Abstract
Vasoactive intestinal peptide (VIP), a neuropeptide, controls multiple functions in exocrine tissues, including inflammation, and relaxation of airway and vascular smooth muscles, and regulates CFTR-dependent secretion, which contributes to mucus hydration and local innate defense of the lung. We had previously reported that VIP stimulates the VPAC1 receptor, PKCϵ signaling cascade, and increases CFTR stability and function at the apical membrane of airway epithelial cells by reducing its internalization rate. Moreover, prolonged VIP stimulation corrects the molecular defects associated with F508del, the most common CFTR mutation responsible for the genetic disease cystic fibrosis. In the present study, we have examined the impact of the absence of VIP on CFTR maturation, cellular localization, and function in vivo using VIP knockout mice. We have conducted pathological assessments and detected signs of lung and intestinal disease. Immunodetection methods have shown that the absence of VIP results in CFTR intracellular retention despite normal expression and maturation levels. A subsequent loss of CFTR-dependent chloride current was measured in functional assays with Ussing chamber analysis of the small intestine ex vivo, creating a cystic fibrosis-like condition. Interestingly, intraperitoneal administration of VIP corrected tissue abnormalities, close to the wild-type phenotype, as well as associated defects in the vital CFTR protein. The results show in vivo a primary role for VIP chronic exposure in CFTR membrane stability and function and confirm in vitro data.
Keywords: CFTR, cystic fibrosis, VIP, epithelium, VIP-knockout mice
vasoactive intestinal peptide (VIP) is a 28-amino-acid neuropeptide with a highly conserved sequence among species, belonging to the secretin/glucagon/pituitary adenylyl cyclase-activating peptide (PACAP) family. It was discovered in the 1970s by Said and Mutt (34, 35). It is released from nerves surrounding exocrine tissues where it exerts multiple functions, including the control of inflammation, relaxation of airway and vascular smooth muscles, and regulation of cystic fibrosis transmembrane conductance regulator (CFTR)-dependent secretions, which contributes to mucus hydration and local innate defense of the lung through mucociliary clearance (36).
CFTR is a tightly regulated ion channel that mediates cAMP-stimulated anion conductance in epithelia and other cell types. It is activated by protein kinase A (PKA)-dependent phosphorylation of its regulatory domain, while protein kinase C (PKC) phosphorylation enhances responsiveness to PKA activation, in part through direct phosphorylation (9, 10). PKC can also enhance CFTR-dependent chloride secretion through other mechanisms such as increasing the number of functional CFTR channels into the apical membrane of epithelial cells. We had previously established that activating the PKCϵ signaling cascade by prolonged VIP stimulation (1–2 h) of the VPAC1 receptor and Gαi/q proteins increases CFTR stability at the cell membrane by reducing its internalization rate by ∼50% (8). VIP stimulation increases the membrane insertion of wild-type CFTR in shark rectal glands (25) and rat small intestinal epithelium preparations (4).
Earlier studies demonstrate that VIPergic innervation of sweat glands and nasal and intestinal mucosa is sparse in cystic fibrosis patients (20, 37, 38, 42), suggesting that a lack of VIP secretion could play a role in cystic fibrosis (CF) development. At the molecular level, we found that prolonged VIP stimulation of nasal epithelial cells derived from a CF patient corrected trafficking defect and membrane instability of CFTR proteins with the F508del mutation (30), the most common mutation found in CF.
In the present study, we have used VIP knockout (KO) C57Bl/6 mice to show that in vivo the absence of VIP induces constitutive CFTR intracellular retention and compromises exocrine epithelial tissue integrity and function. We found pathological changes and molecular defects with some characteristics of cystic fibrosis in the lung and small intestine. VIP administration corrected these pathologies and reverted CFTR membrane expression and function close to a wild-type phenotype.
MATERIALS AND METHODS
Chemicals.
Monoclonal antibodies used were as follow: anti-CFTR MAB1660 was from R and D Systems (Minneapolis, MN); anti-CFTR MM13–4 was from EMD Millipore (Bellerica, MA); and anti-VPAC1 (AS58), anti-VPAC2 (AS69), anti-PAC1 (1B5), anti-actin (sc-8432), anti-pan-cytokeratin (sc-58825), anti-vimentin (sc-73262), and anti-ZO1 (polyclonal, sc-8147) were from Santa Cruz Biotechnology (Santa Cruz, CA). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated goat anti-mouse light chain secondary and Cy3-conjugated goat anti-mouse secondary were from Jackson ImmunoResearch Lab (West Grove, PA). Normal goat serum and Vectashield Mounting Media for Fluorescence were from Vector Laboratories (Burlingame, CA). Low-viscosity tissue mounting medium Cytoseal 60 and 6× loading dye were from Thermo Fisher Scientific (Burlington, ON, Canada). VIP (no. 1911) was from Tocris Bioscience (Bristol, UK). ECL+ chemiluminescence detection kit was from GE Healthcare (Buckinghamshire, UK). iTaq DNA Polymerase kit, iScript cDNA Synthesis kit, and dNTP mix were from Bio-Rad (Mississauga, ON, Canada). UltraPure Agarose and Ethidium Bromide Solution (10 mg/ml) were from Invitrogen (Carlsbad, CA). Protease Inhibitor Cocktail was from Roche (Laval, QC, Canada). Other chemicals were from Sigma (St. Louis, MO) and of the highest grade available.
Animals and tissue preparation.
VIP-KO mice, backcrossed to C57BL/6 wild-type mice, were prepared as described earlier (19). All mice were C57Bl/6 males aged 16–31 wk. Males were selected as their lung phenotype was more pronounced (Said SI and Szema A, unpublished observations). Following euthanasia by intraperitoneal injection of pentobarbital sodium overdose, the upper lobe of the right lung and the proximal 1 cm of the small intestine were collected and rinsed in ice-cold PBS. Tissue samples for protein and cDNA analysis were immediately flash-frozen in liquid nitrogen and stored at −80°C until further processing. For VIP injections, 15–20 μg of VIP diluted in 200 μl of sterile PBS was injected intraperitoneally on alternate days over a 3-wk period. Control animals received injections of sterile PBS alone.
All experimental procedures were in accordance with the principles of the Canadian Council on Animal Care (CCAC) and according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Protocols were approved by Dalhousie University and Stony Brook University Committees on Animal Care and use.
Histology.
Tissues were fixed in 10% formalin buffer and embedded in paraffin blocks before sectioning into 5-μm-thin sections, followed by hematoxylin and eosin (H and E) staining. Slides were mounted with low-viscosity mounting medium, allowed to dry, and stored at room temperature (RT) (Histology Lab, Dept. of Pathology, Dalhousie University).
Isolation and primary culture of epithelial cells from mice tracheas.
Tracheas, obtained at necropsy, were rinsed in sterile PBS before fine dissection. Epithelial cells were recovered by enzymatic digestion (7). Clean tracheas were opened longitudinally and incubated in calcium- and magnesium-free Hanks buffer supplemented with 1.4 mg/ml pronase and 0.1 mg/ml deoxyribonuclease overnight at 4°C with gentle shaking. Epithelial cells were gently dislodged by several inversions and 10% FBS was added to stop enzymatic digestion. Cells were centrifuged for 10 min at 1,000 rpm at 4°C and resuspended in DMEM/Ham's F12 with penicillin, streptomycin, 5% FBS, insulin and 1× nonessential amino acids. Cells were incubated at 37°C for 2 h in uncoated culture dish to remove fibroblasts. Cells in suspension were collected, centrifuged for 10 min at 1,000 rpm at 4°C, and resuspended in PCT Airways Epithelium Medium complete (CnT-17 CellnTEC) + 5% FBS before plating into a collagen I-coated culture dish. Epithelial cells were maintained at 37°C in 5% CO2, 95% humidity until 80–90% confluence. The medium was changed every 2–3 days.
Immunostaining of primary epithelial cells.
After the first passage, epithelial cells were plated at low density on collagen-coated sterile glass coverslips with cnt-17 complete medium and allowed to adhere for 48 h. Cells were fixed in 2% paraformaldehyde/PBS, then blocked and permeabilized in PBS/0.1% Triton X-100/2% BSA with very gentle shaking. We used primary antibodies (CFTR MAB1660 1/1,000; vimentin 1/400 or cytokeratin 1/200) in PBS/0.1% Triton X-100/0.2% BSA overnight at 4°C with very gentle shaking and Cy-3 conjugated secondary antibody (1/100) in PBS/0.1% Triton X-100/0.2% BSA for 1 h at RT. Slides were incubated with DAPI (0.5 μg/ml) for 7 min before mounting. Membrane localization was visualized by staining of the plasma membrane with the Vibrant CM-Dil cell labeling solution (Molecular Probes) according to the manufacturer's instructions.
Tissue immunostaining.
Thin tissue sections (5 μM) annealed to microscopy slides were deparaffinized in xylene and rehydrated prior to antigen retrieval. Tissue sections were blocked (normal goat serum in TBS with 5% milk) inside a humidity chamber before incubation with polyclonal goat anti-ZO1 overnight at 4°C. Tissues were washed with TBS (3×, 5 min) at RT prior to incubation with rabbit anti-goat IgG coupled to Alexa 488 (1:100 in blocking solution) for 45 min at RT inside a humidity chamber. Secondary antibody was removed and tissues washed again with TBS (3×, 5 min) before incubation with anti-CFTR MAB1660 1 h at RT inside a humidity chamber. We used a CY3 conjugated goat anti-mouse (1:100 in blocking solution) as a secondary antibody to detect CFTR. After final wash, slides were mounted with Vectashield Mounting Media for Fluorescence containing DAPI, sealed with nail polish, and stored at −20°C until use. Confocal microscopy was with a Zeiss LSM 510 Meta Confocal Microscope; HeNe 543 nm laser (CMDI facility, Faculty of Medicine, Dalhousie University).
Immunoblotting.
Pieces of flash-frozen tissues (20–30 mg) were thawed in RIPA buffer (0.15 M NaCl, 10 mM Tris, 1 mM EDTA, 1% Triton X-100, 0.08% deoxycholic acid, 0.01% SDS, pH 7.5) supplemented with protease inhibitors and immediately grounded (rotor-stator homogenizer). Total protein concentration was measured using the bicinchoninic acid method (Pierce). Proteins (10–50 μg) were subjected to 7.5% gradient gel SDS-PAGE, transferred to PVDF membranes, and probed with MM13–4 (1/1,000) anti-CFTR antibody. For VIP receptor detection, samples were preboiled (5 min at 95°C) and subjected to 7.5% SDS-PAGE. Anti-VPAC1 (1/500), anti-VPAC2 (1/500), or anti-PAC1 (1/1,000) was used. The secondary antibody (light-chain goat anti-mouse conjugated to peroxidase) was detected by chemiluminescence. To detect actin content, PVDF membranes were stripped, reprobed, and revealed by chemiluminescence.
RNA extraction and RT-PCR.
RNA was extracted from mice tissue samples or primary epithelial cells with the RNeasy minikit following manufacturer's instructions. RNA was recovered in RNase-free water. Concentration and purity of recovered RNA were determined by UV spectrometry. Only RNA preparation with a 260/280 ratio above 1.8 was used. RNA (1 μg) was converted to cDNA with the iScript cDNA Synthesis kit according to manufacturer's instructions. The reaction cycle was 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. PCR was performed using the iTaq DNA Polymerase kit. Reaction mix included 5 μl of 10× iTaq buffer, 1.5 μl MgCl2 (50 mM), 1 μl of forward primer (50 mM), 1 μl of reverse primer (50 mM), 1 μg cDNA template, 1 μl dNTP mix (10 mM), 0.25 μl iTaq DNA polymerase, and sterile ddH2O. DNA amplification protocol was 95°C for 3 min once, followed by 40 cycles of 95°C for 30 s, 55–60°C for 30 s, and 72°C for 50 s, 72°C for 4 min and decrease to 4°C indefinitely. Samples were stored at −20°C.
PCR products were subjected to 0.8–1.5% agarose gel electrophoresis with ethidium bromide. The φx DNA ladder was used to identify sample sizes. Gels were imaged using an ultraviolet imaging box and digital camera in combination with the AlphaDigiDoc software-imaging program. Primers for VPAC1, VPAC2, PAC1, CFTR, and PKCα are listed in Tables 1 and 2.
Table 1.
Primer sequences for CFTR and VIP receptor expression detection by RT-PCR in tracheal epithelial cells
| VIP Receptor | Primer Sequence (5′→3′) | Product Size, bp | Tm, °C | |
|---|---|---|---|---|
| VPAC1 | Forward: CTCATCGGCTGGGGAGTGCC | 910 | 60 | |
| Reverse: CCGACGGGGCGGAACCTTTT | ||||
| VPAC2 | Forward: AGGCGGAGAGGGCGATAGGC | 866 | 60 | |
| Reverse: ACTGGGGATGCCCCATCCGA | ||||
| PAC1 | Forward: GGGCCAACGACCTGATGGGC | 944 | 60.8 | |
| Reverse: GGAGCGGGCCAGCCGTAAGT | ||||
| Positive control (PKCα) | Forward: TGCCGGCCAGTGGATGGTAT | 651 | 57.9 | |
| Reverse: TGCACATCCCAAAGTCGGCG | ||||
| CFTR | Forward: CGCGCAGCAACATGATCGCC | 902 | 60 | |
| Reverse: TGGACTCCTGCCTTCAGATTCCAGT |
Expected product sizes in base pairs (bp) and annealing temperature (Tm) used in RT- PCR are indicated.
Table 2.
Primer sequences for VIP receptor expression detection by RT-PCR
| VIP Receptor | Primer Sequence (5′ →3′) | Product Size, bp | Tm, °C |
|---|---|---|---|
| VPAC1 | Forward Primer: ATTTCGGGTGCTGGGACACCAT | 804 | 61.3 |
| Reverse Primer: TTTGAGGGCAGGCGGTTTGCTT | |||
| VPAC2 | Forward Primer: ACCTTCTGATCGGATGGGGCAT | 969 | 60.7 |
| Reverse Primer: TCCATAGGCATGCGTTGGGTGT | |||
| PAC1 | Forward Primer: AGCATCTACTTCAGCTGCGTGC | 245 | 60.9 |
| Reverse Primer: AGTACAGCCACCACAAAGCCCT | |||
| Positive control (PKC α) | Forward Primer: TGCCGGCCAGTGGATGGTAT | 651 | 57.9 |
| Reverse Primer: TGCACATCCCAAAGTCGGCG |
The expected product size is shown in base pairs (bp), and annealing temperature (Tm) is indicated.
Ussing chamber experiments.
Ileums freshly isolated at necropsy were rinsed in sterile PBS before mounting in Ussing chambers with an aperture size of 0.125 cm2. Krebs buffer was placed on the blood side and a low chloride (NaCl replaced with Na-gluconate) buffer on the luminal side. Buffers were gassed with humidified 95% O2:5% CO2 and maintained at 37°C by water circulated on the outside surface of the chambers. Ag/AgCl electrodes for measuring potential difference and short-circuiting were connected to the luminal- and blood-side compartments by 3 M KCl-2% agar bridges. The short-circuit current (Isc) was measured in voltage-clamp mode using an EVC4000 (World Precision Instruments, Sarasota, FL) interfaced with an ADI powerlab 26T (ADInstruments, Colorado Springs, CO). After allowing the tissue to stabilize for 10 min after mounting into the chamber, CFTR was stimulated by adding a cocktail of forskolin (10 μM), 3-isobutyl-1-methylxanthine (IBMX, 1 mM), and 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate monosodium hydrate (cpt-cAMP, 200 μM) to the lumen side of the tissue. The selective CFTR inhibitor CFTRinh172 (20 μM) was added to the lumen side after reaching a plateau of activation to unmask CFTR-dependent chloride current, and the nonspecific chloride channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS, 200 μM) was subsequently added to inhibit all other chloride channel activity. Traces were generated using LabChart7. Transepithelial resistance (TER) for ileum preparations with Krebs buffer on either side was 40.3 ± 6.2 Ω × cm2.
Statistics.
Results are reported as means ± SE. Statistical differences between groups were calculated using the unpaired, two-tailed Student's t-test. Wherever multiple groups were required to be compared we used ANOVA analysis of variance. P < 0.05 was considered statistically significant. OriginPro 8.5 software was used.
RESULTS
Correction of lung and intestinal pathology in C57Bl/6 VIP-KO mice by VIP administration.
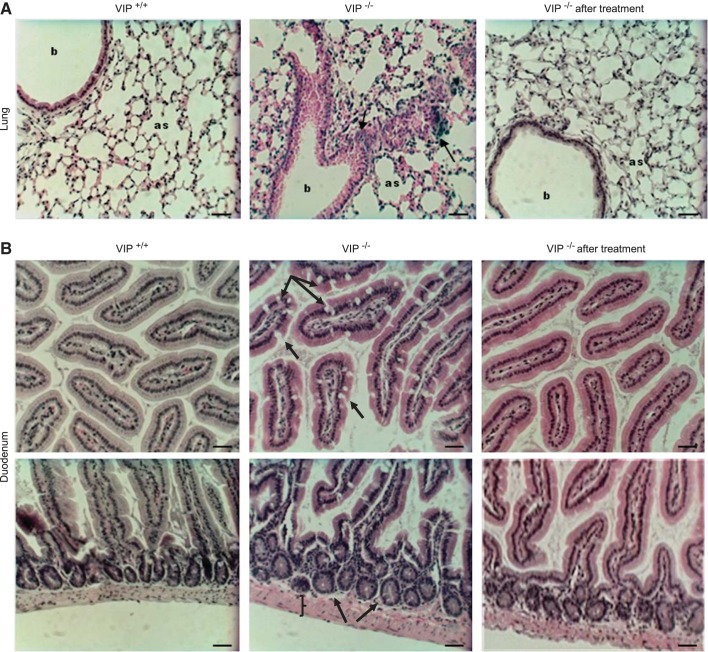
C57Bl/6 VIP-KO mice have been used to characterize the contribution of VIP in the development of respiratory diseases (33, 40) such as bronchial asthma and pulmonary arterial hypertension. Here we confirm the presence of lymphocyte aggregations and alveolar septa thickening due to infiltration of inflammatory cells, increased airway secretion, and alveolar edema (Fig. 1A, Table 3). Some of these features resembled those previously reported in C57Bl/6 cftr−/− mice, a model of long living CF mice (15) that display a typical pathology of obstructive lung disease.
Fig. 1.
Pathology of the lung and small intestine. Light microscopy pictures (20× objective) of hematoxylin and eosin (H and E)- stained tissue sections of the lung (A) and duodenum (B) of wild-type (VIP+/+), VIP-knockout (KO) (VIP−/−), and VIP-KO mice that received intraperitoneal injections of vasoactive intestinal peptide (VIP) for 3 wk (VIP−/− after treatment). A: compared with wild-type tissues, VIP-KO lungs show signs of inflammation (black arrows), inflammatory cells aggregation (dark blue staining), and thickening of the alveolar walls (as: alveolar sacs). b, Bronchioles. B: top panels show increased amount of goblet cells (black arrows) in the upper villi of VIP-KO duodenum. Crypts and muscle layer (bottom panels) are enlarged. Pathological signs observed in VIP-KO tissues were reversed by VIP administration. Scale bars = 50 μm. N = 5–7 mice in each group.
Table 3.
Pathological assessment of the lung by severity ranking
| Mouse | Inflammatory Cells | Alveolar Thickening | |
|---|---|---|---|
| VIP+/+ | 1 | − | − |
| 2 | − | − | |
| 3 | − | − | |
| 4 | − | − | |
| 5 | + | − | |
| VIP−/− | 1 | +++ | ++ |
| 2 | + | − | |
| 3 | + | − | |
| 4 | ++ | + | |
| 5 | ++ | + | |
| 6 | + | − | |
| 7 | ++ | + | |
| VIP−/− after treatment | 1 | + | − |
| 2 | + | − | |
| 3 | + | − | |
| 4 | ++ | ++ | |
| 5 | + | − |
Hematoxylin and eosin (H and E)-stained histological sections of lungs from wild-type (VIP+/+), VIP -KO (VIP−/−) and VIP knockout mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment) were analyzed. Inflammatory cells: <20 cells/section: −; 21–50 cells/section: +; 51–100 cells/section: ++; >100 cells/section: +++. No alveolar thickening: −; minor alveolar thickening: +; severe alveolar thickening: +++.
VIP-KO intestinal tissues that were examined (proximal 1 cm: duodenum) had increased number of goblet cells in the upper villi, and significantly more inflammatory cells than wild-type sections (Fig. 1B, Table 4). This is consistent with other studies on the intestinal pathology of these mice (26).
Table 4.
Pathological assessment of duodenum inflammation level by severity ranking
| Mouse | Goblet Cells | Inflammatory Cells | |
|---|---|---|---|
| VIP+/+ | 1 | − | − |
| 2 | − | − | |
| 3 | − | − | |
| 4 | − | − | |
| 5 | − | − | |
| VIP−/− | 1 | ++ | ++ |
| 2 | +++ | ++ | |
| 3 | +++ | + | |
| 4 | +++ | + | |
| 5 | +++ | ++ | |
| 6 | +++ | + | |
| 7 | + | − | |
| VIP−/− after treatment | 1 | + | − |
| 2 | ++ | + | |
| 3 | + | − | |
| 4 | +++ | + | |
| 5 | ++ | − |
Inflammation was estimated by counting goblet and inflammatory cells in histological sections of wild-type (VIP+/+), VIP-KO (VIP−/−) and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment). −: <20/section; +: 21–50/section; ++: 51–100/section; +++: >100/section.
To confirm the role of VIP gene deletion in these pathological changes, we treated VIP-KO mice with intraperitoneal administration of VIP (500 μg/kg) on alternate days over a 3-wk period as reported earlier (40). Pathological changes observed in lung and small intestine were reversed, close to the wild-type phenotype, following VIP treatment (Fig. 1, Tables 3 and 4), attributing the pathology to lack of VIP chronic exposure.
CFTR membrane localization is lost in VIP-KO epithelia.
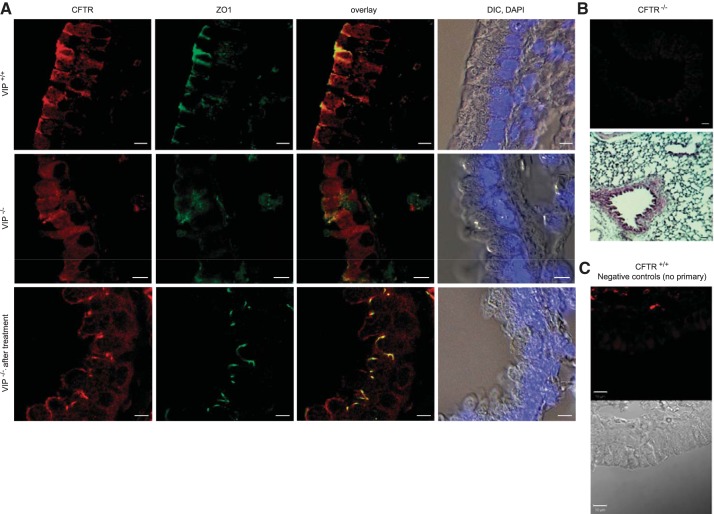
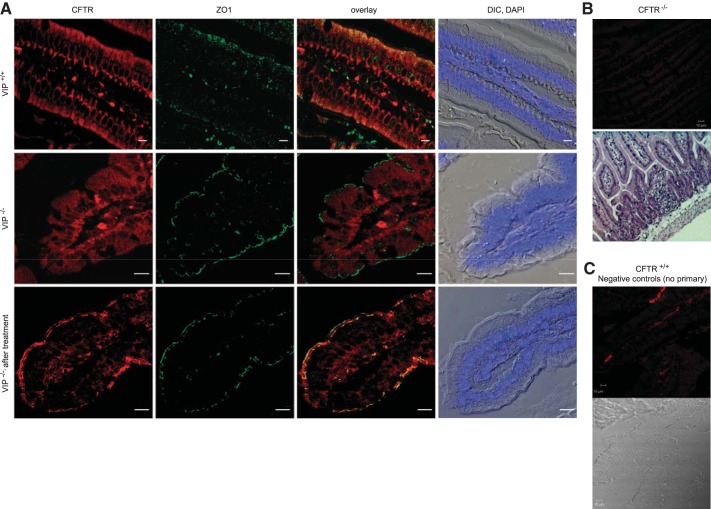
We had previously reported that VIP stimulation of the VPAC1 receptor stabilizes CFTR at the membrane of respiratory epithelial cells (8). To assess the need for chronic VIP stimulation in maintaining CFTR membrane expression in vivo, we examined CFTR localization in the lung (Fig. 2) and duodenum (Fig. 3) of wild-type and VIP-KO mice by immunofluorescence and confocal microscopy. We labeled the apical compartments of the epithelium with an anti-ZO1 antibody, followed by immunostaining for CFTR. In VIP-KO tissue sections, CFTR was largely found intracellularly compared with wild-type tissues, where it was predominantly at the apical surface (Figs. 2A and 3A). However, strong CFTR expression at the surface of the epithelium was restored in tissues from mice that received VIP injections for 3 wk before tissue collection. Negative controls performed with CFTR knockout tissues or wild-type tissues where the primary antibody was omitted confirmed the specificity of CFTR immunostaining.
Fig. 2.
Apical localization of CFTR is compromised in VIP-KO lung and restored after VIP treatment. A: confocal microscopy images of CFTR immunofluorescent signals obtained from tissue sections of paraffin-embedded lungs from wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment). CFTR signal was mainly observed in the apical portion of the epithelium of wild-type tissues where it colocalizes with ZO1, but globally distributed in epithelial cells from VIP-KO tissues. CFTR surface expression was restored in tissues from VIP-KO mice that received VIP injections. Overlay signal from CFTR and ZO1 and differential interference contrast (DIC) pictures with cell nuclei stained with DAPI are also presented. B and C: negative controls for CFTR immunostaining. B, top panels: representative image of CFTR-KO (C57Bl/6 cftr−/−) lung tissue immunostained with the CFTR monoclonal antibody (MAB1660), revealed with a goat anti-mouse secondary antibody coupled to Cy3 as in A. H and E-stained section is also shown. C: negative control for wild-type CFTR (CFTR+/+) immunostaining of the lung in which the primary antibody was omitted. Corresponding DIC picture is shown. Scale bars: 5 μm. N = 5–7 mice in each group.
Fig. 3.
Apical localization of CFTR is compromised in VIP-KO duodenum and restored after VIP treatment. A: confocal microscopy images of CFTR immunofluorescent signals obtained from tissue sections of paraffin-embedded duodenum from wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment). CFTR signal was mainly observed in the apical portion of the epithelium of wild-type tissues where it colocalizes with ZO1, but globally distributed in epithelial cells from VIP-KO tissues. CFTR surface expression was restored in tissues from VIP-KO mice that received VIP injections. Overlay signal from CFTR and ZO1 and DIC pictures with cell nuclei stained with DAPI are also presented. B and C: negative controls for CFTR immunostaining. B, top panels: representative image of CFTR-KO (C57Bl/6 cftr−/−) duodenum tissue immunostained with the CFTR monoclonal antibody (MAB1660), revealed with a goat anti-mouse secondary antibody coupled to Cy3 as in A. H and E-stained section is also shown. C: negative control for wild-type CFTR (CFTR+/+) immunostaining of the duodenum in which the primary antibody was omitted. Corresponding DIC picture is shown. Scale bars: 5 μm. N = 5–7 mice in each group.
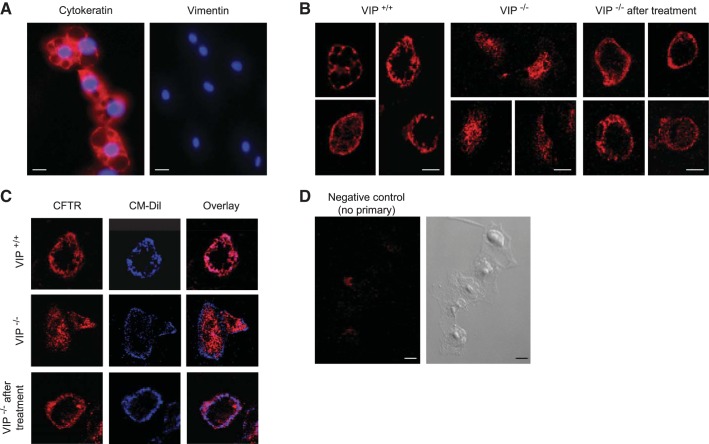
To confirm changes in CFTR localization in VIP-KO mice at the cellular level, we have used tracheal epithelial cells freshly isolated by enzymatic digestion (7). We verified that isolated cells were mainly epithelial by their positive immunoreactivity for cytokeratin, but not for the fibroblastic protein vimentin (Fig. 4A). Overlapping of CFTR signal with the membrane dye CM-Dil confirmed that CFTR immunoreactivity was observed at the cell membrane of wild-type epithelial cells (Fig. 4, B and C). Consistent with our findings in whole tissues, CFTR immunostaining was intracellular and did not colocalize with the membrane dye, in epithelial cells extracted from VIP-KO mice. VIP treatment corrected CFTR membrane localization in tracheal epithelial cells.
Fig. 4.
CFTR localization in primary tracheal epithelial cells. A: cytokeratin and vimentin immunostainings (red) were performed on epithelial cells extracted by enzymatic digestion from mice trachea to confirm the epithelial phenotype of extracted cells. DAPI was used to stain nuclei (blue). Scale bar = 20 μm. B: tracheal epithelial cells were fixed and permeabilized before immunostaining with the MAB1660 anti-CFTR antibody, followed by a goat anti-mouse secondary antibody coupled to Cy3. CFTR immunofluorescent signal was mainly found at the membrane of epithelial cells extracted from wild-type mice tracheas (VIP+/+), intracellularly in VIP-KO cells (VIP−/−), and at the surface of epithelial cells from VIP-KO mice that received VIP injections (VIP−/− after treatment). Scale bars = 10 μm. C: tracheal epithelial cells were labeled with the membrane dye CM-Dil (blue signal) followed by immunostaining for CFTR (red signal) as in B. D: negative control in which the primary antibody was omitted. Corresponding DIC picture is shown. Epithelial cells were extracted from 10 mice in each group and immunostaining repeated 3 times.
These results confirm in vivo the need for chronic VIP exposure to maintain CFTR localization at the membrane of epithelial cells. Importantly, the data suggest that defective cellular localization of mature CFTR can be corrected by VIP treatment.
Intracellular retention of mature CFTR in the absence of VIP.
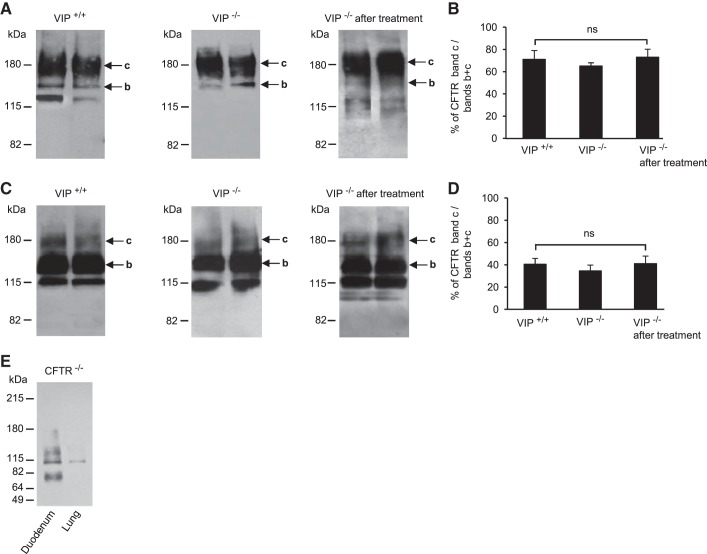
It was previously shown in epithelial cell lines and tissue preparations that CFTR membrane density increases after VIP stimulation (30 min to 2 h), an effect caused by redistribution of the intracellular pool of mature CFTR proteins to the cell surface (8, 25). CFTR is a glycosylated membrane protein with two consensus sequences for N-linked glycosylation in the fourth extracellular loop. The fully glycosylated mature form of CFTR is known to migrate at an apparent molecular mass of ∼160–180 kDa in SDS-PAGE, and is commonly referred to as band c, whereas the incompletely glycosylated form rather migrates at ∼130–150 kDa and is referred to as band b (12, 16). To determine if the lack of CFTR membrane expression in VIP-KO mice was due to intracellular retention of mature CFTR, as opposed to a change in total expression level or maturation, we have compared the amount of fully glycosylated mature CFTR band c with the total amount of CFTR including core-glycosylated immature CFTR band b and band c. Both bands were measured by densitometry of scanned immunoblots from whole tissue lysates (Fig. 5). Although we observed that the amount of band c was more abundant in duodenum than in lung samples (71.08 ± 7.97% and 40.62 ± 5.18% in wild-type duodenum and lung, respectively), we did not find any significant differences in the percentage of mature CFTR (band c) in tissue lysates from VIP-KO and wild-type mice (65.21 ± 2.82%, P > 0.1 and 34.67 ± 5.15%, P > 0.97, in VIP-KO duodenum and lung, respectively). VIP treatment did not modify CFTR expression level and maturation significantly (73.04 ± 7.2%, P > 0.29 and 41.09 ± 6.84%, P > 0.14 in VIP-KO treated duodenum and lung, respectively).
Fig. 5.
CFTR expression and maturation. A and C: representative immunoblots showing similar CFTR expression and maturation level in tissue lysates from duodenum (A) and lung (C), from two different mice in each group: wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment). Mature fully glycosylated band c and immature core-glycosylated band b are indicated by arrows. B and D: histograms showing average values from densitometry measurements of CFTR mature band c (∼180 kDa) expressed as percentage (%) of total CFTR (bands b + c) in duodenum (B) and lung (D) of wild-type, VIP-KO, and VIP-KO mice treated with VIP. ns, not statistically significant. E: negative control immunoblot showing the absence of CFTR signal in tissue lysates from CFTR-KO duodenum and CFTR-KO lung as indicated. Tissue lysates from 5 mice in each group were analyzed and used to calculate average densitometry ratios.
Functional significance of lack of VIP.
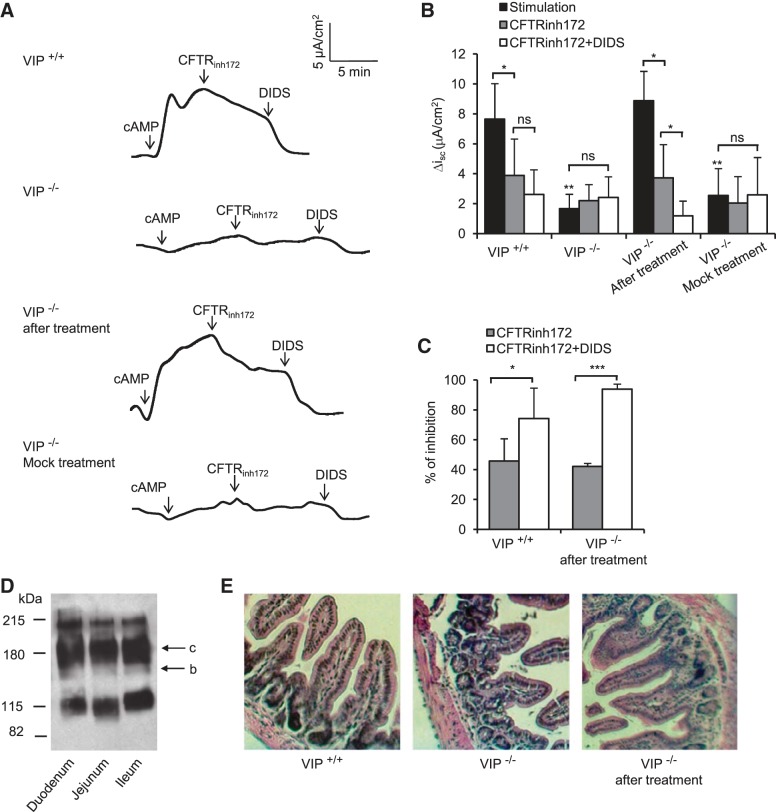
To determine the functional consequence of VIP removal and restoration on CFTR activity, CFTR-dependent chloride current was measured across freshly isolated mouse ileum mounted in Ussing chambers. A basolateral-to-apical chloride gradient was established by replacing NaCl with Na-gluconate in the apical (luminal) compartment to create a driving force for CFTR-dependent chloride secretion. CFTR channels present at the apical surface of the epithelium (lumen side of the tissue) were activated (Fig. 6). In wild-type tissues, the chloride current required to bring the transepithelial potential to zero, i.e., short-circuit current (Isc), was increased by 7.64 ± 2.37 μA/cm2 above the baseline following CFTR stimulation (cpt-cAMP + IBMX + forskolin). The stimulated current was reduced by 45.72% after the addition of a selective CFTR inhibitor (CFTRinhi172), and down to 74.25% following the inhibition of all other chloride channel activity by the nonspecific chloride channel blocker DIDS (Fig. 6, A–C). The cAMP-stimulated current was largely absent in tissues from VIP-KO mice as CFTRinh172 and DIDS had no effect, indicating the absence of functional CFTR chloride channels in tissues from VIP-KO mice. As expected, cAMP-activated CFTR-dependent chloride current was restored in small intestines from mice that received VIP injections for 3 wk, and this current had similar amplitude and sensitivity to chloride channel blockers as that measured in wild-type tissues. Recordings of tissues from VIP-KO mice that received PBS injections instead of VIP resembled those from VIP-KO tissues, confirming the specificity of VIP administration. All three sections of the small intestine (duodenum, jejunum, ileum) of C57Bl/6 mice expressed mature CFTR to a similar level (Fig. 6D). The pathology of VIP-KO ileum was similar to that seen in VIP-KO duodenum and was corrected by VIP treatment (Fig. 6E).
Fig. 6.
CFTR-dependent chloride secretion. A: short-circuit current, measured in voltage-clamp mode, generated by ileums from wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after VIP treatment) or PBS (VIP−/− mock treatment). Ileums were mounted in Ussing chambers with an aperture size of 0.125 cm2. Krebs buffer was placed on the blood side and a chloride-free buffer on the luminal side. After allowing the tissue to stabilize for ∼10 min, CFTR was stimulated by a cAMP cocktail [10 μM forskolin + 1 mM 3-isobutyl-1-methylxanthine (IBMX) + 200 μM 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate monosodium hydrate (cpt-cAMP)] added to the lumen side. The selective CFTR inhibitor CFTRinh172 (20 μM) was added ∼5–10 min after stimulation after reaching a plateau of activation to unmask CFTR-dependent chloride current, and the nonspecific chloride channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS, 200 μM) was subsequently added to inhibit all other chloride channel activity. CFTR-dependent chloride current was observed in wild-type ileum (VIP+/+) but absent from VIP-KO tissues (VIP−/−). The bottom traces show functional recovery after VIP treatment, but not with negative control injections of PBS only. B: histogram showing average values of short-circuit current differences between baseline and plateau of stimulation or inhibition (ΔIsc). C: histogram showing average values of the percentage of inhibition of the cAMP-stimulated Disc current by the CFTRinh172 and after the additive action of CFTRinh172 and DIDS. Asterisks above black bars indicate a significant difference with stimulated ΔIsc measured in wild-type ileum. D: immunoblot showing similar expression level of CFTR in duodenum, jejunum, and ileum. Bands c (mature fully glycosylated) and b (immature core-glycosylated) are indicated by arrow. E: histology sections of corresponding ileums as indicated, showing same pathology as duodenum from VIP-KO mice, and recovery after VIP injections. Ileums from 5 different mice in each group were used. *P < 0.05, **P < 0.01, ***P < 0.001.
The absence of CFTR-dependent chloride current at the apical surface of the small intestine of VIP-KO mice confirms the intracellular retention of functionally mature CFTR proteins in the absence of chronic VIP exposure. This lack of function is reversible by VIP treatment.
VPAC receptor overexpression.
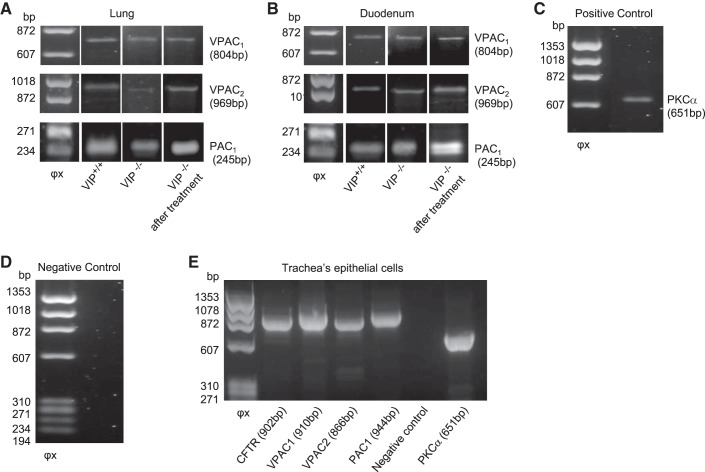
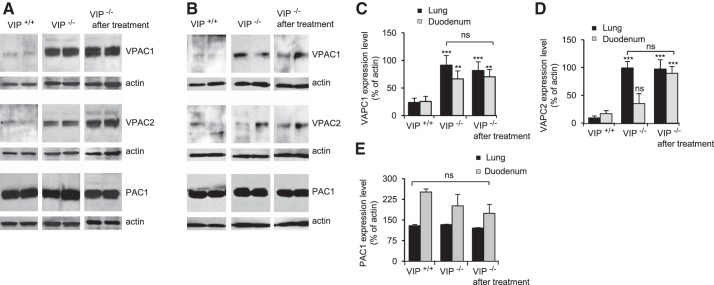
VIP binds to class II seven transmembrane domain G protein-coupled receptors on the basolateral membrane of epithelial cells. The expression of three known VIP receptors, VPAC1 (EC50 < 0.1 nM), VPAC2 (EC50 = 10 nM), and PAC1 (EC50 ∼40 nM) (23), was detected in lung, duodenum, and tracheal epithelial cells by RT-PCR (Fig. 7) and immunoblotting (Fig. 8). VPAC1 and -2 protein expression level was much higher in VIP-KO tissue lysates than in wild-type tissues, regardless of VIP treatment (Fig. 8). PAC1 expression level was unaffected by VIP loss. Deletion of the VIP gene in C57Bl/6 mice has been associated with modification of gene expression (18, 39, 41). However, this is the first report of VPAC receptor overexpression in VIP-KO mice.
Fig. 7.
Detection of VIP receptors expression by RT-PCR. VPAC1, VPAC2, and PAC1 receptor expression was detected by RT-PCR after RNA extraction from whole tissue lysates. PCR products were subjected to 0.8% agarose gel electrophoresis. Left lane: φx DNA ladder. Expected sizes (bp) of amplified products are indicated. A and B: samples from lungs (A) and duodenum (B) of wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment) were amplified with specific primers for each receptor (see materials and methods). C: positive control using PKCα. D: negative control which had cDNA excluded from the reaction. E: CFTR, VPAC1, VPAC2, and PAC1 receptor expression in epithelial cells extracted from wild-type mice tracheas. PCR products were subjected to 1.5% agarose gel electrophoresis. mRNA extracted from primary cultures of epithelial cells were amplified with specific primers (see materials and methods). PKCα was used as positive control. Reaction in which cDNA was excluded was used a negative control.
Fig. 8.
VIP receptor overexpression in VIP-KO mice. A and B: representative immunoblots showing expression of VPAC1, VPAC2, and PAC1 receptors in tissue lysates from lung (A) and duodenum (B) of wild-type (VIP+/+), VIP-KO (VIP−/−), and VIP-KO mice that received intraperitoneal injections of VIP for 3 wk (VIP−/− after treatment). Expression levels in lysates from two different mice in each group are presented. C–E: changes in each receptor's expression were calculated from densitometry measurement of scanned immunoblots and reported as percentage of corresponding actin signal. Values are means ± SE for N = 5. **P < 0.01, ***P < 0.001. ns, not statically significant.
DISCUSSION
Using VIP-KO C57Bl/6 mice, a proven model for several airway diseases (19, 31), our results provide the first in vivo validation of the role of VIP chronic exposure on CFTR membrane expression and function in lung and small intestine. Interestingly, pathological changes and CFTR mislocalization and dysfunction could be reversed, close to a wild-type phenotype, by VIP administration.
In the gastrointestinal tract, VIPergic neurons innervate exocrine glands and epithelial cells, and VIP functions include the relaxation of smooth muscles, mucus and enzyme secretion, and the regulation of electrolyte secretion noteworthy through the activation of CFTR (28). Dysregulation of VIP secretion has been implicated in a number of human disorders of the gut such as the “watery diarrhea syndrome,” inflammatory bowel disease, Type I diabetes, intestinal ileus, and more (36). A loss of VIP-containing neurons was observed in some cases. In a recent study, Lelievre et al. (26) proposed that VIP-KO mice provide an experimental model to study intestinal ileus. They found anatomical alterations that were attributed to the loss of VIP relaxation function and antiproliferative properties. Interestingly, they also reported an increased number of goblet cells, but reduced mucus secretion, potentially attributed to the loss of VIP secretory property with a direct stimulatory role of VIP in goblet cells. The authors did not investigate the molecular mechanism involved, but other studies have suggested that CFTR is expressed in goblet cells (14, 21, 22).
The intestinal blockage attributed to abnormal fluid and mucus secretion in CF mice corresponds to the meconium ileus equivalent human pathology. A failure to propel intestinal content and abrupt blockade with gut stenosis provoked 10–15% mortality rate in the first year of VIP-KO mice (26). Although most CF mice experience a high rate of perinatal mortality, some CF mice strains with less severe mutations and residual CFTR function have higher longevity and fewer propensities to lethal intestinal blockage. In C57Bl/6 cftr−/− CF mice, a model of long-living CF mice, intestinal abnormalities include mucus plugging, defective reabsorption, failure to thrive, goblet cell hyperplasia (ileum), chronic inflammation, and abnormal electrophysiology with strong reduction of CFTR-dependent chloride current (44). These resemble some of the features observed by us and others in VIP-KO mice.
Here we show that pathological signs can be reversed close to the wild-type phenotype after VIP injections (intraperitoneal) on alternate days over a 3-wk period. This treatment was previously reported to efficiently correct VIP-KO lung disease, achieving a similar level of VIP in the lung of VIP-KO mice after VIP treatment to that in wild-type mice (40). Our study also adds to this with the molecular finding that CFTR membrane localization and function are lost in VIP-KO mice, creating a CF-like phenotype.
We found that CFTR localization was largely intracellular in VIP-KO epithelia of the lung and small intestine, as opposed to an apical surface localization in wild-type tissues. We also confirmed the intracellular localization of CFTR in primary trachea epithelial cells. Importantly, the recovery of CFTR membrane localization after VIP treatment confirmed the importance of VIP chronic exposure to the regulation of CFTR localization. CFTR is known to be mainly present in recycling endosomes to form an important intracellular submembranar pool of mature CFTR proteins (6, 43). As no significant changes in CFTR abundance or maturation level were found by immunoblotting of tissue lysates, we conclude that changes in CFTR localization should be due to a redistribution of an existing pool of mature CFTR proteins. These results are consistent with previous studies (3, 5) reporting that CFTR proteins, in highly expressing cells of rat intestinal epithelium, are redistributed from an intracellular pool to the cell's surface in response to VIP stimulation, and back to the intracellular pool after VIP removal. Our immunohistochemistry and immunoblotting data are corroborated by functional assays. Ex vivo analysis of CFTR-dependent chloride current performed in freshly isolated ileums from wild-type, VIP-KO, and VIP-KO mice that received VIP injections for 3 wk confirms that CFTR is absent from the intestinal epithelium of VIP-KO, but can be recovered after VIP treatment. We thus suggest that the loss of secretory function observed in VIP-KO mice intestine can be attributed, at least in part, to a lack of CFTR-dependent secretion, which ultimately depends on VIP stimulation, for both acute and long-term effects.
Except for the gut, VIP is present in nerves supplying exocrine glands, in nerve fibers present in the smooth muscle layer of the airways, and in the microglia innervating the pulmonary structure. It is an airway smooth muscle cell relaxant and a pulmonary vasodilator, with anti-inflammatory and anti-proliferative activities that help control airway remodeling and reduce airway resistance (36). VIP is abundant in inflammatory cells as well. In response to VIP stimulation, submucosal glands, in which CFTR is highly expressed, secrete low level of mucus which participates in the airway's innate defense, noteworthy by enabling mucociliary clearance. As expected from all the above-mentioned important functions of VIP in the airways and immune system, VIP-KO mice display some characteristics of respiratory pathologies, correctable by VIP administration. Increased peribronchial and perivascular infiltration of inflammatory cells, a hyperresponsiveness to the methacholine challenge, and pulmonary arterial hypertension phenotype with thickened pulmonary arteries have been reported (33, 40). Similarly for the intestine, pathological observations and our molecular findings of mature CFTR intracellular retention, correctable by VIP administration, highlight the importance of CFTR regulation by VIP chronic exposure in the lung pathophysiology.
An important and somewhat unanswered question in CF and other diseases for which VIP is suspected to play an important role (CF, bronchial asthma, pulmonary hypertension, certain GI disorders) is the nature and expression level of VIP receptors in tissues affected, especially the lung, small intestine, and pancreas. Interestingly, we found an overexpression of VPAC1 and VPAC2 receptors for VIP in lung and small intestine of VIP-KO mice. Deletion of the VIP gene in C57Bl/6 mice has been associated with the modification of gene expression, but this is the first report indicating VIP receptor overexpression. Desensitization of VPAC receptors involves their phosphorylation by G protein-coupled receptor kinase, followed by internalization and recycling back to the cell surface. For the VAPC1 receptor, however, the link between the phosphorylation level and its internalization is not direct and has not yet been completely clarified (13). Also, VPAC receptors form hetero-oligomers and interact with accessory proteins capable of modifying their activity, membrane expression, and recycling mechanism (24). Possible changes in VPAC interaction with accessory proteins in diseased epithelia could explain the overexpression observed in VIP-KO tissues.
Conflicting results exist in the signaling cascade involved in CFTR membrane expression and recycling. In human nasal epithelial cells (JME/CF15), derived from a F508del-CFTR homozygous patient, VIP treatment (300 nM) for 1 or 2 h induced the maturation and membrane insertion of functional F508del-CFTR proteins by stimulation of the VPAC1 receptor. Both the PKA and PKCϵ-dependent signaling cascade were found to be involved (1, 30). The receptors are widely distributed in the body with some variation in cell type expressing VPAC1 and VPAC2 receptors. Analogs of the VPAC2 receptor, involved in the relaxation of smooth muscle, are being developed for respiratory diseases such as bronchial asthma and chronic obstructive pulmonary disease (COPD) (2, 17). Also, recent studies involved the VPAC1 receptor in animal models of chronic inflammation (13, 23). Further investigations will be necessary to clarify the role of each receptor in CFTR regulation by VIP in the mouse lung and small intestine.
The absence of functional CFTR channels from the cell surface of exocrine epithelial is the hallmark of cystic fibrosis. Although CF patients' mortality is mainly due to respiratory complications, gastrointestinal symptoms commonly precede the pulmonary findings and may suggest the diagnosis in infants with meconium ileus (11). Meconium ileus equivalent or distal intestinal obstruction syndrome (DIOS) occurs in 10–15% of older CF patients. Interestingly, a strong reduction of VIP-immunoreactive nerves was observed in the nasal and intestinal mucosa of CF patients (20, 37, 38, 42), suggesting that a lack of VIP stimulation of CFTR-dependent secretions could play a role in CF development. In favor of this notion are some phenotypic similarities between VIP-KO and CF mice. Genetically modified mice that display abnormalities in the lung and small intestine are largely used to study cystic fibrosis, with a lack of consensus on the most appropriate model to study the human condition (44). Despite considerable variations in the phenotype of CF mice, most studies agree on intestinal manifestations that closely resemble those seen in the human CF pathology with intestinal blockage attributed to abnormal fluid and mucus secretion corresponding to the meconium equivalent human pathology. Also, increase in small intestine weight, goblet cell hyperplasia, and mucus accumulation were reported in the C57Bl/6 CF mice (see 44 for review). These anomalies are somewhat similar to those reported in VIP-KO mice (26 and the present study). Among the differences between VIP-KO and CF mice are survival rates and capacity to thrive. A failure to propel intestinal content and abrupt blockade and gut stenosis are responsible for 10–15% mortality rate in the first year of VIP-KO mice. Although most CF mice experience a high rate of perinatal mortality, some CF mice strains with less severe genetic background, which enable some residual CFTR function, display higher longevity and fewer propensities to lethal intestinal blockage. Our data do not exclude a residual amount of functional CFTR at the surface of epithelial cells, which could explain the difference in survival rate compared with CF mice despite similar intestinal anomalies.
There is large variability in the lung phenotype expressed by CF mice, with generally little sign of lung disease in mice unchallenged with pathogen infection. However, several groups reported inflammatory lung phenotype associated with progressive tissue remodeling in adult (16–19 wk) unchallenged CF mice, mostly in the C57Bl/6 background. Interestingly, their inflammatory phenotype could be attributed to CFTR deficiency (see 44 for review).
With its regulatory effect on multiple organ systems, and its role in the pathogenesis of several human disorders, it is evident that VIP has strong therapeutic potential. Experimental animal models and several clinical trials have shown a beneficial effect of exogenous VIP administration (Aviptadil) in preventing or alleviating phenotypes associated with a number of respiratory diseases, such as acute lung injuries, bronchial asthma, sarcoidosis, and pulmonary arterial hypertension (27, 29, 32). VIP receptors themselves also represent an important therapeutic target, and it is expected that a strong pharmacology will be developed around these receptors in the next few years to provide potent agonists and antagonists with strong potential in human therapy. Our data suggest that VIP or VPAC receptor agonists should now be evaluated as candidate corrector molecules for the treatment of cystic fibrosis. Multiple aspects of the disease could be corrected by a VIP-based treatment. This includes the control of inflammation and the correction of the molecular basis of cystic fibrosis, namely the correction of CFTR mislocalization and dysfunction for the vast majority of cystic fibrosis patients with the F508del mutation.
GRANTS
This work was supported by Cystic Fibrosis Canada, Natural Sciences and Engineering Research Council of Canada (NSERC), and Canada Foundation for Innovation research grants. N. G. Alcolado was supported by a Nova Scotia Health Research Foundation (NSHRF) scholarship, D. J. Conrad by a NSERC scholarship, and D. Poroca by a Capes-SWB-Brazil scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.A., D.J.C., D.P., M.L., W.A., F.G.C., R.M.P., Y.A., Z.X., S.A.H., S.I.S., and V.M.C. performed experiments; N.A., D.J.C., D.P., M.L., W.A., F.G.C., R.M.P., Y.A., Z.X., S.I.S., and V.M.C. analyzed data; N.A., D.J.C., D.P., M.L., W.A., R.M.P., Y.A., Z.X., S.I.S., and V.M.C. interpreted results of experiments; N.A., D.J.C., M.L., F.G.C., R.M.P., and V.M.C. prepared figures; N.A., D.J.C., M.L., R.M.P., Y.A., Z.X., S.I.S., and V.M.C. edited and revised manuscript; N.A., D.J.C., D.P., M.L., W.A., F.G.C., R.M.P., Y.A., Z.X., S.A.H., S.I.S., and V.M.C. approved final version of manuscript; R.M.P., Y.A., S.I.S., and V.M.C. drafted manuscript; S.I.S. and V.M.C. conception and design of research.
ACKNOWLEDGMENTS
We thank P. Colp for expert histology and pathology advice, and help with experiments and students' training at the Dalhousie Pathology and Histology lab; S. Whitefield for expert advice and students' training with confocal microscopy and digital imaging; Staff from Animal Care facilities at Dalhousie and Stony Brook Universities, and especially E. Rogerson who performed VIP injections; and R. Warner and R. Newman from the Department of Mechanical Engineering at Dalhousie University for assistance in the design and construction of Ussing chambers.
REFERENCES
- 1.Alcolado N, Conrad DJ, Rafferty S, Chappe FG, Chappe VM. VIP-dependent increase in F508del-CFTR membrane localization is mediated by PKCε. Am J Physiol Cell Physiol 301: C53–C65, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini F, Thakkar M, Foda HD, Said SI, Lodi R, Pakbaz H, Schraufnagel DE. Vasoactive intestinal peptide enhances lung preservation. Transplantation 56: 964–973, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J, McLaughlin GE. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science 256: 530–532, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Brockman-Schneider RA, Amineva SP, Bulat MV, Gern JE. Serial culture of murine primary airway epithelial cells and ex vivo replication of human rhinoviruses. J Immunol Methods 339: 264–269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappe FG, Loewen ME, Hanrahan JW, Chappe VM. VIP increases CFTR levels in the apical membrane of Calu-3 cells through a PKC-dependent mechanism. J Pharmacol Exp Ther 327: 226–238, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chappe V, Hinkson DA, Howell LD, Evagelidis A, Liao J, Chang XB, Riordan JR, Hanrahan JW. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 101: 390–395, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappe V, Hinkson DA, Zhu T, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol 548: 39–52, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudry G, Navarro OM, Levine DS, Oudjhane K. Abdominal manifestations of cystic fibrosis in children. Pediatr Radiol 36: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol 166: 42–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dray-Charier N, Paul A, Veissiere D, Mergey M, Scoazec JY, Capeau J, Brahimi-Horn C, Housset C. Expression of cystic fibrosis transmembrane conductance regulator in human gallbladder epithelial cells. Lab Invest 73: 828–836, 1995 [PubMed] [Google Scholar]
- 15.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol 164: 1481–1493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol 184: 847–862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groneberg DA, Rabe KF, Fischer A. Novel concepts of neuropeptide-based drug therapy: vasoactive intestinal polypeptide and its receptors. Eur J Pharmacol 533: 182–194, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J 31: 135–139, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Hamidi SA, Szema AM, Lyubsky S, Dickman KG, Degene A, Mathew SM, Waschek JA, Said SI. Clues to VIP function from knockout mice. Ann NY Acad Sci 1070: 5–9, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Heinz-Erian P, Dey RD, Flux M, Said SI. Deficient vasoactive intestinal peptide innervation in the sweat glands of cystic fibrosis patients. Science 229: 1407–1408, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J 6: 169–176, 1993 [PubMed] [Google Scholar]
- 22.Kalin N, Claass A, Sommer M, Puchelle E, Tummler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest 103: 1379–1389, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides 28: 1631–1639, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Langer I. Mechanisms involved in VPAC receptors activation and regulation: lessons from pharmacological and mutagenesis studies. Front Endocrinol (Lausanne) 3: 129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrich RW, Aller SG, Webster P, Marino CR, Forrest JN., Jr Vasoactive intestinal peptide, forskolin, and genistein increase apical CFTR trafficking in the rectal gland of the spiny dogfish, Squalus acanthias. Acute regulation of CFTR trafficking in an intact epithelium. J Clin Invest 101: 737–745, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, Waschek JA. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease. Peptides 28: 1688–1699, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuchte HH, Baezner C, Baumgartner RA, Bevec D, Bacher G, Neurohr C, Behr J. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J 32: 1289–1294, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Murthy KS, Grider JR. VIP. In: Handbook of Biologically Active Peptides (2nd ed.), edited by Kastin AJ. San Diego, CA: Elsevier, 2012, chapt. 184, p. 1354–1360 [Google Scholar]
- 29.Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, Delgado M, Muller-Quernheim J. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med 182: 540–548, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Rafferty S, Alcolado N, Norez C, Chappe F, Pelzer S, Becq F, Chappe V. Rescue of functional F508del-CFTR by VIP in the human nasal epithelial cell line JME/CF15. J Pharmacol Exp Ther 331: 2–13, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Said SI. Animal models of airway hyperresponsiveness. Eur Respir J 33: 217–218, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Said SI. The vasoactive intestinal peptide gene is a key modulator of pulmonary vascular remodeling and inflammation. Ann NY Acad Sci 1144: 148–153, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115: 1260–1268, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science 169: 1217–1218, 1970 [DOI] [PubMed] [Google Scholar]
- 35.Said SI, Mutt V, Yoshida T, Hara N. Vasoactive polypeptides from normal lung. Chest 67, Suppl 2: 44S, 1975 [DOI] [PubMed] [Google Scholar]
- 36.Sami SI, Chappe V, Sayyed HA. VIP. In: Handbook of Biologically Active Peptides (2nd ed.), edited by Kastin AJ. New York: Elsevier, 2012, chapt. 209, p. 1535–1548 [Google Scholar]
- 37.Savage MV, Brengelmann GL, Buchan AM, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol 69: 2149–2154, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Sharma RK, Addis BJ, Jeffery PK. The distribution and density of airway vasoactive intestinal polypeptide (VIP) binding sites in cystic fibrosis and asthma. Pulm Pharmacol 8: 91–96, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Szema AM, Hamidi SA, Koller A, Martin DW. Vasoactive intestinal peptide knockout (VIP KO) mouse model of sulfite-sensitive asthma: upregulation of novel lung carbonyl reductase. BMC Immunol 12: 66–2172-12–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szema AM, Hamidi SA, Lyubsky S, Dickman KG, Mathew S, Abdel-Razek T, Chen JJ, Waschek JA, Said SI. Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol 291: L880–L886, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Szema AM, Hamidi SA, Smith SD, Benveniste H. VIP gene deletion in mice causes cardiomyopathy associated with upregulation of heart failure genes. PLos One 8: e61449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wattchow DA, Furness JB, Gibbins IL, Little KE, Carter RF. Vasoactive intestinal peptide immunoreactive fibres are deficient in intestinal and nasal mucosa affected by cystic fibrosis. J Gastroenterol Hepatol 3: 549–555, 1988 [Google Scholar]
- 43.Webster P, Vanacore L, Nairn AC, Marino CR. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol Cell Physiol 267: C340–C348, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 10, Suppl 2: S152–S171, 2011 [DOI] [PubMed] [Google Scholar]