Abstract
Recent studies show that guidance molecules that are known to regulate cell migration during development may also play an important role in adult pathophysiologic states. One such molecule, semaphorin3A (sema3A), is highly expressed after acute kidney injury (AKI) in mice and humans, but its pathophysiological role is unknown. Genetic inactivation of sema3A protected mice from ischemia-reperfusion-induced AKI, improved tissue histology, reduced neutrophil infiltration, prevented epithelial cell apoptosis, and increased cytokine and chemokine excretion in urine. Pharmacological-based inhibition of sema3A receptor binding likewise protected against ischemia-reperfusion-induced AKI. In vitro, sema3A enhanced toll-like receptor 4-mediated inflammation in epithelial cells, macrophages, and dendritic cells. Moreover, administration of sema3A-treated, bone marrow-derived dendritic cells exacerbated kidney injury. Finally, sema3A augmented cisplatin-induced apoptosis in kidney epithelial cells in vitro via expression of DFFA-like effector a (cidea). Our data suggest that the guidance molecule sema3A exacerbates AKI via promoting inflammation and epithelial cell apoptosis.
Keywords: acute kidney injury, cisplatin, semaphorin3A
acute kidney injury (AKI) is a common condition in humans that is complicated by lack of effective therapies or preventative strategies. Although the incidence varies between definitions and populations, the disorder is estimated to occur in 1–9% of hospital inpatients and over 40% of critically ill patients treated for sepsis in the intensive care unit (3, 7, 12–14). The pathogenesis of AKI is complex and multifactorial in nature. Inflammation, tubular epithelial cell apoptosis, and impaired perfusion are prominent features that promote acute tubular necrosis and subsequent progression to end-stage renal failure (5, 9, 24).
Guidance cues represent a class of molecules that play a key role in development by directing cells to reach target tissues or organs through attraction or repulsion (1, 8, 28). Although these molecules are also expressed in adult tissues, their function in adult organs is poorly understood. Semaphorins compromise the largest family of axon guidance cues yet discovered and play a key role in neural development. They are characterized structurally by a conserved ∼400 amino acid sema domain and classically described as collapsing factors and mediators of axon repulsion, although they may also act as context-dependent chemoattractants (26). Semaphorins are divided into eight classes, with classes 3–7 expressed in vertebrates. Class 3 semaphorins are secreted proteins, classes 4–6 are transmembranous, and class 7 are membrane-associated via glycosylphosphatidylinositol linkages. Semaphorins have also been shown to regulate cardiovascular development, immune cell antigen presentation, and tumor progression (2, 4, 18, 26, 30). Semaphorin3A (sema3A) is a chemorepellent with multiple guidance functions, including axon pathfinding, cardiac patterning, and peripheral vascular patterning and branching morphogenesis. In addition, sema3A is also known to induce apoptosis in T cells, neuronal and endothelial cells (8, 10, 22). Recent studies also suggest that sema3A may be critical for sepsis-induced cytokine storm (34). Both monocyte and dendritic cells mediate injury in ischemia-reperfusion injury by promoting inflammation (19, 20). However, whether sema3A regulates inflammatory response of monocyte and dendritic cells during reperfusion injury is unknown. Sema3A signaling is mediated by a complex of the binding receptor neuropilin 1 and the signaling receptors plexinA1 or A3 (11, 17). Both sema3A and neuropilin 1 are expressed in the developing glomerulus, and sema3A expression persists in adult podocytes and collecting tubules (25, 33). Sema3A inhibits ureteric bud branching by downregulation of glial cell line-derived neurotrophic factor (GDNF) (30). The regulation of sema3A expression and its pathophysiological role in the adult kidney are unknown.
The current studies were undertaken to determine the pathogenic role of sema3A in AKI and whether it mediates its pathogenic activity through increasing epithelial cell apoptosis and inflammation. Our results indicate that genetic inactivation or functional inhibition of sema3A is renoprotective against ischemic-reperfusion injury and suggest that sema3A mediates tissue injury by promoting inflammation and tubular epithelial cell apoptosis.
MATERIALS AND METHODS
Renal ischemia-reperfusion.
C57BL/6J mice, C3H and sema3A mutant mice (C3;B6-Sema3am808Ddg/J) (Jax# 014646) were purchased from Jackson Laboratories. Sema3A mutant mice were created by chemical-induced point mutation, which inactivates sema3A receptor binding. Sema3A mutant mice were transferred into the C3H background by backcrossing at Jackson Laboratories. To induce ischemia-reperfusion injury of the kidneys, 8- to 9-wk-old male mice were anesthetized with pentobarbital sodium (50 mg/kg body wt ip) and placed on a heating pad to maintain body temperature at 37°C. Both renal pedicles were identified through dorsal incisions and clamped for 26 min. Reperfusion was confirmed visually upon release of the clamps. As a control, sham-operated animals were subjected to the same surgical procedure, except the renal pedicles were not clamped. Surgical wounds were closed, and mice were given 1 ml of warm saline intraperitoneally and kept in a warm incubator until they regained consciousness. Some animals received recombinant sema3A inhibitory peptide (50 μg/animal iv, sequence: N-Ac-HAVEHGFMQTLLKVTLE-NH2) (35), LoxBlock-1 (2 μg/animal ip), or vehicle (0.1% BSA) 1 h before renal pedicle clamping. Urine and kidney tissues were collected 24 h after reperfusion and processed for ELISA and RNA isolation. The Institutional Animal Care and Use Committee of the Georgia Regents University approved all protocols and procedures of animal use (approval number BR10-10-369).
Experiments in RAW264.7 macrophage cell line.
RAW264.7 cells (ATCC) were grown to confluence using advanced RPMI medium containing 5% serum in a six-well plate. On the day of treatment, serum-free advanced RPMI medium was added, and cells were treated with 1, 10, or 50 ng of LPS with 100 ng/ml of sema3A or vehicle for a period of 4 h. Supernatant and RNA were isolated at the end of treatment for quantification of cytokine and chemokine expression.
Experiments in renal tubular epithelial cells.
Mouse kidney proximal tubular epithelial cells (TKPTS) were grown to confluence using advanced DMEM/F-12 medium containing 5% serum in a six-well plate. On the day of treatment, serum-free advanced DMEM/F-12 medium was added, and cells were treated with 50 ng of LPS with 100 ng/ml of sema3A or vehicle for a period of 4 h. Sema3A dose was based on previous studies in macrophages (34). Supernatant and RNA were isolated at the end of the experiment for quantification of cytokines and chemokine expression.
Quantification of mRNA by real-time RT-PCR.
Real-time RT-PCR was performed in an Applied Biosystems 7700 Sequence Detection System (Foster City, CA). Total RNA (1.5 μg) was reverse transcribed in a reaction volume of 20 μl using Omniscript RT kit and random primers. The product was diluted to a volume of 150 μl, and 5-μl aliquots were used as templates for amplification using the SYBR Green PCR amplification reagent (Qiagen) and gene-specific primers. The primer sets used were as follows: mouse TNF-α (forward: GCATGATCCGCGACGTGGAA; reverse: AGATCCATGCCGTTG GCCAG), MCP-1 (forward: ATGCAGGTCCCTGTCATG; reverse: GCTTGAGGTGGTTGTGGA), ICAM-1 (forward: AGATCACATTCACGGTGCTG; reverse: CTTCAGAGGCAGGAAACAGG), IL-1β (forward: CTCCATGAGCTTTGTACAAGG; reverse: TGCTGATGTACCAGTTGGGG), IL-18 (forward: ACTGTACAACCGCAGTAATACGG; reverse: AGTGAACATTACAGATTTATCCC), IL-10 primer (forward: ATGCCTGGCTCAGCACTG; reverse: GTCCTGCATTAAGGAGTCG), TGF-β1 (forward: TGACGTCACTGGAGTTGTACGG; reverse: GGTTCATGTCATGGATGGTGC), IL-23 (forward: TGTGCCCCGTATCCAGTGT; reverse: 5′-CGGATCCTTTGCAAGCAGAA), IL-12 (forward: AGAGAGACTTCTTCCACAACAAGAG; reverse: TCTGGTACATCTTCAAGTCCTCATAGA), GDNF (forward: GCCACCATTAAAAGACTGAAAAGG; reverse: GCCTGCCGATTCCTCTCTCT), and IL-6 (forward: GATGCTACCAAACTGGATATAATC; reverse: GGTCCTTAGCCACTCCTTCTGTG). The amount of DNA was normalized to the β-actin signal amplified in a separate reaction (forward primer: AGAGGGAAATCGTGCGTGAC; reverse: CAATAGTGATGACCTGGCCGT).
Fibrotic gene expression was determined using PCR array from SAbiosciences.
Renal function.
Renal function was assessed by measurements of serum creatinine (cat. no.: DZ072B, Diazyme Labs).
Cytokine and chemokine measurements.
Cytokines and chemokines in plasma were measured using ELISA array kit from eBioscience.
Histology and immunostaining.
Kidney tissue was fixed in buffered 10% formalin for 12 h and then embedded in paraffin wax. For assessment of injury, 5-μm sections were stained with periodic acid-Schiff (PAS) followed by hematoxylin. Tubular injury was assessed in PAS-stained sections using a semiquantitative scale (23) in which the percentage of cortical tubules showing epithelial cell necrosis, brush-border loss, cast formation, and apoptotic bodies in the cortex was assigned a score: 0 = normal; 1 = <10%; 2 = 10–25%; 3 = 26–75%; 4 = >75%. Ten fields of ×40 magnification were examined and averaged. The individual scoring of the slides was blinded to the genotype and treatment of the animal. To quantify leukocyte infiltration, sections were stained with rat anti-mouse neutrophil antibody (Abcam, Cambridge, MA; 1:200 dilution) followed by goat anti-rat biotin conjugate. Color was developed after incubation with ABC reagent (Vector Lab). Stained sections were photographed and five ×40 fields of neutrophils were examined for quantification of leukocytes. To determine sema3A-expressing cells in kidney, slides were incubated in the absence or presence of primary antibodies to sema3A (Abcam) in humidified chambers overnight at 4°C, followed by incubation with biotin-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. Color was developed after incubation with ABC reagent (Vector Lab). To determine whether sema3A is induced after injury in proximal tubular epithelial cells, megalin antibody was used to stain the proximal tubules. The stained sections were photographed with an Olympus BX40 microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP12; Olympus America; magnification ×660).
TACS TdT in situ apoptosis detection.
To identify apoptotic cells, tissue sections were stained using TACS TdT in situ Apoptosis Detection kit (R&D Systems) according to the manufacturer's instructions. Briefly, tissue sections were deparaffinized, hydrated, and washed with PBS. Sections were digested with proteinase K for 15 min at 24°C. Slides were then washed, and endogenous peroxidase activity was quenched with 3% H2O2 in methanol. Slides were washed and incubated with TdT labeling reaction mix at 37°C for 1 h and then with streptavidin-horseradish peroxidase. Color was developed using TACS blue label substrate solution. Slides were washed, counterstained, and mounted with Permount. Sections were photographed and labeled cells were counted and quantified.
Bone marrow-derived dendritic cells.
Bone marrow-derived dendritic cells (BM-DC) were obtained using previously described protocol (36). Briefly, mouse tibiae and femurs were flushed with ice-cold PBS through a 70-μm-wide cut-off cell strainer. Cells were pelleted by centrifuging 5 min at 1,400 rpm. The pellet was resuspended in conditioned medium supplemented with 10 μg/ml of GM-CSF (Prospec Biotechnology). Cells were seeded (7 × 106 cells) in 100×20-mm nontreated cell culture plates in 10 ml of conditioned medium and incubated at 37°C 5% CO2 incubator. On days 4 and 7 after being plated, 5 ml of prewarmed conditioned medium were added, and on day 8/9 the BM-DCs were ready for experimental use.
To determine the effects of sema3A on BM-DC, cells were treated with 100 ng/ml of sema3A for 48 h. Cells were harvested for RT-PCR analysis or for in vivo infusion. To determine whether sema3A-treated BM-DC were pathogenic in AKI, 5 × 106 cells were infused via tail vein 1 h before ischemia-reperfusion. Kidney function was monitored by measuring serum creatinine. As a control, vehicle-treated BM-DC were administered.
Cell culture and quantification of apoptosis.
TKPTS were cultured in advanced DMEM/F-12 medium with 5% serum. To determine whether cisplatin-induced cell death is exacerbated in the presence of sema3A, cells were treated with vehicle or 10 μM cisplatin. Twenty-four hours after addition of cisplatin, cells were harvested, stained with PI and annexin V, and analyzed by flow cytometry.
To determine the role of cidea (cell death-inducing DNA fragmentation factor, α subunit-like effector A) in mediating sema3A-induced apoptosis, TKPTS cells were transfected with 50 nM siRNA against cidea. Forty-eight hours after transfection, cells were treated with 10 μM cisplatin for 24 h. Cells were harvested and used for quantification of apoptosis by flow cytometry.
Statistical methods.
All assays were performed in duplicate. The data are reported as means ± SE. Statistical significance was assessed by an unpaired, two-tailed Student's t-test for single comparison or ANOVA for multiple comparisons.
RESULTS
Sema3A and its receptor neuropilin-1 are induced after ischemia-reperfusion in the kidney.
Our previous studies (15) demonstrated that urinary excretion of sema3A is markedly increased after ischemia-reperfusion injury of the kidney compared with sham-operated control. Similarly, a large increase in sema3A excretion was observed following cardiopulmonary bypass surgery in pediatric patients who developed AKI compared with those who did not (15). Consistent with those results, immunohistochemical analysis showed that sema3A expression was localized in the distal and collecting tubules and in podocytes, and expression was primarily upregulated in distal and collecting tubules following ischemia-reperfusion injury (Fig. 1A). Staining for sema3A was also detected in the injured proximal tubular epithelial cells after reperfusion injury.
Fig. 1.
Regulation of expression of semaphorin3A (sema3A) and its receptors in response to ischemia-reperfusion (IR) of the kidney. A: immunohistochemical localization of sema3A and neuropilin-1 in mouse kidney. Sema3A (green) is localized in podocytes, and in distal and collecting duct epithelial cells, in sham-operated kidney, and the intensity of staining was increased following IR. Staining for sema3A was also detected in the injured proximal tubular epithelial cells after reperfusion injury. White asterisk shows megalin (red) and sema3A colocalization (bottom). Scale bar: 100 μM. B: sema3A receptor mRNA expression in wild-type (WT) and sema3A mutant mice in response to renal IR injury. Plexin-A1 was significantly upregulated, whereas plexin-A3 was downregulated in WT mice that were subjected to IR. In contrast, plexin-A2 expression was not altered. Neuropilin-1 expression was increased in WT mice following IR, which was strongly abrogated in sema3A mutant mice, while neuropilin-2 expression was unaffected by IR. *P < 0.05 vs. other groups; n = 4–6. KO, knockout.
RT-PCR analysis demonstrated that expression of the sema3A receptor plexin-A1 increased slightly, whereas plexin-A2 and neuropilin-2 expression were not altered in response to ischemia-reperfusion in wild-type (WT) mice or sema3A mutant mice (Fig. 1B). However, the expression of plexin-A3 was significantly downregulated after ischemia-reperfusion. Consistent with increased protein expression of neuropilin-1 in the kidney, mRNA expression was also increased >8-fold in WT mice kidney after ischemia-reperfusion, which was completely suppressed in sema3A mutant mice (Fig. 1B).
Sema3A mutant mice are resistant to ischemia-reperfusion-induced kidney injury.
The role of sema3A in renal pathophysiology is unknown. To determine the role of sema3A in AKI, WT and sema3A mutant mice were subjected to sham operation or ischemia followed by 24-h reperfusion. As shown in Fig. 2A, WT mice subjected to ischemia-reperfusion developed renal dysfunction indicated by increased serum creatinine, which was significantly reduced in sema3A mutant mice, suggesting a pathogenic role for sema3A in ischemia-reperfusion-induced AKI.
Fig. 2.
Sema3A mutant mice were protected against IR-induced kidney injury. A: creatinine levels (index of renal function). Sema3A mutant or WT mice were subjected to 35 min of ischemia followed by 24 h of reperfusion. Sema3A mutant mice showed significantly lower serum creatinine compared with WT mice after 24 h of reperfusion. *P < 0.001 vs. sham-operated. #P < 0.05 vs. WT IR; n = 8–10. B: tissue histology for acute tubular necrosis, assessed in periodic acid-Schiff (PAS)-stained kidney sections. Sham-operated WT and sema3A mutant mice exhibited normal tubular structure. Following IR, kidneys from WT mice exhibited extensive tubular necrosis, loss of brush border, and cast formation. These changes were strongly abrogated in sema3A mutant mice. Quantification of tubular injury is shown in C. *P < 0.001 vs. sham-operated. #P < 0.001 vs. WT IR; n = 6–8.
Consistent with renal dysfunction in WT mice, PAS-stained kidney sections from WT mice showed tubular necrosis, epithelial cell brush-border loss, nuclear condensation, cytoplasmic swelling, and cast formation in the outer stripe of outer medulla. These pathological changes were strongly abrogated in the sema3A mutant mice (Fig. 2B). Tubular injury score was also significantly lower in sema3A mutant mice compared with WT mice following ischemia-reperfusion (Fig. 2C).
Sema3A inactivation blunts inflammation and apoptosis in response to ischemia-reperfusion injury.
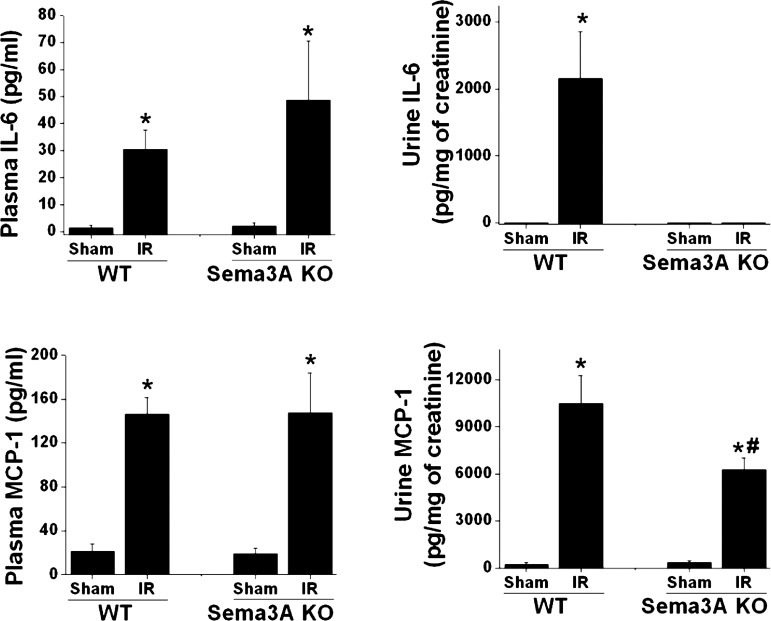
Inflammation is a major mediator of tissue injury in ischemic AKI (16, 29). Since sema3A is also known to regulate inflammatory cell function, we determined the influence of sema3A in ischemia-reperfusion-induced inflammation in the kidney. As shown in Fig. 3, ischemia-reperfusion induced a large increase in the mRNA expression of TNF-α, IL-1β, IL-6, toll-like receptor 4 (TLR4), and ICAM-1 in WT mice, which was blunted in sema3A mutant mice. Expression of MCP-1 was not decreased significantly in sema3A mutant mice compared with WT mice. Ischemia-reperfusion of the kidney is known to upregulate a protective molecule GDNF (27), which was shown to be regulated by sema3A during development (30). To determine whether sema3A-mediated suppression of GDNF contributes to ischemia-reperfusion injury, GDNF expression was quantified. As shown in Fig. 3, ischemia-reperfusion induced a large increase in WT mice which significantly blunted in sema3A mutant mice. However, slight but significant increase was seen at basal GDNF expression in sema3A knockout mice kidney when it was normalized to WT sham and the difference was absent after reperfusion (Fig. 3). This suggests that GDNF expression may be in part negatively regulated by sema3A in normal kidney and the regulation is not prominent after reperfusion. Consistent with increased mRNA expression of cytokines and chemokines, the urinary excretion of IL-6 and MCP-1 was significantly increased in WT mice following ischemia-reperfusion, which was blunted in sema3A mutant mice (Fig. 4). However, plasma levels of IL-6 and MCP-1 did not differ between WT and sema3A mutant mice (Fig. 4), suggesting that urinary MCP-1 and IL-6 are most likely derived from the kidneys as opposed to the circulation. Diminished inflammatory cytokine and chemokine expression were associated with reductions in renal neutrophil infiltration in sema3A mutant mice compared with WT kidney following ischemia-reperfusion (Fig. 5A).
Fig. 3.
IR-induced inflammatory mediators and glial cell line-derived neurotrophic factor (GDNF) expression are suppressed in sema3A mutant mice. IR induced a large increase in mRNA expression of proinflammatory mediators in the kidneys of WT mice, which was significantly suppressed in sema3A mutant mice. *P < 0.001 vs. other groups. #P < 0.001 vs. WT IR; n = 4–6.
Fig. 4.
Sema3A mutant mice exhibited reduced urinary excretion of IL-6 and MCP-1 following IR, while plasma levels were unchanged. IL-6 and MCP-1 in urine were quantified by ELISA as described in materials and methods and expressed as pg/mg of urine creatinine. *P < 0.001 vs. other groups. #P < 0.001 vs. WT IR; n = 5.
Fig. 5.
A: neutrophil infiltration into kidneys of WT and sema3A mutant mice subjected to sham operation or IR. Neutrophil infiltration was assessed by immunohistochemistry (top), and quantitative data are shown in bottom. *P < 0.001 vs. sham-operated. #P < 0.001 vs. WT IR; n = 4–6. B: apoptosis of tubular epithelial cells in kidneys of WT and sema3A mutant mice subjected to sham operation or IR. Apoptosis was assessed by TdT-mediated dUTP nick end labeling (TUNEL) staining. Blue nuclei indicate TUNEL-positive apoptotic cells (top). Quantitative data are shown in bottom. *P < 0.001 vs. sham-operated. #P < 0.001 vs. WT IR; n = 4–6.
Activation of inflammatory pathways may trigger apoptosis of renal tubular epithelial cells. To assess the role of sema3A in mediating ischemia-reperfusion-induced apoptosis of tubular epithelial cells, TdT-mediated dUTP nick end labeling staining was performed. Sections from sham-operated kidneys exhibited no detectable apoptotic nuclei (Fig. 5B). Ischemia-reperfusion induced a large increase in apoptotic nuclei in epithelial cells, which were largely suppressed in sema3A mutant mice.
Pharmacological-based inhibition of sema3A reduced ischemia-reperfusion-induced renal dysfunction and tubular epithelial cell apoptosis.
To further investigate the role of sema3A in ischemia-reperfusion-induced AKI, two additional complementary approaches were employed. First, WT mice were treated with a sema3A signaling blocker, LOXblock-1, or vehicle, and then subjected to ischemia-reperfusion injury. LOXblock-1 blocks 12/15-lipoxygenase, a mediator of sema3A function, and has been demonstrated to inhibit sema3A-induced growth cone collapse (21). As shown in Fig. 6A, treatment with LOXblock-1 prevented renal dysfunction following ischemia-reperfusion and diminished apoptosis of tubular epithelial cells, similar to the findings observed in sema3A mutant mice (Fig. 6, B and C). Since 12/15-lipoxygenase may mediate other effectors, we employed another approach that blocks specifically sema3A binding to its receptor.
Fig. 6.
LOXblock-1, an inhibitor of sema3A signaling, blocked kidney injury and prevented apoptosis in response to IR. A: kidney function was determined by measuring serum creatinine. *P < 0.001 vs. sham-operated. #P < 0.05 vs. IR vehicle treated. B: apoptosis of renal tubular epithelial cells was assessed by TUNEL staining. C: quantitative data for TUNEL-positive nuclei. *P < 0.001 vs. sham-operated. #P < 0.001 vs. IR vehicle treated; n = 4–6.
In the second approach, a sema3A receptor-blocking peptide (35) was used to determine the function of sema3A in ischemia-reperfusion-induced AKI. A scrambled peptide was administered as a control, as described in materials and methods. As shown in Fig. 7, the sema3A receptor-blocking peptide attenuated renal dysfunction and blunted apoptosis of tubular epithelial cells following ischemia-reperfusion. The level of tubular injury seen in the inhibitory peptide and LOXBlock-1 groups was similarly reduced to the sema3A knockout mice. Taken together, these findings strongly support a role for sema3A signaling in the pathogenesis of AKI and suggest that therapies directed against this pathway may be effective at preventing renal injury in susceptible patients.
Fig. 7.
Sema3A-blocking peptide prevented kidney injury and blunted apoptosis in response to IR. A: kidney function was determined by measuring serum creatinine. *P < 0.001 vs. sham-operated. #P < 0.05 vs. IR scrambled peptide treated. B: apoptosis of renal tubular epithelial cells was assessed by TUNEL staining. C: quantitative data for TUNEL-positive nuclei. *P < 0.001 vs. sham-operated. #P < 0.001 vs. IR scrambled peptide treated; n = 6.
Sema3A exacerbates cisplatin-induced epithelial cell apoptosis through cidea in vitro.
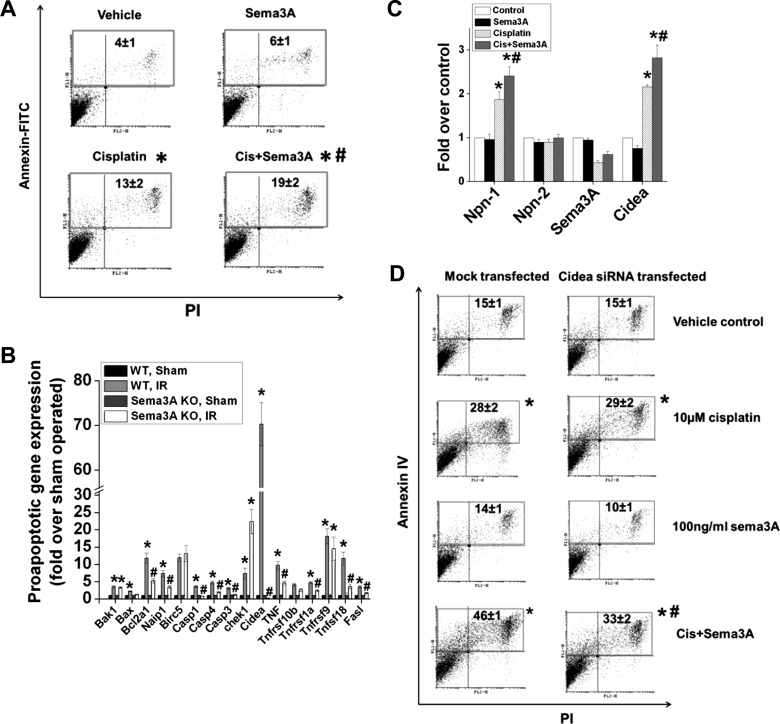
To determine whether sema3A increases epithelial cell apoptosis directly or indirectly, TKPTS were treated with either cisplatin or cisplatin+sema3A for a period of 24 h. Cisplatin induced a significant increase in apoptosis. Addition of sema3A alone did not increase apoptosis. However, when sema3A was added along with cisplatin, apoptosis was enhanced significantly (Fig. 8A).
Fig. 8.
Sema3A exacerbates cisplatin-induced apoptosis in renal tubular epithelial cells. A: quantification of apoptosis (PI and annexin V staining followed by flow cytometry analysis) in mouse proximal tubular epithelial cells (TKPTS) treated with vehicle or 10 μM cisplatin with/without 100 ng/ml sema3A for 24 h. *P < 0.05 vs. vehicle. #P < 0.05 vs. cisplatin. B: expression of proapoptotic genes in kidneys of WT and sema3A mice following IR, determined by PCR microarray analyses. *P < 0.05 vs. sham-operated. #P < 0.05 vs. WT IR. C: effects of cisplatin with/without sema3A on neuropilin, sema3A, and cidea (cell death-inducing DNA fragmentation factor, α subunit-like effector A) expression in TKPTS cells determined by RT-PCR analysis. *P < 0.05 vs. control and sema3A. #P < 0.05 vs. cisplatin; n = 4–6. D: cidea mediates sema3A-induced augmentation of apoptosis in renal epithelial cells (TKPTS). Cells transfected with siRNA against cidea or mock-transfected TKPTS cells were treated with cisplatin + vehicle or cisplatin + sema3A (100 ng/ml) for 24 h. Apoptosis was determined as described above. *P < 0.05 vs. vehicle control. #P < 0.05 vs. mock-transfected cisplatin-treated; n = 6.
To investigate the mechanism of sema3A-induced increase in tubular epithelial cells apoptosis, proapoptotic gene expression was quantified using PCR array. Expression of several proapoptotic genes was increased in kidneys of WT mice after ischemia-reperfusion, which was suppressed in sema3A mutant mice (Fig. 8B). Importantly, cidea gene expression was induced over 70-fold following ischemia-reperfusion in WT mice, which was completely suppressed in sema3A mutant mice. Similar to the in vivo data, cisplatin increased Cidea expression in renal epithelial cells in vitro, which was further increased with sema3A treatment (Fig. 8C).
To determine whether the sema3A-induced increase in epithelial cell apoptosis was mediated by cidea, we transfected cells with siRNA directed against cidea and then treated them with cisplatin with/without sema3A. Cidea knockdown by siRNA was confirmed by RT-PCR. As shown in Fig. 8D, sema3A-induced augmentation of epithelial cell apoptosis was blunted in siRNA-transfected cells, suggesting that cidea mediates sema3A-induced epithelial cell apoptosis.
Sema3A enhances TLR4-induced inflammatory response in epithelial cells, macrophages, and dendritic cells.
Previous studies suggest that sema3A contributes to sepsis-induced cytokine storm and TLR signaling (34). This suggested that sema3A might cooperate with TLR4 signaling to induce inflammation and kidney damage in AKI. To investigate this possibility, epithelial cells, macrophages, and BM-DC were treated with LPS with vehicle or sema3A. The expression of cytokines and chemokines was quantified by RT-PCR and ELISA. As shown in Fig. 9, LPS-induced IL-6 production was significantly enhanced in renal epithelial cells (A) and macrophages (B) in the presence of sema3A, suggesting cross talk between TLR4 and sema3A signaling. Moreover, sema3A induced the expression of TNF-α, IL-1β, IL-6, and IL-23 in dendritic cells, which was further enhanced in the presence of LPS (Fig. 9D). In addition, expression of CD40, an activation marker of dendritic cells, was also enhanced by sema3A treatment (Fig. 9E). Finally, administration of sema3A-treated BM-DC before ischemia-reperfusion exacerbated AKI compared with control (untreated) BM-DC (Figs. 9F and 10, A-D) in conjunction with increased expression of proinflammatory cytokines and chemokines (Fig. 10E).
Fig. 9.
Sema3A synergizes with Toll-like receptor 4 (TLR4) signaling to enhance cellular inflammatory responses and kidney injury. TKPTS (A), macrophages (B), and dendritic cells (C–F) were treated with a low dose of LPS (1 ng/ml) with/without sema3A (100 ng/ml) for 24 h. Control cells were treated with vehicle (0.1% BSA in PBS). Cytokine expression was quantified by RT-PCR and ELISA. Induction of the proinflammatory cytokine IL-6 by LPS was augmented by sema3A treated in TKPTS cells (A), macrophages (B), and dendritic cells (C). *P < 0.05 vs. vehicle-treated. #P < 0.05 vs. LPS- or sema3A-treated. $P < 0.05 vs. LPS-treated. C: flow cytometry analysis of in vitro differentiated bone marrow cells. Ninety-eight percent of cells were positive for CD11c and CD11b, and negative for F4/80 monocyte/macrophage marker, consistent with myeloid-derived dendritic cells (DC). D: upregulation of cytokine expression in LPS-treated DC by sema3A. *P < 0.05 vs. vehicle-treated. #P < 0.05 vs. LPS-treated DC. E: sema3A increased expression of the DC activation marker CD40. *P < 0.001 vs. all other groups. F: administration of sema3A-treated DC before IR exacerbates kidney injury. *P < 0.05 vs. sham. #P < 0.05 vs. vehicle-treated DC; n = 6.
Fig. 10.
Infusion of sema3A-treated bone marrow-derived DC (BM-DC) before IR exacerbated kidney injury. Kidney sections were stained with PAS-hematoxylin, and tubular injury was scored as described in materials and methods. A: sham-operated kidney. B: infusion of vehicle-treated BM-DC before IR, showing tubular necrosis and cast formation. C: infusion of sema3A-treated BM-DC before IR, showing more extensive tubular necrosis and cast formation. D: quantification of tissue injury. *P < 0.01 vs. sham-operated. #P < 0.05 vs. vehicle-treated DC; n = 4. E: inflammatory cytokine and chemokine expression (analyzed by real-time RT-PCR) in kidneys of the 3 groups of mice. *P < 0.001 vs. sham. #P < 0.05 vs. vehicle-treated DC; n = 4.
DISCUSSION
AKI occurs commonly in hospitalized patients in the context of ischemia-reperfusion. Inflammation and epithelial cell apoptosis have been shown to play a critical role in AKI. However, the role of guidance cues such as semaphorins in regulating these processes is unknown. Our results show for the first time that sema3A, a class 3 semaphorin, is highly induced after AKI and mediates tubular epithelial cell apoptosis and inflammation through a cidea-dependent mechanism.
In nondiseased kidneys, sema3A was reported to be expressed in renal collecting ducts and podocytes (15, 33). Consistent with those results, our localization studies show a similar expression pattern in kidneys of healthy mice. Ischemia-reperfusion induced sema3A expression in collecting ducts and injured proximal tubules. However, the induction appears to be at a posttranscriptional level (15). The sema3A staining detected in injured proximal tubules could potentially have resulted from uptake of filtered sema3A or sema3A secreted from podocytes.
Neuropilin-1 was shown to be a major binding receptor for sema3A. Ischemia-reperfusion upregulated neuropilin-1 in the kidney and was shown to be expressed by both epithelial cells and immune cells (31, 33), suggesting that sema3A may function by activating its receptors in both the parenchyma and interstitium. This was further supported by our in vitro studies showing sema3A induced cytokine and chemokine production by macrophages and dendritic cells and upregulated the expression of a DC activation marker. Furthermore, sepsis-induced cytokine storm was reported to require sema3A (34), suggesting molecular cross talk between TLR4 and sema3A signaling. Consistent with this notion, our results show that LPS-induced inflammation in epithelial cells, macrophages, and DC was augmented by sema3A. Moreover, sema3A treatment renders BM-DC more pathogenic in the setting of AKI. We speculate that upregulation of TNF-α and/or IL-23 may mediate sema3A pathogenic activity in BM-DC, although further studies are required to confirm this hypothesis.
Sema3A was reported to induce apoptosis of neuronal cells and endothelial cells (8, 10, 22), and our study sheds new light into the molecular mechanisms. We report that expression of cidea, which mediates epithelial cell apoptosis in response to cisplatin in vitro, was blunted in sema3A mutant mice. In Hela cells, cidea protein is localized in mitochondria under basal conditions, and upon activation, it translocates to the nucleus, where it initiates apoptosis and DNA fragmentation (32). Cidea is also contained in lipid droplets and was reported to induce Fas translocation from lipid droplets to the membrane, thereby increasing apoptosis (22). Our studies using PCR array show that ischemia-reperfusion-induced Fas expression was blunted in kidneys of sema3A mutant mice. Therefore, it is possible that both cidea activation and Fas translocation are linked to sema3A, although further studies are required to confirm this hypothesis. Our data also show that basal expression of GDNF, a prosurvival growth factor (27), was significantly higher in sema3A knockout animal kidneys. Therefore, it is also possible that part of the protective effects may be mediated through GDNF in sema3A knockout mice.
Both activated T cells and DC were reported to produce sema3A (6, 18). However, our in vitro studies suggest that while DC do express receptors for sema3A, they express very little sema3A (data not shown). Moreover, it is not clear whether resident DC in the kidney express sema3A. The contribution of other immune cell types to sema3A expression is also not clear and will require further investigations. Our in vitro studies show sema3A added alone in cultured epithelial cells did not induce apoptosis, but it increased cisplatin-induced epithelial cell apoptosis. Thus, sema3A most likely acts in synergy with cell stress or injurious stimuli to promote apoptosis. This may also explain how sema3A can be highly expressed in healthy kidneys without triggering injury.
In conclusion, our studies demonstrate that sema3A is highly induced after ischemia-reperfusion of the kidneys and plays a pathogenic role by increasing cidea protein-mediated apoptosis of renal epithelial cells and inflammatory response in the kidney. Our studies using a small blocking peptide to inhibit sema3A signaling suggest that these findings could potentially be translated to the treatment of AKI in humans.
GRANTS
G. Ramesh is supported by National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases R01 Grant 1R01DK083379–01A3 and a startup grant from Georgia Health Sciences University. N. L. Weintraub is supported by HL076684 and HL112640 from National Heart, Lung, and Blood Institute. P. Ranganathan and R. Mohamed are supported by a postdoctoral fellowship from American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.R., C.J., and R.M. performed experiments; P.R., R.M., and G.R. analyzed data; P.R., R.M., N.L.W., and G.R. interpreted results of experiments; P.R., C.J., R.M., and G.R. prepared figures; P.R. and G.R. drafted manuscript; P.R., C.J., R.M., N.L.W., and G.R. edited and revised manuscript; P.R., C.J., R.M., N.L.W., and G.R. approved final version of manuscript; G.R. conception and design of research.
REFERENCES
- 1.Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33: 233–248, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383: 525–528, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev 26: 421–431, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 107: 3321–3329, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 8.de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol 71: 249–267, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem 282: 26294–26305, 2007 [DOI] [PubMed] [Google Scholar]
- 11.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Himmelfarb J, Joannidis M, Molitoris B, Schietz M, Okusa MD, Warnock D, Laghi F, Goldstein SL, Prielipp R, Parikh CR, Pannu N, Lobo SM, Shah S, D'Intini V, Kellum JA. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol 3: 962–967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36: S146–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Jayakumar C, Ranganathan P, Devarajan P, Krawczeski CD, Looney S, Ramesh G. Semaphorin 3A is a new early diagnostic biomarker of experimental and pediatric acute kidney injury. PLos One 8: e58446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell 90: 753–762, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci 116: 3463–3470, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte//macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikule K, Gatlin JC, de la Houssaye BA, Pfenninger KH. Growth cone collapse induced by semaphorin 3A requires 12/15-lipoxygenase. J Neurosci 22: 4932–4941, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood 111: 2290–2299, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166–F174, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Robert B, Zhao X, Abrahamson DR. Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol 279: F275–F282, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 66: 649–666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H, Patschan D, Dietz GPH, Bähr M, Plotkin M, Goligorsky MS. Glial cell line-derived neurotrophic growth factor increases motility and survival of cultured mesenchymal stem cells and ameliorates acute kidney injury. Am J Physiol Renal Physiol 294: F229–F235, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sun KLW, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development 138: 2153–2169, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol 185: 3750–3758, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev 125: 558–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadasz Z, Ben Izhak O, Bejar J, Sabo E, Kessel A, Storch S, Toubi E. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus 20: 1466–1473, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Valouskova E, Smolkova K, Santorova J, Jezek P, Modriansky M. Redistribution of cell death-inducing DNA fragmentation factor-like effector-a (CIDEa) from mitochondria to nucleus is associated with apoptosis in HeLa cells. Gen Physiol Biophys 27: 92–100, 2008 [PubMed] [Google Scholar]
- 33.Villegas G, Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev 119, Suppl 1: S149–S153, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Wen H, Lei Y, Eun SY, Ting Y. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J Exp Med 207: 2943–2957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel semaphorin/neuropilin antagonists. J Neurochem 92: 1180–1190, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zanoni I, Ostuni R, Granucci F. Generation of mouse bone marrow-derived dendritic cells (BM-DCs). Protocol Exchange 10.1038/nprot.2009.137 [DOI] [Google Scholar]