Abstract
Circadian rhythms in glucocorticoids are the product of interactions between the hypothalamic-pituitary-adrenal (HPA) axis and the mammalian clock gene system. The adrenal clock can generate the glucocorticoid rhythm that in turn synchronizes other peripheral clocks to maintain homeostasis. Stress acutely activates and chronically upregulates the HPA axis, suggesting that the adrenal clock could be modulated by stress. However, there is no direct evidence that stress affects the adrenal clock rhythm. We tested the hypothesis that a model of chronic subordination stress (CSS) that has a major impact on HPA axis regulation, metabolism, and emotional behavior alters adrenal and pituitary clock gene rhythms. Clock gene rhythms were assessed using mPER2::Luciferase (PER2Luc) knockin mice in which in vitro bioluminescence rhythms reflect the Per2 clock gene expression. PER2Luc mice that experienced CSS for 2 wk showed positive energy balance reflected by increased body weight and food intake. Additionally, CSS phase advanced the adrenal (∼2 h) and the pituitary (∼1 h) PER2Luc rhythm compared with control mice. The activity rhythm was not affected. The adrenal clock phase shift was associated with increased feed conversion efficiency, suggesting that the metabolic phenotype in CSS mice may be related to altered adrenal clock rhythmicity. Interestingly, a single subordination experience followed by 8 h sensory housing also phase advanced the adrenal, but not the pituitary, PER2Luc rhythm. Overall, these data demonstrate a stress-induced phase shift in a peripheral clock gene rhythm and differential stress sensitivity of two peripheral clocks within the HPA axis, suggesting a link between clock desynchrony and individual vulnerability to stress.
Keywords: subordination stress, adrenal clock, circadian rhythm, corticosterone
the circadian clock system and the hypothalamus-pituitary-adrenal (HPA) system interact at multiple levels and are fundamental for survival (29). The circadian system imparts rhythmicity to physiological functions, while the HPA system ensures reactivity to unpredictable environmental stimuli. The mammalian master clock, located in the hypothalamic suprachiasmatic nucleus (SCN), is entrained by light and synchronizes other oscillators or clocks found in most peripheral tissues (27). Circadian clocks are composed of interlocking transcriptional-translational feedback loops that cause oscillating expression of canonical clock genes (Clock, Bma1l, Per, and Cry), that in turn act as transcription factors to regulate clock-controlled genes in a rhythmic fashion (33). The HPA axis is a stress-response circuit that controls the secretion of adrenal glucocorticoids (corticosterone in rodents). Stress-induced activation of discrete brain circuits leads to excitation of neurosecretory neurons in the hypothalamic paraventricular nucleus (PVN), resulting in the release of corticotropin-releasing hormone (CRH) and vasopressin, which in turn induces adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary to activate adrenal secretion of glucocorticoids (10, 44).
Glucocorticoids exhibit a robust circadian rhythm, with a peak occurring at the onset of the daily activity phase. Lesions of the SCN abolish the daily rhythm (1, 32), indicating that SCN activity drives glucocorticoid rhythms. The SCN regulates adrenal corticosteroid secretion by controlling ACTH release via rhythmic release of CRH from the PVN (19) and via sympathetic connections to the adrenal cortex (17, 43). In addition to extrinsic control, an adrenal clock has been implicated in regulation of circadian rhythms in corticosterone. The adrenal cortex expresses canonical clock gene rhythms (8), and selective disruption of the adrenal cortical clock suppresses the daily corticosterone rhythm (30, 40), demonstrating the capability of the adrenal clock to generate a circadian rhythm in corticosterone.
The circadian timing system and the HPA axis interact to affect rhythms in glucocorticoid secretion. For example, daily food restriction results in parallel shifts in HPA clock gene rhythms and functional activity (15). This integration permits rhythmic release of glucocorticoids with precise timing to support daily metabolic and cognitive demands, yet remains highly adaptive to respond to the homeostatic needs of the animal. Since rhythmic glucocorticoids are important for entrainment of peripheral clocks (2, 20, 31) and regulating clock genes in brain nuclei that control motivation and emotional states (37), stress by altering the timing of the adrenal and the anterior pituitary clock could have adverse effects on glucocorticoid-dependent rhythms.
We examined this hypothesis by evaluating adrenal and anterior pituitary clock gene rhythms in mice exposed to chronic subordination stress (CSS), a model in which mice face inescapable defeat and chronic sensory housing with an aggressor (4, 5, 11, 34). To assess whether CSS affects the timing of the adrenal and anterior pituitary clock rhythm, we used mPER2Luc mice in which in vitro rhythms in bioluminescence reflect the response to in vivo manipulation (12, 36). The present results show how the social subordination status over time induced hyperphagia, weight gain, and HPA axis upregulation. Remarkably, CSS induced a phase shift in the adrenal and pituitary gland in absence of changes in the activity rhythm.
MATERIALS AND METHODS
Animals.
Homozygous male mPER2::Luc mice (2–5 mo old) on a C57Bl/6 background bred in-house and male wild-type C57Bl/6 mice (3–4 mo old) obtained from Charles River were used as subordinate mice. Male CD-1 mice (3–4 mo old) obtained from Charles River were used as dominant resident animals. All mice were maintained in a 12:12 h light-dark cycle [Zeitgeber time (ZT)0 = lights on at 0500 h] at 22 ± 2°C. Mice were fed a standard diet (3.1 kcal/g, 18% kcal from fat; 2018 Tecklad, Harlan). Animals were maintained and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” Experimental procedures were approved by the University of Minnesota Animal Care and Use Committee.
CSS protocol.
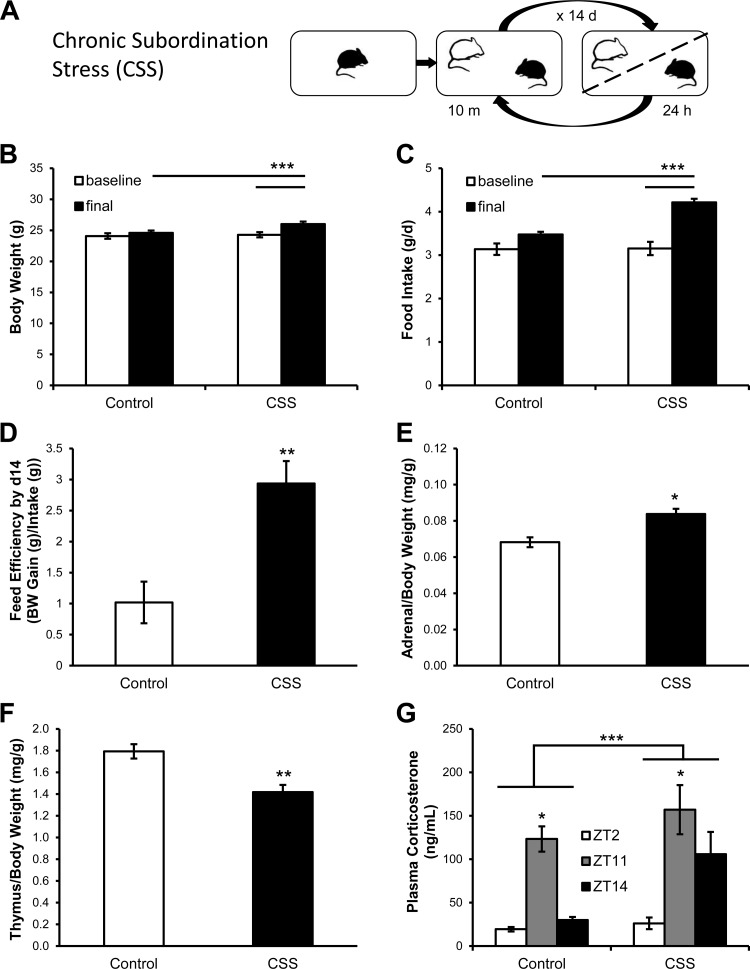
The protocol used is a modified version of our standard procedure (3–5) (Fig. 1A). Stable resident/intruder pairs of adult male mice were established after a baseline period lasting 5 days during which all subjects were individually housed. At the beginning of the 2-wk stress phase, each CD-1 male resident mouse received a PER2Luc or C57Bl/6 intruder mouse and the two animals were allowed to freely interact for a maximum of 10 min between ZT3 and ZT3.5 (0800–0830 h). The 10-min interaction took place on a daily basis throughout the 14-day stress phase. After the interaction, the two animals were separated by a perforated partition, which allowed continuous visual, auditory, and olfactory contact but no physical interaction throughout the remainder of the 24 h. All CD-1 mice become dominant. Age- and weight-matched mice were included as the control group and subjected to daily gentle handling for the duration of the experiment at the same ZT. Food intake was quantified every day for the during the entire experiment. Body weight was measured every other day. The feed conversion efficiency was calculated as the ratio of body weight gain and food intake over the 2-wk stress period. Locomotor activity was determined throughout the experiments by means of an automated system that used small passive infrared sensors positioned on the top of each cage (ActiMeter, TechnoSmart, Rome, Italy). This system allowed a continuous monitoring of mouse locomotor activity, except during the aggressive interaction. The period of the activity rhythm was analyzed using the ActogramJ software package (Department of Neurobiology and Genetics, University of Wuerzburg, Germany). After 2 wk, CSS and control mice were euthanized by decapitation at ZT11; trunk blood was collected for corticosterone analysis, and adrenals and anterior pituitaries were removed and processed for recording bioluminescence.
Fig. 1.
Chronic subordination stress (CSS) altered metabolic function and activated the hypothalamus-pituitary-adrenocortical (HPA) axis. A: schematic of the experimental procedure. Over a 14-day period, subordinate mice (black) undergo social interaction with dominant mice (white) for 10 min daily and then are housed together separated by a partition for the remaining 24 h. CSS increased body weight (B), food intake (C), feed conversion efficiency (D), adrenal weight (E), and thymus involution (F) (control, n = 19; CSS, n = 19). G: CSS increased plasma corticosterone [Zeitgeber time (ZT)2: control, n = 14, CSS, n = 19; ZT11: control, n = 24, CSS, n = 18; ZT14: control, n = 6, CSS, n = 6]. *P < 0.05, **P < 0.01, ***P < 0.0001.
Single subordination stress protocol.
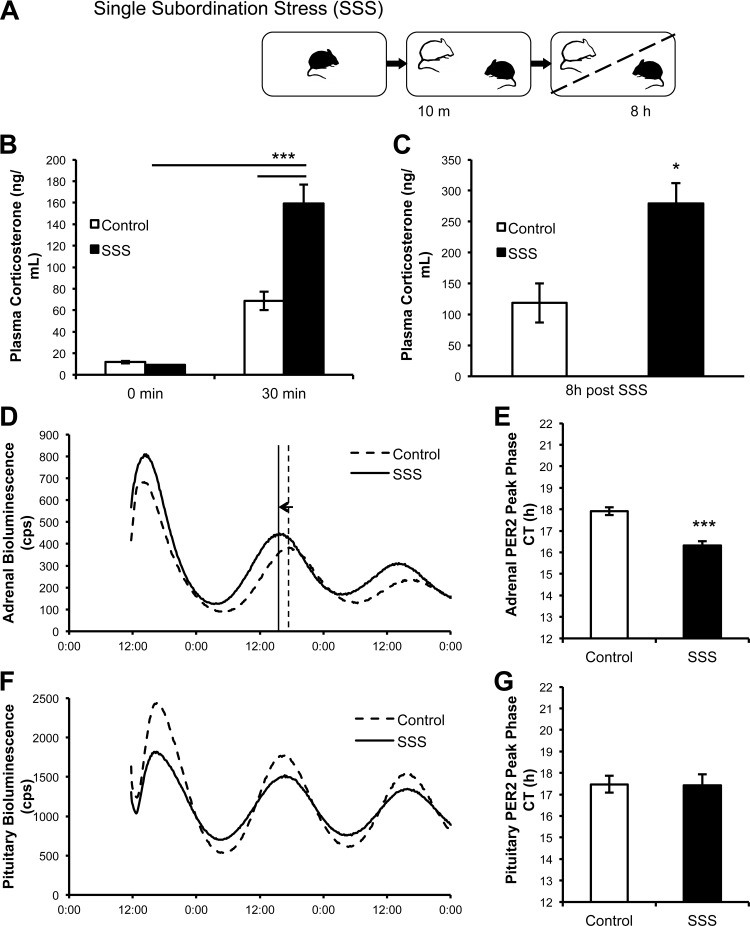
The experiments were conducted exactly as described for the CSS, except that the PER2Luc or the C57BL/6 intruder mice were exposed to a single 10-min social defeat session by a CD1 mouse at ZT3, cohoused in sensory contact for 8 h, and euthanized at ZT11 (see Fig. 4A). Control mice were similarly handled at ZT3 and euthanized at ZT11. Finally, in a separate group of mice, tail vein blood was sampled immediately before the exposure to the social defeat (at ZT3) and at 30 min after the 10-min social interaction. Age and weight-matched C57BL/6 control mice were sampled at ZT3 and at 30 min after handling. Blood samples were used for corticosterone analysis.
Fig. 4.
A single subordination stress (SSS) episode acutely elevates corticosterone and phase advances the adrenal but not the anterior pituitary PER2Luc rhythm. A: schematic of the experimental procedures. Subordinate mouse (black) undergoes social interaction with dominant mouse (white) for 10 min and then mice are housed together separated by a partition for 8 h. B: corticosterone is elevated 30 min after the single defeat exposure (control, n = 8; SSS, n = 6). C: corticosterone remains elevated after a social defeat followed by 8 h sensory contact (control, n = 4; SSS, n = 5). D: PMT tracing of adrenal samples from representative control and subordinate mice. E: adrenal PER2Luc peak phase is advanced in subordinate mice (control, n = 5; SSS, n = 5). F: PMT tracing of pituitary samples from representative control and subordinate mice. G: pituitary PER2LUC peak phase is not altered in subordinate mice (control, n = 5; SSS, n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
Plasma corticosterone analysis.
All blood samples were collected in EDTA-treated tubes (Sarstedt, Nümbrecht, Germany) and centrifuged at 4°C, and the plasma was stored at −20°C. Plasma corticosterone was determined by 125I radioimmunoassay (MP Biomedical, Solon, OH). The intra-assay and inter-assay CVs for plasma corticosterone were 7.6% and 13.3%, respectively.
Real-time monitoring of bioluminescence.
Adrenals and anterior pituitaries were rapidly excised and placed in cold Hank's Balanced Salt Solution. Cleaned and hemisected adrenals or whole pituitaries were cultured on Millicell organotypic inserts in a 35-mm Petri dish with 1.5 ml of warmed culture media (Dulbecco's Modified Eagle Media without phenol red) supplemented with luciferin and penicillin-streptomycin as described previously (47, 48). Dishes were sealed with circular glass coverslips and silicon grease. Cultures were maintained at 36°C, and bioluminescence was measured using photomultiplier tubes in an Actimetrics Lumicycle (Wilmette, IL). The first day of recording was not considered due to transient effects as described by others (12). The remaining data were smoothed and detrended using a 2- and 24-h running average, baseline subtracted, and the pretreatment rhythm fit to a damped sine wave using Lumicycle Analysis software Actimetrics. Only explants showing rhythms with a goodness of fit >85% were accepted. To assess changes in the adrenal and pituitary clock rhythm induced by stress, phase was determined from the peak occurring on the second day of incubation and period was calculated using data from two cycles spanning the second and third days of incubation.
Statistical analysis.
Data were analyzed with one- or two-way ANOVA for repeated measures (Statsoft, Tulsa, OK). Binary comparisons following ANOVA were conducted with Tukey's HSD. Correlations were performed with a Spearman rank R statistic formula. Differences were considered significant if P < 0.05.
RESULTS
CSS induces a positive energy balance and HPA axis upregulation in mice.
Consistent with previous findings (4, 5, 11, 34), CSS induced a robust increase in body weight and food intake [body weight: time, F(1,87) = 29.2, P < 0.0001; stress × time interaction, F(1,87) = 15.63, P < 0.001; food intake: stress, F(1,87) = 10.98, P < 0.01; time, F(1,87) = 73.51, P < 0.0001; stress × time interaction, F(1,87) = 20.02, P < 0.001] (Fig. 1, B and C). Feed conversion efficiency, the ratio of body weight gain to food intake in the 2 wk of chronic stress, was increased in CSS mice [F(1,86) = 11.11, P < 0.01] (Fig. 1D). Subordinate mice also showed an increase in adrenal weight [F(1,86) = 10.87, P < 0.01] (Fig. 1E) and thymic involution [F(1,86) = 16.32, P < 0.001] (Fig. 1F). Plasma corticosterone was elevated at ZT11 compared with ZT2 and ZT14 in control mice; in contrast, plasma corticosterone was elevated at ZT11 compared with ZT2 but not to ZT14 in CSS mice (Fig. 1G). Although corticosterone was increased in CSS mice compared with control mice [stress: F(1,81) = 4.06, P < 0.05], there was no difference at any specific time point.
Chronic subordination stress does not alter the activity rhythm in mice.
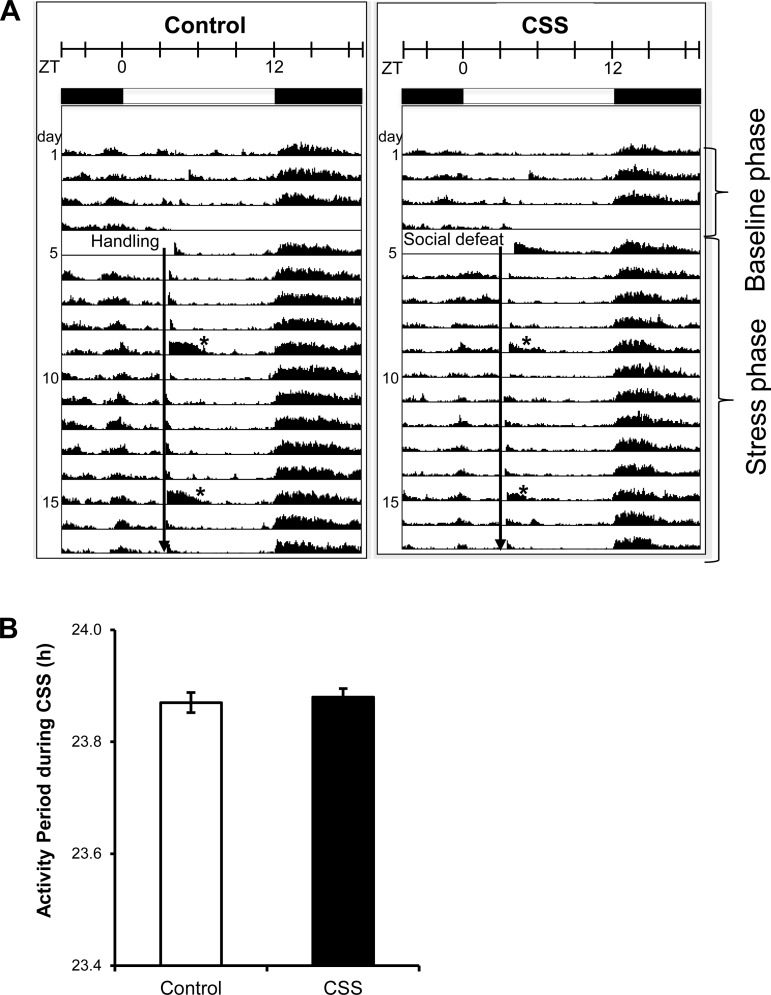
Chronic subordination stress did not disrupt rhythmic locomotor activity. The average actogram shows the expected increase in activity at the onset of the dark phase and reduced activity during the light period in control and CSS mice (Fig. 2A). This activity rhythm was not altered by daily social defeat. In addition, the period was not different in CSS and control mice (Fig. 2B).
Fig. 2.
CSS did not alter the mouse activity rhythms. A: average actograms of control (n = 8) and subordinate (n = 8) animals during the baseline and the stress phase. Animals were exposed to a 12:12 h light-dark cycle with lights on at 0500 h (ZT0). The experimental intervention (indicated by the black vertical arrow), either handling or social defeat encounter, occurred daily between ZT3 (0800 h) and ZT3.5 (0830 h). *Increased activity due to weekly cage change. B: period of the home-cage activity rhythm calculated throughout the stress phase is not different between control and subordinate mice.
CSS phase advances the PER2Luc rhythm in adrenal and pituitary tissues.
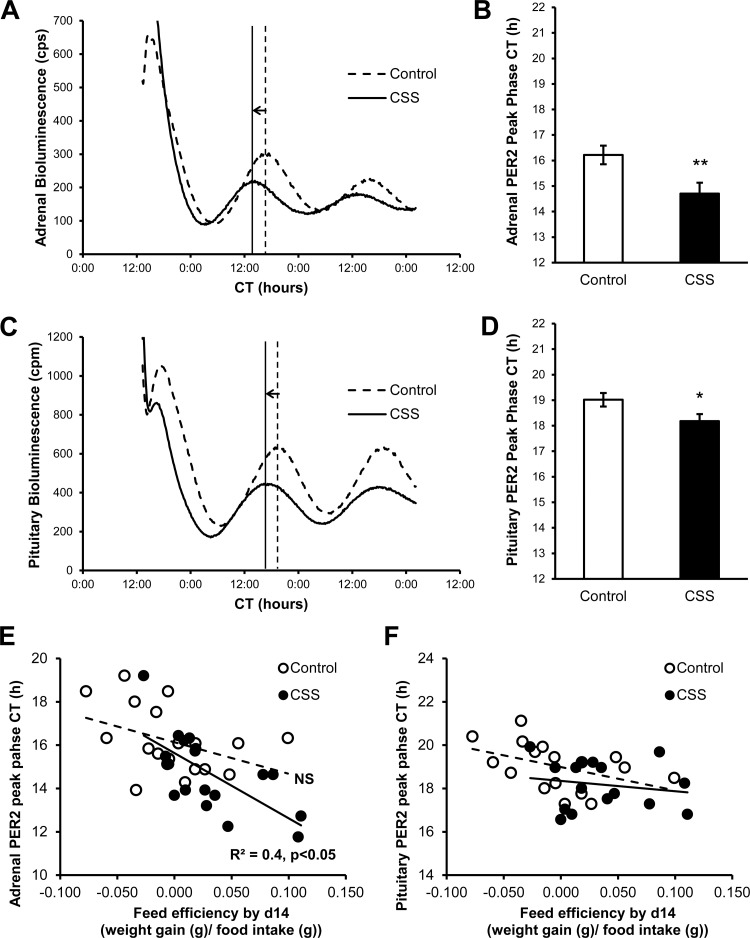
Remarkably, CSS induced a peak phase advance both in the adrenal gland (∼2 h) and in the anterior pituitary (∼1 h) [adrenal: (F1,34) = 7.38, P < 0.05 (Fig. 3, A and B); pituitary: F(1,31) = 4.79, P < 0.05 (Fig. 3, C and D)]. However, CSS did not alter mPER2Luc period in either adrenal or pituitary explants (adrenal: control = 23.9 ± 0.1 h; stress = 23.9 ± 0.3 h; pituitary: control = 23.2 ± 0.2 h; stress = 23.3 ± 0.1). Interestingly, the adrenal (Fig. 3E) but not the pituitary (Fig. 3F) PER2Luc peak phase advance was negatively correlated with feed conversion efficiency, a global index of change in body weight relative to food intake, in subordinate but not in control mice [adrenal: overall population, r = −0.59, P < 0.001; CSS group, r = −0.66, P < 0.01; control group, r = −0.43, not different. Pituitary: all not different].
Fig. 3.
CSS phase advanced the mPER2::Luciferase (PER2Luc) rhythm in adrenal and anterior pituitary explants. Representative experiment showing the PER2Luc rhythm in adrenal (A) and anterior pituitary (C) explants collected from a control and a CSS mouse. Adrenal (B) and pituitary (D) PER2Luc peak phase is advanced in subordinate mice by 2 and 1 h, respectively. The adrenal PER2Luc phase advance was negatively correlated with feed conversion efficiency in subordinate but not in control mice (E). No association was found for the pituitary phase advance and feed efficiency (F). Adrenal explants: control, n = 19, CSS, n = 19; pituitary explants: control, n = 17, CSS, n = 17. *P < 0.05, **P < 0.01.
Single subordination stress phase advances mPER2Luc rhythm in adrenal but not in pituitary tissues.
A single social stress exposure (Fig. 4A) triggered activation of the HPA axis. Plasma corticosterone was increased in subordinate mice 30 min after a 10-min social interaction and defeat experience compared with control mice [stress: F(1,12) = 24.34, P < 0.001; time: F(1,12) = 114.80, P < 0.0001; stress × time interaction: F(1,12) = 23.65, P < 0.001] (Fig. 4B). Furthermore, the 10-min social interaction and defeat followed by 8 h sensory contact with a dominant mouse resulted in elevated plasma corticosterone compared with controls [F(1,7) = 12.19, P < 0.05] (Fig. 4C).
In addition, a single defeat plus 8 h sensory contact induced a phase advance in the adrenal mPER2Luc rhythm of the same order of magnitude shown by CSS mice, i.e., ∼2 h [F(1,8) = 36.92, P < 0.001] (Fig. 4, D and E), whereas no differences were detected in the peak phase of the pituitary mPER2Luc rhythm (Fig. 4, F and G). Similar to that observed after CSS, the periods of the adrenal and pituitary mPER2luc rhythms were not affected by a single subordination stress (adrenal: control = 23.1 ± 0.1 h; stress = 22.9 ± 0.2 h; pituitary: control = 22.9 ± 0.1 h; stress = 22.9 ± 0.1).
DISCUSSION
The major finding of this study is that chronic exposure to subordination stress induced a phase advance of PER2Luc rhythms in both adrenal and anterior pituitary explants. The phase shift occurred in the absence of changes in the period of the PER2Luc rhythms, indicating that chronic subordination stress resets the adrenal and pituitary clock. As expected (4, 5, 11, 34) subordination stress led to hyperphagia, body weight gain, and overall increased feed conversion efficiency. The association between the peak phase of the adrenal PER2Luc rhythm and the increased feed efficiency suggests that the altered adrenal clock rhythm may contribute to the metabolic phenotype. In contrast to CSS, a single episode of subordination stress induced a similar phase advance of the PER2Luc rhythm in the adrenal gland, but not the pituitary, indicating that the adrenal clock is more sensitive to social stress than its pituitary counterpart and that the response of the adrenal clock to chronic stress for 14 days does not habituate.
The CSS model results in a complex syndrome consisting of upregulation of HPA axis function concomitant with autonomic, immune-endocrine, metabolic, and cognitive dysfunction in subordinate mice (4, 5, 11, 34). In the present experiments, the constant exposure to social stress induced activation of the HPA axis. Elevated plasma corticosterone was observed after a single subordination stress that was maintained during exposure to chronic subordination stress. Furthermore, chronic stimulation of the HPA axis by CSS resulted in adrenal hypertrophy and thymic involution, two classic indicators of hypercorticosteronemia (38). Our finding that the adrenal and pituitary PER2Luc rhythms were shifted by CSS adds another component to the neuroendocrine phenotype. The diverse response produced in this stress model would suggest that multiple interconnected mechanisms are disrupted. Recent work showing that adrenalectomy reduces susceptibility to depression in mice exposed to chronic subordination (23) supports a role for elevated glucocorticoids as a causative factor. Although we did not assess the depression-like phenotype in the present study as demonstrated in previous studies (3, 34), the subordinate mice showed hyperphagia and body weight gain that characterize the metabolic phenotype in this model. Since the adrenal clock can regulate the glucocorticoid rhythm (30, 41), it is possible that phase shifts in the adrenal clock rhythm might contribute to the metabolic phenotype displayed in CSS mice by changing the glucocorticoid rhythm. Our data show that a single subordination stress increases corticosterone for at least 8 h and that corticosterone remains elevated after 2 wk of daily stress. We did not collect samples at a sufficient frequency over 24 h to determine whether CSS alters the phase or the amplitude of the glucocorticoid rhythm. However, increases in corticosterone secretion produced by CSS likely would affect the timing of peripheral clocks that respond to glucocorticoid receptor (GR) signaling. For example, the liver clock shifts phase in response to GR activation in a phase-dependent fashion (2), and glucose homeostasis is dependent on direct interactions between the GR and core clock genes including Per2 (40) and Cry (22). The interaction between GRs and clock genes in tissues critical for metabolic control may provide the link between CSS-induced shifts in the adrenal clock and the metabolic phenotype.
The daily rhythm in HPA activity is dependent on the SCN clock (1, 28, 32), so the phase shift in the adrenal and pituitary clock of subordinate mice may be due to CSS altering the SCN clock. However, SCN-dependent rhythms including locomotor activity and body temperature are not phase shifted by repeated social defeat (25) or by other chronic stress paradigms (26, 42); although, the amplitude of the Per mRNA rhythm in the SCN may be blunted by chronic stress (18, 21), a phase shift was not observed in activity rhythms in our study. Our finding that activity rhythms were not changed by CSS would support the conclusion that the SCN clock was not affected. Moreover, since animals were housed under a 12:12 h light-dark cycle, it is likely that light-dark entrainment predominates in maintaining the phase of the SCN clock over CSS related stimuli. These results would suggest that the phase shift in the adrenal and pituitary clocks was not the result of a phase shift in the SCN clock.
As an alternative to SCN-dependent modulation, the phase shift in the adrenal clock rhythm might represent a novel component of the HPA response to stress. We have shown that the adrenal clock is phase shifted by ACTH in vitro and that the phase shift is phase dependent (47). However, our in vitro experiments showed that ACTH produced a phase delay in the adrenal PER2Luc rhythm when administered at a circadian time analogous to the active phase of the rhythm, but no phase advance at the circadian time representing the inactive phase. These findings suggest that the phase advance of the adrenal clock that we have observed in vivo may require factors in addition to ACTH. These factors could include corticosterone, since like other peripheral clocks, adrenal cortical cells express GRs (16); it is possible that increases in corticosterone produced by subordination stress participate in regulating the phase of the adrenal clock rhythm. However, the CSS model consisted of a brief daily period (10 min or less) of physical contact at ZT3 followed by cohabitation for the remainder of the 24-h period. Both daily physical contact and constant exposure to the dominant mouse could produce different factors to affect timing of the adrenal clock. Our finding that a single subordination stress produces a 2-h phase advance in the adrenal PER2Luc rhythm that is maintained after 14 days of CSS argues for a mechanism that limits the magnitude of the phase shift. Additional experiments are required to parse out separate components of the model to determine factors that initiate and maintain the phase shift.
The anterior pituitary clock was also sensitive to the chronic subordination stress. Although it is unclear which pituitary cells are responsible for the PER2Luc rhythm, clock genes are expressed in corticotrophs, the source of ACTH (9, 45) and lactotrophs, the source of prolactin (39). Since both corticotrophs and lactotrophs are stress-responsive cells, the phase advance in the PER2Luc rhythm might reflect changes in clock rhythms associated with these cell types. We showed that a single subordination stress was capable of shifting the adrenal PER2Luc rhythm but not the pituitary PER2Luc rhythm. Responding to a short exposure to social challenge may be a unique feature of the adrenal clock; similar to the pituitary clock rhythm, clock gene rhythms in other peripheral tissues are not shifted by exposure to a single acute challenge (46). Chronic exposure to subordination stress was required to shift the phase of the pituitary clock. The mechanisms that account for differential sensitivity of the adrenal and pituitary clocks to subordination stress are currently unknown. Since rhythmic glucocorticoids are important for entrainment of other peripheral clocks (2, 20, 24, 31), the pituitary clock may be responding to an altered corticosterone rhythm produced by CSS. Although adrenalectomy results in minimal changes in pituitary clock gene rhythms (9, 31), rhythmic corticosteroid replacement in adrenalectomized rats produces a phase advance in the pituitary clock rhythm (31). These results support the possibility that changes in the corticosterone rhythm in CSS mice might underlie the phase shift in the pituitary clock.
Perspectives and Significance
Chronic activation of the HPA axis by various stressors causes somatic and psychiatric disturbances that may result from dysregulation of the clock system (7, 10, 14). It is unclear whether these disorders result from an overstimulated HPA axis acting directly on peripheral clocks or if the HPA axis and the circadian clock systems act via independent pathways. The present study demonstrated that chronic subordination stress not only activates the HPA axis but also produces a phase shift in the adrenal and pituitary PER2Luc rhythm. The magnitude of the phase shift in the adrenal clock was associated with a positive energy balance, an observation that links the adrenal clock rhythm with the metabolic phenotype observed after chronic stress. By altering timing of the adrenal clock, CSS has the potential to desynchronize downstream peripheral clock rhythmicity in other metabolic organs like the liver by phase-shifting the glucocorticoid rhythm to produce the metabolic phenotype characteristic of this model. Although stress-induced alteration in the timing of the adrenal clock appears to be maladaptive, the alteration of peripheral oscillators produced in stress vulnerable subjects needs to be further investigated to understand how these mechanisms are maintained in health and disrupted in pathology.
GRANTS
This work was supported in part by NSF IOS1025199 (to W. C. Engeland), Wallin Neuroscience Discovery Fund (to W. C. Engeland) and University of Minnesota Medical School (to A. Bartolomucci).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R., C.K., J.M.Y., A.B., and W.C.E. conception and design of research; M.R., C.K., J.M.Y., A.B., and W.C.E. performed experiments; M.R., C.K., J.M.Y., and W.C.E. analyzed data; M.R., C.K., J.M.Y., A.B., and W.C.E. interpreted results of experiments; M.R., C.K., J.M.Y., and W.C.E. prepared figures; M.R. and C.K. drafted manuscript; M.R., C.K., J.M.Y., A.B., and W.C.E. edited and revised manuscript; M.R., C.K., J.M.Y., A.B., and W.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jennifer Asturias and Ashish Jain for technical assistance in performing the animal experiments.
REFERENCES
- 1.Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology 29: 119–131, 1979 [DOI] [PubMed] [Google Scholar]
- 2.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell'Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLos One 4: e4331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolomucci A, Palanza P, Gaspani L, Limiroli E, Panerai AE, Ceresini G, Poli MD, Parmigiani S. Social status in mice: behavioral, endocrine and immune changes are context dependent. Physiol Behav 73: 401–410, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29: 899–910, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bartolomucci A, Palanza P, Sacerdote P, Panerai AE, Sgoifo A, Dantzer R, Parmigiani S. Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev 29: 67–81, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 330: 1349–1354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse adrenal tissues. Am J Physiol Regul Integr Comp Physiol 285: R561–R569, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bur IM, Zouaoui S, Fontanaud P, Coutry N, Molino F, Martin AO, Mollard P, Bonnefont X. The comparison between circadian oscillators in mouse liver and pituitary gland reveals different integration of feeding and light schedules. PLos One 5: e15316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10: 213–219, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dadomo H, Sanghez V, Di Cristo L, Lori A, Ceresini G, Malinge I, Parmigiani S, Palanza P, Sheardown M, Bartolomucci A. Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Prog Neuropsychopharmacol Biol Psy 35: 1461–1471, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci 29: 171–180, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Froy O. Metabolism and circadian rhythms-implications for obesity. Endocr Rev 31: 1–24, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional, and clock-related genes throughout the rat H.P.A. axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab 296: E888–E897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol 20: 11–23, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology 59: 97–109, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J, Lu L. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain Res 1399: 25–32, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol 349: 20–29, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Kiessling S, Eickele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120: 2600–2609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita C, Miyazaki K, Ishida N. Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3β phosphorylation in the central clock. Neuroreport 23: 98–102, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480: 552–557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci 33: 2961–2972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20: 7128–7136, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meerlo P, Hoofdakker RHvd Koolhaas JM, Daan S. Stress-induced changes in circadian rhythms of body temperature and activity in rats are not caused by pacemaker changes. J Biol Rhythms 12: 80–92, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki K, Itoh N, Ohyama S, Kadota K, Oishi K. Continuous exposure to a novel stressor based on water aversion induces abnormal circadian locomotor rhythms and sleep-wake cycles in mice. PLos One 8: e55452, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Ann Rev Neurosci 35: 445–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore R, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972 [DOI] [PubMed] [Google Scholar]
- 29.Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 21: 277–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4: 163–173, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Pezük P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 153: 4775–4783, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology 153: 4346–4353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ripperger JA, Jud C, Albrecht U. The daily rhythm of mice. FEBS Lett 585: 1384–1392, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Sanghez V, Razzoli M, Carobbio S, Campbell M, McCallum J, Cero C, Ceresini G, Cabassi A, Govoni P, Franceschini P, de Santis V, Gurney A, Ninkovic I, Parmigiani S, Palanza P, Vidal-Puig A, Bartolomucci A. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the Metabolic Syndrome. Psychoneuroendocrinology 38: 2933–2942, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell 111: 919–922, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol 590: 6213–6226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segall L, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 140: 753–757, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Selye H. Syndrome produced by diverse nocuous agents. Nature 138: 32, 1936 [DOI] [PubMed] [Google Scholar]
- 39.Sheynzon P, Karolczak M, Dehghani Korf HWF. Diurnal variation in CREB phosphorylation and PER1 protein levels in lactotroph cells of melatonin-proficient C3H and melatonin-deficient C57BL mice: similarities and differences. Cell Tissue Res 321: 211–217, 2005 [DOI] [PubMed] [Google Scholar]
- 40.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA 106: 17582–17587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA 105: 20970–20975, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Yamada T, Tsukita S, Kaneko K, Shirai Y, Munakata Y, Ishigaki Y, Imai J, Uno K, Hasegawa Y, Sawada S, Oka Y, Katagiri H. Chronic mild stress alters circadian expressions of molecular clock genes in the liver. Am J Physiol Endocrinol Metab 304: E301–E309, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290: R1128–R1135, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Ulrich-lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wunderer F, Kühne S, Jilg A, Ackermann K, Sebesteny T, Maronde E, Stehle JH. Clock gene expression in the human pituitary gland. Endocrinology 154: 2046–2057, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma M, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem 280: 42036–42043, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Yoder JM, Brandeland M, Engeland WC. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am J Physiol Regul Integr Comp Physiol 306: R387–R393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]