However, the greater influence of the EDH pathway in PAs would decrease tone compared with MCAs, not increase it as we have shown here.
In summary, the increased myogenic tone of PAs compared with MCAs appears to be due to altered ion channel activity in response to pressure, including decreased BKCa channel influence that promotes VSM membrane depolarization at lower pressures. However, we cannot rule out the possibility that the smaller diameter and lower wall tension in PAs compared with MCAs also enhances myogenic constrictions of PAs at any particular cytosolic Ca2+ concentration. Given that PAs are high resistance vessels in the brain that directly connect the pial vessels to the microcirculation, understanding the mechanism by which these vessels are more constricted at lower pressures than MCAs may provide for novel therapeutic targets under conditions in which PAs are pathologically involved, including lacunar stroke and small vessel disease.
GRANTS
We gratefully acknowledge the support of the National Institute of Neurological Disorders and Stroke Grant RO1 NS045940; the National Heart, Lung, and Blood Institute Grants PO1 HL095488, and R01 HL088245; and the Totman Medical Research Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.C., N.I.G., and J.E.B. conception and design of research; M.J.C., J.G.S., S.-L.C., M.J.T., N.I.G., and J.E.B. analyzed data; M.J.C., S.-L.C., N.I.G., and J.E.B. interpreted results of experiments; M.J.C. and J.E.B. prepared figures; M.J.C. and J.E.B. drafted manuscript; M.J.C., N.I.G., and J.E.B. edited and revised manuscript; M.J.C., J.G.S., S.-L.C., M.J.T., N.I.G., and J.E.B. approved final version of manuscript; J.G.S., S.-L.C., and M.J.T. performed experiments.
REFERENCES
- 1.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Chan SL, Sweet JG, Cipolla MJ. Treatment for cerebral small vessel disease: effect of relaxin on the function and structure of cerebral parenchymal arterioles during hypertension. FASEB J 27: 3917–3927, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol 44: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40: 1451–1457, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab 33: 1486–1492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res 110: 285–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Mayhan WG, Heistad DD. Segmental vascular responses to acute hypertension in cerebrum and brain stem. Am J Physiol Heart Circ Physiol 252: H738–H742, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Springer-Verlag: New York, NY, 1993 [Google Scholar]
- 12.Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol 98: 1940–1948, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299: C1195–C1202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl 7: S5–8, 1989 [PubMed] [Google Scholar]
- 16.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 508: 211–221, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelakis E, Tewari K, Simard JM. Calcium channels in smooth muscle cells from cerebral precapillary arterioles activate at more negative potentials than those from basilar artery. Pflügers Arch 426: 459–461, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Nelson MT, Standen NB, Brayden JE, Worley JF., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature 336: 382–385, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA 104: 365–370, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol 300: H803–H812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res 68: 359–367, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Palomares SM, Cipolla MJ. Myogenic tone as a therapeutic target for ischemic stroke. Curr Vasc Pharmacol, in press [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflügers Arch 460: 571–581, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990 [PubMed] [Google Scholar]
- 26.Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res 55: 336–348, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 77: 8–18, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Singer HA, Baker KM. Calcium dependence of phorbol 12,13-dibutyrate-induced force and myosin light chain phosphorylation in arterial smooth muscle. Pharmacol Exp Ther 243: 814–821, 1987 [PubMed] [Google Scholar]