Abstract
The twenty-five known matrix metalloproteases (MMPs) and their endogenous inhibitors, tissue inhibitors of metalloproteases (TIMPs), mediate cell invasion through the extracellular matrix (ECM). In a comparative three-dimensional assay, we analyzed human and mouse satellite cells' competence to invade an artificial ECM (collagen I). We identified a single MMP that 1) is expressed by human muscle satellite cells; 2) is induced at the mRNA/protein level by adhesion to collagen I; and 3) is necessary for invasion into a collagen I matrix. Interestingly, murine satellite cells neither express this MMP, nor invade the collagen matrix. However, exogenous human MMP-14 is not sufficient to induce invasion of a collagen matrix by murine cells, emphasizing species differences.
Keywords: skeletal muscle, muscle regeneration, satellite cells, extracellular matrix, MMP-14, cellular invasion, collagen remodeling
mammalian adult skeletal muscle is composed of parallel, syncytial myofibers derived from the fusion of differentiated myocytes during fetal and postnatal development. Because the resulting myonuclei are terminally postmitotic, muscle growth, repair, or regeneration requires muscle satellite cells, a population of muscle-specific precursor cells located between the sarcolemma and the basal lamina in uninjured muscle (35, 51, 53, 71). Satellite cells are the obligate stem cell of skeletal muscle (45, 54, 68): after activation by damage-induced local or systemic signals they will expand to generate a pool of differentiation-competent myocytes, which will then fuse to each other or to existing myofibers to replace or repair damaged muscle tissue. Although the soluble factors influencing satellite cell activation, proliferation, and (to a lesser extent) motility are increasingly well described (18), our understanding of the roles of signals derived from cell-matrix interactions and matrix remodeling in vivo is limited. The extracellular matrix (ECM) in skeletal muscle is made up of both interstitial matrix (composed of different multiple collagen isoforms, fibronectin, hyaluronic acid, and proteoglycans such as perlecan) and myofiber-associated basement membrane (composed of organized layers of collagen IV, collagen VI, and laminin) (29, 33, 69). Collagen type I in particular is dynamically expressed in muscle by multiple different resident cell types, including fibroblasts, satellite cells, and differentiated myofibers (30, 32). During skeletal muscle regeneration, satellite cells first exit their niche between the lamina and the sarcolemma, then transit on the myofiber lamina as they reposition to the site of injury. This would suggest that satellite cells have the potential to traverse the interstitial matrix, and indeed satellite cells have been observed protruding from the lamina into the interstitial matrix as well as relocating between myofibers (39).
Cell-matrix interactions are frequently bidirectional: binding of ECM proteins by cellular adhesion receptors such as integrins activates intracellular signaling pathways, leading to changes in cell proliferation, cell shape, and cell motility (40), but the ECM is also actively remodeled by secreted and cell surface proteases during processes such as wound healing, fibrotic diseases, and scar formation. This remodeling not only changes the physical properties of the ECM, it liberates cryptic cleavage products that are then capable of signaling to local cells, including satellite cells (1). In addition to degradation and remodeling, deposition of new matrix proteins by local cells will modify the local signaling environment, and thus change the kinetics of tissue regeneration and/or remodeling. For example, recent work suggests that active production of fibronectin by satellite cells modulates their activity and stem cell status by altering their local niche signaling (8).
Matrix metalloproteases (MMPs) are zinc-dependent endopepsidases that play a major role in the constructive proteolysis of the ECM, including collagen type I (13, 85), and are required for cellular invasion through the ECM (77, 88). Twenty-five members of the MMP family have been identified; most are secreted, but six are membrane-type MMPs (MT-MMPs). MMPs are historically grouped as collagenases, gelatinases, stromelysins and matrilysins based on their respective specificity for ECM substrates (22). MMPs are responsible for the activation and processing of secreted molecules during ECM remodeling, including chemokines, cytokines, and growth factors (65, 83), which can in turn reciprocally activate MMPs (2, 37, 75, 89). In general, pathological activation of MMPs is implicated in inflammation, angiogenesis, and cell death, as well as in kidney disease, tumor proliferation and metastasis (14, 89). In skeletal muscle, MMP activity is implicated in both homeostasis and regeneration, and impinges on myofiber integrity, satellite cell activation and the adult as well as myoblast proliferation, migration and fusion during development (16, 24, 26, 42, 57, 84, 91).Therefore, as would be expected, overall MMP activity increases upon injury, correlating with a decrease in intact ECM proteins (16), and inhibition of MMPs also inhibits skeletal muscle regeneration (84). In cell transplant studies, MMP activity has been implicated in migration of exogenous myoblasts, and is therefore a potential therapeutic target (24, 81).
In this study we show that human, but not mouse, satellite cells are competent to invade a three-dimensional (3D) collagen type I matrix in vitro. We also show that expression of the membrane-type metalloprotease MMP-14 (also known as MT1-MMP) is necessary for this invasion. Interestingly, primary murine satellite cells neither express MMP-14 nor invade a collagen I matrix, highlighting the importance of MMP-14 activity for ECM invasion and myoblast migration in vivo. These data highlight one of several differences between species, and may identify an area of potential concern for the translation of murine results into human therapeutic perspective. In particular, these data might be useful in interpretation of satellite cell transplant experiments in cases in which human and murine grafts have given differing results.
METHODS
All methods involving live animals were performed under approval from the University of Missouri Animal Care and Use Committee.
Murine cell culture.
C2C12 cells were cultured in DMEM (GIBCO) supplemented with 15% fetal bovine serum. Mouse satellite cells were harvested from hindlimb muscles of adult (90–120 days) females using our established protocol (19). Mouse muscle fibroblasts were harvested simultaneously and enriched by differential plating 24 h after harvest. Primary cells and MM14s were cultured on gelatin-coated plates (Nunc) in Ham's F-12 media (GIBCO) supplemented with 10% horse serum (HS) and 5 nM fibroblast growth factor 2 (FGF2) (58).
Human cell culture.
Immortalized human satellite cells were originally isolated from a muscle biopsy taken from a 25-yr-old male donor, obtained from Myobank, affiliated with EuroBioBank, in accordance with the French legislation and EU regulation (10). They were grown in a one-fourth volume of 199-DMEM media respectively supplemented with 2.5 ng/ml hepatocyte growth factor (HGF; Miltenyi), 10−7 M dexamethasone (Invitrogen), and 20% fetal calf serum (GIBCO) as previously described (50). Primary human satellite cells were grown under the same conditions, except for the 5-yr-5-day-old clone, which was not supplemented with HGF or dexamethasone. All human cultures were also challenged in an invasion assay in which they were preconditioned for 24 h then plated on the 3D collagen matrix in murine growth medium (Ham's F-12 + 10% HS + FGF2); this did not affect their invasion.
3D collagen culture.
One hundred microliters of acid-extracted rat-tail type I collagen (Sigma) (2 mg/ml in growth medium) was added per well in 96-well plates (Corning) and allowed to rapidly polymerize at 37°C. When used, recombinant tissue inhibitors of metalloproteases (TIMPs) 1, 2, or 3 (R&D Systems) at a final concentration of 3.5 nM (IC50 provided by the supplier is 2.2, 2.5, and 3.0 nM, respectively) or GM6001 (Calbiochem) at a final concentration of 10 μM was added to the collagen prior to polymerization. Satellite cells (50,000) suspended in 100 μl growth medium were added and cultured for 90 h. All conditions in each experiment were repeated in triplicate, in at least five independent runs.
The matrices were fixed in 4% paraformaldehyde, equilibrated into 50% sucrose, and snap frozen. Cryosections (40 μm) were labeled with 1 μg/ml phalloidin-594 or -635 (Invitrogen) and Vectashield containing DAPI (Vector) for analysis. Invading cells were quantified using the cell counting function of μManager (www.micro-manager.org); criteria to score a cell in later statistical analysis required the entire nucleus and cytoplasm to be present in the section.
Live-cell imaging.
C2C12 myoblasts adhered to a 48-well plate (Corning) and overlaid with 3D collagen type I (previously described) copolymerized with DQ collagen type I (1 mg/ml; Life Technologies) were assayed via time-lapse microscopy. Images were automatically collected from each field every 15 min for 6 h using IPLab (Scanalytics) and analyzed via μManager. Images are representative of triplicate wells.
Immunostaining.
Cells were immunostained as described previously (79). Rabbit monoclonal (EP1264Y) anti-MMP-14 (Abcam) was used at 1:250; this antibody recognizes both the inactive and active forms of both human and mouse MMP-14. Mouse monoclonal anti-Pax7 (Developmental Studies Hybridoma Bank) was used neat. Samples were stained with fluorescently labeled secondary antibodies (Invitrogen) at 1:500 and/or fluorescently labeled phalloidin (Invitrogen) at 1 μg/ml concentration. Nuclei were visualized with DAPI (Vector.)
Slides were imaged with an Olympus BX61 microscope using SlideBook (Intelligent Imaging Innovations) and μManager software. Z-stacks were generated for each image, and Z-projections were used for analysis and quantification of cell invasion.
Injury.
Adult mice were anesthetized with 2,2,2-tribromoethanol (Avertin; Sigma) and injected intramuscularly with 50 μl of 1.2% barium chloride in sterile solution. Images shown are of muscles harvested 5 days after injury.
RT-PCR.
Total RNA from human (25-yr-old male clone) or primary mouse satellite cells cultured on collagen-coated 10-cm plates was reverse-transcribed into cDNA (SuperScriptIII, Invitrogen). Four hundred nanograms of each cDNA sample was used as template; primer sequences used were as follows: GAPDH forward (F), 5′-CAAGGTCATCCATGACAACTTTG-3′, reverse (R), 5′-GGGCCATCCACAGTCTTCTG-3′; mouse MMP-2 F 5′-GGAGAAGGCTGTGTTCTTCG-3′, R 5′-GCATCTACTTGCTGGACATCAG-3′; mouse MMP-9 F 5′-CAGAGGTAACCCACGTCAGC-3′ R 5′-GGGATCCACCTTCTGAGACTT-3′; mouse MMP-14 F 5′-GGACTGAGATCAAGGCCAAT-3′, R 5′-GCCCACCTTAGGGGTGTAAT-3′; mouse TIMP-1 F 5′-TACGCCTACACCCCAGTCAT-3′, R 5′-ATGTGCAAATTTCCGTTCCT-3′; mouse TIMP-2 F 5′-AGGTACCAGATGGGCTGTGA-3′, R 5′-GTCCATCCAGAGGCACTCAT-3′; mouse TIMP-3 F 5′-CCACGTGCAGTACATTCACAC-3′, R 5′-TGTACATCTTGCCTTCATACACG-3′; human MMP-2 F 5′-TATGGCTTCTGCCCTGAGAC-3′, R 5′-CACACCACATCTTTCCGTCA-3′; human MMP-9 F 5′-TCGTCATCCAGTTTGGTGTC-3′, R 5′-ATGGGCGTCTCCCTGAAT-3′; human MMP-14 F 5′-GGCAAATTCGTCTTCTTCAAA-3′, R 5′-GAGCAGCATCAATCTTGTCG-3′; human TIMP-1 F 5′-CTGTTGTTGCTGTGGCTGAT-3′, R 5′-AACTTGGCCCTGATGACG-3′; TIMP-2 F 5′-AGAAGAGCCTGAACCACAGG-3′, R 5′-TGACCCAGTCCATCCAGAG-3′; TIMP-3 F 5′-CCCATGTGCAGTACATCCATAC-3′, R 5′-CCATCATAGACGCGACCTG-3′.
siRNA transfection.
MMP-14 siRNA (15, 25, or 50 μM/well; Invitrogen) or control siRNA was transfected into human satellite cells cultured on gelatin-coated six-well plates (Nunc) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocols prior to seeding on 3D collagen I matrices. All samples were cotransfected with Alexa Fluor 555-labeled Red Fluorescent Oligo (Invitrogen) to identify transfected cells. Ninety hours after transfection, cells were fixed with 4% paraformaldehyde for analysis.
Immunoblotting.
Ninety hours after siRNA transfection, total cell lysates were collected in Allen buffer (50 mM Tris, 10 mM EDTA, 5 mM EGTA pH 7.4 with 1 × Roche Protease Inhibitor, 1 mM sodium orthovanadate, 20 mM sodium fluoride, 1 μg/μl pepstatin A, and 1% Triton X-100.) Ten micrograms of each lysate were loaded onto 4–8% gradient polyacrylamide gel (Invitrogen), transferred to polyvinylidene difluoride membranes, and blocked in StartingBlock (TBS) blocking buffer (Fisher). Membranes were incubated overnight at 4°C with primary antibodies to MMP-14 (EP1264Y, Abcam) at 1:2,000 and IP90 (AB10286, Abcam) at 1:2,000 in StartingBlock, washed, and incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz) for 1 h at room temperature. Chemiluminescent substrate (Pierce SuperSignal West) was detected with a LAS3000 imager (Fujifilm).
MMP-14 overexpression.
Ninety-six hours after isolation, primary mouse satellite cells on gelatin-coated 10-cm plates (Nunc) were transfected with 5 μg of human MMP-14 expression vector (GenBank accession no. BC064803 cloned into pCMV-SPORT6, Open BioSystems) and 5 μg pCMV-Tomato reporter using 15 μl Fugene HD (Promega); control cells were transfected with 5 μg pCDNA4 and 5 μg pCMV-Tomato reporter. After 8 h the cells were seeded onto the 3D collagen type I matrices and cultured for an additional 90 h. Collagen matrices were then fixed, sectioned, immunostained, and imaged for analysis as above.
Zymography.
Forty micrograms of protein lysate were separated on 8% acrylamide gels polymerized with 2 mg/ml gelatin. After electrophoresis, gels were renatured in 2.5% Triton X-100 for 1 h at room temperature then incubated in developing buffer (50 mM Tris·HCl, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij 35) at 37°C for 12 h. Gels were rinsed in distilled H2O and stained with Coomassie R-250 for 30 min, then destained in methanol:acetic acid:water (50:10:40) and imaged on a flatbed scanner.
RESULTS
Human, but not mouse, satellite cells invade a 3D collagen type I matrix.
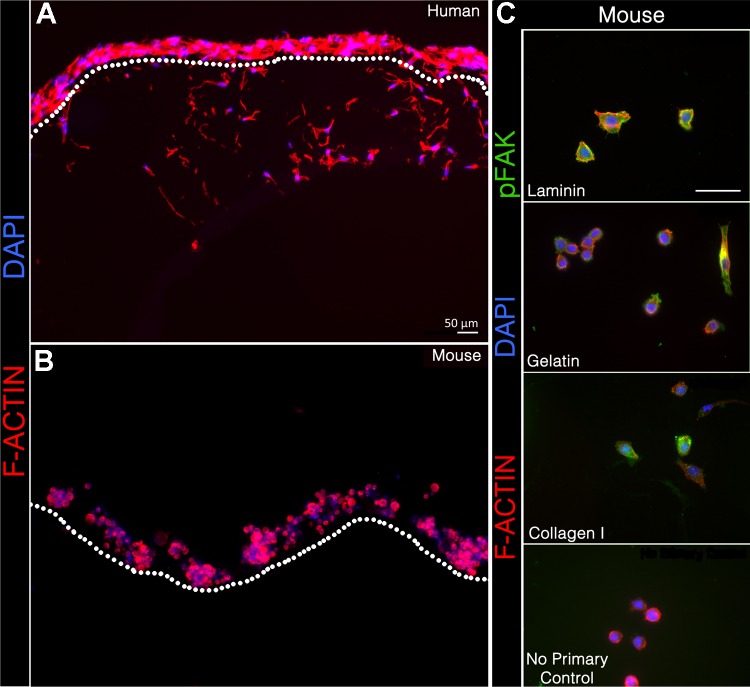
We compared the potential of primary adult murine satellite cells and adult human satellite cells immortalized by expression of telomerase and cdk4 (93) to invade a 3D collagen matrix, as an in vitro model of cellular movement through muscle tissue in vivo. Murine and human satellite cells were seeded on the top of a 2% collagen type I matrix and cultured for 90 h, after which the matrices were fixed, sectioned, and stained with Alexa 594-phalloidin to visualize satellite cell cytoarchitecture. Under these conditions, we observed that human satellite cells invade the collagen matrix an average of 87 μm (Fig. 1A) based on at least five sets of independent runs (see methods). In contrast, primary murine satellite cells failed to invade any detectable distance (Fig. 1B). The same result was seen in both cell types when the cells were first adhered to the culture plate then overlaid with 3D collagen I and challenged to invade “up” (data not shown).
Fig. 1.
Human, but not mouse, satellite cells invade a three-dimensional (3D) collagen type I matrix. A and B: human (A) and mouse (B) satellite cells after 90 h on a 3D collagen type I matrix; F-actin (Alexa 594-phalloidin, red) and nuclei (DAPI, blue). Human satellite cells invade the 3D collagen type I matrix, while mouse cells do not. Dotted line indicates the upper boundary of the 3D collagen. C: immunocytochemistry of primary murine satellite cells for phospho-focal adhesion kinase (green), phalloidin 594 (red), and DAPI (blue) on laminin, gelatin, and 2D collagen I. The negative no primary antibody control was performed on laminin.
To test whether poor adhesion to the collagen I prevented murine cells from initiating invasion, we compared adhesion of human and murine cells to both collagen and laminin. We have previously shown that murine satellite cells detectably express mRNAs for all integrin monomers except αE and αL, and that while laminin is the preferred substrate for primary mouse satellite cells as well as immortalized muscle cell lines, they adhere to and migrate over collagen I as well (72). Consistent with these data, murine satellite cells plated on collagen have a rounder morphology and fewer focal adhesions on collagen or gelatin than on laminin (Fig. 1C), but are nonetheless adhered. Murine cells also failed to invade matrices composed either of collagen I copolymerized with 50% or 75% laminin, or of Matrigel, which is ∼60% laminin (data not shown).
Invasion of collagen I by human satellite cells is MMP dependent.
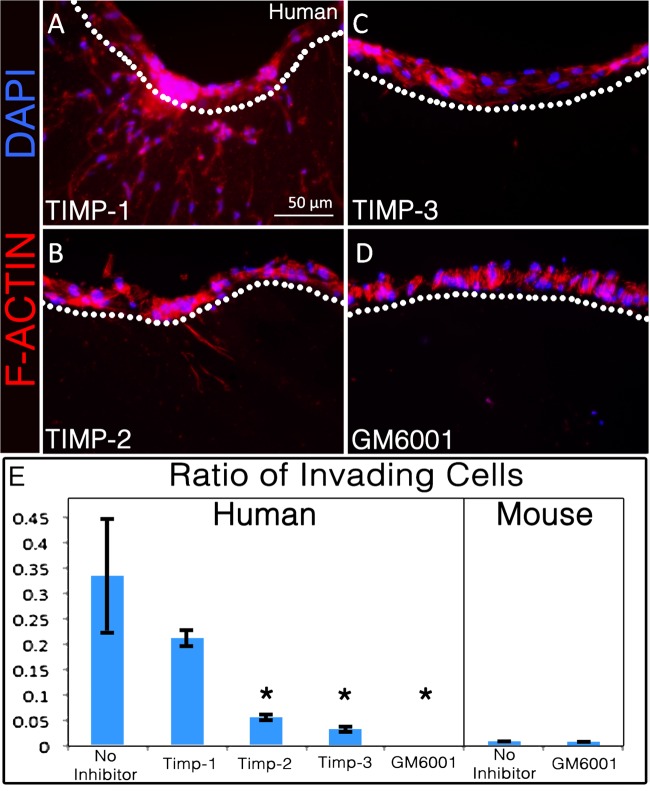
While the primary mechanism of mesenchymal cell invasion is via MMPs, other proteases are expressed in muscle and could be active in remodeling the ECM and in the process of invasion of the matrix. To determine the specific participation of MMPs in satellite cell invasion, we treated human satellite cell cultures as above with a global MMP inhibitor, GM6001. This treatment completely prevented their invasion into the collagen matrix (Fig. 2), indicating that one or more MMPs are essential for invasion. To validate that the invasion is MMP dependent, we treated human satellite cells in 3D cultures with recombinant tissue inhibitors of MMPs (TIMP-1, TIMP-2, and TIMP-3), the endogenous inhibitors of MMPs (80). Each TIMP binds to specific MMPs at a different rate and affinity and blocks their catalytic activity: in general, TIMP-1 inhibits soluble MMPs, while TIMP-2 and TIMP-3 inhibit both soluble MMPs and membrane-type MMPs. TIMP-1 did not significantly inhibit invasion, but TIMP-2 and TIMP-3 both did, implicating membrane-type rather than soluble MMPs in this process.
Fig. 2.
Matrix metalloprotease (MMP) inhibitors differentially inhibit human satellite cell invasion. A–D: human satellite cell invasion in the presence of tissue inhibitors of metalloprotease-1 (TIMP-1; A), TIMP-2 (B), TIMP-3 (C), or GM6001 (D); all but TIMP-1 significantly inhibit invasion. E: quantitative comparison of human and mouse satellite cell invasion based on the fraction of cells invading beneath the surface of the collagen matrix, compared with all cells in the section (n ≥ 3). *P < 0.05. Statistically significant P values were based on comparison of protease inhibitors to the no inhibitor positive control.
Human, but not mouse, satellite cells express MMP-14 when adhered to collagen I.
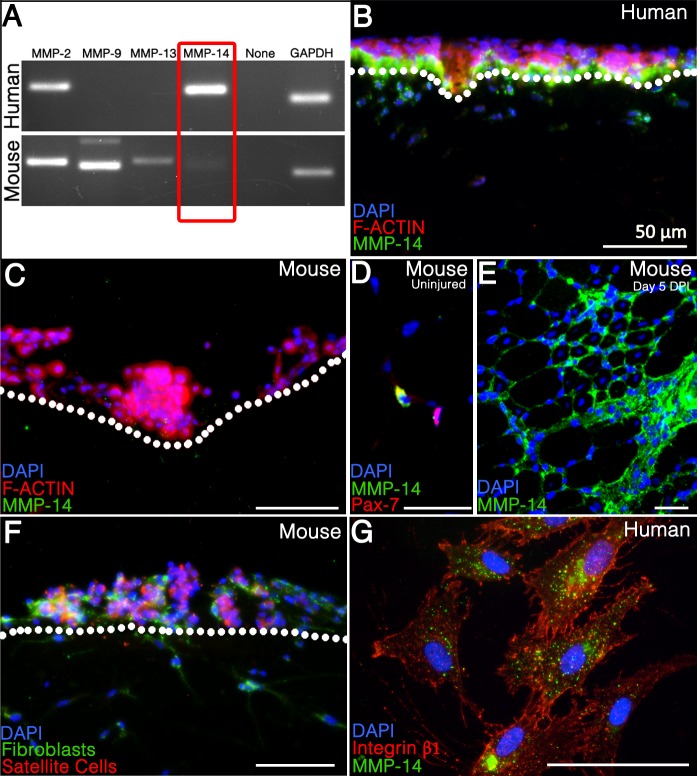
The primary MMPs expressed in skeletal muscle are MMP-2, MMP-9, and MMP-14 (6, 16). In other systems, MMP-2 and MMP-9 are required for invasion of angiogenic, endothelial, and metastatic cells through the ECM (17, 23, 24, 92). MMP-14 (also known as MT1-MMP) is more broadly associated with invasion by fibroblasts, endothelial cells, cancer cells, inflammatory cells, and leukocytes in addition to myogenic cells (23, 27, 44, 76, 79) and acts not only to degrade the ECM but also to activate other MMPs including MMP-2 (87) and MMP-9 (82) in trans. When we screened human and murine satellite cells for their expression of MMP-2, MMP-9, MMP-13, and MMP-14 mRNA using primers designed to amplify sequences from both species, we found that while both express MMP-2, only mouse cells express MMP-9 and MMP-13, and only human satellite cells express MMP-14 mRNA (Fig. 3A).
Fig. 3.
Human, but not mouse, satellite cells express MMP-14; MMP-14 expression in human satellite cells is dependent on contact with collagen I. A: RT-PCR for candidate MMP mRNAs. GAPDH was used as a positive control. B and C: cross sections of collagen I matrices on which human (B) or mouse (C) satellite cells were seeded and allowed to invade for 90 h. Sections were stained for MMP-14 (green) and then labeled with phalloidin 594 (red) and DAPI (blue); note the differential MMP-14 expression in human cells based on location adjacent to the collagen matrix. D: cross section of uninjured mouse tibialis anterior (TA) stained for Pax7 (red), MMP-14 (green), and DAPI (blue). E: cross section of mouse TA 5 days after BaCl injury showing high local expression between nascent myofibers in the area of injury. MMP-14 (green), DAPI (blue). F: coculture of ROSAmTmG mouse satellite cells (red) and unlabeled mouse fibroblasts on a collagen I matrix; all cells were stained for MMP-14 expression (green.) G: human satellite cells were seeded onto gelatin-coated coverslips and analyzed for MMP-14 (green) and integrin-β1 (red) colocalization.
Because of this exclusive expression of MMP-14 by human cells, we considered it a potential mediator of their invasive phenotype. To confirm protein expression and test for its localization, we stained sectioned matrices as above for MMP-14 by immunohistochemistry. Consistent with the mRNA results, human cells on 3D collagen I show robust expression of MMP-14 protein (Fig. 3B) but murine satellite cells do not (Fig. 3C). We confirmed that the antibody detects murine MMP-14 on uninjured muscle sections (Fig. 3D) costained with antibodies to MMP-14 (green) and the satellite cell marker Pax7 (red), as well as on sections of hindlimb muscle five days after barium chloride-induced injury (Fig. 3E).
We noted Pax7-negative, MMP-14-positive interstitial cells, possibly muscle fibroblasts, in the uninjured sections, raising the possibility that nonmyogenic cells might act as a local source of MMP-14 in regenerating mouse muscle to compensate for the lack of expression by satellite cells. In vitro, primary mouse fibroblasts express MMP-14 protein (64), so we asked whether coculture with fibroblasts would enhance murine satellite cells' ability to invade collagen I. We isolated primary adult mouse muscle fibroblasts (54) from a wild-type mouse and cocultured them with genetically labeled satellite cells isolated from a ROSAmTmG mouse (55) constitutively expressing membrane-localized tdTomato, to differentiate between the two cell types. The mixed cell population was plated as above on a collagen I matrix, and while the muscle fibroblasts both express MMP-14 and invade, the satellite cells do neither (Fig. 3F). This suggests that primary mouse satellite cells do not invade collagen type I even in the presence of secreted MMPs and cleaved collagen fibers generated by other cell types.
Interestingly, the MMP-14 protein expression in human cells is not homogeneous in that only cells in direct contact with the collagen matrix show significant protein expression. 3D collagen-dependent MMP-14 expression has previously been described in vivo and in vitro in lung fibroblasts, endothelial cells, and metastatic cells (66, 67, 82); in at least one cell type where it was tested this expression is independent of both integrin activity and substrate stiffness (67). Consistent with those data, we found that MMP-14 and β1-integrin do not colocalize on human satellite cells (Fig. 3G). In addition, neither antibody neutralization of β1-integrin nor treatment with cyclic arginine-glycine-aspartic acid (RGD) peptides significantly inhibited invasion (data not shown). Thus, our data support an integrin-independent mechanism for invasion of 3D collagen by human satellite cells.
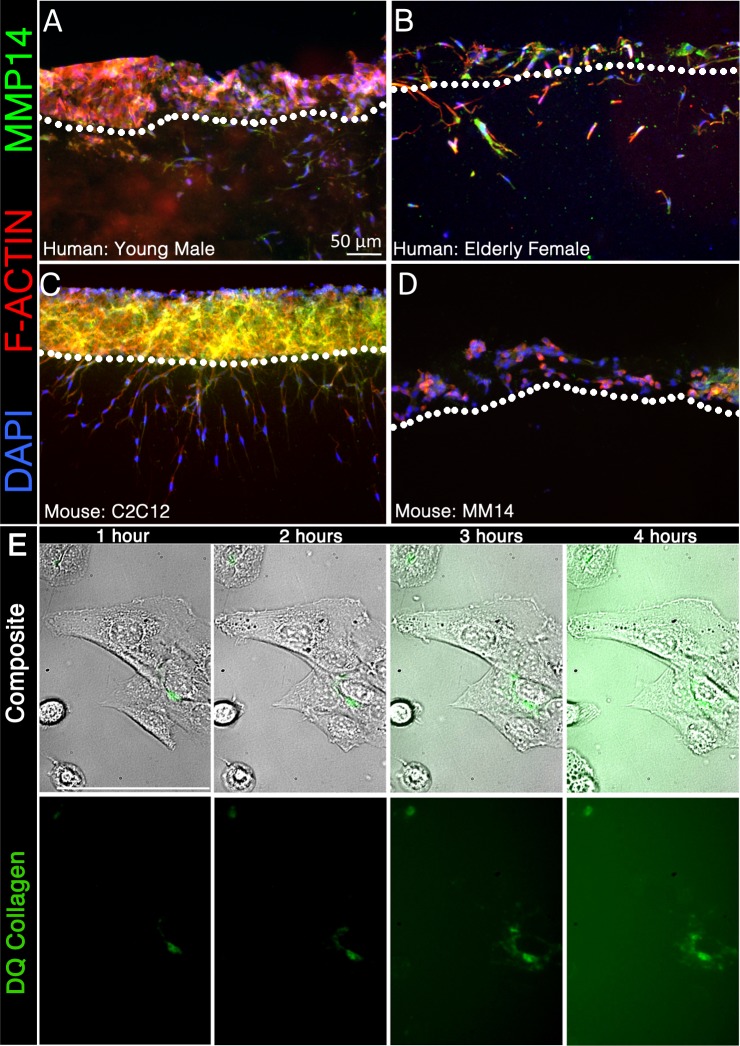
To ensure that the difference we observed between human and mouse satellite cells is not an artifact of the immortalized human satellite cell line, we repeated invasion assays using two different samples of nonclonal, nontransformed human primary myoblasts, as well as two immortalized murine myoblast cell lines. Primary human myoblasts generated at the Institute of Myology (http://www.institut-myologie.org/), one from a 5-yr-5-day-old male (Fig. 4A) the other from a 73-yr-old female (Fig. 4B), both express MMP-14 and invade into the collagen matrix. C2C12 myoblasts, the most commonly used murine myoblast cell line (90), also express MMP-14 and display a remarkable capacity for invasion into collagen (Fig. 4C). MM14 myoblasts (47), which on the basis of morphology, gene expression, and myogenic capacity are the most similar to primary mouse satellite cells, did not invade although they do express low levels of MMP-14 (Fig. 4D). C2C12 cells rapidly proteolyze 3D collagen I, as indicated by cleavage-induced activation of fluorescent collagen I (Fig. 4E and Supplemental Video S1; Supplemental Material for this article is available at the Journal website). These characteristics may correlate with the tendency of C2C12s to form tumors when engrafted into host muscle (61).
Fig. 4.
All human satellite cell populations tested express MMP-14 and invade collagen I, while murine cell lines differ from primary cells and each other. Two primary (nonclonal, nontransformed) human myoblast samples from a 5-yr, 5-day-old male (A) and a 73-yr-old female (B) and two immortalized murine myoblast cell lines, C2C12 (C) and MM14 (D), were seeded on a 3D collagen type I matrix for 90 h and stained for MMP-14 (green) with phalloidin 635 (red) and DAPI (blue). E: images are reference stills from Supplemental Video S1. The panel illustrates collagen proteolysis (green) by C2C12 myoblasts over 4 h. A composite (top) of the FITC (bottom) and brightfield channels shows localized proteolysis respective to the cell. C2C12 satellite cells adhered to the bottom of a 48-well plate were challenged with 3D collagen I copolymerized with DQ Collagen I (1 mg/ml; green.)
MMP-14 is necessary but not sufficient for invasion of collagen I.
To determine whether MMP-14 activity is necessary for human satellite cell invasion, we knocked down MMP-14 expression in human cells using targeted siRNA (Invitrogen). Knockdown was confirmed by immunocytochemistry and Western blotting, and transfection efficiency (98%) was verified through BLOCK-iT Alexa Fluor Red Fluorescent Control Oligo. Transfection with MMP-14-specific (Fig. 5, A and B) but not scrambled (Fig. 5, C and D) siRNA significantly reduced expression of MMP-14 protein as well as human satellite cell invasion through collagen I, and this reduction is dose dependent (Fig. 5, E-G). These data indicate that MMP-14 is necessary for invasion of collagen I by human satellite cells, which could be either direct cleavage of matrix substrates or proteolytic activation of other collagenases, or both. Reduction of MMP-14 activity also reduces the level of active MMP-2 when assayed by gelatin zymography (Fig. 6A), raising the possibility that the requirement for MMP-14 is through MMP-2. Human satellite cells also displayed an elevated amount of TIMP-2 mRNA when compared with primary murine satellite cells (Fig. 6B), and it is important to note that TIMP-2 is required for activation of MMP-2 in conjunction with MMP-14 dimerization (9).
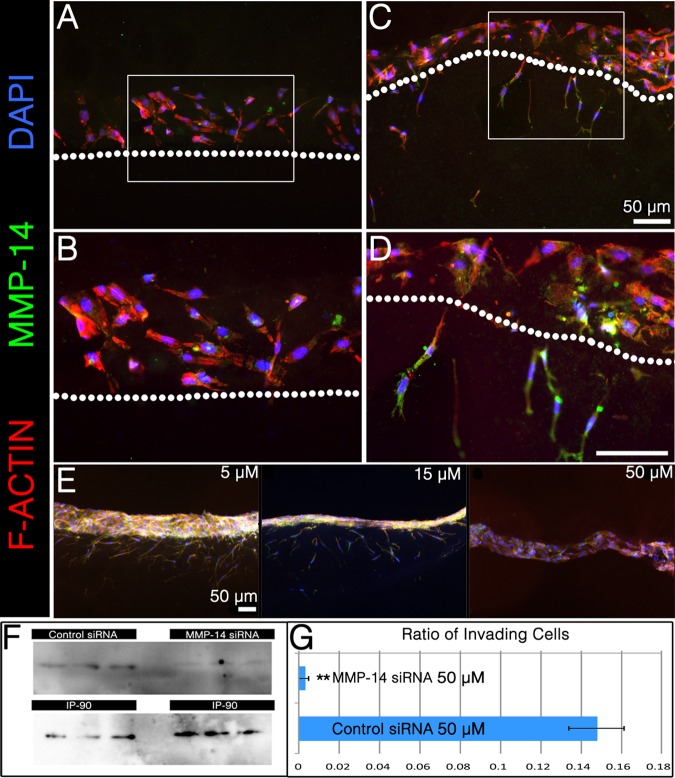
Fig. 5.
MMP-14 is necessary for invasion by human satellite cells. A-D: transfection with MMP-14 siRNA (A and B; B is inset from A), but not scrambled control siRNA (C and D; D is inset from C), reduced invasion by human satellite cells. E: this decrease in invasion was dose dependent. F: knockdown of MMP-14 protein was confirmed by Western blotting; IP90 was used as a loading control. Protein lysates from three separate experiments are shown. G: the ratios of invading cells for control siRNA and MMP-14 siRNA were quantified as described above in 3D collagen culture. **P < 0.005.
Fig. 6.
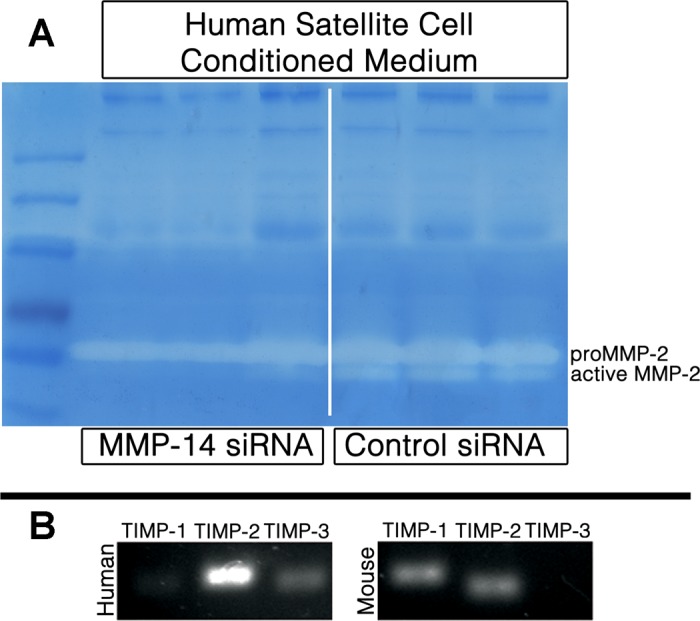
MMP-2 activity in human satellite cells is correlated with MMP-14 expression. Conditioned medium from human satellite cells transfected with either MMP-14 or scrambled control siRNA was run in triplicate onto a gelatin zymogram. Medium from cells treated with MMP-14 siRNA contained reduced MMP-2 activity (A). On the basis of RT-PCR for TIMP-1, TIMP-2, and TIMP-3, human cells express higher levels of TIMP-2 mRNA compared with primary murine satellite cells (B).
To test whether MMP-14 is sufficient to permit invasion of 3D collagen, we asked whether ectopic expression of MMP-14 in primary murine satellite cells would confer an invasive phenotype. Interestingly, even primary murine satellite cells expressing human MMP-14 did not invade (Fig. 7). Coupled with the lack of invasion by MMP-14-expressing MM14 myoblasts noted above, we conclude that MMP-14 is necessary but not sufficient in this system. It is possible that the decreased levels of TIMP-2 we noted in murine cells may contribute to the lack of invasion.
Fig. 7.
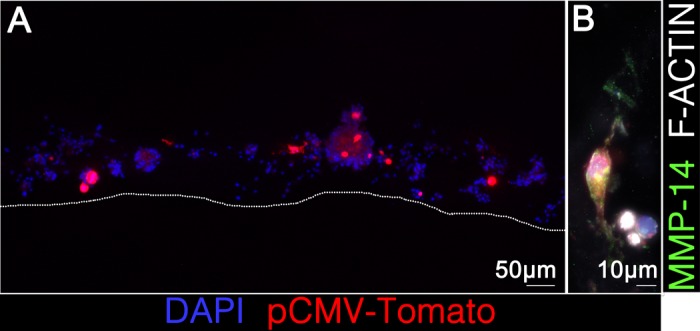
Human MMP-14 is not sufficient to confer an invasive phenotype on primary murine satellite cells. Primary murine satellite cells were transfected with human MMP-14 or empty vector (control) with pCMV-TOMATO (red) as a transfection marker. Phalloidin 633 (white; B) and DAPI (blue; A and B) were used to visualize whole cells seeded onto the matrix.
DISCUSSION
Surprisingly, given the key role played by satellite cell-ECM interactions during muscle homeostasis and repair (48), analysis of the suite of proteins secreted by satellite cells for matrix modification in vivo is not yet well characterized. To date, the only published secretome for murine myoblasts was made using the C2C12 cell line (36), which are not an ideal model for primary myoblasts (31, 46). The secretome from differentiating human myocytes was recently published (43): of the 253 soluble secreted proteins identified by gel-free nano-flow LC-MS/MS analysis, 72 are matrix-modifying enzymes or structural matrix proteins; for comparison, in the same set, there are only 25 secreted growth factors represented. The presence of both constructive and destructive modulators of the ECM suggests a dynamic interplay between the local matrix and satellite cells, which we interpret to be remodeling and not simply proteolysis.
Matrix remodeling by MMPs contributes to adult tissue homeostasis and stem cell-mediated regeneration (3, 16, 21, 25, 38, 85); MMP-14 in particular is required for regeneration in multiple tissues (4, 15, 21, 57, 86). In the context of skeletal muscle, other membrane-type MMPs (MMP-2 and MMP-9 as well as MMP-14) have been implicated in muscle homeostasis (57) as well as in satellite activation after damage (26, 42) and motility in vivo (24). MMP-14 is directly or indirectly required for activation of membrane-type proteases (i.e., MMP-2 and MMP-9) and secreted proteases (i.e., MMP-13) (66, 70, 90) and also activates multiple chemokines, cytokines, and growth factors that would be expected to impinge on muscle regeneration (37, 75). Thus, MMP-14 has the potential to affect muscle regeneration by multiple distinct mechanisms and would not be limited to directly promoting matrix invasion.
It is important to note that these in vitro experiments do not fully represent multiple aspects of muscle regeneration in vivo, and accordingly care should be taken in their interpretation. We did not assess satellite cell activity on or in response to a bona fide muscle ECM in vivo, which is not simply a more complex mix of proteins but is also organized, cross-linked, and polarized in patterns that are difficult to replicate in vitro (48). Attempts to modify the experimental system by using Matrigel (a more complex ECM mixture) instead of collagen I produced the same results (data not shown.) However, our own data suggest that murine satellite cells that have been continually in contact with a native matrix or their host myofiber may transit through 3D collagen (72). This may be a contributing factor to the dramatically enhanced engraftment and spread of satellite cells when single fibers, rather than monoculture satellite cells, are injected into host muscles (34).
Muscle connective tissue fibroblasts, which are a local source of both extracellular matrix components and matrix-modifying enzymes in vivo and are necessary for robust satellite cell-mediated muscle regeneration (54), were included in coculture experiments to ask whether they provided a key component of the in vivo environment. Although they did not enhance myoblast invasion in our experimental system, a role for fibroblast-specific effects on the matrix in vivo is still strongly suggested by the work of other groups. Inflammatory cells including leukocytes and macrophages express MMPs including MMP-14 (7, 56), and pericytes, which may participate in muscle regeneration independently of satellite cells (20), require MMP-14 for key processes during tissue growth and remodeling (78); these cell types were not tested in our in vitro model. Our inability to induce invasion by murine cells with ectopic expression of MMP-14 also does not rule out the possibility that other membrane proteins are necessary in an MMP-14 containing proteolytic complex, and thus MMP-14 alone would not be sufficient.
Our results also serve to reinforce the need for caution in generalizing from animal models to human physiology. It is well established that muscle regeneration differs between mice and humans, particularly in the context of disease (reviewed in Ref. 11) or therapy (5, 12, 28, 59). Potential cell-based therapies are frequently tested either by engrafting murine satellite cells into immunodeficient mice (24, 52, 63) or by doing the same with human cells (62, 73, 74); our data suggest caution both in interpreting the results of these experiments and in generalizing mouse-mouse or human-mouse grafts to human cells engrafted in human patients. One of the confounding difficulties in developing such therapies to date has been failure of engrafted cells to spread away from the point of injection (60); thus a key area of interest is in enhancing migration and invasion. In published studies of simple 2D motility, murine satellite cells are significantly more motile than human (41, 72); however, in the current study murine cells are also significantly less competent to invade a three-dimensional matrix; neither set of results adequately explains the differences observed between species in satellite cell transplantation studies.
We have not tested for species-specific differences in MMP-14 expression and activity in nonmuscle cell types. Because both its matrix remodeling and intracellular signaling activities play key roles in multiple physiological and pathological processes including angiogenesis and tumor metastasis (14, 89), it would be important to know whether any such differences exist in other contexts. If the phenotype is widespread, it may necessitate a reexamination of existing research outside the muscle field as well.
GRANTS
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-062836 (to DDW Cornelison).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.K.L., V.M., D.C. conception and design of research; D.K.L., performed experiments; D.K.L., D.C. analyzed data; D.K.L., V.M., D.C. interpreted results of experiments; D.K.L., D.C. prepared figures; D.K.L., drafted manuscript; D.K.L., V.M., D.C. edited and revised manuscript; D.C. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A 17: 2435–2443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DL, Teitelbaum DH, Kurachi K. Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro. Am J Physiol Cell Physiol 284: C805–C815, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Alwayn IP, Verbesey JE, Kim S, Roy R, Arsenault DA, Greene AK, Novak K, Laforme A, Lee S, Moses MA, Puder M. A critical role for matrix metalloproteinases in liver regeneration. J Surg Res 145: 192–198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson JJ, Toennies HM, Holmbeck K, Senior RM. Membrane type 1 matrix metalloproteinase is necessary for distal airway epithelial repair and keratinocyte growth factor receptor expression after acute injury. Am J Physiol Lung Cell Mol Physiol 293: L600–L610, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci USA 101: 3581–3586, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balcerzak D, Querengesser L, Dixon WT, Baracos VE. Coordinate expression of matrix-degrading proteinases and their activators and inhibitors in bovine skeletal muscle. J Anim Sci 79: 94–107, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 126: 2738–2749, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12: 75–87, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo MM, Fridman R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J 374: 739–745, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigot A, Klein AF, Gasnier E, Jacquemin V, Ravassard P, Butler-Browne G, Mouly V, Furling D. Large CTG repeats trigger p16-dependent premature senescence in myotonic dystrophy type 1 muscle precursor cells. Am J Pathol 174: 1435–1442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem 58: 941–955, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells 30: 1971–1984, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29: 191–197, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Chan KM, Wong HL, Jin G, Liu B, Cao R, Cao Y, Lehti K, Tryggvason K, Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev Cell 22: 1176–1190, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr 3: 337–341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res 4: 507–515, 1998. [PubMed] [Google Scholar]
- 18.Cornelison DD. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J Cell Biochem 105: 663–669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev 18: 2231–2236, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147: 539–553, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174, 2002. [DOI] [PubMed] [Google Scholar]
- 23.El Bedoui J, Oak MH, Anglard P, Schini-Kerth VB. Catechins prevent vascular smooth muscle cell invasion by inhibiting MT1-MMP activity and MMP-2 expression. Cardiovasc Res 67: 317–325, 2005. [DOI] [PubMed] [Google Scholar]
- 24.El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res 258: 279–287, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Filous AR, Miller JH, Coulson-Thomas YM, Horn KP, Alilain WJ, Silver J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev Neurobiol 70: 826–841, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda S, Ikeda S. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord 8: 54, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 15: 678–687, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 174: 231–243, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg B, Green H. Collagen synthesis on polyribosomes of cultured mammalian fibroblasts. J Mol Biol 26: 1–18, 1967. [DOI] [PubMed] [Google Scholar]
- 31.Grabowska I, Szeliga A, Moraczewski J, Czaplicka I, Brzoska E. Comparison of satellite cell-derived myoblasts and C2C12 differentiation in two- and three-dimensional cultures: changes in adhesion protein expression. Cell Biol Int 35: 125–133, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Grounds MD. Towards understanding skeletal muscle regeneration. Pathol Res Pract 187: 1–22, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Grounds MD, Sorokin L, White J. Strength at the extracellular matrix-muscle interface. Scand J Med Sci Sports 15: 381–391, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Hall JK, Banks GB, Chamberlain JS, Olwin BB. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci Transl Med 2: 57ra83, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics 9: 2482–2496, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Semin Cancer Biol 12: 131–138, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci 28: 13467–13477, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes SM, Blau HM. Migration of myoblasts across basal lamina during skeletal muscle development. Nature 345: 350–353, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol 9: M33–37, 1999. [PubMed] [Google Scholar]
- 41.Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol 174: 403–413, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205: 158–170, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Le Bihan MC, Bigot A, Jensen SS, Dennis JL, Rogowska-Wrzesinska A, Laine J, Gache V, Furling D, Jensen ON, Voit T, Mouly V, Coulton GR, Butler-Browne G. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J Proteomics 77: 344–356, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Sodek KL, Hwang Q, Brown TJ, Ringuette M, Sodek J. Phagocytosis of collagen by fibroblasts and invasive cancer cells is mediated by MT1-MMP. Biochem Soc Trans 35: 704–706, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindsell CE, Shawber CJ, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell 80: 909–917, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Linkhart TA, Clegg CH, Hauschka SD. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struct 14: 483–498, 1980. [DOI] [PubMed] [Google Scholar]
- 48.Lund DK, Cornelison D. Enter the matrix: shape, signal and superhighway. FEBS J 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, Wolff A, Kandalla PK, Marie S, Di Santo J, St Guily JL, Muntoni F, Kim J, Philippi S, Spuler S, Levy N, Blumen SC, Voit T, Wright WE, Aamiri A, Butler-Browne G, Mouly V. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle 1: 34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan J, Rouche A, Bausero P, Houssaini A, Gross J, Fiszman MY, Alameddine HS. MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve 42: 584–595, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170: 421–435, 1971. [DOI] [PubMed] [Google Scholar]
- 54.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 28: 2108–2114, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci 119: 3822–3832, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol 118: 631–639, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J 280: 4177–4186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther 15: 867–877, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125: 1275–1287, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riederer I, Negroni E, Bencze M, Wolff A, Aamiri A, Di Santo JP, Silva-Barbosa SD, Butler-Browne G, Savino W, Mouly V. Slowing down differentiation of engrafted human myoblasts into immunodeficient mice correlates with increased proliferation and migration. Mol Ther 20: 146–154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riederer I, Negroni E, Bigot A, Bencze M, Di Santo J, Aamiri A, Butler-Browne G, Mouly V. Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc 40: 624–630, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Rowe RG, Keena D, Sabeh F, Willis AL, Weiss SJ. Pulmonary fibroblasts mobilize the membrane-tethered matrix metalloprotease, MT1-MMP, to destructively remodel and invade interstitial type I collagen barriers. Am J Physiol Lung Cell Mol Physiol 301: L683–L692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 27: 5287–5297, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol 167: 769–781, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakai K, Nakamura T, Suzuki Y, Imizu T, Matsumoto K. 3-D collagen-dependent cell surface expression of MT1-MMP and MMP-2 activation regardless of integrin beta1 function and matrix stiffness. Biochem Biophys Res Commun 412: 98–103, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278: 12601–12604, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Seomun Y, Kim JT, Joo CK. MMP-14 mediated MMP-9 expression is involved in TGF-beta1-induced keratinocyte migration. J Cell Biochem 104: 934–941, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev 20: 1692–1708, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27: 2527–2538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva-Barbosa SD, Butler-Browne GS, de Mello W, Riederer I, Di Santo JP, Savino W, Mouly V. Human myoblast engraftment is improved in laminin-enriched microenvironment. Transplantation 85: 566–575, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Silva-Barbosa SD, Butler-Browne GS, Di Santo JP, Mouly V. Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant 14: 457–467, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200: 448–464, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103: 1237–1241, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9: 541–573, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal 18: 68–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114: 237–247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takino T, Sato H, Seiki M. [Molecular biology of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), and the regulation of these genes in tumor tissues]. Nihon Rinsho 53: 1791–1797, 1995. [PubMed] [Google Scholar]
- 81.Torrente Y, El Fahime E, Caron NJ, Bresolin N, Tremblay JP. Intramuscular migration of myoblasts transplanted after muscle pretreatment with metalloproteinases. Cell Transplant 9: 539–549, 2000. [PubMed] [Google Scholar]
- 82.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun 308: 386–395, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 82: 1375–1381, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol 279: 86–98, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe T, Niioka M, Ishikawa A, Hozawa S, Arai M, Maruyama K, Okada A, Okazaki I. Dynamic change of cells expressing MMP-2 mRNA and MT1-MMP mRNA in the recovery from liver fibrosis in the rat. J Hepatol 35: 465–473, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell 91: 439–442, 1997. [DOI] [PubMed] [Google Scholar]
- 88.Werb Z, Mainardi CL, Vater CA, Harris ED., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med 296: 1017–1023, 1977. [DOI] [PubMed] [Google Scholar]
- 89.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5: 2145–2154, 1991. [PubMed] [Google Scholar]
- 90.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977. [DOI] [PubMed] [Google Scholar]
- 91.Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature 377: 652–656, 1995. [DOI] [PubMed] [Google Scholar]
- 92.Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. Downregulation of the Notch signaling pathway inhibits hepatocellular carcinoma cell invasion by inactivation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor. Oncol Rep 28: 874–882, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, Wright WE. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 6: 515–523, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.