Abstract
The present study tested the hypothesis that the multiligand endocytic receptor megalin is partially involved in the uptake of ANG II and downstream signaling responses in mouse proximal tubule cells (mPCT) by interacting with AT1a receptors. mPCT cells of wild-type (WT) and AT1a receptor-deficient (AT1a-KO) mice were treated with vehicle, the AT1 receptor blocker losartan (10 μM), or a selective megalin small interfering (si) RNA for 48 h. The uptake of fluorescein (FITC)-labeled ANG II (10 nM, 37°C) and downstream signaling responses were analyzed by fluorescence imaging and Western blotting. AT1a receptors and megalin were abundantly expressed in mPCT cells, whereas AT1a receptors were absent in AT1a-KO mPCT cells (P < 0.01). In WT mPCT cells, FITC-ANG II uptake was visualized at 30 min in the cytoplasm and in the nuclei 1 h after exposure. Losartan alone completely blocked the uptake of FITC-ANG II, whereas megalin siRNA inhibited only 30% of the response (P < 0.01). The remaining FITC-ANG II uptake in the presence of megalin siRNA was completely abolished by losartan. ANG II induced threefold increases in phosphorylated MAP kinases ERK1/2 and a onefold increase in phosphorylated sodium and hydrogen exchanger 3 (NHE3) proteins, which were also blocked by losartan and megalin-siRNA. By contrast, losartan and megalin siRNA had no effects on these signaling proteins in AT1a-KO mPCT cells. We conclude that the uptake of ANG II and downstream MAP kinases ERK1/2 and NHE3 signaling responses in mPCT cells are mediated primarily by AT1a receptors. However, megalin may also play a partial role in these responses to ANG II.
Keywords: angiotensin II, AT1 receptor-mediated endocytosis, kidney, megalin, proximal tubule
the proximal tubule plays a critical role in maintaining basal arterial blood pressure and body salt and fluid homeostasis under physiological conditions by reabsorbing >65% of filtered sodium and water from the kidney (14, 34, 35, 45, 52). Increases in sodium and fluid reabsorption by the proximal tubule may contribute to salt and fluid retention and therefore the development of hypertension (10, 12, 18). It is now well established that sodium and fluid transport in the proximal tubule is promoted by ANG II via acting on its type 1 receptors (AT1 or AT1a) on apical and basolateral membranes (11, 19, 36, 49). ANG II levels are much higher in the proximal tubule than in the circulation (3, 37, 41, 50). Two major mechanisms have been suggested to contribute to high levels of ANG II in the proximal tubule (37, 51). First, the proximal tubule expresses all major components of the renin-angiotensin system, including the substrate angiotensinogen and the enzymes renin and angiotensin-converting enzyme (ACE) necessary for the formation of ANG II (4, 5, 37, 51). This strongly suggests that ANG II may be generated in proximal tubule cells. However, we and others have demonstrated that the proximal tubule takes up the circulating endocrine and local paracrine ANG II from the extracellular fluid compartments via the AT1 (AT1a) receptor-dependent mechanism (29, 31, 42, 44, 50, 53). In vitro, fluorescein-labeled ANG II (FITC-ANG II) is clearly taken up by cultured rabbit and wild-type mouse proximal tubule cells, which is mediated by AT1 (AT1a) receptors (25, 27, 30). In vivo, we have previously shown that the proximal tubule of the kidney accumulates systemically infused unlabeled and [125I]-labeled ANG II (29, 31). These studies confirm that the proximal tubule of the kidney has the unique capacity to accumulate the circulating or extracellular ANG II.
In addition to the AT1 (AT1a) receptor-dependent mechanism, little is known about other molecular and cellular mechanisms underlying the uptake of extracellular ANG II and its downstream signaling responses in the proximal tubule. In previous studies, we reported that colchicine and specific siRNAs for microtubule-associated proteins 1A and 1B significantly inhibited AT1a receptor-mediated uptake of FITC-ANG II, suggesting that the cytoskeleton microtubule proteins may be involved in the AT1a receptor-mediated uptake of ANG II by cultured proximal tubule cells (25, 27). However, it is likely that other cellular or molecular mechanism(s) may also be involved. One of these additional mechanisms may be the endocytic receptor megalin. Megalin is a multiligand endocytic receptor, which is abundantly expressed in apical membranes of the proximal tubule of the kidney (2, 8, 9). Megalin plays a crucial role in mediating the uptake of low-molecular-weight (LMW) proteins, peptides, amino acids, vitamins, and nutrients in the proximal tubule (13, 22, 43, 48). Global genetic deletion of megalin is prenatally lethal due to defective forebrain and lung development (46) and severe LMW proteinuria in mice (23). There is early evidence that megalin may bind and internalize ANG II and its metabolite ANG (1–7) in an immortalized rat yolk sac carcinoma cells and in mouse kidney cortical brush-border membrane vesicles (16, 17). However, whether megalin is involved in AT1a receptor-mediated uptake of ANG II has not been investigated in mouse proximal tubule cells with or without expression of AT1 (AT1a) receptors. Similarly, whether megalin is involved in the ANG II-induced MAP kinases ERK1/2 and sodium and hydrogen exchanger 3 (NHE3) signaling responses has not been investigated in proximal tubule cells previously.
In the present study, we used immortalized mouse proximal tubule cells (mPCT) from wild-type C57BL/6J mice and mutant mice with deficiency of ANG II AT1a receptors (AT1a-KO) to test the hypothesis that megalin mediates the uptake of ANG II and downstream signaling responses in mPCT cells by interacting with AT1a receptors. To determine the physiological relevance of megalin-mediated uptake of ANG II in the proximal tubule, we further determined whether molecular knockdown of megalin expression alters AT1a receptor-mediated uptake of ANG II and ANG II-induced signal responses in mPCT cells.
MATERIALS AND METHODS
Chemicals.
DMEM, nutrient mixture Ham's F-12 (DMEM/F-12), heat-inactivated FBS, trypsin, penicillin, and streptomycin were obtained from ATCC. Fluorescein (FITC)-labeled ANG II was purchased from Invitrogen, whereas unlabeled ANG II was purchased from Bachem. The AT1 receptor blocker losartan and the AT2 receptor blocker PD 123319 were purchased from Tocris Bioscience. The specific megalin small interfering (si) RNA (h), a pool of three target-specific 19- to 25-nucleotide siRNAs designed to maximally knock down megalin expression, and an affinity-purified goat polyclonal antibody raised against the C terminus of megalin of human origin (sc-16476) were obtained from Santa Cruz Biotechnology (sc-40103). The specificity and effectiveness of the megalin siRNA and megalin antibody were previously reported (24, 30). The mouse monoclonal antibody targeting phosphorylated MAP kinases ERK1/2, the goat polyclonal antibody targeting total and phosphorylated NHE3 were obtained from Santa Cruz Biotechnology and Millipore, respectively. The rabbit polyclonal antibody targeting total MAP kinases ERK 1/2 was purchased from Cell Signaling. Western blot supplies were purchased from Life Sciences, whereas a BCA protein assay kit was obtained from Thermo Fisher Scientific.
Proximal tubule cell culture.
Wild-type (C57BL/6J) and AT1a-KO mPCT cells were kindly provided by Dr. Ulrich Hopfer of Case Western Reserve University. To generate immortalized wild-type and AT1a-KO mPCT cells, Dr. Hopfer and colleagues bred C57BL/6J or AT1a-KO mice with an Immortomouse, which harbored a thermolabile SV40 large-T antigen (Tag) (47). Since S1 proximal convoluted tubules express the most abundant megalin among all tubular segments, they were microdissected from wild-type and AT1a-KO mouse kidneys to generate matched sets of AT1a receptor-expressing wild-type or AT1a-KO mPCT cells. Woost et al. (47) have previously confirmed that mPCT cells retain characteristic morphological and functional properties of the proximal tubular epithelium, including uniformed cobblestone-shaped monolayers with extensive brush borders, well-defined tight junctions, and primary cilia. These mPCT cells have intact transepithelial electrolyte transport, short-circuit current activities, and show normal responses to ANG II in vitro (47). For all experiments in the present study, mPCT cells of passages 8–12 were subcultured to 80% confluence in six-well plates or glass coverslips, as appropriate, in the complete DMEM/F-12 growth medium at 37°C, supplied with 95% O2-5% CO2, 50 nM hydrocortisone, 5% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, as we described previously (27, 30, 33, 47). Untreated wild-type and AT1a-KO mPCT cells were used as controls in all experiments.
AT1a receptor expression in mPCTs.
The expression of AT1 (AT1a) receptors in wild-type mPCT cells or the lack of AT1a receptor expression in AT1a-KO mPCT cells was recently characterized using [125I]-ANG II receptor binding assays, RT-PCR, and Western blotting, respectively (25, 31, 32, 47).
Immunofluorescent imaging and Western blot analysis of megalin protein expression in mPCT cells.
mPCT cells were split cultured on glass coverslips to ∼80% confluence. After the medium was removed and mPCTs were washed with PBS twice, the cells were first incubated with a specific megalin primary antibody (sc-16476, 1:100) for 3 h, followed by incubation with a secondary donkey anti-goat FITC-labeled antibody (sc-2024, 1:100) for 3 h, respectively, for immunofluorescent imaging of megalin proteins, as we described (30). For negative control of megalin immunofluorescence, mPCT cells grown on glass coverslips were incubated only with the donkey anti-goat FITC-IgG without a first incubation with a primary megalin antibody. A blocking peptide was also previously used to verify the specificity of the first megalin antibody (30). Megalin immunofluorescence was visualized using a Nikon-Eclipse TE2000-U inverted fluorescence microscope and a FITC-specific filter (26, 27, 30). Western blot analysis was also performed to quantitate megalin protein expression in WT and AT1a-KO mPCTs.
Knockdown of megalin expression in mPCT cells.
To determine the role of megalin in mediating ANG II or AT1a-dependent uptake of ANG II in mPCT cells, wild-type or AT1a-KO mPCTs were transfected with a selective 19- to 25-nucleotide megalin siRNA (sc-40103, 4 μg/well), or a scrambled siRNA (sc-37007, 4 μg/well) for 48 h using Lipofectamine 2000 (27, 30). The specificity and the effectiveness of megalin siRNA in knocking down megalin expression were determined by measuring megalin mRNA expression in mPCT cells by RT-PCR and live cell fluorescent imaging of megalin expression. The forward and reverse primers for PCR of megalin mRNA expression were provided by Santa Cruz Biotechnology (sc-40103-PR), and the annealing and extending temperatures were 55°-60°C and 68°-70°C, respectively (24). The effect of specific megalin siRNA on megalin protein knockdown was further determined using a specific megalin primary antibody (sc-16476, 1:100).
Live cell fluorescent imaging of megalin- and AT1a receptor-mediated FITC-labeled ANG II in mPCT cells.
Wild-type and AT1a-KO mPCT cells were grown to 60% confluence on glass coverslips. Under these conditions, mPCT cells did not have the entire lateral and basal membranes exposed to ligands or drugs. Only brush border or apical membranes were exposed to ligands or drugs. Untreated, the AT1 receptor antagonist losartan-, AT2 receptor antagonist PD123319-, megalin siRNA-, or scrambled siRNA-treated wild-type and AT1a-KO mPCT cells were incubated with FITC-ANG II (1 nM, Molecular Probes) at 37°C without exposure to light for 30 min to 1 h. After incubation, the medium was removed and mPCT cells were washed twice with warm PBS and counterstained with the nuclear acid marker 4,6-diamidino-2-phenylindole (DAPI; 300 nM) for 10 min. Glass coverslips were mounted on a temperature-controlled perfusion chamber (37°C), which was further mounted on a Nikon-Eclipse TE2000-U inverted fluorescence microscope for live cell fluorescent imaging. Green (FITC-ANG II) and blue (DAPI) fluorescent images were captured sequentially, and the relative fluorescence levels of internalized (or intracellular) FITC-ANG II in mPCT cells were analyzed using the MetaMorph Imaging System (Molecular Devices) as we described previously (25, 27, 28, 30). For better visualization of internalized FITC-ANG II in mPCT cells, DAPI-stained nuclei (blue) were converted to red to ensure that the colocalization would be viewed as yellow or orange in merged fluorescent images.
Effects of AT1 receptor blockade and AT1a-KO on ANG II-induced MAP kinases ERK1/2 activation and NHE3 phosphorylation in mPCT cells.
Activation of MAP kinases ERK1/2 and NHE3 may be used as markers of AT1a receptor stimulation by ANG II in proximal tubule cells (27, 30, 33). To determine these signaling mechanisms in response to ANG II, wild-type and AT1a-KO mPCT cells were treated with losartan, as described above. Protein samples were extracted for Western blot analysis of total and phosphorylated MAP kinases ERK1/2 or total and phosphorylated NHE3 as described (27, 30, 33).
Effects of siRNA knockdown of megalin expression on ANG II-induced activation of MAP kinases ERK1/2 and NHE3 phosphorylation in mPCT cells.
To determine the role or physiological relevance of megalin-mediated uptake of ANG II, wild-type and AT1a-KO mPCT cells were first transfected with a specific megalin siRNA or a scrambled siRNA for 48 h using Lipofectamine 2000. These mPCT cells were then stimulated with ANG II (10 nM) for 1 h in the presence or absence of losartan (10 μM) to block AT1 receptors, respectively. Protein samples were extracted for Western blot analyses of total and phosphorylated MAP kinases ERK1/2 and NHE3, respectively, as we described previously (27, 30, 33).
Statistical analysis.
All results are expressed as means ± SE. The levels of internalized FITC-ANG II in wild-type and AT1a-KO mPCT cells were measured as arbitrary fluorescence units (AFU) above background or autofluorescence levels with the uptake in control wild-type mPCT cells being arbitrarily set at 100. At least 20 individual live cell fluorescent images from at least 6 samples and from 3 experiments were analyzed. Cell surface receptor-bound FITC-ANG II was excluded from analysis. For Western blot analysis of signal protein responses, at least four to six samples from three experiments were performed. One-way ANOVA was used to compare the differences between control and ANG II-, losartan, PD123319-, megalin siRNA-, or scrambled siRNA-treated samples and Student's unpaired t-test was used to determine the differences between wild-type and AT1a-KO mPCT cells. P < 0.05 was considered significant for all comparisons.
RESULTS
AT1a receptor expression and AT1a receptor-mediated uptake of FITC-ANG II in wild-type and AT1a-KO mPCT cells.
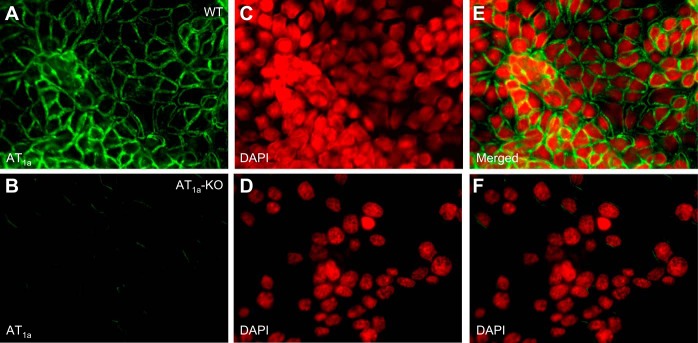
Figure 1 shows two unique mouse proximal tubule cell models that were used in the present study. Our previous study already showed that wild-type mPCT cells expressed a 300-base pair PCR product representing AT1a receptor mRNA, which was not detectable in mPCT cells derived from AT1a-KO mice (28). As expected, live cell fluorescent imaging found that FITC-ANG II bound wild-type mPCT cells primarily on the apical membrane surface at high levels when incubated at 4°C for 3 h (Fig. 1, A and E). By contrast, there was little FITC-ANG II binding observed on the cell membranes of AT1a-KO mPCT cells (Fig. 1, B and F). This remaining, very low level of FITC-ANG II binding to the apical membrane surface may represent the remaining AT1b and/or AT2 receptors. These results suggest that wild-type and AT1a-KO mPCTs cells serve as two uniquely different cell models in the present study.
Fig. 1.
Live cell fluorescent imaging of the binding of FITC-ANG II to AT1 receptors in mouse proximal convoluted tubule (mPCT) cells of wild-type (WT; A, C, and E) and AT1a knockout (AT1a-KO) mice (B, D, and F). Monolayers of mPCT cells grown on glass coverslips were incubated with FITC-ANG II (10 nM) at 4°C for 3 h, washed, and imaged (see materials and methods for details). FITC-ANG II-bound apical membranes were shown in green, whereas 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei were converted to red to facilitate better contrast and visualization. Note that AT1a-KO mPCT cells showed minimal FITC-ANG II binding. Magnification: ×40.
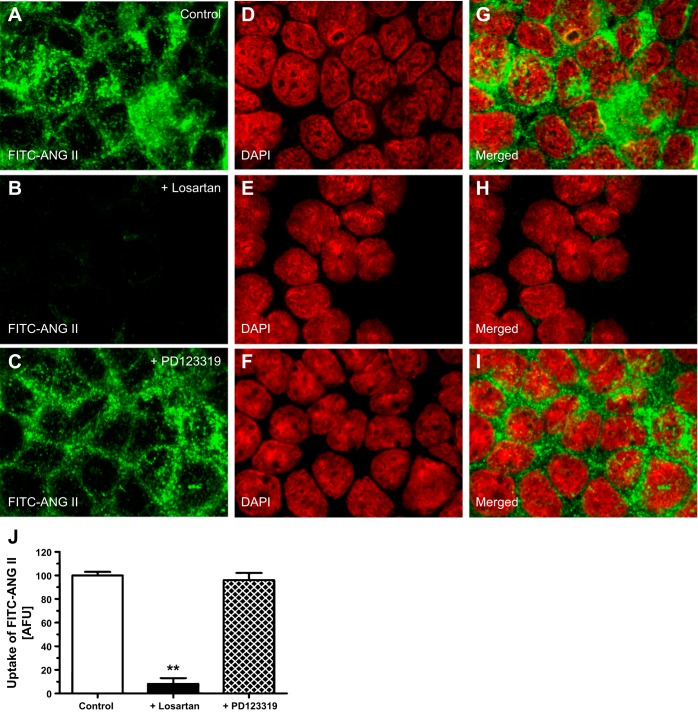
AT1a receptor-mediated uptake (or endocytosis) of FITC-ANG II in wild-type mPCT cells is shown in Fig. 2. When incubated at 37°C for 30–60 min, FITC-ANG II was internalized into wild-type mPCT cells from the apical membranes (see Fig. 2, A and G), which was readily observed in the cytoplasm with some clearly overlapping the nuclei (Fig. 2, A and G). Pretreatment of wild-type mPCT cells with the AT1 receptor blocker losartan (10 μM) largely blocked the binding and the subsequent uptake of FITC-ANG II by wild-type mPCT cells (Fig. 2, B, H, and J). As it was reported previously that the AT2 receptor does not internalize in response to ANG II in both nonrenal and renal cells (20, 30), pretreatment of wild-type mPCT cells with the AT2 receptor blocker PD123319 (10 μM) appeared to have minimal effects on the uptake of FITC-ANG II (Fig. 2, C, I, and J). These results support the concept that the uptake of FITC-ANG II in wild-type mPCT cells is predominantly mediated by AT1 (AT1a) receptors.
Fig. 2.
AT1 receptor-mediated uptake of FITC-ANG II in wild-type mPCT cells. Monolayers of wild-type mPCT cells grown on glass coverslips were incubated with FITC-ANG II (10 nM) at 37°C for 30 min, washed, and imaged (see materials and methods for details). A, D, and G: FITC-ANG II (A; green)-, DAPI-stained (D; red), or merged image (G) of A and D. Uptake of FITC-ANG II was seen in the cytoplasm and pronuclear regions of the cells. B, E, and H: effect of losartan, which largely abolished the uptake of FITC-ANG II. C, F, and I: effect of PD123319, which did not significantly block the uptake of FITC-ANG II. J: semiquantitated data on the uptake of FITC-ANG II in control (untreated), losartan- or PD123319-pretreated mPCT cells, expressed as arbitrary fluorescence units (AFU). Magnification: ×100. **P < 0.01 vs. untreated mPCT cells.
Expression of megalin and megalin-mediated uptake of FITC-ANG II in wild-type mPCT cells.
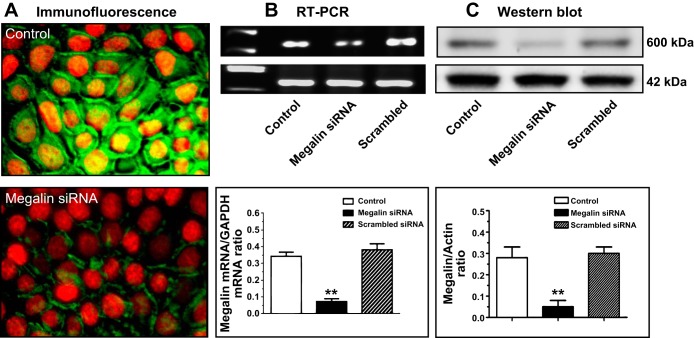
Expression of megalin mRNAs and proteins in wild-type mPCT cells is shown in Fig. 3. Under basal conditions, abundant megalin proteins (green immunofluorescence) were primarily expressed in apical membranes of mPCT cells, with low levels also seen in the cytoplasm (Fig. 3A). Semiquantitative RT-PCR analysis detected high levels of mRNAs for megalin as a 636-base pair PCR product (Fig. 3B). Transfection of mPCT cells with a megalin-specific siRNA for 48 h decreased megalin mRNA and protein expression by ∼87% (Fig. 3C). Pretreatment of mPCT cells with a scrambled siRNA had no effect on megalin mRNA and protein expression (Fig. 3, B and C). However, there were no significant differences in megalin mRNA and protein expression between WT and AT1a-KO mPCT cells (not shown).
Fig. 3.
Expression of megalin mRNA and proteins in mPCT cells and effects of megalin siRNA. A: immunofluorescent imaging of megalin protein expression. B: RT-PCR of megalin mRNA expression. C: Western blot of megalin protein expression. Magnification for A and B: ×100. **P < 0.01 vs. untreated/control mPCT cells.
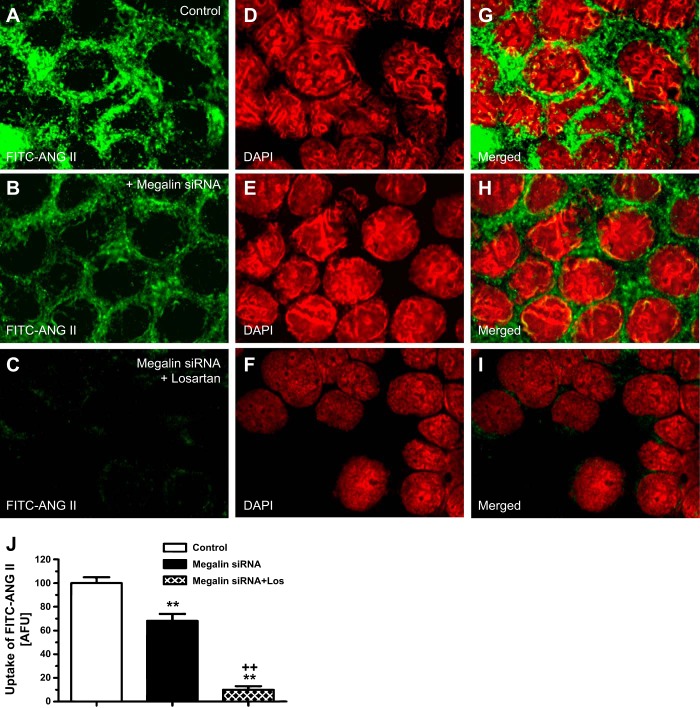
In contrast to the effects of AT1 receptor blockade with losartan in wild-type mPCT cells (Fig. 2, B and H), knockdown of megalin mRNA expression with a specific siRNA caused ∼30% inhibition of the FITC-ANG II uptake in wild-type mPCT cells (Fig. 4, B, H, and J) compared with the level of FITC-ANG II uptake in untreated wild-type mPCT cells (Fig. 4, A, G, and J). The remaining ∼70% of FITC-ANG II uptake was blocked by supplemental losartan treatment (Fig. 4, C, I, and J).
Fig. 4.
Megalin-mediated uptake of FITC-ANG II and effect of AT1 receptor blockade by losartan in WT mPCT cells. Monolayers of WT mPCT cells grown on glass coverslips were unpretreated or pretreated with megalin small interfering (si) RNA alone or with losartan. mPCT cells were then incubated with FITC-ANG II (10 nM) at 37°C for 30 min, washed, and imaged (see materials and methods for details). A, D, and G: FITC-ANG II (A; green)-, DAPI-stained (D; red), or merged image (G) of A and D. Uptake of FITC-ANG II was seen in the cytoplasm and pronuclear regions of the cells. B, E, and H: effect of megalin siRNA, which inhibited the uptake of FITC-ANG II by ∼30%. C, F, and I: combined effects of losartan and megalin siRNA on uptake of FITC-ANG II. J: semiquantitated data on the uptake of FITC-ANG II in control (untreated), megalin siRNA- or megalin siRNA plus losartan-treated mPCT cells, expressed as AFU. Magnification: ×100. **P < 0.01 vs. untreated or control mPCT cells. ++P < 0.01 vs. megalin siRNA-treated mPCT cells.
Effects of losartan or megalin siRNA on ANG II-induced phosphorylation of ERK1/2 (p-ERK1/2) and NHE3 in wild-type mPCTs.
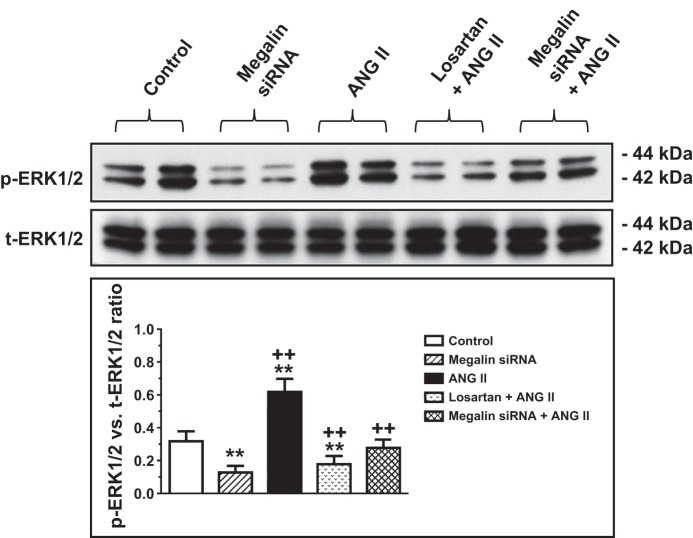
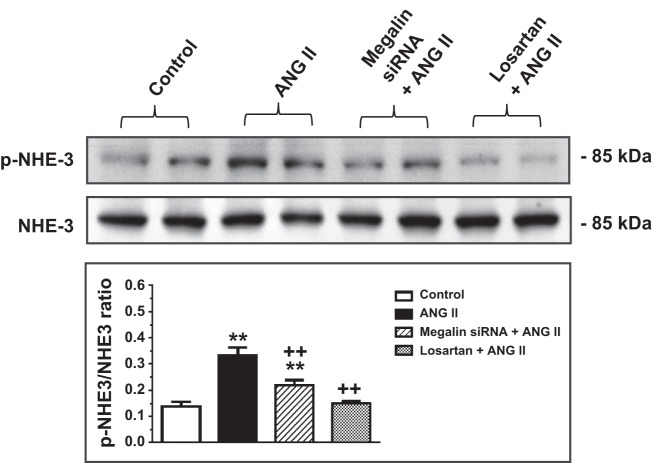
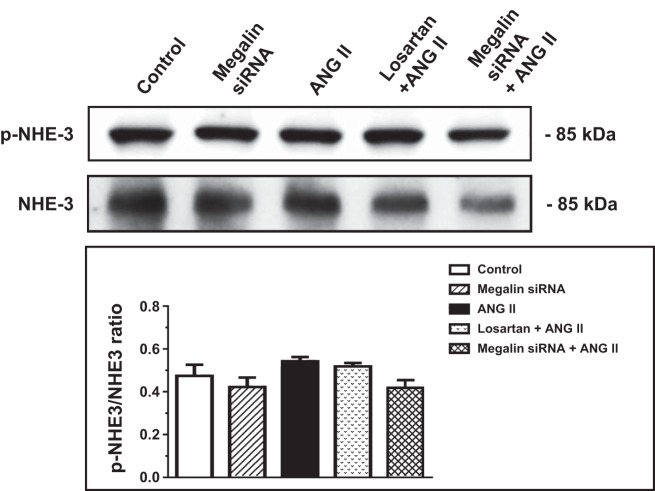
We previously showed that AT1a receptor-mediated ANG II uptake was associated with the activation of MAP kinases ERK1/2 signaling, which was blocked by losartan and AT1a receptor knockdown or knockout (25, 27, 30). As expected, incubation of wild-type mPCT cells with ANG II (10 nM) for 1 h significantly increased levels of phosphorylated MAP kinases ERK1/2 (p-ERK1/2; P < 0.01), which was completely inhibited by losartan (Fig. 5). The increases in p-ERK1/2 signaling responses to ANG II were associated with significant increases in phosphorylated NHE3 (p-NHE3; P < 0.01), which were also completely blocked by losartan (Fig. 6). Interestingly, pretreatment of mPCT cells with megalin siRNA to knock down megalin expression significantly attenuated ANG II-induced increases in p-ERK1/2 and p-NHE3 signaling proteins. However, the effects of megalin siRNA on ANG II-induced p-ERK1/2 and p-NHE3 signaling responses were statistically smaller than those of losartan (Figs. 5 and 6). Scrambled siRNA had no effect on ANG II-induced increases in MAP kinases ERK1/2 (not shown).
Fig. 5.
Effects of megalin siRNAs and/or AT1 receptor blocker losartan on ANG II-induced activation of MAP kinases ERK1/2 in wild-type mPCT cells. mPCT cells pretransfected with or without megalin siRNAs or cotreated with megalin siRNAs and losartan for 48 h were incubated with ANG II (10 nM) at 37°C for 1 h before proteins were extracted for Western blots of total and phosphorylated MAP kinases ERK1/2. *P < 0.05 or **P < 0.01 vs. control mPCT cells not transfected with megalin siRNAs. ++P < 0.01 vs. preceding group.
Fig. 6.
Effects of megalin siRNAs and/or AT1 receptor blocker losartan on ANG II-induced Na/H exchanger 3 (NHE3) expression in wild-type mPCT cells. mPCT cells pretransfected with or without megalin siRNAs or pretreated with losartan for 48 h were incubated with ANG II (10 nM) at 37°C for 1 h before proteins were extracted for Western blots of total and phosphorylated NHE3 proteins. **P < 0.01 vs. control mPCT cells not transfected with megalin siRNAs. ++P < 0.01 vs. the preceding group.
Effects of megalin siRNA on AT1a receptor-mediated uptake of FITC-ANG II in AT1a-KO mPCT cells.
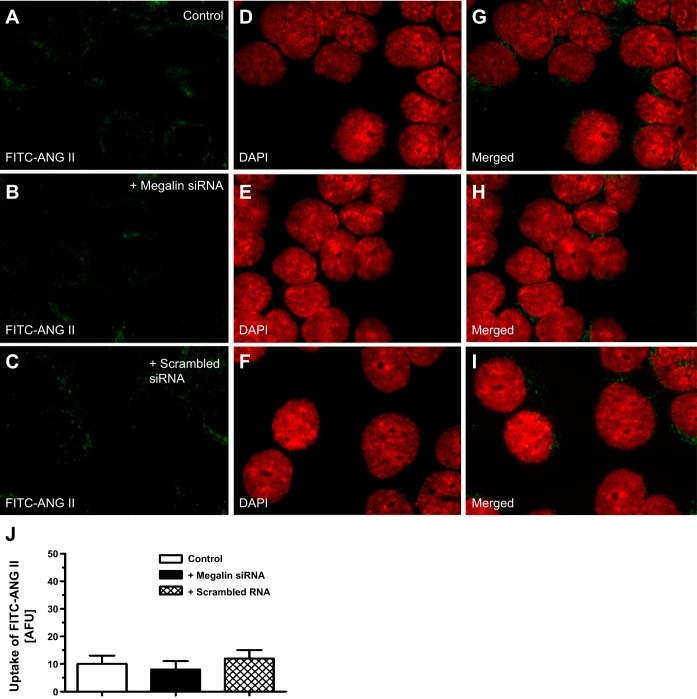
In the absence of AT1a receptors, the uptake of FITC-ANG II was remarkably decreased in AT1a-KO mPCT cells to <10% of wild-type counterparts (Fig. 7, A and G; P < 0.01). Pretreatment with megalin siRNA to knock down megalin expression slightly, but not significantly, further reduced FITC-ANG II uptake in AT1a-KO mPCT cells (Fig. 7, B, H, and J). Similarly, pretreatment with a scrambled siRNA had no effect on FITC-ANG II uptake (Fig. 7, C, I, and J).
Fig. 7.
Effects of megalin siRNA on the uptake of FITC-ANG II in AT1a-KO mPCT cells. Monolayers of AT1a-KO mPCT cells grown on glass coverslips were transfected with a specific megalin siRNA or a control scrambled siRNA for 48 h before being incubated with FITC-ANG II (10 nM) at 37°C for 30 min, washed, and imaged (see materials and methods for details). A, D, and G: FITC-ANG II- (A; green), DAPI-stained (D; red), or merged image (G) of A and D in control AT1a-KO mPCT cells. B, E, and H: effect of specific megalin siRNA, which slightly inhibited the remaining uptake of FITC-ANG II AT1a-KO mPCT cells. C, F, and I: effect of nonspecific scrambled siRNA, which had no significant effect. J: semiquantitated data on the uptake of FITC-ANG II in control (untreated), megalin siRNA-, or scrambled siRNA-treated AT1a-KO mPCT cells, expressed as AFU. Magnification: ×100.
Effects of megalin siRNA on ANG II-induced activation of MAP kinases ERK1/2 and NHE signaling responses in AT1a-KO mPCTs.
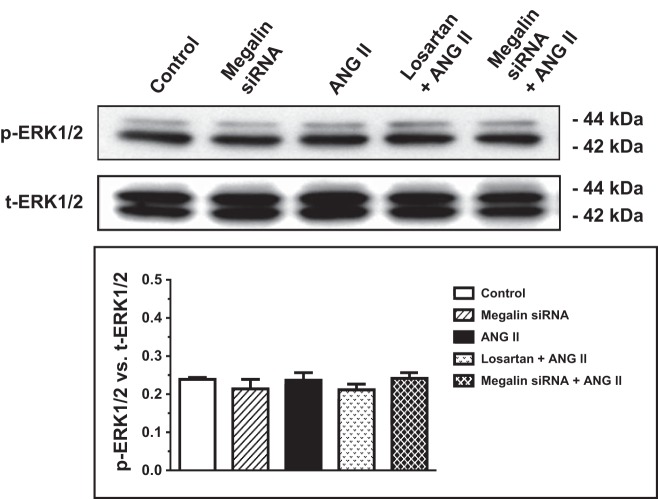
In contrast to wild-type mPCT cells, in which ANG II substantially increased phosphorylated MAP kinases ERK1/2 and NHE3 proteins, whereas losartan and megalin siRNA markedly attenuated these effects (Figs. 5 and 6), ANG II had no further significant effects on p-ERK1/2 and p-NHE3 signaling responses in AT1a-KO mPCT cells (Figs. 8 and 9). Similarly, ANG II with or without megalin siRNA or losartan pretreatment had no significant effects on p-ERK1/2 and p-NHE3 signaling responses in AT1a-KO mPCT cells (Fig. 9).
Fig. 8.
Effects of megalin siRNAs and/or AT1 receptor blocker losartan on ANG II-induced activation of MAP kinases ERK1/2 in AT1a-KO mPCT cells. AT1a-KO mPCT cells pretransfected with or without megalin siRNAs or pretreated with losartan for 48 h were incubated with ANG II (10 nM) at 37°C for 1 h before proteins were extracted for Western blots of total and phosphorylated MAP kinases ERK1/2. No significant effects of ANG II, megalin siRNA, or losartan were observed in AT1a-KO mPCT cells.
Fig. 9.
Effects of megalin siRNAs and/or AT1 receptor blocker losartan on ANG II-induced activation (phosphorylation) of NHE3 in AT1a-KO mPCT cells. AT1a-KO mPCT cells pretransfected with or without megalin siRNAs or pretreated with losartan for 48 h were incubated with ANG II (10 nM) at 37°C for 1 h before proteins were extracted for Western blots of total and phosphorylated NHE3 proteins. No significant effects of ANG II, megalin siRNA, or losartan were observed in AT1a-KO mPCT cells.
DISCUSSION
The major objectives of the present study were to explore the potential role of the multiligand endocytic receptor megalin in mediating ANG II uptake (or endocytosis), its interactions with AT1 (AT1a) receptors, and downstream signaling responses to ANG II in cultured murine proximal tubule cells. Megalin is a 600-kDa type I cell surface receptor protein with a large extracellular domain, a single transmembrane domain, and a short cytoplasmic carboxy terminal (9). The extracellular domain contains the ligand-binding regions, which are important for binding of LMW proteins to megalin and initiation of endocytosis (9). The cytoplasmic tail contains three NPXY motifs, which may probably be involved in signaling functions associated with ligand endocytosis (9). Megalin is widely expressed in various absorptive epithelia, including the kidney, brain, ears, intestines, and placenta (2, 6, 21, 39, 40). In the kidney, megalin is primarily localized in apical membranes of the proximal tubule, which explains the observed LMW proteinuria in the rarely surviving megalin-KO mice (9, 23). Global deletion of the megalin gene is lethal due to severely impaired lung inflation, abnormal formation of the forebrain, and LMW proteinuria (23, 46). However, the role of megalin in mediating ANG II uptake and its downstream signaling responses in the proximal tubule of the kidney remain poorly understood.
It should be recognized that our study is not the first to determine whether megalin is involved in mediating ANG II internalization in epithelial cells. The potential role of megalin in mediating the internalization of ANG II and its metabolite ANG (1–7) has been studied previously in immortalized BN-16 cells and purified mouse kidney cortical brush-border membrane vesicles by Gonzalez-Villalobos et al. (16, 17). BN-16 cells are rat yolk sac carcinoma cells, which express abundant megalin and cubilin and have been used as a cultured cell model for studying cellular uptake of LMW proteins (8, 9, 17). Gonzalez-Villalobos et al. showed that both ANG II and ANG (1–7) bound megalin, which could be inhibited by anti-megalin antibodies, but not by the AT1 receptor antagonist candesartan, suggesting that megalin may be a distinct receptor for ANG II and ANG (1–7) in BN-16 cells independent of the AT1 receptor (17).
The results of the present study are conceptually consistent with the general conclusion of Gonzalez-Villalobos et al. that megalin binds and internalizes ANG II and ANG (1–7) in epithelial cells (17). However, our study differs from previous studies with respect to the cell models used, the specific role of megalin, and the downstream signaling responses to the knockdown of megalin expression. For example, we used mouse proximal tubule cells derived from wild-type C57BL/6J and AT1a receptor-deficient (AT1a-KO) mice as the novel cell models in the present study. Second, we used live cell fluorescent imaging to determine whether megalin directly or indirectly mediates the uptake of ANG II in mPCT cells with or without the expression of the AT1a receptor. Third, we determined whether the mPCT cells we used express abundant megalin mRNAs and proteins and used megalin siRNA silencing in mPCT cells to determine whether megalin-mediated ANG II uptake is biologically (and/or physiologically) relevant to alter p-ERK1/2 and p-NHE3 signaling responses to ANG II in mPCT cells. Previously, the specificity of anti-megalin antibodies to inhibit megalin remains an issue of debate among investigators (2, 8, 9, 17). As an alternative approach to antibodies or inhibitors, siRNAs are widely used to knock down the expression of a specific target gene. The ∼87% of knockdown of megalin expression by this specific siRNA, while a scrambled control had no effect, is consistent with those we reported previously involving AT1 (AT1a) receptors, heavy and light chains of clathrin proteins, and microtubule-associated proteins in proximal tubule cells (25, 27, 30).
Our live cell fluorescent imaging analysis of FITC-ANG II uptake suggests that megalin may be involved in ∼30% of ANG II uptake in wild-type mPCT cells. The magnitude of the contribution by megalin may be physiologically significant in the proximal tubule of the kidney. However, it is much smaller than the reported role in mediating the uptake of a majority of LMW proteins, amino acids, nutrients, vitamins, and transferrin in the proximal tubule of the kidney (8, 9). The role of megalin in mediating ANG II uptake in mPTC cells is also substantially smaller than that of AT1 (AT1a) receptors. Furthermore, our results obtained in wild-type mPCT cells are different from those reported previously in BN-16 cells in several aspects (17). Gonzalez-Villalobos et al. (17) suggested that megalin is the major pathway for ANG II uptake in BN-16 cells, which was largely independent from AT1 receptors, since the AT1 receptor blocker candesartan had no effect. In the present study, the AT1 receptor blocker losartan completely blocked the uptake of FITC-ANG II in wild-type mPCT cells. This finding was reproduced in parallel experiments using AT1a-KO mPCT cells, further confirming a dominant and specific role of AT1 (AT1a) receptors. It is interesting to note that subsequent losartan treatment completely blocked FITC-ANG II uptake, which persisted in the presence of megalin siRNA treatment. There are several explanations underlying the differences between the current and above-referenced studies. The first reason is most likely due to different cell models used between studies. BN-16 cells are rat yolk sac carcinoma cells, whereas mPTC cells are murine proximal tubule cells; both may express different levels of megalin and AT1 (AT1a) receptors (and major components of the RAS) and have different epithelial transport activities for ANG II. Indeed, mPCT cells were not cultured under strictly polarized conditions in the present study to expose lateral and basal membranes, which likely alters megalin and AT1a receptor expression and responses in apical membranes. The second reason may be that the present study only followed the uptake response for the first 30–60 min, whereas the other study studied the uptake responses for up to 24 h (17). Third, megalin is a massive, nonspecific, multiligand endocytic receptor with a molecular mass of 600 kDa, whereas ANG II is a small peptide of ∼1 kDa. It is likely that other proteins in the medium may bind megalin and compete with ANG II for megalin binding (8, 9, 17). Finally, since losartan completely blocked ANG II uptake whereas megalin blocked only 30% of the uptake, our results suggest that ANG II may first bind to apical membrane AT1a receptors and internalize with the receptor; 30% of internalized ANG II may be subsequently shuttled to the megalin-dependent pathway. The latter possibility may be further investigated using different approaches.
The physiological role of megalin-mediated ANG II uptake in the proximal tubule of the kidney remains unknown. There is no question that megalin mediates the uptake of LMW albumin in renal proximal tubules (13). Global deletion of megalin leads to LMW proteinuria in mice and is lethal (23). Recently, Pohl et al. (38) showed that megalin may also mediate the uptake of AGT in early proximal tubule by comparing AGT uptake in wild-type and megalin-KO mice. Li et al. (24) has reported that silencing megalin and cubilin genes ameliorated nephrotoxicity induced by myeloma light chains in human renal proximal tubule cells. There is evidence that megalin and NHE3 may interact with each other in the proximal tubule. For example, Biemesderfer et al. (1) showed that a significant pool of NHE3 exists in a complex with megalin in apical membranes of the proximal tubule, whereas Gekle et al. (15) suggested that in opossum kidney cells, megalin-mediated endocytosis may require NHE3 since NHE3 deficiency impaired receptor (megalin)-mediated endocytosis. However, Christ et al. (7) found that the soluble intracellular domain of megalin does not affect renal proximal tubular function in vivo in a mouse model. Thus whether megalin interferes with ANG II/AT1a receptor interactions and downstream signaling responses in mPTC cells has not been studied previously.
The present study shows that the knockdown of megalin expression by a specific megalin siRNA was associated with an inhibition of ANG II-induced activation or phosphorylation of MAP kinases ERK1/2 and NHE3 in wild-type mPCT cells. As expected, ANG II stimulates, whereas losartan blocked AT1 (AT1a) receptor-mediated activation of downstream p-ERK1/2 and p-NHE3 in wild-type mPTC cells (25, 30). However, megalin siRNA alone also attenuated ANG II-induced p-ERK1/2 and p-NHE3 signaling responses, although to smaller extents than losartan. Interestingly, although megalin is equally expressed in AT1a-KO mPCT cells (not shown), the effects of megalin silencing observed in wild-type mPCT cells were no longer observed in AT1a-KO mPCT cells. This suggests that megalin may play an important role in AT1a receptor-mediated downstream signaling responses to ANG II in the proximal tubule. How megalin knockdown interferes with the ANG II-induced p-ERK1/2 and NHE3 responses in mPCT cells is not clear at present. In a previous study in BN-16 cells, Gonzalez-Villalobos et al. (17) hypothesized that there are two pathways for ANG II uptake, namely, megalin and AT1 receptor mediated, in BN-16 cells and possibly in proximal tubule cells. According to the hypothesis, megalin targets ANG II to degradation whereas AT1 receptors target ANG II to intracellular accumulation under physiological conditions. In ANG II-induced hypertension, ANG II upregulates AT1 receptor but downregulates megalin expression, further promoting AT1 receptor-mediated accumulation of ANG II (17). However, this hypothesis may not adequately explain the inhibitory effects of megalin silencing on ANG II uptake and p-ERK1/2 and p-NHE3 signaling responses in mPCT cells.
In summary, the present study demonstrates that megalin and AT1 (AT1a) receptors are abundantly expressed in apical membranes of wild-type mPCT cells. In addition to further confirming that the uptake of FITC-ANG II in mPCT cells is primarily AT1 (AT1a) receptor dependent, our results indicate that the multiligand endocytic receptor protein megalin may be responsible for ∼30% of AT1a receptor-mediated uptake of ANG II in these cells. It is likely that in mPCT cells, ANG II first binds to AT1 (AT1a) receptors on the apical membranes, internalizes with the receptor, and is subsequently sorted to the megalin-dependent pathway. Since knockdown of megalin expression with a specific siRNA inhibited 30% of FlTC-ANG II uptake, but significantly blocked ANG II-induced downstream p-ERK1/2 and p-NHE3 signaling responses in mPCT cells, our study indicates that these responses to ANG II may partly involve the multiligand endocytic receptor megalin.
GRANTS
This work was supported in part by National Institute of Diabetes, Digestive, and Kidney Diseases Grants (5RO1DK067299, 2R56DK067299, and 2RO1DK067299), an American Society of Nephrology M. James Scherbenske grant, and a Hearin Foundation Medical Research Scholar Award from the University of Mississippi Medical Center to J. L. Zhuo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.C.L. and J.L.Z. provided conception and design of research; X.C.L. and J.L.Z. performed experiments; X.C.L. and J.L.Z. analyzed data; X.C.L. and J.L.Z. interpreted results of experiments; X.C.L. and J.L.Z. prepared figures; X.C.L. and J.L.Z. drafted manuscript; X.C.L. and J.L.Z. edited and revised manuscript; X.C.L. and J.L.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ulrich Hopfer of Case Western Reserve University for providing wild-type and AT1a-KO mPCT cells for the present study.
REFERENCES
- 1.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274: 17518–17524, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Birn H, Verroust PJ, Nexo E, Hager H, Jacobsen C, Christensen EI, Moestrup SK. Characterization of an epithelial approximately 460-kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and binds receptor-associated protein. J Biol Chem 272: 26497–26504, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F891–F898, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24: 261–271, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol 2: 2733–2752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatelet F, Brianti E, Ronco P, Roland J, Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol 122: 500–511, 1986 [PMC free article] [PubMed] [Google Scholar]
- 7.Christ A, Terryn S, Schmidt V, Christensen EI, Huska MR, Andrade-Navarro MA, Hubner N, Devuyst O, Hammes A, Willnow TE. The soluble intracellular domain of megalin does not affect renal proximal tubular function in vivo. Kidney Int 78: 473–477, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17: 1402–1409, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Crowley SD, Zhang J, Herrera M, Griffiths RC, Ruiz P, Coffman TM. The role of AT1 receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol 301: F1124–F1130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 271: F900–F907, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. II. Impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gekle M, Serrano OK, Drumm K, Mildenberger S, Freudinger R, Gassner B, Jansen HW, Christensen EI. NHE3 serves as a molecular tool for cAMP-mediated regulation of receptor-mediated endocytosis. Am J Physiol Renal Physiol 283: F549–F558, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin-(1–7). Am J Physiol Renal Physiol 290: F1270–F1275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physio l 288: F420–F427, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PJ, Navar LG. Tubular transport responses to angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 248: F621–F630, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 11: 1266–1277, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci USA 79: 5557–5561, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, utry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci USA 98: 12491–12496, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Balamuthusamy S, Simon EE, Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am J Physiol Renal Physiol 295: F82–F90, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Li XC, Carretero OA, Navar LG, Zhuo JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol 291: F375–F383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 300: F1076–F1088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through the microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol 297: F1342–F1352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 303: F1617–F1628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of angiotensin II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 293: F586–F593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-Ang II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol 293: C367–C378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125]-Val5-angiotensin II in the kidneys and adrenal glands of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 294: F293–F302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XC, Zhuo JL. Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int 80: 620–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddox DA, Gennari FJ. The early proximal tubule: a high-capacity delivery-responsive reabsorptive site. Am J Physiol Renal Fluid Electrolyte Physiol 252: F573–F584, 1987 [DOI] [PubMed] [Google Scholar]
- 35.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navar LG, Carmines PK, Huang WC, Mitchell KD. The tubular effects of angiotensin II. Kidney Int Suppl 20: S81–S88, 1987 [PubMed] [Google Scholar]
- 37.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahali D, Mulliez N, Chatelet F, Dupuis R, Ronco P, Verroust P. Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med 167: 213–218, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seetharam B, Levine JS, Ramasamy M, Alpers DH. Purification, properties, and immunochemical localization of a receptor for intrinsic factor-cobalamin complex in the rat kidney. J Biol Chem 263: 4443–4449, 1988 [PubMed] [Google Scholar]
- 41.Siragy HM. Angiotensin II compartmentalization within the kidney: effects of salt diet and blood pressure alterations. Curr Opin Nephrol Hypertens 15: 50–53, 2006 [DOI] [PubMed] [Google Scholar]
- 42.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 19: 583–589, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Vegt E, Melis M, Eek A, de Visser M, Brom M, Oyen WJ, Gotthardt M, de Jong M, Boerman OC. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur J Nucl Med Mol Imaging 38: 623–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Thun AM, Vari RC, El Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol 266: F120–F128, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens 18: 412–420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA 93: 8460–8464, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, Coffman TM, Hopfer U. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim 42: 189–200, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust PJ, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int 58: 1523–1533, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Zhuo JL, Thomas D, Harris PJ, Skinner SL. The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J Physiol 453: 1–13, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: Focus on intracrine/intracellular angiotensin II. Peptides 32: 1551–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuo JL, Li XC. Proximal nephron. Compr Physiol 3: 1079–1123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens 11: 570–578, 1998 [DOI] [PubMed] [Google Scholar]