Abstract
Background/Aims
The aim of the present study was to evaluate the relationship between thyroid hormone levels and infarct severity in patients with ST-elevation myocardial infarction (STEMI).
Methods
We retrospectively reviewed thyroid hormone levels, infarct severity, and the extent of transmurality in 40 STEMI patients evaluated via contrast-enhanced cardiac magnetic resonance imaging.
Results
The high triiodothyronine (T3) group (≥ 68.3 ng/dL) exhibited a significantly higher extent of transmural involvement (late transmural enhancement > 75% after administration of gadolinium contrast agent) than did the low T3 group (60% vs. 15%; p = 0.003). However, no significant difference was evident between the high- and low-thyroid-stimulating hormone/free thyroxine (FT4) groups. When the T3 cutoff level was set to 68.3 ng/dL using a receiver operating characteristic curve, the sensitivity was 80% and the specificity 68% in terms of differentiating between those with and without transmural involvement. Upon logistic regression analysis, high T3 level was an independent predictor of transmural involvement after adjustment for the presence of diabetes mellitus (DM) and the use of glycoprotein IIb/IIIa inhibitors (odds ratio, 40.62; 95% confidence interval, 3.29 to 502; p = 0.004).
Conclusions
The T3 level predicted transmural involvement that was independent of glycoprotein IIb/IIIa inhibitor use and DM positivity.
Keywords: Triiodothyronine, Myocardial infarction, Magnetic resonance imaging
INTRODUCTION
The thyroid hormone system can be suppressed in patients with severe illness of any kind. Under such circumstances (termed the sick euthyroid syndrome), the typical feedback control for thyroid hormone synthesis (thyroid homeostasis) is altered [1,2]. Conversion of the prohormone (free thyroxine [FT4]) into triiodothyronine (T3) is compromised, resulting in buildup of an inactive metabolite, a process termed reverse T3. Low T3 syndrome occurs in approximately 20% to 30% of patients with heart failure (HF) [3,4,5] and has also been described in patients with acute myocardial infarction (AMI) [6,7,8,9].
The physiological properties of high T3 syndrome are well-documented and include increased energy expenditure during both rest and exercise, elevated heart rate, a sensation of nervousness, and palpitations [10,11,12]. T3 increases cardiac contractility and reduces systemic vascular resistance [13,14]. However, many investigators have commented on the paradox that thyroid hormone is protective of rather than harmful to the ischemic heart, even though oxygen consumption is increased (due to accelerated heart rhythm and increased cardiac contractility). Under such conditions, the heart becomes depleted of glycogen [15].
Plasma T3 levels 6 months after myocardial infarction are strongly associated with improved heart function [9]. Experimental animal studies suggest that thyroid hormone treatment may be beneficial in cases of HF occurring after AMI [16,17,18].
Contrast-enhanced cardiac magnetic resonance (CE-CMR) imaging detects infarcts and yields information on size and transmurality, the myocardial area at risk (AAR), myocardial viability, microvascular obstruction (MVO), and myocardial hemorrhage [19,20,21,22,23]. CE-CMR imaging can assess and quantify the extent of myocardial salvage [24]. Moreover, variations in CE-CMR imaging parameters seem to be associated with abnormal electrocardiographic findings in patients with anterior AMI [25].
The aim of the present study was to explore the association between baseline thyroid hormone level and infarct transmurality using CE-CMR imaging of patients with ST-elevation myocardial infarction (STEMI).
METHODS
Study population
Between November 2010 and July 2012, a total of 137 consecutive STEMI patients whose baseline thyroid hormone levels had been determined were enrolled in the present study with the approval of the Chosun University Hospital Research Ethics Committee (approval no. CHOSUN 2012-09-003). Patients were included if they were over 18 years of age and underwent primary percutaneous coronary intervention (PCI) within 12 hours of symptom onset. Patients who did not consent to CE-CMR imaging or who exhibited contraindications to such imaging were excluded. Ultimately, 40 patients were included.
Definition of ST elevation myocardial infarction
STEMI was defined as ST-segment elevation of at least 1 mm in two or more standard leads, or of at least 2 mm in two or more neighboring precordial leads, or a (presumed) new-onset left-bundle branch block.
Percutaneous coronary intervention
All patients received dual oral antiplatelet treatment, consisting of aspirin (300 mg) and clopidogrel (600 mg) prior to intervention followed by daily aspirin (100 to 200 mg) and clopidogrel (75 mg) subsequently. Coronary angiography and stent implantations were performed using standard intervention techniques. Glycoprotein IIb/IIIa receptor antagonists were administered intravenously as judged appropriate by attending physicians.
Blood collection and measurement of thyroid hormones
Venous blood samples were collected into serum-separating tubes (BD Vacutainer Systems, Franklin Lakes, NJ, USA) on each index day. Thyroid-stimulating hormone (TSH), T3, and FT4 levels were measured by an immunoradiometric assay (TSH) or radioimmunoassays (RIAs; T3 and FT4), using RIA-gnost FT4, TSH, and T3 kits (CISbio International, Cedex, France) and a Cobra E 5005 gamma counter (Packard, Ramsey, MN, USA). All assays were performed within 2 hours of sample collection. The normal ranges are as follows: TSH, 0.25 to 4 mIU/L; FT4, 0.7 to 1.8 ng/dL; and T3, 60 to 190 ng/dL.
CE-CMR imaging protocol and analysis
We used a 1.5-T magnetic resonance (MR) scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany) f itted with a SENSE cardiac coil. The magnetic resonance imaging (MRI) protocol consisted of T2-weighted imaging, cine-imaging, first-pass perfusion, evaluation under adenosine-induced stress, rest perfusion, and delayed (5 and 15 minutes) enhancement imaging. Cine-MRI images were obtained using a fast-gradient echo sequence (steady-state free precession) after scout and localizer image acquisition. The slice thickness was 8 mm (with no gap), and short-axis left ventricular (LV) images were acquired from the apex to the base (thus including the entire LV volume). Repeated breath holds were required to acquire adequate images. The temporal resolution was 25 to 30 frames per RR interval. T2-weighted MRI was performed in the cardiac short-axis direction using a dark-blood T2-weighted inversion-recovery fast-spin echo sequence. Next, 0.2 mmol/kg gadolinium-diethylenetriaminepentaacetic acid (Magnevist, Bayer Schering Pharma, Berlin, Germany) was injected intravenously at 3 mL/sec followed 4 minutes later by a saline flush under adenosine infusion. The first pass of the contrast agent (after intravenous bolus injection) through the myocardium was recorded using a T1-weighted dynamic sequence. The slice thickness was 8 mm, the field of view 35 × 35 cm, and the image matrix 128 × 128. Images were taken on 40 occasions of four locations (the apex, the lower mid-axis, the upper mid-axis, and the basal short axis) every two heart beats. Delayed hyperenhancement and the extent of MVO were evaluated 5 and 15 minutes after contrast administration in 10 to 12 contiguous slices (each 8 mm thick; no gap) using a phase-sensitive inversion recovery-spoiled gradient-echo sequence (echo time 4 ms; repetition time 8 ms; and flip angle 30°). The field of view and image matrix were 35 × 35 cm and 256 × 256, respectively. The inversion delay time was varied within the range of 200 to 300 ms.
All measurements were performed at our MRI core laboratory. After the short-axis images were acquired at end-diastole and end-systole and the endocardial borders traced, the left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and ejection fraction (EF) were computed using Simpson's algorithm (Argus, Siemens Medical Solutions). Myocardial mass was calculated by multiplying the myocardial volume by the myocardial density (1.05 g/mL). Each infarct volume was quantified based on visual border detection using a manual drawing method with a commercial software package (INFINITT PACS, INFINITT Healthcare, Seoul, Korea). The infarct volume was calculated as the sum of the areas exhibiting delayed hyperenhancement within each segment of the short-axis images multiplied by the slice thickness over the entire left ventricle. The volume and extent of the MVO, defined as late-hypoenhanced regions within the infracted myocardium evident on delayed enhancement images, were calculated in the same manner. Endocardial and epicardial borders were delineated by planimetry to allow calculation of myocardial areas and were summed as described above to calculate LV myocardial volume. The infarct volume (i.e., infarct size) and the extent of MVO were expressed as percentages of the LV myocardial volume. T2-weighted images were used to explore whether myocardial hemorrhage was evident; this presented as a central hypointense signal core within the area of increased T2 signal intensity [26]. The myocardial AAR, defined as myocardium with a signal intensity greater than two standard deviations (SDs) above the mean signal intensity of remote normal myocardium, was quantified on T2-weighted images using a similar algorithm and expressed as a percentage of the LV myocardial volume. The ratio between infarct size and the AAR was calculated for each patient. The myocardial salvage index was computed as follows: myocardial salvage index = (AAR - infarct size) × 100/AAR [27]. To allow regional analysis, the LV was divided into 17 myocardial segments, as described previously [28]. The extent of infarct transmurality in each segment was calculated by dividing the maximal hyperenhanced thickness by the full thickness of the affected myocardium. Transmural infarction was considered to be present when the extent of infarct transmurality was > 75%.
Statistical analysis
All values are expressed as means ± SDs, medians (with interquartile ranges [IQRs]), or numbers (with percentages). The Mann-Whitney test was used to compare continuous variables and the chi-square test to compare noncontinuous variables, to establish the baseline characteristics of the various groups.
Receiver operating characteristic (ROC) analysis was used to determine the sensitivities and specificities (with 95% confidence intervals [CIs]) of thyroid hormone cutoff values. Multivariable logistic regression analysis of the factors associated with the extent of transmural infarction (> 75% of infarct transmurality) was conducted using a forward conditional stepwise model. Baseline clinical factors with p values < 0.1 in the univariable analysis were entered into this model. The independent variables were the presence of diabetes mellitus (DM), a T3 level ≥ 68.3 ng/dL, and the use of glycoprotein IIb/IIIa inhibitor(s). The relationships between thyroid hormone levels and other clinical variables were evaluated using the aid of Pearson correlation analysis. All statistical analyses were performed using SPSS version 15 (SPSS Inc., Chicago, IL, USA), and a p value < 0.05 was considered to reflect statistical significance.
RESULTS
Baseline characteristics of the entire cohort
The mean patient age was 57.8 years, and 82.5% of patients were male. The clinical, angiographic, and CE-CMR imaging characteristics of the entire cohort are shown in Tables 1, 2, and 3, respectively. The overall mean T3, FT4, and TSH levels were 73 ± 23.7 ng/dL (median, 68.3; IQR, 59.2 to 85.1; normal range, 60 to 190); 1.09 ± 0.28 ng/dL (median, 1.07; IQR, 0.90 to 1.25; normal range, 0.7 to 1.8); and 1.137 ± 1.14 mIU/L (median, 0.71; IQR, 0.38 to 1.63; normal range, 0.25 to 4), respectively.
Table 1.
Patient baseline characteristics in terms of T3 level
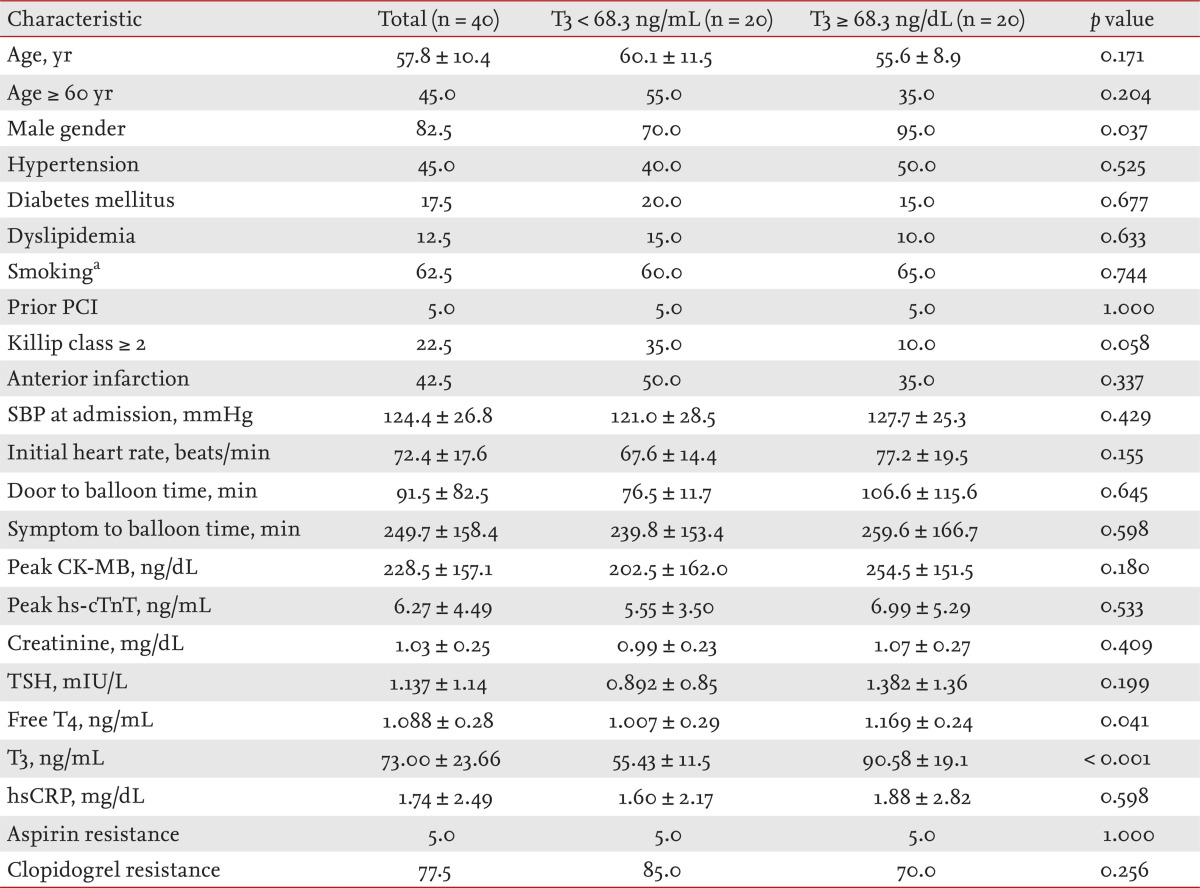
Values are presented as mean ± SD or percentage.
T3, triiodothyronine; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; CK-MB, creatine kinase-MB; hs-cTnT, high-sensitivity cardiac troponin T; TSH, thyroid-stimulating hormone; T4, thyroxine; hsCRP, high-sensitivity C-reactive protein.
aSmoking denotes active smokers as well as ex-smokers who stopped smoking less than 1 year before enrollment.
Table 2.
Angiographic and procedural findings in terms of T3 levels
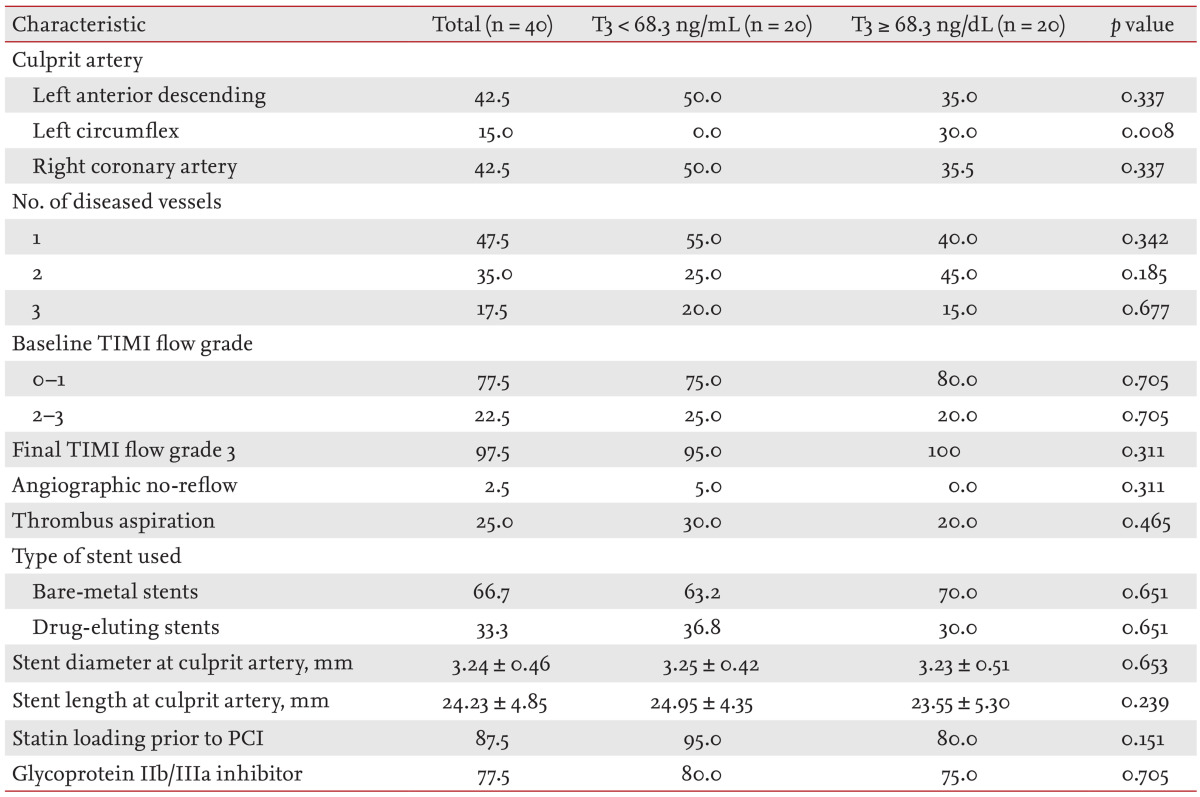
Values are presented as percentage or mean ± SD.
T3, triiodothyronine; TIMI, thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention.
Table 3.
Results of cine-magnetic resonance imaging (MRI), T2-weighted MRI, and contrast-enhanced MRI, in terms of T3 level
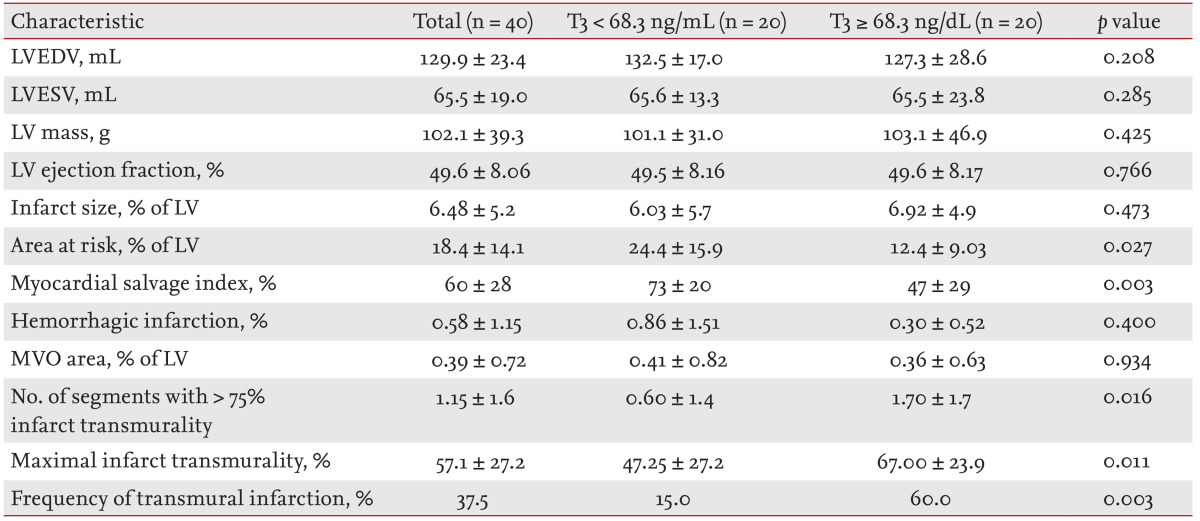
Values are presented as mean ± SD.
T3, triiodothyronine; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LV, left ventricle; MVO, microvascular obstruction.
Relationship between thyroid hormone levels and infarct transmurality
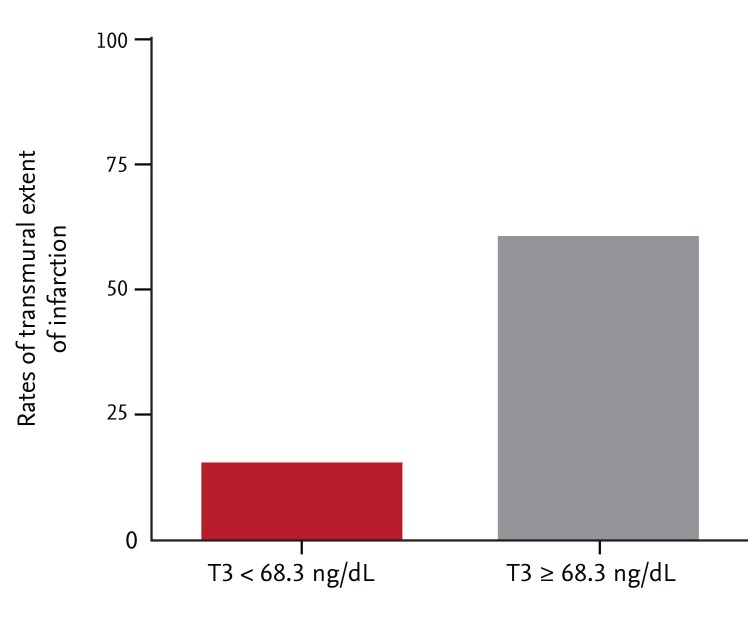
Compared with patients with T3 levels < 68.3 ng/dL, the mean extent of infarct transmurality was higher in those with T3 levels ≥ 68.3 ng/dL (47% ± 27.2% vs. 67% ± 23.9%; p = 0.02). Furthermore, the group with higher T3 levels exhibited a greater extent of transmural infarction than did the lower T3 group (p = 0.003) (Fig. 1). However, no significant difference in the mean levels of infarct transmurality or the transmural extent of infarction was evident between those with higher and lower levels of TSH or FT4.
Figure 1.
The extent of transmural infarction with respect to the median triiodothyronine (T3) values.
The T3 cutoff value predicting the extent of transmural infarction
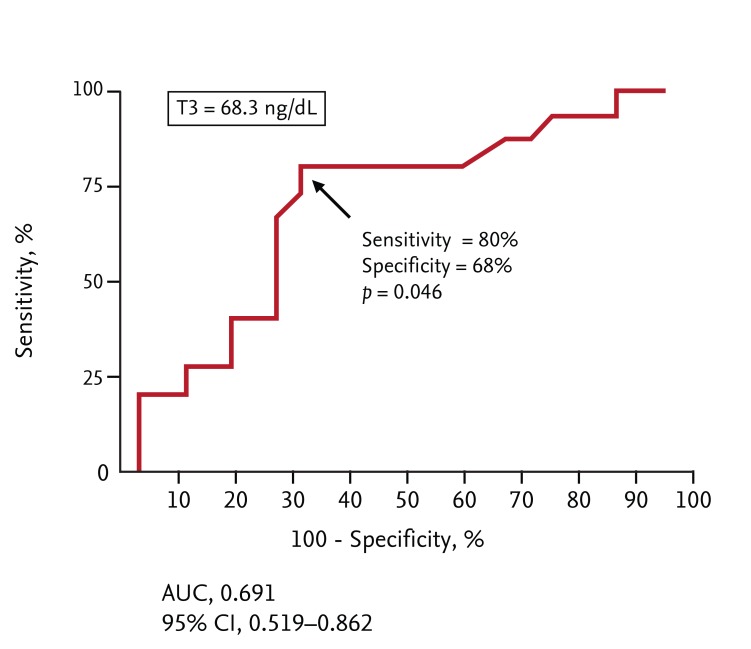
The cutoff T3 level predicting the extent of transmural infarction (> 75% of infarct transmurality) was evaluated via ROC analysis. The T3 cutoff value was 68.3 ng/mL, with 80% sensitivity (95% CI, 51.9 to 95.7) and 68% specificity (95% CI, 46.5 to 85.1) (area under the curve [AUC] = 0.691, p = 0.046) for detection of transmural infarction (Fig. 2).
Figure 2.
The receiver operating characteristic curve for triiodothyronine (T3). AUC, area under the curve; CI, confidence interval.
Clinical characteristics of patients with T3 values below and above the cutoff
The clinical and demographic characteristics of these two groups are shown in Table 1. Patients with high T3 levels were more likely to be male, showed a lower incidence of Killip class ≥ 2 disease, were more likely to have a higher initial heart rate, and exhibited a greater probability of a greater free T4 level. No significant difference in any other baseline clinical characteristic was evident between the two groups.
Angiographic and procedural data on patients with T3 values below and above the cutoff
Table 2 shows the angiographic and procedural results. No patient with a low T3 level had disease of the left circumflex artery. No other difference in angiographic or procedural characteristics was evident between the two groups.
CE-CMR imaging data on patients with T3 values below and above the cutoff
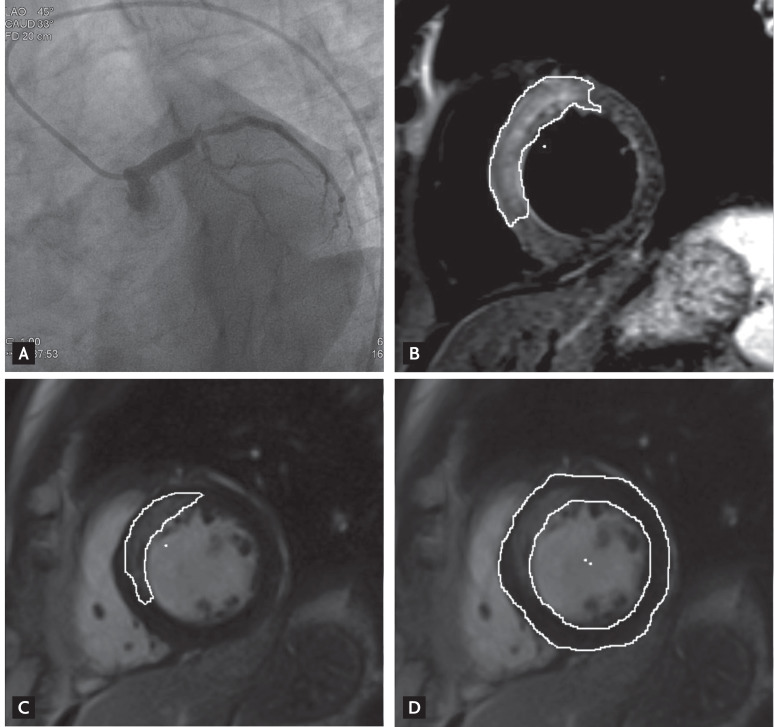
CE-CMR imaging was performed at a median interval of 41 days after the index event (IQR, 25 to 61 days). The interval from the procedure to CE-CMR tended to be greater in the high T3 group (49.5 days [IQR, 23 to 80.5]) than in the low T3 group (40.5 days [IQR, 25.8 to 55.5]). Fig. 3 shows representative coronary angiographic images before reperfusion and CE-CMR images of reperfused STEMI patients. The results of CE-CMR are shown in Table 3. No significant difference in LVEDV, LVESV, LV mass, left ventricular ejection fraction (LVEF), infarct size, the extent of hemorrhagic infarction, or MVO area was evident between the two groups. The AAR was significantly larger and the myocardial salvage index higher in the low T3 group than the high T3 group upon CE-CMR imaging. However, the numbers of segments exhibiting > 75% infarct transmurality as well as the maximal infarct transmurality were higher in the high T3 group. In addition, transmural infarction was detected more frequently in the high T3 than the low T3 group.
Figure 3.
(A) A ST-elevation myocardial infarction patient with total occlusion of the proximal left anterior descending coronary artery. (B) A T2-weighted short axis image shows the contour of the edema and (C) the corresponding delayed enhancement. (D) The myocardial mass is derived from stacked images showing the endocardial and epicardial contours.
The cutoff peak level values of cardiac enzymes predicting the transmural extent of infarction
The relationships between cardiac enzyme peak levels and the extent of transmurality were evaluated using Pearson correlation analysis. Peak creatine kinase-MB (CK-MB) and peak high-sensitivity cardiac troponin T (hs-cTnT) levels were significantly (positively) associated with the extent of transmurality (r = 0.392, p = 0.012; r = 0.465, p = 0.002, respectively).
The cutoff levels of cardiac enzymes predictive of the transmural extent of infarction (> 75% of infarct transmurality) were evaluated by ROC analysis. The cutoff values were 137.15 ng/dL for CK-MB, with 86.7% sensitivity (95% CI, 59.5 to 98.3) and 52% specificity (95% CI, 31.3 to 72.2; AUC = 0.637, p = 0.150), and 4.485 ng/dL for hs-cTnT, with 86.7% sensitivity (95% CI, 59.5 to 98.3) and 60% specificity (95% CI, 38.7 to 78.9; AUC = 0.725, p = 0.018).
Independent predictors of the transmural extent of infarction
Univariate analysis showed that DM positivity, a T3 level ≥ 68.3 ng/dL, and use of glycoprotein IIb/IIIa inhibitors were significantly associated with the extent of transmural infarction. We included the significant univariate variables in the multivariate logistic regression analysis. The only variable that remained as an independent risk factor for transmural infarction was a high T3 level (≥ 68.3 ng/dL) (Table 4).
Table 4.
Univariate and multivariate logistic regression analyses revealing significant independent predictors of the extent of transmural infarction
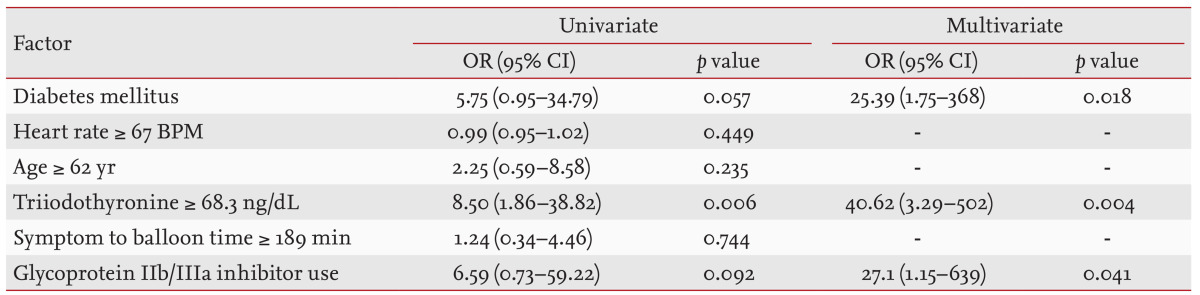
The reference group had the following features: heart rate < 67 BPM, age < 62 years, triiodothyronine level < 68.3 ng/dL, and symptom to balloon time < 189 minutes. The cutoff values for the extent of transmural infarction, determined by receiver operating characteristic analysis, are shown.
OR, odds ratio; CI, confidence interval; BPM, beats per minute.
Independent predictors of a low myocardial salvage index or AAR
Univariate analysis showed that a high peak CK-MB level (≥ 137.15 ng/dL), a high peak hs-cTnT level (≥ 4.485 ng/dL), a long interval between the index day and the day of CE-CMR imaging (> 41 days; the median value), the transmural extent of infarction, and a T3 level ≥ 68.3 ng/dL were all significantly associated with a low median myocardial salvage index when the entire cohort was evaluated. We included the significant univariate variables in the multivariate logistic regression analysis. The only variable that remained as an independent risk factor for a low myocardial salvage index was a long median interval from the index day to the CE-CMR imaging day.
Univariate analysis showed that only a longer symptom to balloon time (> 189 minutes; the median value) was significantly associated with a high AAR (> 13.3%; the median value).
Correlations between the T3 level and other clinical variables
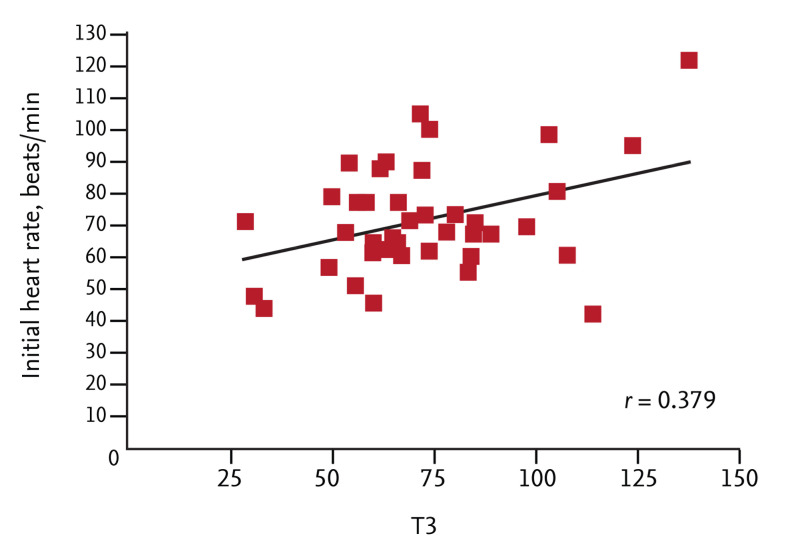
The relationships between the T3 level and other clinical variables were evaluated using Pearson correlation analysis. All continuous variables, including age, Killip class, systolic blood pressure at admission, initial heart rate, door to balloon time, symptom to balloon time, peak levels of cardiac enzymes (CK-MB and hs-cTnT), levels of creatinine and high-sensitivity C-reactive protein, number of diseased vessels, "baseline thrombolysis in myocardial infarction" flow grade, and LVEF, were evaluated. Of these, only the initial heart rate was significantly associated with the T3 level (r = 0.379, p = 0.016) (Fig. 4). Age tended to correlate negatively with the T3 level (r = -0.302, p = 0.058).
Figure 4.
A scatter plot showing the relationship between initial heart rate and triiodothyronine (T3) level.
To determine if initial heart rate and age served as surrogate markers of T3 levels, we determined the cut-off values predictive of a high T3 level (≥ 68.3 ng/dL) for both variables using ROC analysis. The cutoff initial heart rate was 67 beats per minute, with 75% sensitivity (95% CI, 50.9 to 91.3) and 55% specificity (95% CI, 31.5 to 76.9; AUC = 0.631, p = 0.156), and the cutoff age was 62 years, with 80% sensitivity (95% CI, 56.3 to 94.3) and 50% specificity (95% CI, 27.2 to 72.8; AUC = 0.626, p = 0.172), with regard to predicting a high T3 level (≥ 68.3 ng/dL). However, neither heart rate nor age was predictive of the extent of transmural infarction (Table 4).
DISCUSSION
The principal finding of the present study was that a high T3 level was an independent predictor of the extent of transmural infarction in STEMI patients. Even after adjustment for DM positivity and the use of glycoprotein IIb/IIIa inhibitors, the odds ratio (OR) remained significant in the high T3 group. CE-CMR imaging is very sensitive and reliable when used to identify morphological and functional sequelae of AMI, and it has substantial potential to improve risk stratification and guide patient management after AMI.
To our knowledge, this is the first study to explore the predictive utility of thyroid hormone levels (especially that of T3) as biomarkers of the extent of transmural infarction in STEMI patients.
CMR imaging can differentiate viable from infarcted myocardium and can accurately determine the extent of transmurality after myocardial infarction. Transmurality of an infarction is important in predicting development of LV remodeling, changes in LV function, and clinical prognosis [23,29,30,31,32]. Most studies using delayed-enhancement imaging have found an inverse relationship between transmural involvement > 75% and improved regional LV contractile function, but minimal or no improvement in the numbers of dysfunctional segments was found [33].
We suggest that the low T3 syndrome develops in AMI patients to protect the myocardium. The physiological features of those with high T3 levels are well-documented and include increased resting and exercise energy expenditure and an elevated heart rate [10,11,12]. In addition, T3 increases cardiac contractility [13,14]. Therefore, transient low T3 status in AMI patients may protect the myocardium, resulting in low transmurality evident upon CE-CMR imaging and reducing energy expenditure, heart rate, cardiac contractility, and oxygen consumption.
In the present study, we found no significant difference in the mean extent of infarct transmurality or the transmural extent of infarction between high and low TSH and FT4 level groups. One explanation is that T3 is the biologically active form of thyroid hormone, and T3 levels are correlated with functional status and serve as an index of disease outcome [5,8,9,34].
Sick euthyroid syndrome is often observed in patients with chronic HF and AMI, and it has been associated with increased mortality in both types of patients [5,6,7,8,35]. Recently, Ozcan et al. [36] showed that this syndrome was linked to both in-hospital and long-term mortality in STEMI patients undergoing primary PCI. Although we did not choose mortality as a primary endpoint, our results appear to differ from those of previous studies. It is presumed that the effect of sick euthyroid syndrome on the mortality of STEMI patients ref lects not only the extent of transmurality in such patients but also the involvement of other (unknown) intracellular signaling pathways associated with the response to stress and cardiac remodeling [37,38].
Our results suggest that biomarkers such as thyroid hormone levels have predictive value when used to assess the extent of transmural infarction in patients with STEMI. A high T3 level was an independent predictor of the extent of such infarction, revealing the clinical relevance of T3 levels in terms of risk stratification of STEMI patients. Such stratification should be used in conjunction with the ACC/AHA recommendations on β-blocker prescriptions for STEMI patients [39] with high T3 levels, because β-blockers reduce conversion of T4 to T3 [40,41].
Limitations
The present study was performed on a relatively small sample size, rendering imprecise ORs and wide CIs. Furthermore, the small sample size limited our ability to consider additional (possibly relevant) factors. In addition, the investigation was not prospective, and our conclusions are thus subject to the limitations inherent in such analyses.
Selection bias was possible because we included only STEMI patients in whom thyroid hormone levels had been measured and infarct transmurality assessed via CE-CMR imaging.
In conclusion, a high T3 level (> 68.3 ng/mL) was an independent predictor of the extent of transmural infarction in patients with STEMI. This predictive power was independent of glycoprotein IIb/IIIa inhibitor use and DM status.
KEY MESSAGE
The high T3 group (≥ 68.3 ng/dL) had a significantly higher transmural involvement (extent of late transmural enhancement of gadolinium agent > 75%) rate in patients with ST-elevation myocardial infarction underwent primary percutaneous coronary intervention.
T3 was a predictive marker for transmural involvement.
Its predictive power for transmural involvement was independent of glycoprotein IIb/IIIa inhibitor use and diabetes mellitus.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 2.Sypniewski E. Comparative pharmacology of the thyroid hormones. Ann Thorac Surg. 1993;56(1 Suppl):S2–S6. doi: 10.1016/0003-4975(93)90548-v. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91–95. doi: 10.1016/0735-1097(90)90462-x. [DOI] [PubMed] [Google Scholar]
- 4.Opasich C, Pacini F, Ambrosino N, et al. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur Heart J. 1996;17:1860–1866. doi: 10.1093/oxfordjournals.eurheartj.a014804. [DOI] [PubMed] [Google Scholar]
- 5.Pingitore A, Landi P, Taddei MC, Ripoli A, L'Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med. 2005;118:132–136. doi: 10.1016/j.amjmed.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Eber B, Schumacher M, Langsteger W, et al. Changes in thyroid hormone parameters after acute myocardial infarction. Cardiology. 1995;86:152–156. doi: 10.1159/000176862. [DOI] [PubMed] [Google Scholar]
- 7.Franklyn JA, Gammage MD, Ramsden DB, Sheppard MC. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond) 1984;67:585–590. doi: 10.1042/cs0670585. [DOI] [PubMed] [Google Scholar]
- 8.Friberg L, Werner S, Eggertsen G, Ahnve S. Rapid down-regulation of thyroid hormones in acute myocardial infarction: is it cardioprotective in patients with angina? Arch Intern Med. 2002;162:1388–1394. doi: 10.1001/archinte.162.12.1388. [DOI] [PubMed] [Google Scholar]
- 9.Lymvaios I, Mourouzis I, Cokkinos DV, Dimopoulos MA, Toumanidis ST, Pantos C. Thyroid hormone and recovery of cardiac function in patients with acute myocardial infarction: a strong association? Eur J Endocrinol. 2011;165:107–114. doi: 10.1530/EJE-11-0062. [DOI] [PubMed] [Google Scholar]
- 10.Dimitriadis G, Baker B, Marsh H, et al. Effect of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. Am J Physiol. 1985;248(5 Pt 1):E593–E601. doi: 10.1152/ajpendo.1985.248.5.E593. [DOI] [PubMed] [Google Scholar]
- 11.Lovejoy JC, Smith SR, Bray GA, et al. A paradigm of experimentally induced mild hyperthyroidism: effects on nitrogen balance, body composition, and energy expenditure in healthy young men. J Clin Endocrinol Metab. 1997;82:765–770. doi: 10.1210/jcem.82.3.3827. [DOI] [PubMed] [Google Scholar]
- 12.Lebon V, Dufour S, Petersen KF, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemperer JD, Zelano J, Helm RE, et al. Triiodothyronine improves left ventricular function without oxygen wasting effects after global hypothermic ischemia. J Thorac Cardiovasc Surg. 1995;109:457–465. doi: 10.1016/S0022-5223(95)70276-8. [DOI] [PubMed] [Google Scholar]
- 14.Gomberg-Maitland M, Frishman WH. Thyroid hormone and cardiovascular disease. Am Heart J. 1998;135(2 Pt 1):187–196. doi: 10.1016/s0002-8703(98)70081-x. [DOI] [PubMed] [Google Scholar]
- 15.Pantos CI, Malliopoulou VA, Mourouzis IS, et al. Long-term thyroxine administration protects the heart in a pattern similar to ischemic preconditioning. Thyroid. 2002;12:325–329. doi: 10.1089/10507250252949469. [DOI] [PubMed] [Google Scholar]
- 16.Dyke CM, Yeh T, Jr, Lehman JD, et al. Triiodothyronine-enhanced left ventricular function after ischemic injury. Ann Thorac Surg. 1991;52:14–19. doi: 10.1016/0003-4975(91)91410-w. [DOI] [PubMed] [Google Scholar]
- 17.Hsu RB, Huang TS, Chen YS, Chu SH. Effect of triiodothyronine administration in experimental myocardial injury. J Endocrinol Invest. 1995;18:702–709. doi: 10.1007/BF03349792. [DOI] [PubMed] [Google Scholar]
- 18.Mahaffey KW, Raya TE, Pennock GD, Morkin E, Goldman S. Left ventricular performance and remodeling in rabbits after myocardial infarction: effects of a thyroid hormone analogue. Circulation. 1995;91:794–801. doi: 10.1161/01.cir.91.3.794. [DOI] [PubMed] [Google Scholar]
- 19.Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson. 2012;14:68. doi: 10.1186/1532-429X-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugander M, Bagi PS, Oki AJ, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim RJ, Shah DJ. Fundamental concepts in myocardial viability assessment revisited: when knowing how much is "alive" is not enough. Heart. 2004;90:137–140. doi: 10.1136/hrt.2003.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klem I, Kim RJ. Assessment of microvascular injury after acute myocardial infarction: importance of the area at risk. Nat Clin Pract Cardiovasc Med. 2008;5:756–757. doi: 10.1038/ncpcardio1373. [DOI] [PubMed] [Google Scholar]
- 23.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 24.Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc Imaging. 2009;2:825–831. doi: 10.1016/j.jcmg.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Yang HS, Lee CW, Hong MK, et al. Terminal QRS complex distortion on the admission electrocardiogram in anterior acute myocardial infarction and association with residual flow and infarct size after primary angioplasty. Korean J Intern Med. 2005;20:21–25. doi: 10.3904/kjim.2005.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mather AN, Fairbairn TA, Ball SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart. 2011;97:453–459. doi: 10.1136/hrt.2010.202028. [DOI] [PubMed] [Google Scholar]
- 27.O'Regan DP, Ariff B, Neuwirth C, Tan Y, Durighel G, Cook SA. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart. 2010;96:1885–1891. doi: 10.1136/hrt.2010.200634. [DOI] [PubMed] [Google Scholar]
- 28.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 29.Tarantini G, Razzolini R, Cacciavillani L, et al. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–1089. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 31.Baks T, van Geuns RJ, Biagini E, et al. Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J. 2005;26:1070–1077. doi: 10.1093/eurheartj/ehi131. [DOI] [PubMed] [Google Scholar]
- 32.Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 33.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 34.Pantos C, Dritsas A, Mourouzis I, et al. Thyroid hormone is a critical determinant of myocardial performance in patients with heart failure: potential therapeutic implications. Eur J Endocrinol. 2007;157:515–520. doi: 10.1530/EJE-07-0318. [DOI] [PubMed] [Google Scholar]
- 35.Friberg L, Drvota V, Bjelak AH, Eggertsen G, Ahnve S. Association between increased levels of reverse triiodothyronine and mortality after acute myocardial infarction. Am J Med. 2001;111:699–703. doi: 10.1016/s0002-9343(01)00980-9. [DOI] [PubMed] [Google Scholar]
- 36.Ozcan KS, Osmonov D, Toprak E, et al. Sick euthyroid syndrome is associated with poor prognosis in patients with STEMI undergoing primary percutaneous intervention. Cardiol J. 2014 Mar 27; doi: 10.5603/CJ.a2013.0108. [Epub]. http://dx.doi.org/10.5603/CJ.a2013.0108. [DOI] [PubMed] [Google Scholar]
- 37.Pantos C, Mourouzis I, Cokkinos DV. Thyroid hormone and cardiac repair/regeneration: from Prometheus myth to reality? Can J Physiol Pharmacol. 2012;90:977–987. doi: 10.1139/y2012-031. [DOI] [PubMed] [Google Scholar]
- 38.Pantos C, Mourouzis I, Cokkinos DV. New insights into the role of thyroid hormone in cardiac remodeling: time to reconsider? Heart Fail Rev. 2011;16:79–96. doi: 10.1007/s10741-010-9185-3. [DOI] [PubMed] [Google Scholar]
- 39.Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Trepanier LA. Medical management of hyperthyroidism. Clin Tech Small Anim Pract. 2006;21:22–28. doi: 10.1053/j.ctsap.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Wiersinga WM. Propranolol and thyroid hormone metabolism. Thyroid. 1991;1:273–277. doi: 10.1089/thy.1991.1.273. [DOI] [PubMed] [Google Scholar]