Abstract
Initial studies found that female Dahl salt-sensitive (DS) rats exhibit greater blood pressure (BP) salt sensitivity than female spontaneously hypertensive rats (SHR). On the basis of the central role played by NO in sodium excretion and BP control, we further tested the hypothesis that blunted increases in BP in female SHR will be accompanied by greater increases in renal inner medullary nitric oxide synthase (NOS) activity and expression in response to a high-salt (HS) diet compared with DS rats. Gonad-intact and ovariectomized (OVX) female SHR and DS rats were placed on normal salt (NS; 0.4% salt) or HS (4% salt) diet for 2 wk. OVX did not alter BP in SHR, and HS diet produced a modest increase in BP. OVX significantly increased BP in DS rats on NS; HS further increased BP in all DS rats, although OVX had a greater increase in BP. Renal inner medullary NOS activity, total NOS3 protein, and NOS3 phosphorylated on serine residue 1177 were not altered by salt or OVX in either strain. NOS1 protein expression, however, significantly increased with HS only in SHR, and this corresponded to an increase in urinary nitrate/nitrite excretion. SHR also exhibit greater NOS1 and NOS3 protein expression than DS rats. These data indicate that female sex hormones offer protection against HS-mediated elevations in BP in DS rats but not SHR. We propose that the relative resistance to HS-mediated increases in BP in SHR is related to greater NOS expression and the ability to increase NOS1 protein expression compared with DS rats.
Keywords: nitric oxide, Dahl-salt sensitive rats, ovariectomy, spontaneously hypertensive rats
salt sensitivity of blood pressure (BP) is associated with an increased risk for the development of hypertension and cardiovascular disease. Among women, the prevalence of salt sensitivity increases following both surgical and natural menopause (14, 38), suggesting that the loss of ovarian hormones renders women prone to salt sensitivity and increases their risk of developing hypertension (38). This is further supported by studies in experimental animals; surgical ovariectomy (OVX) increases the BP response to salt in many rat strains, including Dahl salt-sensitive (DS) and Dahl salt-resistant rats (59), mRen(2). Lewis rats (7), Sprague-Dawley rats treated with aldosterone (57), and spontaneously hypertensive rats (SHR) (10). These data suggest that female sex hormones are critical in influencing sodium handling and BP regulation; however, the mechanisms responsible continue to be investigated.
Salt-sensitive hypertension has been linked to the renin-angiotensin system, as well as a decrease in NO production and bioavailability (8, 19, 53). There is ample evidence in the literature supporting a role for increased ANG II and AT1 receptor activation in female experimental animals following ovariectomy or high-salt diet that is blocked by estrogen supplementation (12, 15, 39, 59). However, less is known regarding the role of the NO/NOS pathway in females following a high-salt diet, despite the fact that numerous studies have suggested that one of the primary mechanisms underlying the cardiovascular protective effect of estrogen is via increased NO production (31).
The renal inner medulla (IM) is the site of the final regulation of sodium reabsorption and, therefore, plays an important role in sodium and water homeostasis and in the long-term control of BP. Moreover, NO is an important regulator of medullary water and sodium excretion (18, 27). The renal inner medulla contains the highest amounts of NO synthase (NOS) protein in the kidney, with the greatest amount of NOS activity present in the inner medullary collecting duct (18, 56). More recently, renal inner medullary collecting duct NOS has been directly demonstrated to impact sodium excretion to regulate BP (16).
DS rats have been suggested to be an NO-deficient rat strain compared with SHR, and infusion of the NOS substrate l-arginine into the medulla of male DS rats prevents the development of salt-sensitive hypertension (28). Our initial studies in the current article confirmed that female DS rats exhibit greater BP salt sensitivity than female SHR. There is a lack of information in the literature regarding the mechanisms regulating BP control in hypertensive females and whether these mechanisms are similar in salt-sensitive and essential models of hypertension. Therefore, additional studies tested the hypothesis that blunted increases in BP in female SHR will be accompanied by an increase in renal inner medullary NOS activity and expression in response to a HS diet, which will not be evident in female DS rats. OVX increases BP in female DS rats, even when maintained on a normal salt diet (12, 15, 59). In contrast, BP in female SHR tends to be resistant to OVX-mediated alterations in BP (12, 49). Therefore, we further hypothesized that the effects of a high-salt diet on NOS and BP would be sex hormone-dependent in DS rats, but not SHR.
MATERIALS AND METHODS
Animals.
Gonad-intact and OVX female SHR (Taconic Farms, Germantown, NY) and DS rats (Georgia Regents University breeding colony) were studied. Rats were housed in temperature- and humidity-controlled, light-cycled quarters. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the Georgia Regents University Institutional Animal Care and Use Committee. At 10 wk of age, a subset of female SHR and DS rats underwent OVX. Average body weight at the time of OVX was 158 ± 3 g in SHR and 174 ± 4 g in DS rats. The efficacy of OVX was confirmed by measuring uterus and body weights at the time of the experiment, as previously described (5). At 12 wk of age, all rats were placed on a phytoestrogen-free normal-salt diet (NS; 0.4% NaCl, Harlan Teklad). At 14 wk of age, rats were randomized to either remain on the NS diet for an additional 2 wk or were placed on a phytoestrogen-free high-salt diet (HS; 4% NaCl, Harlan Teklad) for 2 wk. Rats were placed in metabolic cages weekly for 24-h urine collection. A subset of gonad-intact and OVX SHR and DS rats, randomized to receive HS treatment, were implanted with telemetry devices (Data Sciences, St. Paul, MN) at 11 wk of age for the continuous monitoring of BP, as previously described (36, 42). Rats were allowed 1 wk of recovery before initiating phytoestrogen-free NS diet as discussed above. At 16 wk of age, all rats were anesthetized with ketamine/xylazine (48 mg/kg and 6.4 mg/kg ip, respectively; Phoenix Pharmaceuticals, St. Joseph, MO), kidneys were removed, and the renal IM were isolated and snap-frozen in liquid nitrogen.
A separate set of intact and OVX SHR were instrumented with telemetry devices and randomized to receive either phytoestrogen-free NS (0.4% NaCl, Harlan Teklad) or phytoestrogen-free HS (4% NaCl, Harlan Teklad) diet for 8 wk. At 22 wk of age, rats were anesthetized with ketamine/xylazine (48 mg/kg and 6.4 mg/kg ip, respectively; Phoenix Pharmaceuticals), kidneys were removed and the renal IM were isolated and snap-frozen in liquid nitrogen.
Total NOS activity.
Renal IMs were homogenized in buffer in the presence of protease inhibitors, as previously described (46), and the whole homogenate was used in the NOS activity assay, as previously described (43). Briefly, total NOS activity was determined on the basis of the rate of l-[3H]citrulline formation from l-[3H]arginine and defined as l-[3H]arginine to l-[3H]citrulline conversion inhibited by the nonselective NOS inhibitor Nω-nitro-l-arginine (1 mmol/l).
Western blot analysis.
Renal inner medullary homogenates were also used in Western blot analysis, as previously described, with 50 μg of protein loaded per well (3, 48). Two-color immunoblots were performed using primary antibodies to NOS1, NOS3, and NOS3 phosphorylated on serine residue 1177 (pNOS3 Ser-1177) (1:500; Transduction Laboratories, Lexington, KY), or monoclonal antibody to actin (A1978, 1:10,000; Sigma, St. Louis, MO). Protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using BSA as the standard. Actin was used to verify equal protein loading, and data are reported normalized to actin.
Urinary analysis.
Urinary total nitrate/nitrite concentration (NOx) was measured as an index of NO using the Griess assay, according to the manufacturer's instructions (Cayman Chemicals, Ann Arbor, MI). Urinary sodium excretion was measured after diluting samples at a 1:20 ratio with deionized water using a sodium analyzer, according to the manufacturer's instructions (ELISE, Beckman Instruments, Fullerton, CA) (52).
Statistical analysis.
All data are expressed as means ± SE. Telemetry data and metabolic cage data within each group were compared using repeated-measures ANOVA; BP values between intact and OVX rats were compared using a Student's t-test. Between-group comparisons within each strain for metabolic parameters, total NOS activity, NOS protein expression, and urinary NOx excretion data were compared using a two-way ANOVA. NOS enzymatic activity and NOx excretion between strains was compared in intact or OVX SHR and DS rats using a two-way ANOVA. For all comparisons, P < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism version 5.04 software (GraphPad Software, La Jolla, CA).
RESULTS
Metabolic parameters.
In agreement with previous reports (47), OVX increased body weight in SHR, and OVX SHR remained heavier than intact females throughout the study (Table 1). Intact female SHR gained more weight when switched to a HS diet than when maintained on NS diet; a HS diet did not impact weight gain in OVX rats. Food intake was consistent in intact and OVX SHR over the course of the treatment regardless of diet, although OVX ingested more NS chow than intact females. As expected, water intake, urine output, and sodium excretion increased among intact and OVX SHR on a HS diet.
Table 1.
Metabolic parameters in SHR
| SHR | Intact-NS (10) | OVX-NS (11) | Intact-HS (14) | OVX-HS (14) |
|---|---|---|---|---|
| Body weight, g | ||||
| Baseline | 205 ± 2 | 244 ± 4† | 209 ± 3 | 243 ± 5† |
| Week 1 | 209 ± 2‡ | 265 ± 4†‡ | 223 ± 3*‡ | 267 ± 4†‡ |
| Week 2 | 217 ± 3‡ | 277 ± 4†‡ | 230 ± 2*‡ | 271 ± 4† |
| Food Intake, g/day | ||||
| Baseline | 15 ± 1 | 23 ± 1† | 19 ± 1 | 22 ± 1† |
| Week 1 | 17 ± 0 | 21 ± 2 | 18 ± 1 | 18 ± 1 |
| Week 2 | 17 ± 1 | 21 ± 0 | 17 ± 1 | 18 ± 1 |
| Water intake, ml/day | ||||
| Baseline | 28 ± 6 | 31 ± 2 | 26 ± 6 | 21 ± 1 |
| Week 1 | 31 ± 4 | 36 ± 2 | 50 ± 4*‡ | 50 ± 5*‡ |
| Week 2 | 32 ± 3 | 32 ± 3 | 50 ± 2* | 48 ± 3* |
| Urine output, ml/day | ||||
| Baseline | 20 ± 5 | 23 ± 1 | 18 ± 1 | 21 ± 2 |
| Week 1 | 24 ± 3 | 26 ± 2 | 39 ± 3*‡ | 41 ± 4*‡ |
| Week 2 | 22 ± 3 | 25 ± 2 | 48 ± 3* | 39 ± 2* |
| Urinary Na+ excretion, mmol/day | ||||
| Baseline | 0.64 ± 0.1 | 0.96 ± 0.3† | 0.78 ± 0.7 | 0.99 ± 0.1† |
| Week 1 | 0.70 ± 0.5 | 0.78 ± 0.5 | 11.73 ± 0.6*‡ | 11.66 ± 0.3*‡ |
| Week 2 | 0.76 ± 0.1 | 0.83 ± 0.1 | 11.04 ± 0.6* | 10.78 ± 0.5* |
At baseline, all rats were maintained on phytoestrogen-free normal salt diet (NS; 0.4% NaCl, Harlan Teklad). Rats were then randomized to remain on NS or were switched to phytoestrogen-free high-salt diet (HS; 4% NaCl) for an additional 2 wk. All data are expressed as means ± SE.
Significant difference from NS control of the same group [intact or ovariectomized (OVX)].
Significant difference from gonad-intact of the same treatment group (NS or HS).
Significant difference from the previous week within the same group. Numbers in parentheses refer to n values.
Similar to SHR, OVX increased body weight in DS rats, and OVX DS rats remained heavier than intact females throughout the study (Table 2). However, there were no group, diet, or temporal differences in weight among DS rats when compared using a two-way ANOVA. Food intake was comparable in all groups. Water intake, urine output, and sodium excretion increased among intact and OVX DS rats on a HS diet.
Table 2.
Metabolic parameters in Dahl SS rats
| Dahl-SS | Intact-NS (7) | OVX-NS (7) | Intact-HS (7) | OVX-HS (7) |
|---|---|---|---|---|
| Body weight ratio, g | ||||
| Baseline | 230 ± 6 | 254 ± 4† | 228 ± 4 | 260 ± 3† |
| Week 1 | 249 ± 8 | 271 ± 6† | 244 ± 6 | 282 ± 6† |
| Week 2 | 253 ± 9 | 280 ± 7† | 240 ± 5 | 280 ± 6† |
| Food intake, g/day | ||||
| Baseline | 21 ± 1 | 20 ± 1 | 21 ± 1 | 21 ± 1 |
| Week 1 | 17 ± 1 | 19 ± 1 | 17 ± 1 | 16 ± 1 |
| Week 2 | 14 ± 1 | 16 ± 1 | 14 ± 1 | 13 ± 1 |
| Water intake, ml/day | ||||
| Baseline | 20 ± 1 | 16 ± 2 | 16 ± 1 | 21 ± 1 |
| Week 1 | 19 ± 2 | 20 ± 2 | 43 ± 3*‡ | 49 ± 3*‡ |
| Week 2 | 19 ± 2 | 20 ± 2 | 45 ± 3* | 44 ± 4* |
| Urine output, ml/day | ||||
| Baseline | 14 ± 1 | 11 ± 1 | 9 ± 1 | 14 ± 1 |
| Week 1 | 12 ± 2 | 19 ± 4 | 36 ± 3*‡ | 44 ± 3*‡ |
| Week 2 | 11 ± 1 | 15 ± 2 | 27 ± 3* | 37 ± 3*† |
| Urinary Na+ excretion, mmol/day | ||||
| Baseline | 0.79 ± 0.1 | 0.78 ± 0.5 | 0.64 ± 0.1 | 0.81 ± 0.1 |
| Week 1 | 0.68 ± 0.1 | 0.93 ± 0.1 | 11.28 ± 0.8*‡ | 11.06 ± 0.1*‡ |
| Week 2 | 0.69 ± 0.1 | 0.80 ± 0.1 | 10.05 ± 0.6* | 10.96 ± 0.3* |
At baseline, all rats were maintained on phytoestrogen-free NS diet (0.4% NaCl; Harlan Teklad). Rats were then randomized to remain on NS or were switched to phytoestrogen-free HS diet (4% NaCl) for an additional 2 wk. All data are expressed as means ± SE.
Significant difference from NS control of the same group (intact or OVX).
Significant difference from gonad-intact of the same treatment group (NS or HS).
Significant difference from the previous week within the same group. Numbers in parentheses refer to n values.
Blood pressure response to 2 wk HS in SHR and DS rats.
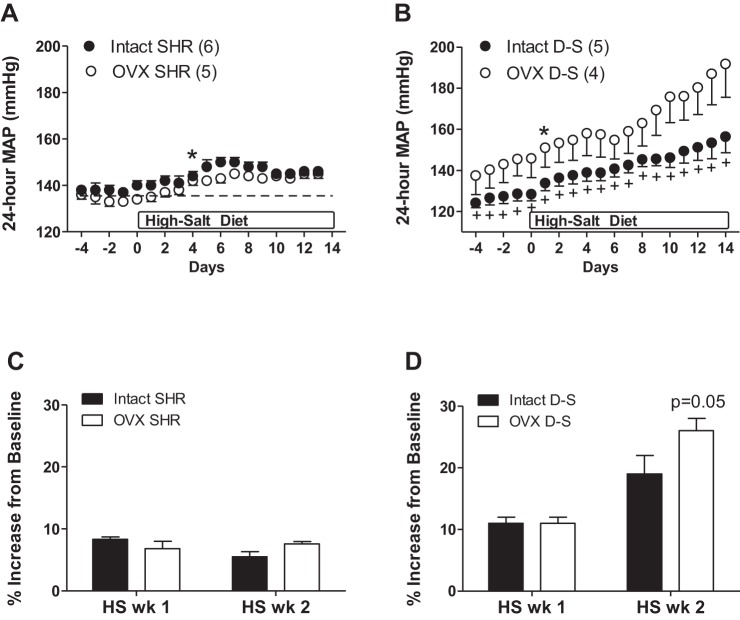
BP was comparable in intact and OVX female SHR at baseline (Fig. 1A). HS diet resulted in a slight, but significant, increase in BP in both intact and OVX SHR following 4 days of HS diet. There were no further increases in BP for the remainder of the 2 wk of HS diet and the increase in BP was comparable between intact and OVX SHR at the end of 2 wk (Fig. 1C). In contrast, OVX DS rats exhibited a higher BP than intact rats at baseline, which was maintained throughout the study (Fig. 1B). A HS diet significantly increased BP in both intact and OVX DS rats following 1 day of HS. Following 1 wk of HS treatment, there was a comparable percent increase in BP between the two groups; however, OVX rats had a greater further increase in BP in the second week of HS compared with intact rats (Fig. 1D).
Fig. 1.
Twenty-four-hour mean arterial pressure (MAP) in response to a high-salt (HS) diet in gonad-intact and ovariectomized (OVX) spontaneously hypertensive rats (SHR; A) and Dahl salt-sensitive rats (DS; B). C and D: percent increase in MAP from baseline in response to 1 and 2 wk of HS diet. In D, P = 0.05 indicates significant difference from OVX DS following 2 wk of HS. *Significant increase from baseline MAP. +Significant difference from same-strain OVX. Numbers in parentheses refer to n values.
Total NOS enzymatic activity and protein expression in the renal IM.
To test the hypothesis that rat strain impacts the effect of salt on NOS protein expression and activity, renal inner medullary NOS enzymatic activity and expression were measured in intact and OVX SHR and DS rats maintained on a NS or HS diet for 2 wk. Total NOS activity was comparable in intact and OVX SHR (Fig. 2A) and DS rats (Fig. 2B) regardless of diet. NOS enzymatic activity was also compared in intact or OVX SHR and DS rats. For both comparisons, SHR have greater NOS activity than DS rats (intact rats: strain, P = 0.03; OVX rats: strain, P = 0.003). There was no significant effect of salt (intact rats: salt, P = 0.8; OVX rats: salt, P = 0.4) or interaction (intact rats: interaction, P = 0.8; OVX rats: interaction, P = 0.5).
Fig. 2.
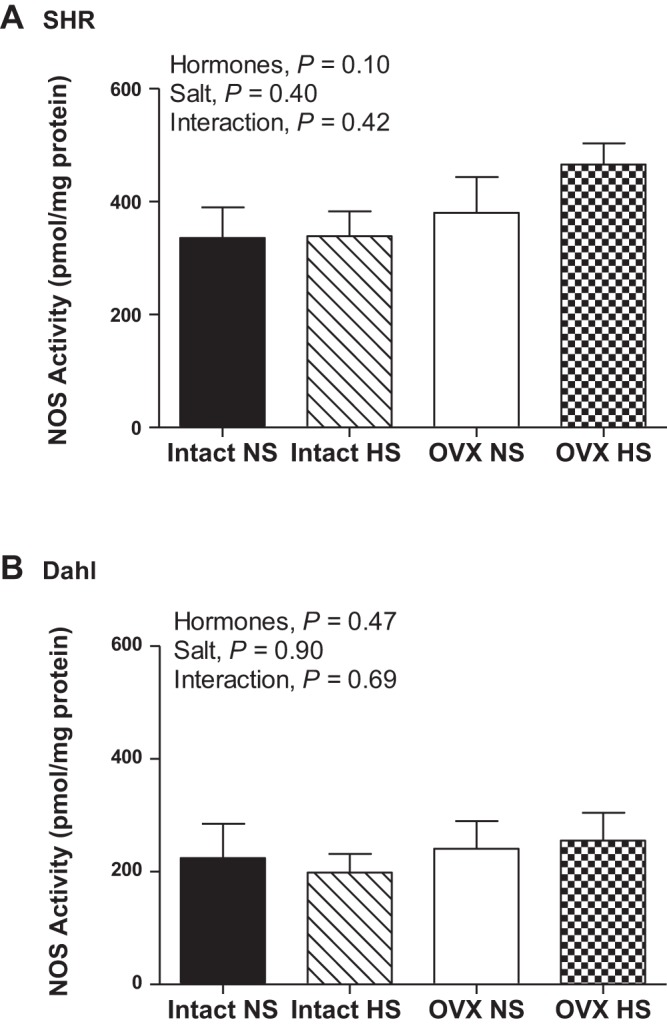
Total nitric oxide synthase (NOS) enzymatic activity in the renal inner medulla (IM) following 2 wk of normal-salt (NS) or high-salt (HS) diet in intact and OVX SHR (A; n = 8–11) and DS rats (B; n = 6 or 7).
Despite no changes in NOS enzymatic activity, NOS1 protein expression was significantly increased by HS in intact and OVX SHR (Fig. 3A). NOS1 protein expression was not altered by OVX or HS in DS rats (Fig. 3B). NOS3 protein expression was not significantly altered by HS or OVX in either SHR or DS rats (Fig. 4). Phosphorylation of NOS3 on serine residue 1177 has been identified as an estrogen-sensitive phosphorylation site (13); however, neither OVX nor HS significantly impacted pNOS3 Ser-1177 protein expression in either SHR or DS rats either when normalized to actin (Fig. 5) or to total NOS3 protein expression (SHR: effect of hormones, P = 0.7; salt: P = 0.8; interaction: P = 0.5; and DS: effect of hormones: P = 0.3; salt: P = 0.9; interaction: P = 0.9).
Fig. 3.
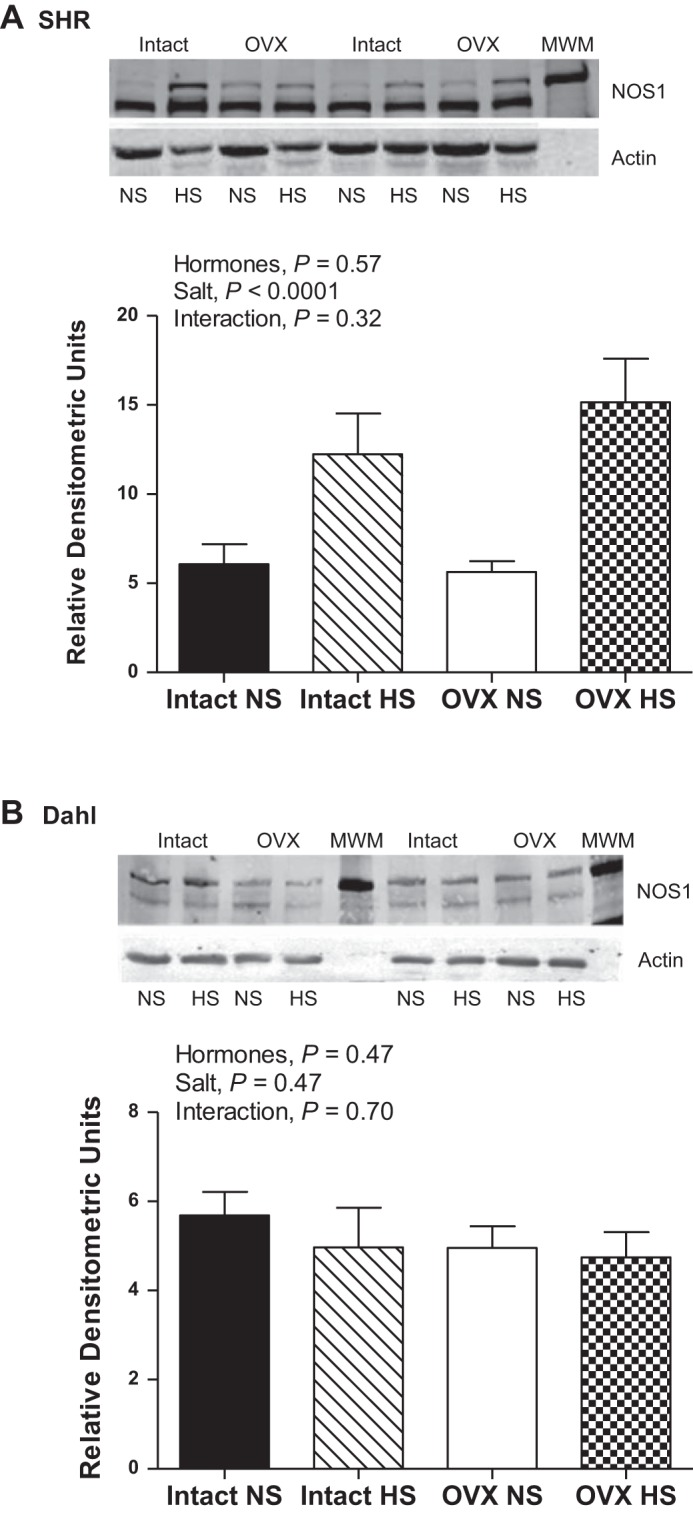
NOS1 protein expression in renal IM following 2 wk of NS or HS diet in intact and OVX SHR (A; n =11–13) and DS rats (B; n = 6 or 7). The top immunoreactive NOS1 band corresponds to NOS1α (160 kDa) and the bottom immunoreactive band corresponds to NOS1β (130 kDa).
Fig. 4.
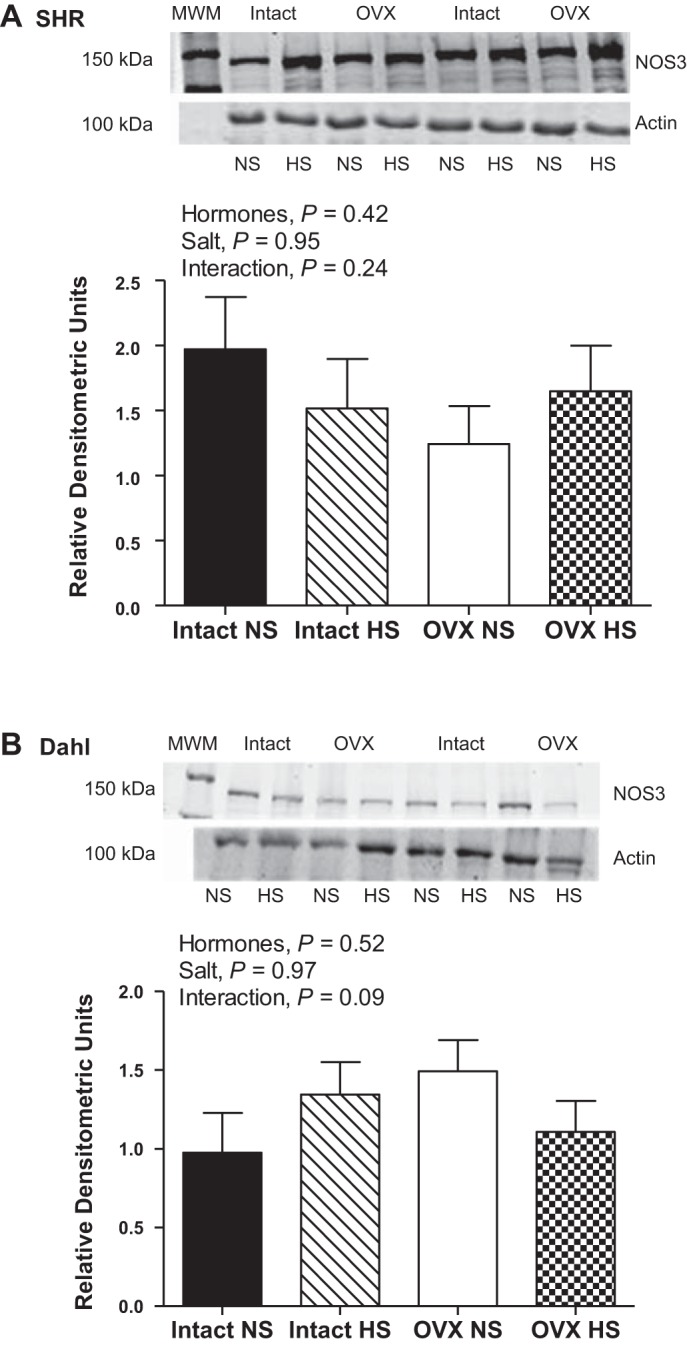
NOS3 protein expression in renal IM following 2 wk of normal-salt (NS) or high-salt (HS) diet in intact and ovariectomized (OVX) SHR (A; n = 10 or 11) and DS rats (B; n = 6 or 7).
Fig. 5.
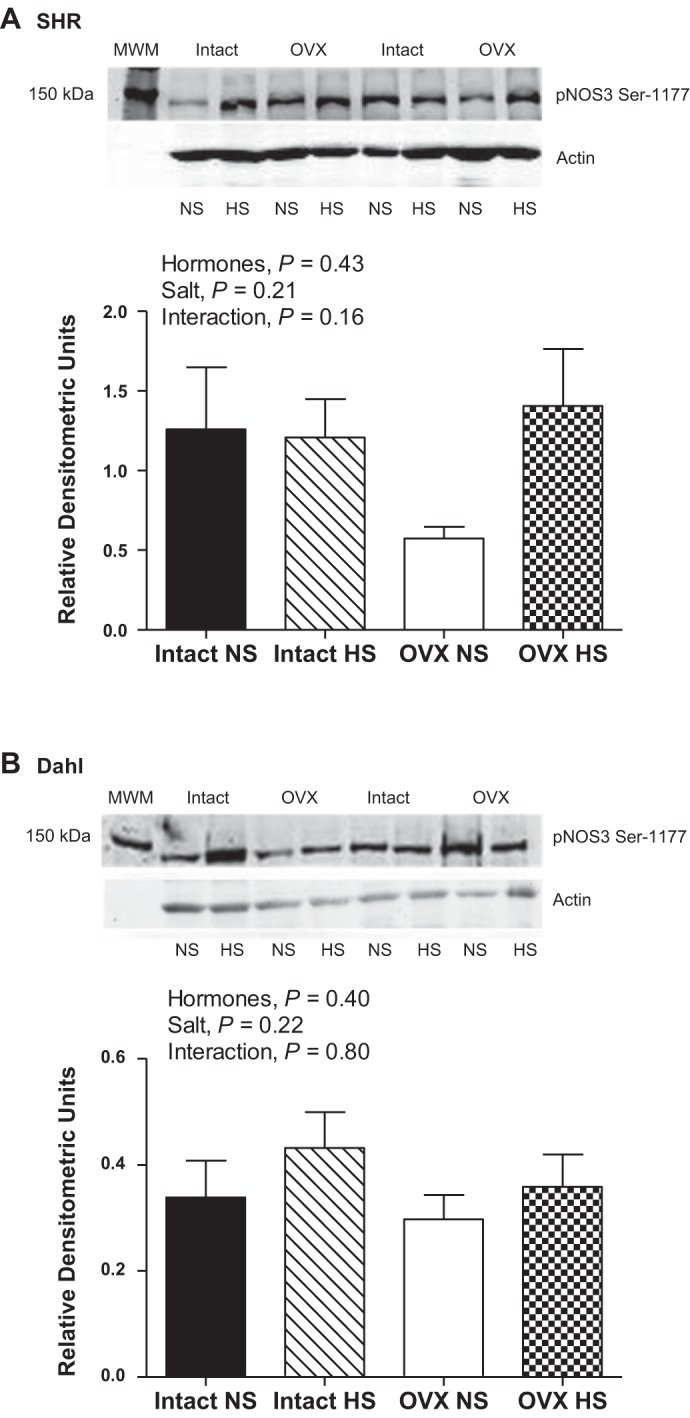
Protein expression of NOS3 phosphorylated on serine residue 1177 (pNOS3 Ser-1177) in renal IM following 2 wk of NS or HS diet in intact and OVX SHR (A: n = 6 or 7) and DS rats (B; n = 6).
Urinary NOx excretion.
Nitrate and nitrite are stable breakdown products of NO and can be used to assess NO bioavailability. HS diet induced an increase in NOx excretion in both gonad-intact and OVX SHR, although OVX alone had no impact on NOx excretion (Fig. 6A). In contrast, HS diet did not alter NOx excretion in DS rats and OVX decreased NOx excretion (Fig. 6B). NOx excretion was also compared in intact or OVX SHR and DS rats. For both comparisons, SHR have greater NOx excretion than DS rats (intact rats: strain, P = 0.002; OVX rats: strain, P = 0.01) and HS increased urinary NOx only in SHR (intact: interaction, P = 0.01; OVX: interaction, P = 0.01).
Fig. 6.
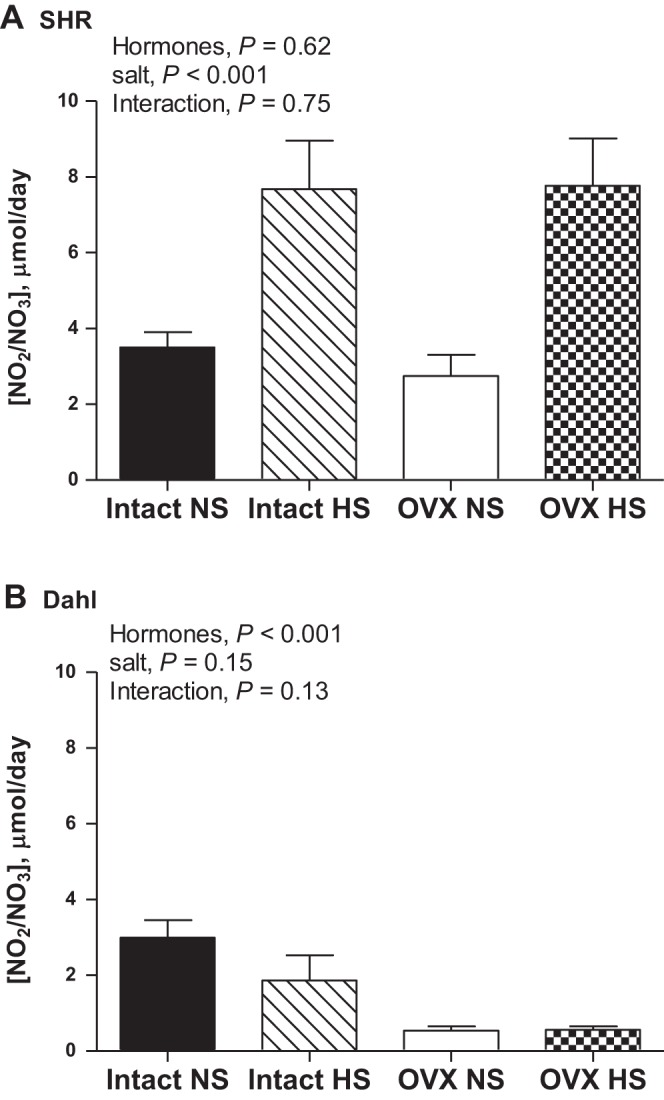
Urinary nitrate/nitrite (NOx) excretion following 2 wk of NS or HS diet in intact and OVX SHR (A; n = 10) and DS rats (B; n = 6 or 7).
NOS expression in SHR vs. DS.
NOS1 and NOS3 protein expression was also directly compared in the renal IM of female SHR and DS rats on a NS diet. Female SHR exhibited greater NOS1 (P = 0.001; Fig. 7A) and NOS3 (0.006; Fig. 7B) protein expression than DS rats.
Fig. 7.

NOS1 (A) and NOS3 (B) protein expression in renal IM of gonad-intact female SHR and DS rats on a NS diet; n = 6. The top immunoreactive NOS1 band corresponds to NOS1α (160 kDa), and the bottom immunoreactive band corresponds to NOS1β (130 kDa). MWM indicates the molecular weight marker.
Blood pressure response to 8 wk HS in SHR.
To determine whether the attenuated salt-sensitive hypertension in OVX female SHR relative to DS rats was due to a delayed increase in BP, additional intact and OVX SHR were maintained on a HS diet for 8 wk. Similar to what was observed in the 2-wk study, BP was comparable in intact and OVX SHR, and BP was significantly increased following 4 days of HS (Fig. 8A). However, maintaining female SHR on a HS diet for an additional 6 wk did not significantly increase BP beyond that observed in 2 wk. Intact females exhibited a 4 ± 1% increase from baseline MAP following 2 wk and a 6 ± 1% increase following 8 wk. Similarly, OVX rats exhibited a 5 ± 1% increase from baseline MAP following 2 wk and a 6 ± 1% increase following 8 wk. Furthermore, 8 wk on a HS diet did not reveal an impact of female sex hormones on renal inner medullary NOS activity (Fig. 8B).
Fig. 8.
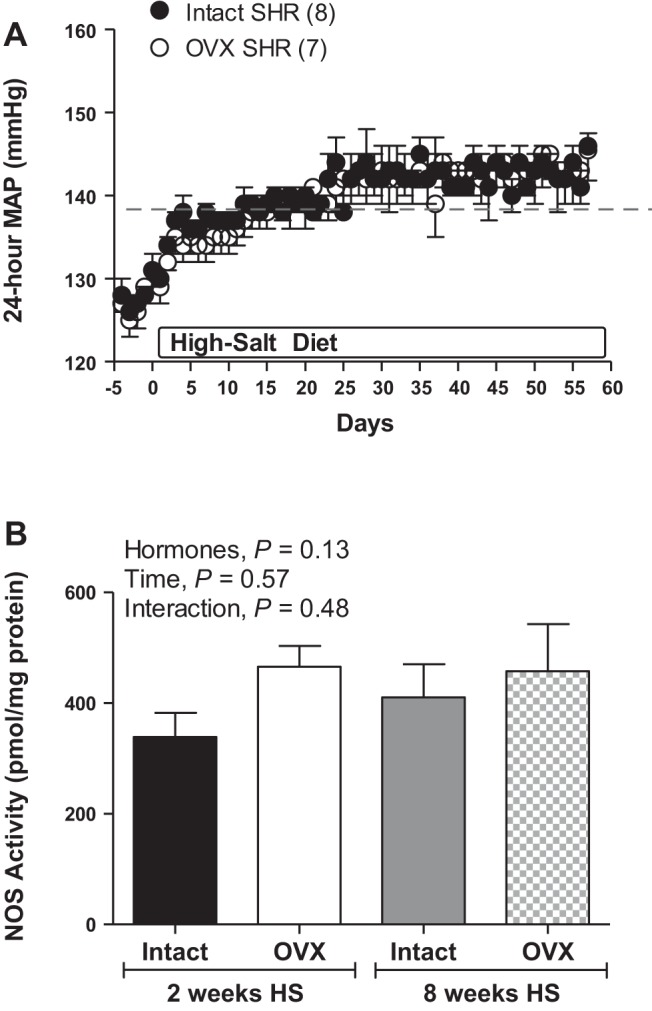
Twenty-four-hour MAP in response to a HS diet in gonad-intact and OVX SHR (A; n = 7). The dashed line represents the average increase in MAP after 2 wk. Total NOS enzymatic activity in renal IM following 2 or 8 wk of HS diet in intact and OVX SHR (B; n = 7–10).
DISCUSSION
The current study confirmed that, as hypothesized, female DS rats have greater BP salt sensitivity than female SHR. To gain insight into the mechanisms by which female SHR resist salt-induced increases in BP, we examined the impact of sex hormones on HS diet-induced changes in NOS expression, activity, NOx excretion, and BP. The major novel findings of the present study are 1) female sex hormones attenuate salt-induced increases in BP and increase NOx excretion in DS rats, but not SHR; 2) despite no change in NOS enzymatic activity in either strain by hormones or salt, female SHR exhibit a sex hormone-independent increase in renal inner medullary NOS1 protein expression and urinary NOx excretion following a HS diet that was not apparent in DS rats; and 3) female SHR have greater renal inner medullary NOS protein expression and activity than female DS rats. We propose that female SHR upregulate NOS1 expression and NO production in response to HS, which contributes to their lower BP sensitivity to a HS diet. Taken together, our data suggest that the mechanisms by which these two hypertensive rat strains modulate BP differ and underscore the importance of expanding our understanding of how females modulate BP under both physiological and pathological situations.
Female sex hormones have been widely suggested to protect against cardiovascular disease and the development of salt sensitivity in premenopausal women and experimental animals (35, 40). Consistent with previous reports in the literature, OVX increased basal BP in female DS rats but not in SHR (12, 15, 59). Initiation of a HS diet increased BP in both strains, although as expected, DS had a faster and more pronounced increase in BP than SHR. Also consistent with previous reports, OVX of DS rats exacerbated HS-mediated increases in BP (59); BP in SHR on a HS diet was not altered by OVX. Interestingly, it has been reported that early OVX of female SHR prior to sexual maturation (3 wk of age) resulted in a measurable increase in BP not seen in rats maintained on a phytoestrogen-replete diet (10). Gonad-intact female SHR also exhibited an increase in BP when the HS diet was initiated at 3 wk of age, although the increase was attenuated compared with the OVX rats (10). These data suggest that the timing of OVX and the age when a HS diet is started are critical determinants of the impact of a HS diet on BP in female SHR.
Renal medullary NOS is a key regulator of medullary blood flow and sodium and water balance, and inhibition of medullary NOS1 in male rats results in hypertension (25–27). It was recently shown that selective deletion of NOS1 from principal cells of the renal collecting duct in male mice results in a salt-sensitive BP phenotype (16), directly implicating renal NOS1 in BP salt sensitivity. The two immunoreactive NOS1 bands detected via Western blot analysis in the rat kidney correspond to NOS1α and NOS1β (16, 20). HS diet-induced increases in renal NO production in male Sprague-Dawley rats is associated with greater increases in NOS1β than NOS1α, suggesting a critical role for NOS1β, in particular, in responding to a HS diet in male rats (17, 20). NOS1 densitometric values reported in the current study include both NOS1 splice variants.
We previously reported that female SHR have greater renal inner medullary NOS activity and NOS1 protein expression than males, and female SHR are more dependent on NOS to maintain their BP than males (2, 45). Relative to SHR, DS rats have been suggested to be an NO-deficient rat strain (1, 4, 59), although few studies have directly compared NOS in the two strains. On the basis of these data, we hypothesized that female SHR would have a greater increase in renal inner medullary NOS in response to a HS diet compared with DS rats. The results of the current study support an important role for NOS1 to limit BP sensitivity to HS in female SHR. Similarly, the inability of female DS rats to increase NOS1 protein expression or urinary NOx levels in response to HS likely contributes to their exacerbated increase in BP. It should be noted that the majority of data in the literature supporting a link between NO and salt has been performed in males, yet the current study only examined females. Potential strain differences in male SHR and DS rats in NOS and BP responses to HS have yet to be examined; however, consistent with our findings, NOS1 mRNA levels are less in male DS rats compared with Dahl salt-resistant (DR) rats and the HS diet increased NOS1 only in DR rats (6). Moreover, the authors suggested that increased NOS1 activity in DR rats prevented the development of salt-sensitive hypertension.
OVX was not associated with changes in NOS expression or activity in either strain regardless of the diet. Female sex hormones consistently increase NO production in vitro (32), although in vivo studies have yielded conflicting results (11). Consistent with our current results, we have previously published that manipulation of female sex hormones in SHR does not alter NOS enzymatic activity or expression in the renal IM (45) or cortex (44). In agreement with our results, OVX in Sprague-Dawley rats (33), DS rats (on 0.1% NaCl) (22), or Fischer-344 rats (37) does not alter renal NOS expression. OVX was also not associated with significant changes in NOS3 phosphorylation on serine residue 1177. This result is consistent with previous reports in SHR directly measuring NOS3 phosphorylation and studies in female DS rats, which found that expression of phospho-Akt is not altered by OVX or estrogen supplementation (22, 44). The protein kinase Akt increases NOS3 activity by enhancing its phosphorylation on Ser-1177 (9). However, in contrast to these findings, renal NOS protein expression decreases following OVX of hypertensive congenic female mRen(2). Lewis rats and Sprague-Dawley rats (33, 58). On the basis of differences in the age at which animals were ovariectomized and the duration of time allowed to pass post-OVX before study, these data suggest that the age at OVX and strain may also be critical determinants of the impact on NOS.
OVX differentially impacted BP and NOS in DS rats. BP was altered independent of NOS expression and activity in female DS rats, although OVX decreased NOx excretion. On the basis of the lack of changes in NOS activity and expression, this decrease may reflect an increase in oxidative stress and scavenging of NO. Indeed, HS has been shown to increase renal and vascular superoxide production in male and female DS rats (21). These data support studies in the literature indicating that the DS rat strain is a NOS-deficient strain, and, as a result, female DS rats are likely less dependent on NO for BP control. Interestingly, increases in BP following OVX in DS rats were accompanied by a decrease in NOx excretion that was not apparent in SHR. Therefore, while DS rats have lower levels of NOS expression, activity, and NOx excretion than SHR, female sex hormone-mediated NO production may contribute to basal BP control in DS rats.
We previously published that BP in female SHR is extremely sensitive to NOS inhibition, and salt-induced increases in BP in the current study were accompanied by an increase in NOS1 expression; however, this increase was hormone-independent. The mechanism by which salt induced NOS1 protein expression in female SHR was not examined. However, high-salt intake has been consistently shown in male mice and rats to increase urinary and plasma NOx (16, 17, 54). In addition, NO plays an important role in salt excretion by increasing medullary blood flow (24, 27) and by direct inhibition of sodium and chloride reabsorption (34, 41). Although NOS3 is highly expressed in endothelial cells throughout the kidney, thick ascending limb, inner medullary collecting duct, and proximal tubules (30), NOS3 expression and phosphorylation were not altered by HS in either strain. Consistent with our results in the current study, data from our laboratory and others have shown that a HS diet does not alter renal inner medullary NOS3 expression, total renal NOS3 expression, or NOS3 phosphorylation in male Sprague-Dawley rats, male DR rats, or male DS rats (6, 29, 50). Therefore, we suggest that increased expression of renal NOS1 in SHR represents an adaptive response to maintain NO production to increase salt excretion and maintain BP.
The increase in NOS1 protein expression in female SHR with a HS diet was not accompanied by an increase in total NOS enzymatic activity. Indeed, NOS activity was equivalent between SHR on NS and HS diets, despite marked differences in NOS1 protein expression. Since both protein expression and activity are reported normalized to total protein expression, this may suggest that HS actually reduced NOS enzymatic capacity in renal IM, although this would not be supported by the increase in urinary NOx excretion with HS in the female SHR. It should be noted that the NOS activity assay reflects the potential of all NOS isoforms present to produce NO and is not a measurement of in vivo NO production. Moreover, isoform-specific activity was not assessed, so it is unknown whether there were isoform-specific changes in NOS activity. The NOS activity assay was performed in the presence of optimal concentrations of cofactors, which may not be the case in vivo. For example, Dahl rats have been demonstrated to have high levels of the endogenous NOS inhibitor ADMA relative to SHR (23). In addition, although NOS typically catalyzes the conversion of l-arginine to NO and l-citrulline, the enzyme can also be uncoupled to produce superoxide, and this would not be differentiated in our assay. In particular, when levels of the cofactor tetrahydrobiopterin (BH4) are limited, NOS is uncoupled (55), and male DS rats have decreased BH4 availability and uncoupled NOS in the renal medulla (51).
Perspectives and Significance
Both the increase in salt consumption and the loss of female sex hormones are known to contribute to the progression of hypertension in humans. In this study, we confirmed that there are different mechanisms modulating the response to a HS diet in two different models of hypertension. DS rats are widely used as a model of salt sensitivity, often representing the phenotype of hypertensive African Americans, and SHR are a major model of primary hypertension. Close to half of the clinical population with hypertension are women, yet the majority of basic science studies continue to focus on males. The results of the current study provide insight into differences by which female models of salt-sensitive and essential hypertension differentially respond to a HS challenge. With consumption of salt continuing to increase in the Western world, understanding how females respond to a salt challenge mechanistically will provide needed insight into increasing BP control rates in all hypertensive individuals.
GRANTS
The authors acknowledge funding from the American Heart Association and the NIH (to JCS: Scientist Development Grant and HL093271-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.N.B. and J.C.S. conception and design of research; K.N.B. performed experiments; K.N.B., O.R., and J.C.S. analyzed data; K.N.B., O.R., and J.C.S. interpreted results of experiments; K.N.B. and J.C.S. prepared figures; K.N.B., O.R., and J.C.S. drafted manuscript; K.N.B., O.R., and J.C.S. edited and revised manuscript; K.N.B., O.R., and J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge Hiram Ocasio for his excellent technical expertise.
REFERENCES
- 1.Barton M, Vos I, Shaw S, Boer P, D'Uscio LV, Grone HJ, Rabelink TJ, Lattmann T, Moreau P, Luscher TF. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. J Am Soc Nephrol 11: 835–845, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T-cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol 305: R701–R710, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy CM, Knoepp L, Xin J, Pollock JS. Functional expression of NOS 1 in vascular smooth muscle. Am J Physiol Heart Circ Physiol 278: H991–H997, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Mu JJ, Fang Y, Yuan ZY, Liu FQ. Impact of high-salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of Dahl salt-sensitive rats. Int J Mol Sci 14: 8062–8072, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther 291: 524–530, 1999 [PubMed] [Google Scholar]
- 6.Castrop H, Kurtz A. Differential nNOS gene expression in salt-sensitive and salt-resistant Dahl rats. J Hypertens 19: 1223–1231, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2). Lewis rat. Am J Physiol Regul Integr Comp Physiol 291: R1557–R1563, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Cubeddu LX, Alfieri AB, Hoffmann IS, Jimenez E, Roa CM, Cubeddu R, Palermo C, Baldonedo RM. Nitric oxide and salt sensitivity. Am J Hypertens 13: 973–979, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl-sensitive hypertension in female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 281: R1934–R1939, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hamilton CA, Groves S, Carswell HV, Brosnan MJ, Graham D, Dominiczak AF. Estrogen treatment enhances nitric oxide bioavailability in normotensive but not hypertensive rats. Am J Hypertens 19: 859–866, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension 42: 1157–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hernandez Schulman I, Raij L. Salt sensitivity and hypertension after menopause: role of nitric oxide and angiotensin II. Am J Nephrol 26: 170–180, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats. Clin Exp Pharmacol Physiol 40: 233–239, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kopkan L, Cervenka L. Renal interactions of renin-angiotensin system, nitric oxide and superoxide anion: implications in the pathophysiology of salt-sensitivity and hypertension. Physiol Res 58 Suppl 2: S55–S67, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD, Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning RD, Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand 179: 243–250, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 β-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med 5: 147–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, Yasukawa H, Iwami G, Okuda S, Imaizumi T. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension 29: 242–247, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Mattson DL, Bellehumeur TG. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension 28: 297–303, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr. Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol Heart Circ Physiol 266: H1918–H1926, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Mattson DL, Roman RJ, Cowley AW., Jr. Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension 19: 766–769, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr. Renal medullary interstitial infusion of l-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 275: R1667–R1673, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Mount PF, Fraser SA, Watanabe Y, Lane N, Katsis F, Chen ZP, Kemp BE, Power DA. Phosphorylation of neuronal and endothelial nitric oxide synthase in the kidney with high and low salt diets. Nephron Physiol 102: 36–50, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 187: 433–446, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev 12: 293–300, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nechmad A, Merin G, Schwalb H, Shimon DV, Borman JB, Milgalter E, Mosseri M. Estrogen induces nitric oxide-mediated vasodilation of human mammary arteries in vitro. Nitric Oxide 2: 460–466, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol 8: 1240–1246, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Pallone TL, Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens 11: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens 17: 994–1001, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sasser JM, Akinsiku O, Moningka NC, Jerzewski K, Baylis C, LeBlanc AJ, Kang LS, Sindler AL, Muller-Delp JM. Sexual dimorphism in development of kidney damage in aging Fischer-344 rats. Gend Med 9: 219–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension 47: 1168–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Shenoy V, Grobe JL, Qi Y, Ferreira AJ, Fraga-Silva RA, Collamat G, Bruce E, Katovich MJ. 17β-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides 30: 2309–2315, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Sowers JR, Lester M. Hypertension, hormones, and aging J Lab Clin Med 135: 379–386, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan JC, Giulumian AD, Pollock DM, Fuchs LC, Pollock JS. Functional NOS 1 in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 283: H658–H663, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Sullivan JC, Pardieck JL, Brinson K, Kang KT. Effects of estradiol on renal cyclic guanosine monophosphate and oxidative stress in spontaneously hypertensive rats. Gend Med 6: 498–510, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension 39: 597–602, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 45: 406–411, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R764–R768, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JC, Smart EJ, Pollock DM, Pollock JS. Influence of salt on subcellular localization of nitric oxide synthase activity and expression in the renal inner medulla. Clin Exp Pharmacol Physiol 35: 120–125, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr. NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension 41: 657–662, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Varagic J, Ahmad S, Brosnihan KB, Habibi J, Tilmon RD, Sowers JR, Ferrario CM. Salt-induced renal injury in spontaneously hypertensive rats: effects of nebivolol. Am J Nephrol 32: 557–566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem 274: 26736–26742, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Xue B, Badaue-Passos D, Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17β-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaleyeva LM, Gallagher PE, Vinsant S, Chappell MC. Discoordinate regulation of renal nitric oxide synthase isoforms in ovariectomized mRen2. Lewis rats. Am J Physiol Regul Integr Comp Physiol 292: R819–R826, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Zheng W, Ji H, Maric C, Wu X, Sandberg K. Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 294: H1508–H1513, 2008 [DOI] [PubMed] [Google Scholar]