Abstract
Background/Aims
Gastroesophageal reflux disease is one of the most common causes of chronic cough and is a potential risk factor for the exacerbation of chronic obstructive pulmonary disease (COPD). The aim of this study was to investigate the prevalence and risk factors for reflux esophagitis (RE) in COPD patients.
Methods
From our hospital database, between September 2006 and April 2010, we searched for subjects who were 40 years old or older and had undergone both postbronchodilator spirometry and esophagogastroduodenoscopy (EGD). COPD was defined as having a ratio of forced expiratory volume in 1 second to forced vital capacity < 0.7 in postbronchodilator spirometry and no abnormality causing airway obstruction, except emphysematous changes, on a chest X-ray. The diagnosis of RE was based on a mucosal break surrounding the distal esophageal sphincter through EGD.
Results
In total, 253 patients with COPD were enrolled. The prevalence of RE in COPD was 30% (76/253). Multiple logistic regression analyses revealed that age (odds ratio [OR], 0.950; 95% confidence interval [CI], 0.918 to 0.983; p = 0.003), smoking pack-years (OR, 1.015; 95% CI, 1.004 to 1.025; p = 0.006), and inhaled anticholinergics (OR, 0.516; 95% CI, 0.271 to 0.982; p = 0.044) were independently associated with RE in COPD patients.
Conclusions
The prevalence of RE in our COPD patients was higher than that reported previously in the Korean general population. In COPD, smoking increased the risk of RE, whereas inhaled anticholinergics may be associated with a reduced risk of RE.
Keywords: Pulmonary disease, chronic obstructive; Esophagitis, peptic; Prevalence; Risk factors
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is not only a lung disease but also a systemic inflammatory disease [1]. Exacerbations of COPD are associated with a rapid decline in lung function, worsening quality of life, a substantial economic burden to society, and, eventually, increased mortality [1,2]. Preventing exacerbations remains a primary goal of management but is difficult because the cause(s) of acute exacerbations of COPD remain(s) largely unknown [1].
Gastroesophageal reflux (GER) is one of the most common causes of chronic cough [3] and has been reported more frequently in COPD patients than in those without COPD [4,5,6,7]. GER has been suggested to be a risk factor for COPD exacerbations [4,8,9,10,11]. In an animal model, micro-aspiration due to GER induced a bronchiolitis obliterans-like reaction [12]. A study in Taiwan reported that COPD was an independent factor associated with reflux esophagitis (RE) [13]. The gold standards for the diagnosis of gastroesophageal reflux disease (GERD) are esophagogastroduodenoscopy (EGD) and 24-hour esophageal pH monitoring [14]. EGD allows direct visualization of an esophageal mucosal break, which is direct evidence of RE [14]. However, because symptomatic diagnosis is simple and readily applicable in clinical practice, most studies have investigated the prevalence of GER in COPD patients using questionnaires [4,6,8,9,10,11]. Some have used 24-hour pH monitoring [7,15].
In Korea, diseases of the upper gastrointestinal (UGI) tract are more common than in Western countries, possibly due to the relatively spicy and salty food, and the incidence of stomach cancer has been the highest among all types of cancer. Thus, under a health screening program of the Korean National Health Insurance System, all people can receive EGD or a UGI series every 2 years from the age of 40 [16].
The aim of this study was to investigate the prevalence and risk factors for RE in COPD patients.
METHODS
Study subjects and design
We collected data retrospectively from patients with COPD, aged 40 and older, who had undergone both postbronchodilator spirometry and EGD at Ewha Womans University Mokdong Hospital between September 2006 and April 2010. The Institutional Review Board of Ewha Womans University Mokdong Hospital approved the analysis of the data.
COPD was defined as a ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) of < 0.7 in postbronchodilator spirometry, according to the criteria of the Global Initiative for Obstructive Lung Disease (GOLD) [1]. We excluded those who had an abnormality affecting lung function, such as old tuberculosis scars, bronchiectasis, pulmonary fibrosis on a chest X-ray, and those who had a UGI malignancy, a previous history of UGI surgery, or a hiatal hernia. Also, patients with a body mass index > 30 kg/m2 were excluded because obesity is a well-known risk factor for RE [17]. Finally, 253 patient files were reviewed retrospectively and data on age, gender, height, weight, smoking history, comorbidities, medication records, and UGI symptoms were collected.
Postbronchodilator spirometry and esophagogastroduodenoscopy
Postbronchodilator spirometry was performed as recommended by the American Thoracic Society using the Vmax 22 (Sensor Medics, Yorba Linda, CA, USA) [18]. The following values were evaluated: postbronchodilator FEV1, FVC, and the ratio of FEV1 to FVC (FEV1/FVC). RE was diagnosed as a mucosal break surrounding the distal esophageal sphincter through EGD by an endoscopy specialist in the Division of Gastroenterology, which was performed within 6 months of the postbronchodilator spirometry.
Statistical analysis
Statistical analyses were performed using the SPSS version 18.0 (IBM Co., Armonk, NY, USA). Data are expressed as mean ± SD, medians (interquartile range), or number (%). The assumption of the normal distribution of data was tested with the Shapiro-Wilk statistic. Differences between the groups were tested using Student t test for parametric variables, the Mann-Whitney U test for nonparametric variables, or the chi-square test. A multiple logistic regression analysis was used to identify independent factors associated with RE in COPD patients. Statistical significance was accepted for p values < 0.05.
RESULTS
The prevalence of RE in COPD patients was 30% (76/253) (Table 1). The clinical characteristics and comparisons according to the presence or absence of RE are shown in Table 1. One or more of heartburn, regurgitation, dyspepsia, or epigastric pain, were reported in 79% (200/253) (Table 2). Among those symptoms, heartburn had the highest specificity (97%) and predictive value for RE (odds ratio [OR], 3.490; 95% confidence interval [CI], 1.071 to 11.370; p = 0.038). Although COPD patients with RE showed higher mean FVC (p = 0.002), FVC % predicted (p = 0.017), and FEV1 than those without RE (p = 0.010), there was no difference in FEV1% predicted or the severity of airflow limitation by GOLD (Table 3). COPD patients without RE used more inhaled anticholinergics than those with RE (p = 0.014) (Table 4).
Table 1.
Clinical characteristics of patients with chronic obstructive pulmonary disease according to the presence or absence of RE
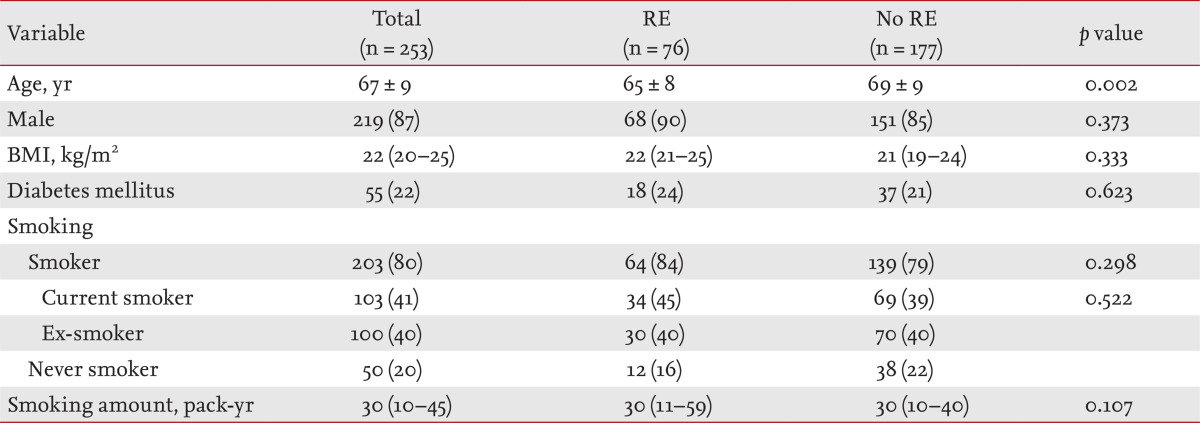
Values are presented as mean ± SD, number (%), or medians (interquartile range).
RE, reflux esophagitis; BMI, body mass index.
Table 2.
Gastrointestinal symptoms and EGD findings in chronic patients with chronic obstructive pulmonary disease according to the presence or absence of RE
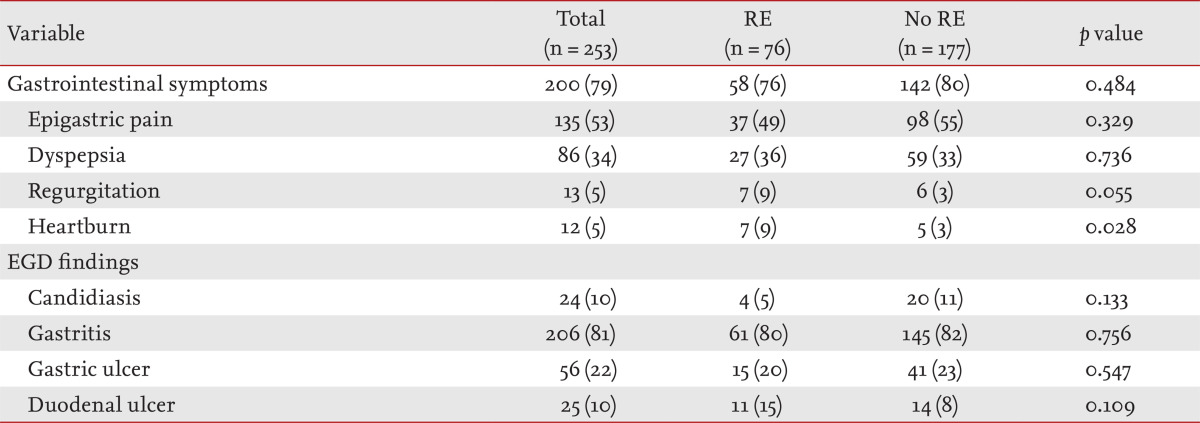
Values are presented as number (%).
RE, reflux esophagitis; EGD, esophagogastroduodenoscopy.
Table 3.
Postbronchodilator spirometry in patients with chronic obstructive pulmonary disease according to the presence or absence of RE
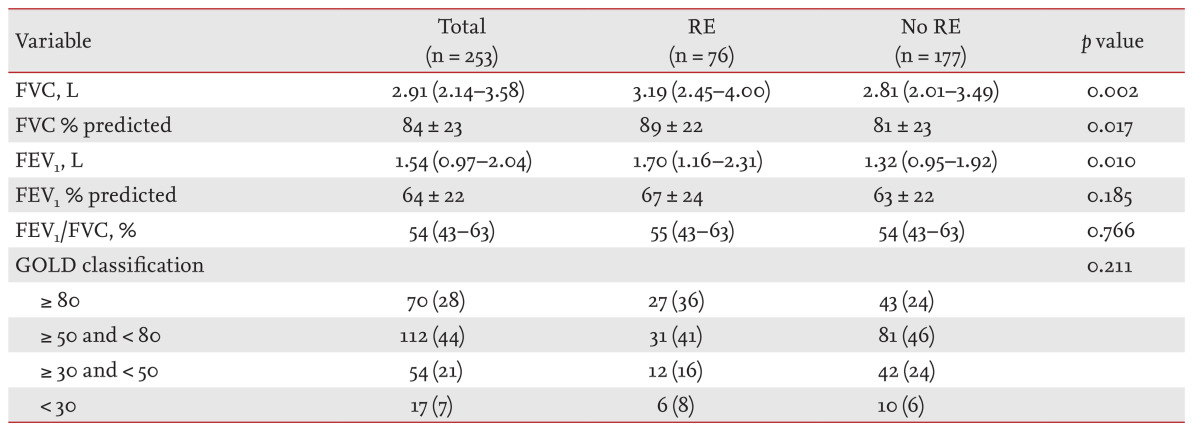
Values are presented as medians (interquartile range), mean ± SD, or number (%).
RE, reflux esophagitis; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Obstructive Lung Disease.
Table 4.
Medication history in patients with chronic obstructive pulmonary disease according to the presence or absence of RE
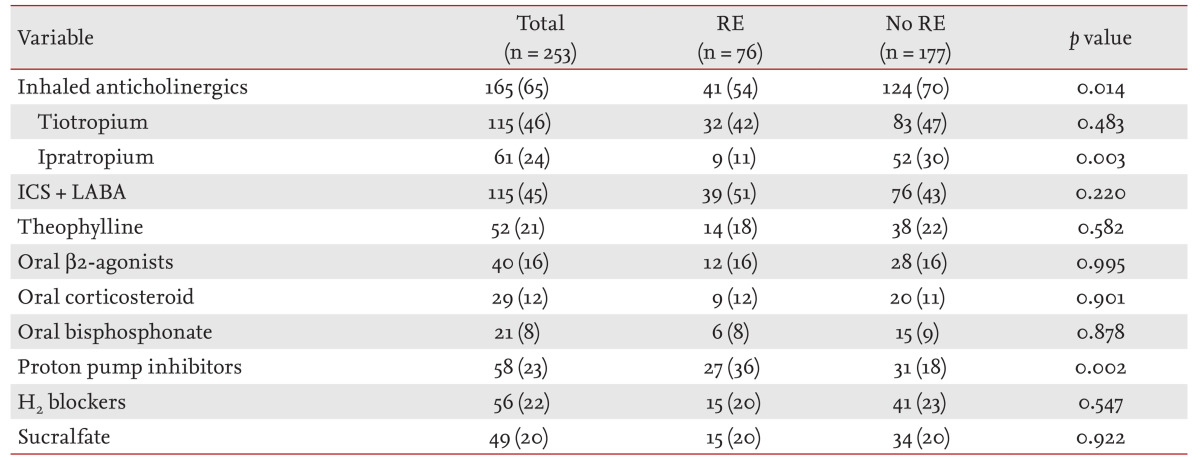
Values are presented as number (%).
RE, reflux esophagitis; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.
Multiple logistic regression analyses revealed that age (OR, 0.950; 95% CI, 0.918 to 0.983; p = 0.003), smoking pack-years (OR, 1.015; 95% CI, 1.004 to 1.025; p = 0.006), and inhaled anticholinergics (OR, 0.516; 95% CI, 0.271 to 0.982; p = 0.044) were independent factors associated with RE in COPD (Table 5).
Table 5.
Risk factors for reflux esophagitis in patients with chronic obstructive pulmonary disease by multiple logistic regression analyses
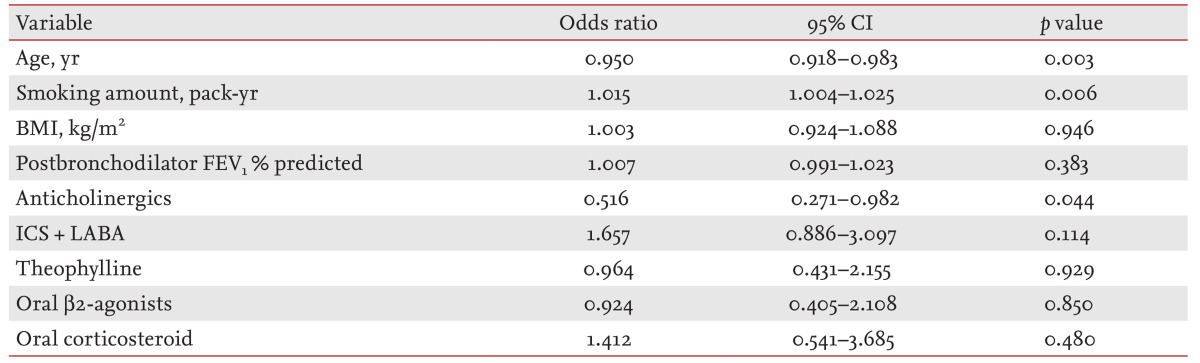
CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.
DISCUSSION
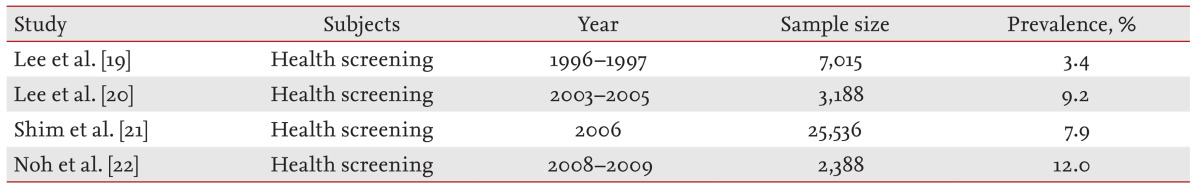
In our study, the prevalence of RE in COPD was 30%, which is higher than that reported previously in the Korean general population (Table 6) [19,20,21,22] and Asia [23]. Generally, the prevalence of GERD in Asia is known to be lower than that in Western countries [17,23]. Dent et al. [17] reported that the prevalence of GERD was 15% to 20% in America and Europe, but < 5% in Asia through a systematic review. Reasons for the difference may include the fact that Asians generally have lower body mass indices, lower secretion of gastric acid, and higher pressure in the lower esophageal sphincter than Westerners. However, the prevalence of GERD has been increasing, possibly due to the recently more westernized diet and weight gains in Korea [16]. The prevalence of RE has also increased [16,19,20,21,22].
Table 6.
Prevalence of reflux esophagitis in Korea
The diagnosis of GERD only requires distinct reflux symptoms, whereas RE requires mucosal changes in the esophagus, and GER is confirmed by measuring esophageal pH through 24 hour ambulatory pH monitoring [14,16]. Most studies of GERD in COPD have used self-reported questionnaires [4,6,8,9,10,11] though some have measured esophageal pH [7,15]. The prevalence of GERD in patients with pulmonary disease, including COPD, was 20% higher than those with other conditions except pulmonary disease [6]. Several studies measuring esophageal pH have shown similar results [7,15]. However, patients with symptoms suggesting GER do not always have RE [24], and even if reflux of gastric acid at the esophagogastric junction occurs, it does not always cause symptoms [7,15] or mucosal changes in the esophagus. Although we did not use a prospectively designed systematic questionnaire, we collected data as to whether patients presented with one of four symptoms-heartburn, regurgitation, epigastric pain, and dyspepsia-by reviewing medical records. More than three-quarters of the subjects complained of one or more of those symptoms. Although heartburn and regurgitation are known to be specific for RE [14], only heartburn predicted RE statistically in this study. There are many patients with UGI diseases in Korea, and diseases other than RE were identified in 81% of our subjects. Most COPD patients are elderly and current or ex-smokers. Because symptoms do not always reflect GERD [24] and old age and smoking are well-known risk factors for GERD [17], the prevalence of GERD may be overestimated using a self-questionnaire in COPD patients. Thus, EGD may be an accurate diagnostic tool for GERD in COPD patients.
In the multivariate analysis, smoking was a significant independent risk factor, similar to the results of other studies [13,16]. Smoking is a well-known risk factor for RE and reduces the pressure of the lower esophageal sphincter [25,26]. Advanced age is another known risk factor for RE [24], whereas our study apparently showed the opposite. One reason may be that all the subjects were at least middle-aged; indeed, the mean age was 67 years. Another point is that EGD is too invasive for routine use in very elderly persons. Casanova et al. [7] also failed to show an association between age and GER in COPD patients.
In this study, use of inhaled anticholinergics seemed to decrease the risk of RE. A previous study reported that relative risk of GERD increased with use of inhaled anticholinergics [5]. Both centrally and peripherally acting anticholinergic drugs reduce lower esophageal sphincter pressure. However, tiotropium reduced lung inflammation in a mouse model of chronic GER [27]. Inhaled anticholinergics, which block the muscarinic receptors from the larynx to the smallest airway and alveoli, are prescribed as a bronchodilator for COPD patients. However, they have also been used for chronic cough, particularly chronic bronchitis [28]. Chronic cough is one of the most common symptoms in COPD patients. Chronic bronchitis and GERD are the most common causes of chronic cough [3]. Vigorous coughing can result in vomiting, and may aggravate GER. Ipratropium is effective even for cough hypersensitivity induced by upper respiratory tract infection [29]. Although inhaled corticosteroids are known to be effective in chronic cough, especially asthma [3], our subjects had COPD exclusively. Tiotropium inhibits cough reflex sensitivity to capsaicin in subjects with acute viral upper respiratory tract infection [30]. The antitussive effect of tiotropium may occur through a mechanism other than bronchodilation. Thus, inhaled anticholinergics may reduce GER through suppressing chronic cough. If so, they may be more effective in patients with predominantly chronic bronchitis rather than emphysema. The effects of inhaled anticholinergics on RE should be evaluated in further well-designed studies.
In this study, there was no difference in use of inhaled corticosteroids according to the presence or absence of RE in COPD. However, Garcia Rodriguez et al. [5] reported that the prevalence of GER increased according to the use of inhaled corticosteroids. In another study, the effect of medication was not statistically significant or hard to evaluate because COPD patients are usually treated with multiple medications and have both pulmonary and other systemic problems [7]. When a patient takes multiple drugs at the same time, as well as COPD medication(s), drug interactions are likely.
In one study, COPD patients with FEV1 ≤ 50% predicted showed a high prevalence of GERD [6,7]. They did not use EGD. Our subjects showed no difference according to the severity of airway obstruction.
The present study had several limitations. It was a retrospective cohort study of a limited number of subjects. We did not use a systemic questionnaire for GERD. There may have been selection bias. If a COPD patient underwent EGD, this indicates that the patient already had symptoms or at least was concerned about UGI disease. Thus, the prevalence would be overestimated. Nevertheless, because there has been no report of RE using EGD in COPD patients, this may also be a strength. We found a negative association between the presence of RE and the use of inhaled anticholinergics. However, whether inhaled anticholinergics can truly reduce the risk of RE should be answered by well-designed prospective studies.
In conclusion, physicians should consider GER as one of the most important comorbidities because it is one of the most common causes of chronic cough as well as a risk factor of COPD exacerbation.
KEY MESSAGE
1. Because reflux esophagitis (RE) was found in 30% of our subjects with chronic obstructive pulmonary disease (COPD), active evaluat ion and management of RE are needed to improve quality of life and possibly reduce exacerbations.
2. In COPD, use of inhaled anticholinergics may be associated with a decreased risk of reflux esophagitis.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2010-0027945).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Diagnosis, Management, and Prevention of COPD [Internet] London (UK): Global Initiative for Chronic Obstructive Lung Disease; 2014. [cited 2013 May 7]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. [Google Scholar]
- 2.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molassiotis A, Bryan G, Caress A, Bailey C, Smith J. Pharmacological and non-pharmacological interventions for cough in adults with respiratory and non-respiratory diseases: a systematic review of the literature. Respir Med. 2010;104:934–944. doi: 10.1016/j.rmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 5.Garcia Rodriguez LA, Ruigomez A, Martin-Merino E, Johansson S, Wallander MA. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest. 2008;134:1223–1230. doi: 10.1378/chest.08-0902. [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119:1043–1048. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 7.Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23:841–845. doi: 10.1183/09031936.04.00107004. [DOI] [PubMed] [Google Scholar]
- 8.Terada K, Muro S, Sato S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63:951–955. doi: 10.1136/thx.2007.092858. [DOI] [PubMed] [Google Scholar]
- 9.Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–1101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 10.Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastrointestin Liver Dis. 2010;19:253–256. [PubMed] [Google Scholar]
- 11.Takada K, Matsumoto S, Kojima E, et al. Prospective evaluation of the relationship between acute exacerbations of COPD and gastroesophageal reflux disease diagnosed by questionnaire. Respir Med. 2011;105:1531–1536. doi: 10.1016/j.rmed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Oue K, Mukaisho K, Higo T, et al. Histological examination of the relationship between respiratory disorders and repetitive microaspiration using a rat gastro-duodenal contents reflux model. Exp Anim. 2011;60:141–150. doi: 10.1538/expanim.60.141. [DOI] [PubMed] [Google Scholar]
- 13.Chen TS, Chang FY. The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan. J Clin Gastroenterol. 2007;41:819–822. doi: 10.1097/01.mcg.0000225658.30803.79. [DOI] [PubMed] [Google Scholar]
- 14.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 15.Kempainen RR, Savik K, Whelan TP, Dunitz JM, Herrington CS, Billings JL. High prevalence of proximal and distal gastroesophageal reflux disease in advanced COPD. Chest. 2007;131:1666–1671. doi: 10.1378/chest.06-2264. [DOI] [PubMed] [Google Scholar]
- 16.Cho YK, Kim GH, Kim JH, et al. Diagnosis of gastroesophageal reflux disease: a systematic review. Korean J Gastroenterol. 2010;55:279–295. doi: 10.4166/kjg.2010.55.5.279. [DOI] [PubMed] [Google Scholar]
- 17.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Song CW, Jeen YT, et al. Prevalence of endoscopsic reflux esophagitis among Koreans. J Gastroenterol Hepatol. 2001;16:373–376. doi: 10.1046/j.1440-1746.2001.02464.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–675. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 21.Shim KN, Hong SJ, Sung JK, et al. Clinical spectrum of reflux esophagitis among 25,536 Koreans who underwent a health check-up: a nationwide multicenter prospective, endoscopy-based study. J Clin Gastroenterol. 2009;43:632–638. doi: 10.1097/MCG.0b013e3181855055. [DOI] [PubMed] [Google Scholar]
- 22.Noh YW, Jung HK, Kim SE, Jung SA. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil. 2010;16:148–156. doi: 10.5056/jnm.2010.16.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14–27. doi: 10.5056/jnm.2011.17.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Liu J, Zhang H, Yu CH, Li YM. Risk factors for gastroesophageal reflux disease, reflux esophagitis and non-erosive reflux disease among Chinese patients undergoing upper gastrointestinal endoscopic examination. World J Gastroenterol. 2007;13:6009–6015. doi: 10.3748/wjg.v13.45.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag H, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther. 2009;29:470–480. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 26.Ruigomez A, Garcia Rodriguez LA, Wallander MA, Johansson S, Graffner H, Dent J. Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharmacol Ther. 2004;20:751–760. doi: 10.1111/j.1365-2036.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Devillier P, Kuang X, et al. Tiotropium reduction of lung inflammation in a model of chronic gastro-oesophageal reflux. Eur Respir J. 2010;35:1370–1376. doi: 10.1183/09031936.00139909. [DOI] [PubMed] [Google Scholar]
- 28.Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):104S–115S. doi: 10.1378/chest.129.1_suppl.104S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry R, Wood A, Higenbottam T. The effect of anticholinergic bronchodilator therapy on cough during upper respiratory tract infections. Br J Clin Pharmacol. 1994;37:187–191. doi: 10.1111/j.1365-2125.1994.tb04259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dicpinigaitis PV, Spinner L, Santhyadka G, Negassa A. Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung. 2008;186:369–374. doi: 10.1007/s00408-008-9114-6. [DOI] [PubMed] [Google Scholar]


