Abstract
Much is known about the mechanotransducer (MT) channels mediating transduction in hair cells of the vertrbrate inner ear. With the use of isolated preparations, it is experimentally feasible to deliver precise mechanical stimuli to individual cells and record the ensuing transducer currents. This approach has shown that small (1–100 nm) deflections of the hair-cell stereociliary bundle are transmitted via interciliary tip links to open MT channels at the tops of the stereocilia. These channels are cation-permeable with a high selectivity for Ca2+; two channels are thought to be localized at the lower end of the tip link, each with a large single-channel conductance that increases from the low- to high-frequency end of the cochlea. Ca2+ influx through open channels regulates their resting open probability, which may contribute to setting the hair cell resting potential in vivo. Ca2+ also controls transducer fast adaptation and force generation by the hair bundle, the two coupled processes increasing in speed from cochlear apex to base. The molecular intricacy of the stereocilary bundle and the transduction apparatus is reflected by the large number of single-gene mutations that are linked to sensorineural deafness, especially those in Usher syndrome. Studies of such mutants have led to the discovery of many of the molecules of the transduction complex, including the tip link and its attachments to the stereociliary core. However, the MT channel protein is still not firmly identified, nor is it known whether the channel is activated by force delivered through accessory proteins or by deformation of the lipid bilayer.
I. INTRODUCTION
Ion channels are proteinaceous pores that regulate the flow of cations or anions across the plasma membrane, the resulting current flow initiating the electrical signals that are the hallmark of neural activity in animals. Common examples include the voltage-gated Na+ and K+ channels, underlying the nerve action potential, and ligand-gated channels, such as the glutamate and GABA receptors, which mediate excitatory and inhibitory synaptic transmission. Both types of ion channel have been extensively characterized at a biophysical and molecular level over the last 20 years (98). They are typically composed of four to five membrane-spanning protein subunits clustered around a central aqueous conduit that can be opened or closed by the appropriate electrical or chemical stimulus. The basis of the stimulus specificity resides within the primary subunits, either as a voltage-sensing or a ligand-binding domain. A third more heterogeneous and less well understood variety of ion channel comprises the mechanically sensitive channels that detect or transduce mechanical forces occurring during cellular deformation and motion. These belong to an ancient class of channels, examples of which have been documented in bacteria and fungi (141) as well as in invertebrate and vertebrate animals (6). They may have first evolved as a sensor of membrane stretch during osmotic shock as exemplified by the bacterial MscL channel or vacuolar transient receptor potential TRP-Y1 channel (141). However, in animals ranging from nematode worms to mice, mechanosensitive channels unrelated to MscL are present in sensory cells specialized to detect stretching of the skin or distention of internal tissues such as the vasculature, muscle fibers, or membranes of the inner ear. Two aspects of specialization can be noted. The channels may be part of a multimolecular complex that includes accessory proteins in the internal cytoskeleton and in the extracellular matrix, the role of which may be to focus force on the ion channel to ensure rapid activation. The prime example is the array of MEC proteins named from touch-insensitive mutations in the worm Caenorhabditis elegans. Multiple accessory proteins, including MEC-2 and MEC-6, are linked directly or indirectly to an ion channel fashioned from two pore-forming subunits, MEC-4 and MEC-10 (6, 7). The transduction apparatus in C. elegans touch neurons is currently the most completely described of all animal mechanoreceptors. Second, the extracellular matrix may be elaborated by addition of supporting structures to direct the mechanical stimulus to the receptor cells, and to provide the equivalent of an impedance matching device, to adjust the magnitude of the incident forces to those required to operate the ion channel itself. The best examples are the mechanoreceptors in the vertebrate skin, where some afferent terminals are cloaked in nonneuronal structures forming specialized endings such as Pacinian corpuscles or Merkel disks, each being designed to optimize the afferent response for a tactile stimulus of given size and speed (252). Thus the Merkel disks respond best to textured stimuli slowly grazing the skin, whereas the onion-like capsules of Pacinian corpuscles transmit only transient stimuli (170) and are tuned to vibrations of several hundred Herz.
Nowhere is the structural refinement more evident than in auditory receptors, for which an essential prerequisite is fast transduction to encode high-frequency sound vibrations. A striking case is Johnston's chordotonal organ in Drosophila in which the auditory neuron projects a distal sensory cilium (thought to be the site of transduction) that is inserted into the antennal joint and ensheathed by specialized supporting cells. In the vertebrate inner ear, the sensory hair cells also possess an external projection of 50 or more interconnected microvilli, the hair bundle, the structure of which varies according to the location in the organ (72, 52). In the auditory division of the inner ear, known as the cochlea, motion of the hair bundles is driven by attachment to an acellular matrix, the tectorial membrane (Figure 1); in the vestibular division, including the saccule and utricle, force is applied to the bundle via an otolithic membrane that is mass loaded with calcium carbonate crystals. Auditory hair cells are probably the fastest and most sensitive of all vertebrate mechanoreceptors, and those in the mammalian cochlea may respond to vibrations (elicited by sound stimuli) with amplitudes of 1 nm and in some species, such as mice or bats, at frequencies as high as 100 kHz. Adaptation to these exacting stimuli is likely to involve the accessory apparatus, the external and internal attachments of the mechanotransducer (MT) channel within each stereocilium, and the nature of the MT channel protein itself.
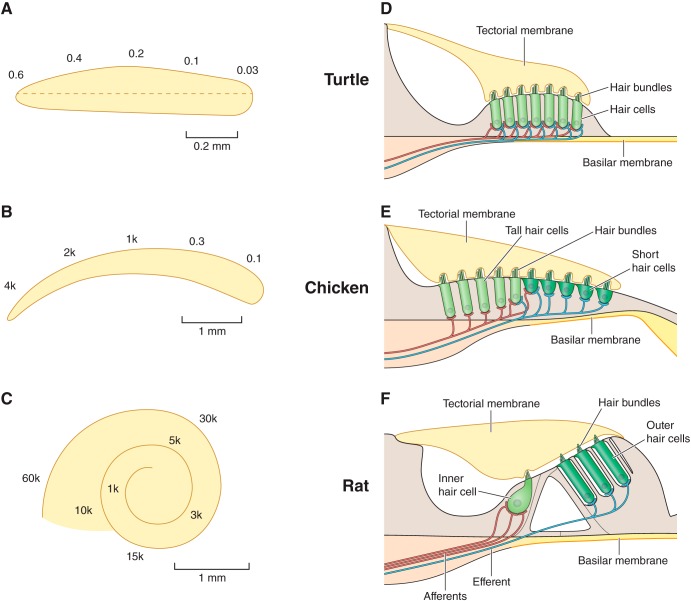
FIGURE 1.
The auditory organ in three vertebrate classes. The spatial map of best or characteristic frequencies (CF) of neurons along the basilar membrane, often referred to as the tonotopic map, is illustrated for members of three vertebrate classes: A, basilar papilla of the red-eared turtle, Trachmemys scripta elegans; B, basilar papilla of the chicken, Gallus gallus domesticus; C, cochlea of the rat, Rattus norvegicus. The CFs are expressed in kHz, with the frequency range being ∼30–600 Hz in turtle (47); 100 Hz to 4 kHz in chicken (33, 118), and 1–60 kHz in rat (175). Transverse sections across the hair cell epithelium are schematized on the right. D: turtle papilla contains a single type of hair cell innervated by both afferent (red) and efferent (blue) nerve fibers. E: chicken papilla contains two extremes of hair cell: on the neural aspect tall hair cells innervated mainly by afferents and on the abneural aspect short hair cells innervated predominantly by efferents. Although not shown, in both turtle and chicken, each afferent collects from only one or two hair cells. F: rat cochlea, similar to other mammals, contains three rows of outer hair cells with efferent innervations, and one row of inner hair cells innervated by 95% of the auditory afferents.
Among the known mechanoreceptors, the need to fine-tune channel activation to the attributes of the mechanical stimulus to be detected may go some way to explaining the diversity of channel proteins employed. These range from the pentameric MscL channel, which forms large 3-nm-wide pores in E. coli membrane; the trimeric MEC channels in C. elegans, closely related to the vertebrate epithelial Na+ channel (ENaC), which may be reused as ASIC1 channels in some vertebrate cutaneous afferents; and the recently discovered piezo2, an enormous, 1.2-MDa protein tetramer that may too be employed in fast-adapting mammalian cutaneous endings (43, 44), including the Merkel disks (268). The molecular identity of the hair cell MT channel is still not firmly established. The heterogeneity of the mechanosensitive channels across taxa has undermined attempts to isolate the hair cell MT channel by homology with proteins of equivalent function from other taxa, a technique that proved successful in cloning voltage-gated K+ channels (98). Thus ion channels thought to be involved in transduction in Johnston's organ are transient receptor potential channels, NOMPC (TRPN) and NANCHUNG and INACTIVE (both TRPV channels) (6), none of which is directly implicated in hair cell transduction (36). The most powerful approach for identifying mechanosensitive channels in all animals has been by studying specific mutants lacking touch or auditory sensation; it is expected that this approach will ultimately uncover the hair-cell MT channel.
Despite incomplete information on the makeup of the hair-cell transduction complex, the physiological setting and biophysical properties of the MT channel have been better characterized than for all other vertebrate mechanoreceptors. The accessory apparatus, organization of the stereociliary bundle, and mechanism of channel activation are addressed in the first two sections, and the physiological implications and biophysical properties are in the next two sections. Available evidence on the molecular under pinning is reviewed in the last section. Many of the seminal conclusions have stemmed from study of frog saccular hair cells (41, 107, 109, 228), and these are described where relevant. However, the review will focus primarily on auditory hair cells, since their physiological context in responding to sounds of different frequencies is best understood, and the molecular foundations have been illuminated by studying genetic deafness in mice and humans.
II. THE HAIR CELL AND ITS ENVIRONMENT
A. Organization of the Cochlea
The auditory epithelium of the vertebrate inner ear, homologous in reptiles, birds, and mammals (160), consists of hair cells firmly anchored to nonsensory supporting cells and sandwiched between a fibrous basilar membrane and an acellular tectorial membrane (Figure 1). The submicron relative motion of these two structures elicited by sound stimuli is detected by the hair cells. It is important to consider at the outset the functional specializations of the epithelium because these influence the mechanotransduction process. First, it is a tight epithelium separating fluid compartments of different composition. Perilymph bathing the basolateral aspect of the hair cell in adult rats contains 138 mM Na+, 7 mM K+, and 0.7 mM Ca2+ (25, 26), which, with adequate Ca2+ and Na+, is conducive to synaptic transmission from the hair cell and for action potential generation in the afferent dendrites. In contrast, the top surface of the hair cell and the hair bundle are immersed in endolymph, which in rats has a composition of 154 mM K+, 1 mM Na+, 0.03 mM Ca2+, and 0.01 mM Mg2+ (25, 26); the low concentrations of Ca2+ and Mg2+ enhance operation of mechanotransduction since at high levels these two divalent ions block the MT channels (42, 46, 207). In addition, in mammals there is up to a 100 mV positive potential (rat, 25; mouse, 235), the endolymphatic potential, between the endolymph and perilymph, which is absent in nonmammals. Second, with the evolution of the inner ear from early reptiles to mammals, the epithelium has been progressively elaborated to include more than one type of hair cell accompanied by diversification of supporting cells, changes associated with extension of the upper limit of the frequency range (160). Thus, in reptiles like the turtle, there is a single morphological class of hair cell having common afferent and efferent innervation. At the other extreme, placental mammals possess two distinct categories of hair cell: a single row of flask-shaped inner hair cells (IHCs), contacting the majority of the afferent fibers, and three to four rows of columnar outer hair cells (OHCs); the latter play a role in amplifying the mechanical stimulus (see sect. IVB) and have a sparse afferent but strong efferent innervation. This division of labor is also seen in egg-laying mammals, the monotremes, and in birds and crocodilians (160, 259), although more rows of each cell type are present and the dichotomy between these two categories is less marked than in mammals (246). A third feature of the auditory end-organ, known as the cochlea in mammals, is its ability to separate sound frequencies along its length; as a consequence, nerve fibers emanating from different regions are tuned to different frequencies, high-pitched tones at the proximal end of the organ nearest the saccule, and low frequencies at the distal end (67; Figure 1). The mapping of frequency onto longitudinal position is referred to as the tonotopic organization and is reflected in gradients in numerous cellular properties, including epithelial and cellular dimensions and the ion channel complement of the hair cells. Of particular note here is the variation in hair bundle structure and MT channel properties depending on the animal or frequency range detected.
B. The Stereociliary Bundle
The stereociliary (hair) bundle, projecting from the top face of the hair cell, is the organelle through which all mechanical stimuli are focused for detection by the transduction machinery. This sensory specialization is composed of a cohort of modified microvilli known as stereocilia, arranged in a staircase, with successive ranks increasing in height across the bundle (Figure 2; Ref. 72). Abutting the tallest rank is a microtubule-containing cilium, the kinocilium, which is present in vestibular hair cells and immature auditory hair cells, but not in the adult mammalian cochlea (72, 196). The shaft of each component stereocilium is buttressed by a core of actin filaments that are cross-bridged by a number of proteins including plastin-1 and fascin-2 (the two most abundant; Ref. 226) and espin (277), and extend from its very top to the narrowed ankle region; only a fraction of the filaments traverses the ankles into the cell body (68, 249), rendering the ankles the site of least stiffness. Here, the actin filaments are packed more densely than in the stereociliary shaft and are interconnected by the actin bundling protein TRIOBP, which probably increases the strength and durability of this region (134). With application of force to the tip of the bundle, the stereocilia bend at their ankles and, because of the extracellular links connecting adjacent ranks, the entire array bends as one (45, 135). Transduction is polarized so that deflection of the bundle towards its tallest edge opens MT channels, motion in the opposite direction closes these channels, and orthogonal displacements are relatively ineffective (228). A variety of interciliary links endows the bundle with cohesion (91), the most important in the adult being the horizontal top connectors in the contact region between the top of one stereocilium and its taller neighbor (260), and the tip links (Figure 3A). The top connectors, of which stereocilin may be a component, appear during maturation of mouse cochlear hair cells between postnatal days 9 and 14, around the onset of hearing (260). The top connectors replace other interciliary links including transient lateral links and ankle links (84). In stereocilin-null mice, the top connectors do not form, leading to loss of bundle cohesion after postnatal day 15, and the concomitant disappearance of the tip links (260). In intact wild-type bundles, displacements of the stereocilia are believed to be transmitted to the MT channels by a change in tension in the tip links (71, 193), which stretch from the top of one stereocilium to the side wall of its taller neighbor. Various models have been suggested to explain transmission of force from the tip links to the channels (e.g., Ref. 194). At one extreme, the channels float free in the plasma membrane and are activated by a change in membrane tension (Figure 3B); at the other extreme, the channels are immobilized by connections to the tip link externally and the cytoskeleton internally (Figure 3C), and act as force sensors between these two attachments. There are intermediate models in which the channels are connected only to the tip links or only to the cytoskeleton.
FIGURE 2.
Structure of the stereociliary bundle. A: scanning electron micrograph of the hair bundle from a turtle auditory hair cell exhibiting nine rows of stereocilia of incremental heights and a kinocilium, arrowed (90). B: rat OHC showing only three rows of stereocilia. C: rat IHC illustrating the tall first row of stereocilia that can behave like a “flag” to fluid flow in the subtectorial space and the shorter and more numerous second, third, and fourth rows (18). D: bat inner hair cell from the high-frequency region of the cochlea showing only two rows of stereocilia. Scale bar = 1 μm for A and 2 μm for B–D. (Micrographs in A, B, and D courtesy of C. M. Hackney and D. N. Furness.)
FIGURE 3.
The tip link and connections. A: transmission electron micrograph of a guinea pig outer hair cell illustrating the tip link and the electron-dense regions at the upper and lower ends, denoted as the upper tip-link density (UTLD) and lower tip-link density (LTLD), respectively. Note the membrane has pulled away from the LTLD where it attaches to the tip link, and there is an electron-dense “contact region” where the shorter stetereocilium abuts the taller one. This may be the site of the top connectors. Scale bar = 0.2 μm. B: one theoretical scheme for the connections of the MT channel, where two channels float free in the plasma membrane. C: another scheme where each MT channel is attached to one strand of the tip link and is also anchored to the internal cytoskeleton by an “adaptation spring.” Two intermediate arrangements have been proposed in which the MT channels are connected only to the tip link or only to the internal cytoskeleton (194).
The tip links are helical extracellular filaments ∼150 nm in length and, in transmission electron micrographs, the end points of attachment to the stereocilia are marked by electron-dense regions referred to as the upper tip-link density (UTLD) and lower tip-link density (LTLD) (73, 121). Detailed evidence for the role of the tip links and the MT channel localization at the lower end of the tip links (20) will be described in section VB. The directionality of the transduction mechanism can be largely accounted for by the uniform orientation of the tip links which to a first approximation run parallel to the bundle's plane of symmetry (193). The directionality conforms to a cosine rule (228), the probability of MT channel opening being proportional to cosθ, where θ is the angle between the direction of the stimulus and the bundle's axis of symmetry. For perspective, if θ = 45°, cosθ = 0.71, so the directionality is not highly tuned, and there is some variance in the polarity of the tip links further weakening the directional tuning. Nevertheless, in the adult cochlea the hair bundles, all have a common orientation. The “V”-shaped bundles point away from the neural limb, so the hair cells are maximally excited by upward motion of the cochlear partition towards the scala media, producing radial deflection of the tectorial membrane and hair bundles. The uniform bundle orientation and the molecular events that establish it during development are collectively referred to as “planar cell polarity” and involve multiple signaling pathways (169). The directionality to transduction is a feature of mature hair bundles, and there are exceptions to this rule in immature mammals (265). Another important property that emerges from the structure of the bundle is that it behaves as a transformer. For small stimuli within the physiological range, displacements occurring at the channel are reduced relative to those at the bundle's tip by a geometrical factor γ (77, 166, 192), given approximately by the ratio of s, the distance along the axis of symmetry between the pivot points at the stereociliary ankles (<1 μm) to the mean stereociliary height h (1–10 μm); γ depends on the bundle dimensions and in most cases lies between 0.1 and 0.5 (166). Detailed analysis of several hair bundle morphologies, ranging from those with many rows, as in the bullfrog saccule, to these with only two or three rows, such as guinea pig and bat IHCs (see Figure 2), showed that the gain γ was approximately constant across the bundle if the interciliary coupling was tight. Furthermore, despite differences in the step height from one row to the next, γ is largely determined by the separation of adjacent rootlets and the overall height of the bundle (192).
The dimensions and organization of the hair bundle vary considerably depending on the end organ and the frequency range detected. The height of the tallest rank extends from ∼1 to 10 μm, and the number of stereocilia ranges from a few dozen to a few hundred. In vestibular and nonmammalian auditory hair cells, there may be up to 10 ranks of stereocilia across the bundle (Figure 2A), but in the mammalian cochlea, only two to three rows are present (Figure 2, B–D). In auditory hair cells there are also variations along the tonotopic axis (turtle, Ref. 90; rat, Ref. 216; chicken, Ref. 248). In the chicken auditory papilla, the number of stereocilia per bundle increases and the bundle height decreases, both by a factor of five, in progressing from the low-frequency to high-frequency end of the papilla (248); together these changes make the bundle stiffness vary 100-fold from one end of the papilla to the other (sect. IVB). Similar though smaller variations are seen in mammalian cochlear OHCs, where maximum bundle height decreases from 6 to 1.5 μm and the number of stereocilia increases from ∼60 to 100 (18, 153, 216). As discussed later (see sect. IVB), hair bundle mechanical stiffness is inversely proportional to the square of the bundle height, so changes in height occurring in the cochlea can have a large effect on bundle stiffness.
The diversity in bundle morphology will have a number of outcomes for the response properties of the bundles. For example, an increase in the number of stereocilia per bundle (and by implication number of MT channels) will augment the sensitivity of high-frequency hair cells. More interestingly, the reduction in the number of stereociliary ranks and the greater increment between adjacent ranks in mammalian compared with nonmammalian auditory hair cells is likely to minimize viscous resistance between adjacent ranks and enhance the mechanical frequency response of the bundle. In IHCs, the second and third ranks are also much shorter than the tallest rank so the degree of overlap between adjacent ranks is reduced, which might also minimize viscous resistance (Figure 2C). The most extreme example is seen in IHCs at the base of the bat cochlea (Figure 2D; Ref. 258), where the stereocilia are very short and the number of ranks is reduced to two, the minimum possible for gating MT channels. Such modifications in bundle morphology may be part of an evolutionary trend accompanying the expansion of the upper frequency limit of mammalian hearing. Changes in bundle height and stereociliary number will also have consequences for hair bundle amplification and force-feedback onto the tectorial membrane (35). The morphological diversity will be assessed again in considering the MT channel currents, but it may signify a matching of the accessory apparatus to the coding demands during the course of evolution.
The hair bundles in auditory organs of most vertebrates are coupled to the tectorial membrane, an acellular 25- to 50-μm-thick sheet the motion of which provides the immediate drive to the hair cells in vivo. In mammals, the tectorial membrane consists of radial bundles of collagen embedded in a matrix of glycoproteins, α-tectorin, β-tectorin, and otogelin (148, 210, 211). The mechanical stiffness of the tectorial membrane increases up to 20-fold from apex to base (212) in parallel with the stiffness of the hair bundles, which increases the shorter they are (21, 241). The importance of the tectorial membrane structure for OHC excitation is highlighted by the large loss in auditory sensitivity resulting from targeted deletion of α-tectorin (147). The tectorial membrane in nonmammals has a similar physical appearance and in reptiles such as the turtle, and in birds, the bundles are encased in pockets on the underside of the membrane, strands of which are tightly attached to the tallest row of stereocilia and to the kinocilium (90, 247). The connection is different in mammals where OHCs of adults lack a kinocilium and the stereocilia in the tallest row are inserted into individual pits visible in scanning electron micrographs of the underside of the membrane (153, 227); the points of attachment are associated with the protein stereocilin (260). The mechanical strength of this attachment point is unknown, but the histology suggests a tight coupling between the OHC bundles and the tectorial membrane so the two vibrate in synchrony, which might be essential for forward transduction and for feedback amplification especially at high frequencies. The viscoelastic coupling to the overlying membrane differs for IHCs, where a ridge known as Hensen's stripe on the undersurface of the tectorial membrane is closely apposed but not firmly connected to the IHC bundles. It is usually supposed that fluid flow in the gap between the tectorial membrane and the upper surface of the organ of Corti is the stimulus for the IHCs (89, 114, 181) the bundles of which are therefore velocity-coupled to basilar membrane motion. However, such fluid flow requires the edge of the tectorial membrane to be fixed to the spiral limbus and not to float freely above the reticular lamina. Mutation of the limbal anchoring protein otoancorin causes hearing loss thought to result from a failure in the excitation of the IHCs (156).
III. MECHANOTRANSDUCER CURRENTS
A. Overview: Methods of Stimulation
Mechanoelectrical transduction in hair cells was first studied by recording receptor potentials with sharp microelectrodes (49, 109), and also more indirectly by measuring extracellular microphonic currents (41, 42) in response to manipulation of the hair bundle. More direct and precise information about the MT channels was subsequently derived by patch-clamping either dissociated hair cells (11, 45, 46, 183) or cells in the whole epithelium (18, 54, 78, 96, 101, 114, 127, 139, 206, 233, 261). Recordings in the intact epithelium generally yielded larger MT currents than those from solitary hair cells, probably due to reduced mechanical damage to the hair bundle during tissue preparation. The initial work was done on nonmammals such as frogs, turtles, and chickens, but more recent experiments were performed on cochlear and vestibular hair cells of mice, rats, or gerbils. Most MT channel properties, such as ion selectivity, unitary conductance, and blocking agents, are similar across species, but activation kinetics are faster in mammals as might be expected because of the higher frequency limit of their hearing. All demonstrate an inward current at negative membrane potentials indicative of a nonselective cation conductance elicited by deflections of the hair bundle towards its taller edge.
An important difference between the reports is the type of stimulator employed for deflecting the hair bundle. The two main methods use either a fluid jet aimed at the bundle or a stiff glass probe pressed against the bundle and driven by a linear piezoelectric device. The fluid jet, although less destructive on the bundle, has the drawback that the force generated is low-pass filtered, and it cannot impose movements with rise times faster than a few milliseconds (57, 139, 245, 261); moreover, the flow is directed at the entire bundle and may therefore cause movements at the bundle's base as well as its tip. In the other method, a fire-polished glass probe, a few microns in diameter, is placed against the tallest or intermediate ranks of stereocilia. The piezoelectrically driven glass probe can generate very fast submillisecond bundle deflections but suffers from the disadvantage that it may not contact all the stereociliary ranks causing them to be differentially deflected. The method is also less satisfactory for negative stimuli that pull the bundle back towards its shorter edge (where the probe may lose contact with the bundle). For mammalian hair cells, the glass probe must be fashioned to fit the V-shape of the bundle (127, 233), a task difficult to implement with very short but wide hair bundles such as those of IHCs in the mouse cochlea. The fluid jet is good for assaying maximum MT currents, whereas the piezoelectrically driven probe is more suitable for measuring the kinetics of the MT current. In each case, the potential limitations should be considered, with neither method strictly reproducing the in vivo stimulus. A third method, best approximating the physiological mode of stimulation, has been to use a piezoelectrically driven glass probe attached to the kinociliary bulb which is enlarged in the frog saccule (101); this method has not been widely employed because the bulb is small or the kinocilium absent in most auditory hair cells.
The two main methods of stimulation yield similar values for the maximum current (Imax), but differ in the range of displacements encoded as seen in the representative results for mouse OHCs (Figure 4). With step deflections of the bundle using an attached glass probe, the inward current developed rapidly and then adapted to a reduced level (Figure 4, A–C). The response was graded with bundle deflection, and the relationship between the peak current (I) and displacement (X) could be described with a single (two-state) Boltzmann equation
| (1) |
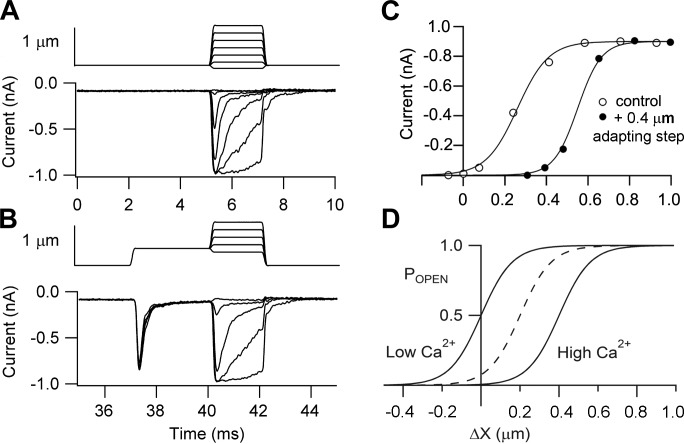
FIGURE 4.
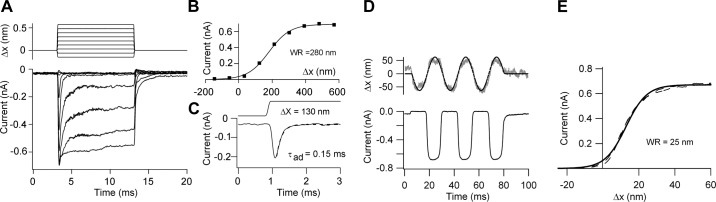
MT currents in mouse apical OHCs obtained using two different methods of hair bundle stimulation. A: deflections with a glass probe attached to a piezoelectric actuator, MT currents for step displacements of bundle, ΔX, shown at top. B: relationship between the peak MT current and bundle displacement, ΔX; the experimental points were fitted with a single-stage Boltzmann equation with 10–90% working range (WR) of 280 nm. C: onset of MT current to a 130-nm step showing that the current develops with the same time course as the stimulus but then undergoes fast adaptation with a time constant of 0.15 ms. D: hair bundle stimulation with a fluid jet, MT currents shown below and bundle motion above; the smooth curve is the 40-Hz sinusoidal driving voltage, and the noisy gray trace is the photodiode signal used to calibrate the movements. E: current-displacement relationship for first cycle of response in D, fitted with a single-stage Boltzmann equation with WR = 25 nm. In both sets of recordings, the holding potential was −84 mV, and the Ca2+ concentration in the extracellular solution was 1.5 mM.
where XO is the displacement to half activate the current and XS is the slope factor. Use of the fluid jet (Figure 4, D and E) gave a similar maximum current and an I-X relationship also described by a single Boltzmann equation, but with a 10-fold smaller slope factor XS, implying a substantially narrower working range. The precise form of the I-X relationship has been interpreted as reflecting the channel gating scheme (41, 45, 101). A single Boltzmann fit is expected for a two-state channel in which mechanical energy modulates the transition between a closed state and an open state
| (2) |
with forward and backward rate constants, β and α, respectively, depending on the force applied to the channel. The channel opens and the current develops with a time constant α/β. The open probability of the channel (PO) can be specified as
| (3) |
where Z, the single-channel gating force, is a measure of the sensitivity of transduction, kB is Boltzmann's constant, and T is absolute temperature. Z can be related to Λ, the range of hair bundle displacements (166) over which the channel open probability changes from 10 to 90%
| (4) |
Displacements of the hair bundle are assumed to exert force on the MT channel by extending a “gating spring” in series with the channel (106), just as force can be applied by stretching a macroscopic spring. The gating force can then be expressed in terms of the microscopic stiffness (kg) of the gating spring and channel motion in response to bundle deflection, usually referred to as the gating swing (b), which is the distance moved by the channel's gate during opening
| (5) |
Typical values for the parameters can be derived experimentally on the basis of the gating spring model: stiffness of the gating spring (kg) = 0.5–1.5 mN/m; the gating swing (b) = 1–4 nm; single-channel gating force (Z) = 0.25–0.7 pN (21, 34, 106, 166, 168, 251). Occasionally, values outside this range have been required to reproduce experimental data, particularly for the gating swing (e.g., 5–11 nm; Refs. 168, 251); such values seem unrealistically large if representing a change in the dimensions of an ion channel or the movement of a protein subcomponent. Also, larger values for the gating spring stiffness are needed for mammalian cochlear hair cells (21, 251). The introduction of the geometrical factor γ transforms the force at the tip of the bundle to that occurring at the channel, with Z being the single-channel gating force experienced at the tip of the bundle. Because of the geometrical factor, forces are larger and displacements smaller at the channel than they are at the tip of the bundle.
While the single Boltzmann equation provides an adequate approximation for many I-X relationships, some require a (3-state) double Boltzmann equation to fit more accurately the results. This formulation produces an asymmetric I-X relationship with a curvature less pronounced at the upper end than at the lower end of the sigmoid. A double-Boltzmann fit was needed to describe the I-X relationship in nonmammalian hair cells (42, 45, 101) and in mammalian hair cells (139) using both methods of bundle stimulation, and has been linked theoretically to a small delay in the current onset. The double-Boltzmann fit suggests a more complex gating scheme with multiple closed-state transitions with mechanically sensitive rate constants, all consistent with a delay incurred by transition through the closed states. While not questioning the validity of the double-Boltzmann fits, it should be noted that not all I-X relationships have required a double-Boltzmann equation and some, especially for cochlear outer hair cells, are adequately described by a single Boltzmann equation (18, 96, 116). In addition, there is a concern that the need for a double Boltzmann fit (and reduced curvature at the upper end) may sometimes be an artifact of the mechanical stimulus, especially with an attached glass probe. The shallower corner and greater working range at the upper end of the curve could be partly due to a nonsynchronized deflection and splaying of the stereociliary ranks, or to slipping of the probe up the back of the bundle, both effects causing a proportion of the MT channels to be recruited only for larger stimuli. This is particularly the case for IHCs which, because of their wide and flat hair bundles, are difficult to stimulate, their I-X relationships often requiring a double-Boltzmann fit (18, 233), but not always (114).
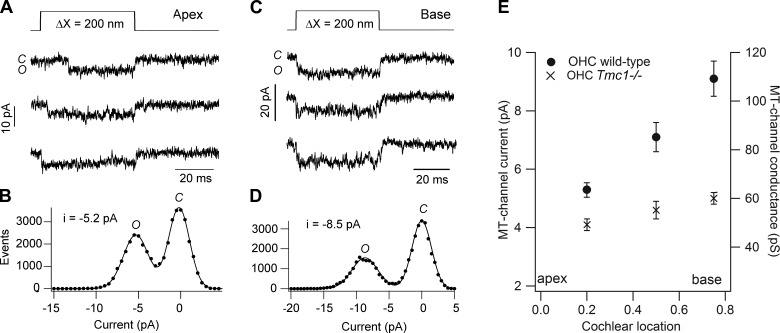
Whatever the method of stimulation, maximum currents of several hundred picoamps up to several nanoamps (typically at −80 mV holding potential) have been reported for MT currents in a variety of hair cell types. It seems reasonable to assume that the largest currents are most representative of the in vivo state, since any damage to the cells or bundles while the tissue is being prepared will be likely to reduce the maximum current. For OHCs, the largest currents recorded at the apex are ∼1.0 nA, and this amplitude increases by up to twofold in progressing towards the high-frequency base (18, 96, 116, 130). A tonotopic gradient of similar magnitude and polarity has been observed in auditory hair cells of turtle (205) and chicken (246), and in all cases is thought to result from an increase in both the number of stereocilia per bundle, and the single-channel conductance (see sect. IVB). In contrast, no gradient has been found in MT currents of IHCs (18, 23, 114).
B. Working Range of Mechanotransduction
The working range of hair cell transduction as specified by “Λ” is important because it provides a measure of mechanical sensitivity and can be applied empirically to both two-state and three-state Boltzmann fits. It can also be used to derive a value for the Z (Eq. 4) in the two-state model. Unfortunately, the substantial spread of values depending on the mode of stimulation undermines precise analysis. Nevertheless, several conclusions can be drawn from existing results. The narrowest working range, Λ, inferred using stimulation with an attached stiff glass probe is 0.2–0.4 μm for turtle auditory hair cells (206), frog saccular hair cells (101), chicken auditory hair cells (246), rat apical OHCs (18, 265), and mouse apical OHCs (233; Figure 4A); all species listed have maximum bundle heights of 4–6 μm, with the mouse being at the lower end of this range. Significantly narrower activation curves have been observed for turtle (Λ= 50 nm, Ref. 204), chicken (Λ = 50 nm, Ref. 22), rat OHCs (Λ = 75 nm, Ref. 116), and mouse OHCs (Λ = 80 nm, Refs. 78, 219, 220; Λ = 32 nm, Figure 4B) when force stimuli were applied with a flexible glass fiber or water jet. Taken at face value, this discrepancy suggests that measurements with an attached stiff glass probe overestimate the working range. A useful comparison is with the values obtained in the hemi-cochlea preparation under more physiological conditions (96). Here, stimuli were delivered to the basilar membrane, with force being transmitted to the bundles via displacements of the tectorial membrane; these measurements gave Λ = 50 nm for basal OHCs and 190 nm for apical OHCs, the stimulus in each case being expressed as a displacement of the basilar membrane. With the assumption of a unitary gain between displacements of the basilar membrane and the tip of the hair bundle (50), the apical-basal difference largely reflects the fivefold shorter bundle heights at the base. Thus the values of Λ for hair cells in the hemi-cochlea are more similar to those with fluid jet deflection of exposed bundles in rodent OHCs.
It is conceivable that the stiff glass probe causes more damage to the hair bundles than does the fluid jet, including destroying or weakening interciliary links. However, the simplest explanation for the difference between the two methods of stimulation is that the stiff probe does not contact all stereociliary ranks equally so that when it advances, the ranks are differentially recruited, thus smearing the stimulus to the MT channels and broadening the I-X relationship. Such differential motion might occur along the axis of symmetry so that the tallest edge of the bundle is displaced more than the shortest edge. But laser imaging of the two edges of bundles in frog hair cells argues against this with all ranks moving synchronously (135). The motion could also be distributed across the bundle so that the central stereocilia are displaced further than those on the periphery, producing a bell-shaped profile of displacement amplitudes across the bundle. The latter problem will be most acute for the mammalian cochlear hair cells where the glass probe is unlikely to fit perfectly into the back of the bundle, particularly if the shape of the bundle varies somewhat from one cell to another. The IHC bundles (with the tallest stereociliary row being much longer than the two shorter rows, and arrayed almost linearly) are likely to be the most problematic so that deflections of the central stereocilia will pull the peripheral ones via lateral interciliary links. Consistent with this notion, the I-X relationships of IHCs are most asymmetric when assayed with a stiff probe and exhibit a larger working range (Λ ≥ 400 nm; Refs. 18, 233, 238) than more physiological stimuli (Λ = 60–130 nm; Ref. 114). IHC bundles in vivo are probably stimulated by fluid motion in the subtectorial space (89, 181), so use of a fluid jet would provide the most physiological stimuli.
The value of Λ represents the displacements at the tip of the bundle that modulate the MT channels through their working range. The Λ values assayed from fluid jet stimuli (30–80 nm) may be taken as the best estimate for those occurring in vivo, and can be compared with the movements of the basilar membrane that activate the MT channels through their working range as measured in intact animals. The latter can be inferred from the range of basilar membrane movements over which the nonlinearity between sound pressure and displacement is observed; this nonlinearity arises from OHC somatic contractions caused by the receptor potentials generated by forward transduction. With this idea, vibrations of the basilar membrane are only amplified by OHC motility for displacements over which the MT channel open probability varies. A number of complete measurements are available, and the amplitudes, interpolated from the upper extent of the nonlinearity, vary inversely with the characteristic frequency (CF) of the location assayed. (The CF is defined as the sound frequency to which the hair cells and auditory nerve fibers are most sensitive at a given position in the cochlea; variation in the CF with location specifies the tonotopic map.) They extend from ∼100 nm at the apex (chinchilla CF = 600–800 Hz; Ref. 200), through 30 nm for the mid-frequency region (chinchilla: 6–10 kHz; refs.199, 217) to 10 nm for the high-frequency region (gerbil: 35 kHz, Ref. 185; cat: 30–40 kHz, Ref. 38). Although no data are available for mouse or rat, the range for other animals largely encompasses measurements on OHCs. Both imply that the operating range of hair cell MT channels must be smaller in basal OHCs than in apical ones, attributable to the shorter OHC bundles at the base. About a fivefold difference in OHC bundle height between base and apex has been reported for rat (216) and chinchilla (153).
C. Kinetics of the MT Current: Activation and Adaptation
Despite the limitations of the stiff probe for deflecting the hair bundle, it has been the principal means of characterizing the time course of MT current activation and adaptation. The kinetics have been well documented for nonmammalian hair cells, showing that in response to a step deflection of the bundle, the current develops with a submillisecond time constant and then declines despite a sustained stimulus. The decline is referred to as adaptation rather than inactivation because the current can be recovered by increasing the stimulus amplitude; the end result is a shift in the I-X relationship to larger displacements without change in shape or diminution in the maximum current (Figure 5; Refs. 61, 94, 233, 261). Adaptation occurs over several distinct time frames from tens of microseconds to seconds. Two major processes are commonly recognized: fast adaptation operating on a millisecond or submillisecond time scale and slow adaptation acting over tens of milliseconds (261, 270). A third process, conceivably metabolic and mediated by cAMP, may occur over a time scale of seconds or more (206). The exact mechanisms underlying these different forms of adaptation are still controversial, but all may ultimately be regulated by intracellular Ca2+. Evidence supporting this notion includes: 1) the fast adaptation time constant (τAf) is slowed by lowering extracellular [Ca2+], (τAf)−1 being proportional to Ca2+ influx via the MT channels (207). 2) Adaptation is abolished by depolarizing to +80 mV, a membrane potential approaching the Ca2+ equilibrium, which therefore minimizes Ca2+ influx (11, 46). 3) The position of the I-X relationship on the displacement axis is sensitive to the extracellular Ca2+ concentration and intracellular Ca2+ buffering (94, 206, 209); as a consequence, the probability of MT channel opening at the bundle's resting position (PO,r) depends on external Ca2+ and internal Ca2+ buffering. PO,r is determined from the ratio of the MT current active at the bundle's unstimulated position to the maximum MT current achievable with a saturating stimulus. It is an important parameter that will be discussed in more detail later.
FIGURE 5.
Two-step analysis of fast adaptation in a rat OHC. A: family of MT currents (controls) in response to 2-ms step deflections of the hair bundle with a glass probe driven by a piezoactuator. B: MT currents for 2-ms step deflections of the hair bundle superimposed on a 0.4-μm maintained adapting step; these stimuli were interleaved with the control measurements as indicated by the time axis at a stimulus repetition of 30 Hz. C: current-displacement (I-X) relationship for the control measurements (○) and the adapted measurements (●). Note the effect of adaptation is to reset rapidly the working range by shifting the I-X relation along the displacement axis by approximately the same amount as the adapting step; holding potentials, −84 mV. D: schematic of the effects of intracellular Ca2+ on the position of the I-X relationship along the displacement axis. Elevated Ca2+, as occurs during adaptation, shifts the I-X relationship from its resting position, denoted by dashed curve, to larger displacements. Reduction in intracellular Ca2+ during lowering external Ca2+ or depolarization translates the I-X relationship to smaller displacements.
The prevailing hypothesis is that changes in stereociliary Ca2+ concentration reset the range of bundle displacements over which the MT channel is activated. Elevating the Ca2+ influx, as occurs during sustained channel opening, shifts the I-X relationship positive to larger displacements causing desensitization; on the other hand, reducing Ca2+ influx, as may result from prolonged channel closure or lowering the extracellular Ca2+ concentration, shifts the I-X relationship in the opposite direction. The site(s) at which binding of Ca2+ triggers adaptation are specific for this ion, and other alkaline earth cations do not substitute for Ca2+ (94), even though some, such as Sr2+, permeate the channel with comparable ease (183).
In terms of molecular mechanisms, it has been suggested that slow adaptation involves a change in the attachment point of the elastic element connected to the MT channels (105). A specific proposal is that during a positive bundle deflection, the upper end of the tip link slips down the side of the stereocilium to reduce the tension in the elastic element (105). It is further suggested that the resting tension in the tip link is maintained by an isoform of myosin (e.g., myosin Ic; Ref. 81) ascending the actin backbone of the stereocilium. Support for this mechanism has come from generation of transgenic mice expressing a mutant form of myosin Ic that can be inhibited by intracellular ADP analogs (100). Utricular hair cells from mice possessing the transgene Y61G-myosin Ic (in addition to wild-type myosin Ic) demonstrated a slow adaptation (time constant τA = ∼50 ms) that could be blocked by addition of the ADP analog to the whole cell recording pipette; this maneuver also abolished any adaptive shift of the I-X relationship along the displacement axis in the transgenic but not in wild-type hair cells. These experiments were extended by constructing a transgenic mouse in which only mutant Y61G-myosin Ic was present, and showing that a faster form of adaptation (τA = 15–20 ms) in mouse vestibular hair cells was also slowed or blocked by the ADP analog (234). While this confirmed that a component of the adaptation employed myosin Ic, it is unlikely that this component represents fast adaptation; fast adaptation has a time constant of <5 ms in frog and mouse vestibular hair cells (261) and in auditory hair cells of turtle (τA = 0.5–3 ms; Refs. 45, 206) and rat (τA = 0.05–0.5 ms; Refs. 127, 208). The millisecond time constant of fast adaptation argues against its being mediated via an ATPase like that in any myosin isoform, which typically has cycling rates of <20 s−1 (103) but is more consistent with adaptation occurring at a site very close (<30 nm) to the MT channel (45, 207, 270); a specific hypothesis, based on fast adaptation being reflected in hair bundle mechanics (203), is that it stems from a Ca2+-induced change in the force sensitivity of the MT channel making the channel more difficult to open (34).
Despite the elegance of these experiments and the accompanying hypotheses, the precise mechanism of either kinetic form of adaptation remains unclear. Furthermore, several important questions linger particularly with regard to mammalian cochlear hair cells. 1) There have been no studies on whether introduction of the ADP analog in Y61G-myosin Ic mutants affects adaptation in cochlear OHCs; it is conceivable that these cells are unusual and lack slow adaptation, but it would be useful to provide definitive evidence one way or the other. 2) If the MT channels are confined to the lower end of the tip link, as is now supposed (19), myosin Ic motors localized at the upper end of the tip link will have little access to Ca2+ entering via the MT channel; how then are they regulated and what determines the sliding of the attachment point with an increase in tension? 3) Immunolabeling has demonstrated that myosin Ic is localized to both upper and lower ends of the tip links in frog vestibular hair cells (76). However, no discrete foci for myosin Ic are visible in cochlear hair cells, and labeling for the myosin Ic isoform is distributed along the entire length of the stereocilium (224). The diffuse labeling contrasts sharply with that for three other myosins examined: myosin IIIa and myosin XVa are both confined to the stereociliary tips (224), whereas myosin VIIa clusters in a complex with sans and harmonin-b at the upper end of the tip link (85). Functional evidence for the role of myosin VIIa in cochlear hair cells comes from the observation that several mutations of this motor protein affect adaptation of the MT current (138). Myosin VIIa climbing up the actin filaments in the stereocilium may also be responsible for maintaining the resting tip-link tension in mammalian OHCs (85). 4) Fast adaptation may involve myosin XVa too (238). In IHCs of shaker-2 mice lacking this isoform (Myo15 sh2/sh2), the stereocilia are short, and there is no increment in height between adjacent ranks of stereocilia. Large MT currents were still recordable from IHCs of shaker-2 mice, but the current lacked all signs of fast adaptation and was insensitive to external Ca2+. In contrast, fast adaptation and its dependence on external Ca2+ remained intact in OHCs of shaker-2 mice. The reasons for these differences are unclear. 5) Even the role of intracellular Ca2+ in adaptation has lately been questioned for cochlear hair cells (190), because none of the three lines of evidence cited above for substantiating involvement of Ca2+ in adaptation is definitive in mammalian cochlear hair cells. Step deflections of the hair bundle evoke an MT current exhibiting fast adaptation (τA = 45–85 μs depending on cochlear location), and these time constants do slow on reducing extracellular Ca2+ from 1.5 mM to (endolymphatic) 0.02 mM, but the increase in time constant is small, no more than a factor of two (208). Depolarization to +80 mV approaching the Ca2+ equilibrium potential, where Ca2+ influx ought to be minimal, diminishes the extent of adaptation but never totally abolishes it, suggesting that either the remnant is Ca2+ insensitive or a stimulation artifact (e.g., slipping of the probe). Furthermore, in recordings with pipette solutions containing high millimolar intracellular Ca2+, fast adaptation still occurred. Finally, lowering external Ca2+ to an endolymphatic concentration shifts the I-X curve leftward and increases PO,r to ∼0.45, but whether this shift depends on the intracellular Ca2+ buffer is contentious (20, 116, 190). Taken together, these observations on adaptation in mammalian cochlear hair cells do not fit with the simple notion, derived from experiments on nonmammals, that adaptation, and the position of the I-X relationship are regulated by changes in intracellular Ca2+.
A further puzzle is the potential involvement of cAMP in mechanotransduction and adaptation (206), for which neither is the effect confirmed in mammals, nor is the signaling pathway identified. An obvious route analogous to other forms of adaptation might entail regulation by Ca2+: entry of the ion through the MT channels could lead to activation of a Ca2+/calmodulin-dependent adenylate cyclase (AC), production of cAMP, and phosphorylation of the channel or other protein linked to transduction. Of the nine membrane-bound isoforms, AC1 and AC8 are stimulated by Ca2+ and AC5 and AC6 are inhibited by Ca2+, and there is evidence for the occurrence of at least one of each category, AC1 and AC6, in cochlear hair cells (59, 140, 172). Renewed interest in this signaling pathway has stemmed from the recent discovery that a mutation in adenylate cyclase 1 (ADCY1) is linked to hearing imparimen in humans (223). ADCY1 was found to be localized to the cochlea and vestibule of mice, including hair cells and their stereocilia (223). Furthermore, mutation of the equivalent protein in zebrafish caused a defect in hair cell mechanotransduction (223). These results indicate the mechanism of MT channel regulation by cAMP warrants further investigation.
The time course of MT current activation, as with adaptation, has been most thoroughly documented in nonmammalian hair cells (42, 45, 208). It is best quantified by recording in low external Ca2+ where distortion of the response onset by the development of adaptation is minimized (208). Under these conditions, it was shown that the activation time constant decreased with stimulus amplitude from 1 to <0.1 ms, as might be expected if the mechanical stimulus energy modulates the MT channel opening and closing rate constants. In turtle auditory hair cells, the activation time constant at low levels is roughly sixfold faster than the adaptation time constant, so the two processes impose single-pole low-pass and high-pass filters, together creating a band-pass filter, albeit weak, on transduction. Because both time constants vary systematically with distance along the basilar membrane, the center frequency of the transduction filter approximately matches the CF of the location (208).
It has been difficult to quantify the activation kinetics of the MT current in mammalian auditory hair cells mainly because the kinetics are much faster than in nonmammalian hair cells, and as a consequence, the speed of the piezoelectric stimulator becomes rate limiting. The turtle has a behavioral upper-frequency limit of ∼0.6 kHz, so for MT-channel kinetics not to be limiting, the activation time constant should be no more than 0.25 ms, which is in range of measurements. For the mouse, with an upper frequency limit of 90 kHz, the corresponding time constant is <2 μs. Because of intrinsic under-damped oscillations in the conventional piezoelectric stimulator, the driving voltage must be low-pass filtered at 10 kHz, achieving a rise time at best of 30 μs (208, 233, 238). As a consequence, the MT current onset kinetics are not resolvable, the rise time of the current being contemporaneous with that of the stimulus. However, a new solid state nanoscale cantilever has recently been developed that can produce deflections with <10 μs rise time (58). Provided the recording bandwidth can also be expanded, this stimulation technique promises to improve measurements to the point where the kinetics of cochlear hair-cell MT channels may be directly quantifiable.
D. Development of the Hair Bundle and MT Currents
Precocial mammals such as guinea pigs are born with a mature cochlea and able to hear at birth, but in altricial rodents, like mice, rats, and gerbils, the auditory system, in parallel with the visual system, does not develop fully until 2–3 wk after birth. Mice of the first two neonatal weeks in age are often employed for hair cell study because their temporal bones are not fully ossified, which enables cochlear access while minimizing mechanical damage. Hearing commences around postnatal day (P) 12, but a number of properties are not fully mature until the end of the third week (196). The hair bundle structure and mechanotransduction develop during the first week, and the final differentiation of IHCs and OHCs, with acquisition of adult voltage-dependent ion channels (163, 164), OHC prestin-based electromotility (rat, Refs. 15, 184; mouse, Ref. 1), maturation of the exocytotic machinery at the IHC synapse (117), and pattern of differential innervation, (type I afferents to IHCs and efferents to OHCs, Ref. 196) proceeds during the second and third weeks. The endolymphatic potential does not achieve its adult value until P16 (25, 235). A critical juncture is the appearance of the tunnel of Corti, the heads of the pillar cells separating the top surfaces of the IHCs and OHCs, at around P11 just prior to the onset of hearing. Even after the onset of hearing, auditory sensitivity grows and does not reach an adult level in mice for several months (63). These developmental changes have been reviewed in detail elsewhere (84, 196, 210), so only those features linked to transduction will be described as they bear on the properties measured in neonates that can be generalized to the adult.
The distinctive arrangement of the OHC hair bundle develops during the first few postnatal days with emergence of the three stereociliary rows out of a carpet of microvilli, their elongation and formation of the staircase in heights, and attainment of the characteristic “V” or arrowhead shape (265). These morphological changes accompany acquisition of the MT current which achieves maximum amplitude by P2 to P4 in mice, hair cells at the base of the cochlea preceding the apex by ∼2 days (130, 149). Rats exhibit a similar progression but are delayed about 1 day relative to mice (265). The peak amplitude then remains constant up until the onset of hearing (127), there being no evidence of a further increase in the current into adulthood; a rigorous test of this notion is not available as the experiments become increasingly difficult at later stages and the current size tends to be underestimated. Despite no further increase in the MT current amplitude, there are probably subsequent variations in the molecular structure of the channel. For example, the relative Ca2+ permeability of the OHC MT channel decreases after P6 (130). The mechanism of this transformation may be related to a change in the expression of transmembrane channel like proteins, with downregualtion of TMC2 after postnatal day 6 (see sect. VIB and Refs 123, 130), but it occurs with no major change in the magnitude of the MT current.
In altricial rodents there are also changes in the interstereociliary connections over the first two neonatal weeks (84, 91, 210). From birth, OHC bundles possess tip links (enabling mechanotransduction), but in addition they have ankle links and “transient lateral links,” including those coupling the tallest stereocilia to the kinocilium. A protein component of the ankle links is the very large G protein-coupled receptor (VLGR1), which occurs along with the membrane proteins usherin and vezatin (159, 172). VLGR1 and usherin are both defective in Usher syndrome type II (see sect. VIA). In the cochlea, the ankle links and most lateral links are eventually lost along with reabsorption of the kinocilium (196); the top connectors appear after P9, signifying the mature condition (84, 260). Despite the transient appearance of the ankle links, mutations in VLGR1 are associated with early disorganization of the hair bundle and loss of mechanotransduction in cochlear hair cells (159, 172). In the vestibular organs, unlike the cochlea, the kinocilium and ankle links are retained into adulthood. In the adult, some lateral links between stereocilia in the same row must persist to ensure bundle cohesion in both OHCs and IHCs (253, 254). During early maturation of the bundle, as the MT current achieves its full amplitude, a bidirectional response is sometimes apparent, with MT currents being elicited by deflections both towards and away from the tall edge of the bundle (172, 265). The origin of such bidirectional selectivity is unclear, but it usually disappears with pruning of the lateral links during the first few days (265), and may therefore be partly attributable to nonuniform polarization of the tip links. Zebrafish lateral-line hair cells also display a response to reversed-polarity bundle motion early in development prior to formation of tip links. In this case, the response has been ascribed to the presence of the kinocilium since it disappears in specific mutants lacking the kinocilium (133).
In charting early postnatal hair cell development in altricial rodents, an important question is the extent to which a functional MT channel is necessary for maturation of hair bundle structure. Indeed, why does mechanotransduction precede by more than a week the maturation of the bundle, and the onset of hearing which is ultimately limited by sound transmission through the middle ear? A clue may derive from the observation that in mice with mutations in proteins comprising the tip link and its attachments, the bundles are deformed and, from P1 to P5, growth of the stereocilia, especially those in the middle and shortest rows, is stunted (28, 146, 271). Two possible mechanisms may underlie this process (28, 250). First, the polymerization of actin that is required for stereociliary elongation may be stimulated by mechanical tension applied by intact tip links to cytoskeletal proteins at the tops of the two shorter rows of stereocilia. Alternatively, the rate of actin polymerization might be increased by Ca2+ influx via functional MT channels (250). Stereociliary Ca2+ handling matures during the early neonatal period in rodents as indicated by upregulation of the plasma membrane Ca2+-ATPase pump, which appears contemporaneously with the onset of MT channel function (32). The Ca2+ pump, the PMCA2a isoform (60, 240), has fast kinetics, is present at high concentrations in the stereociliary membrane (32), and its absence in various mutants causes hair cell degeneration and deafness (e.g., Ref. 240). Both mechanical stress and intracellular Ca2+ may act cooperatively to regulate actin polymerization, so investigating the link between the two developmental mechanisms in hair bundle maturation and stereociliary growth will be an important line of investigation.
IV. REVERSE TRANSDUCTION
A. MT Currents In Vivo: MT Channel Set Point and Hair Cell Resting Potential
Besides converting a sound stimulus into an electrical replica, auditory hair cells also participate in decomposing the stimulus into its constituent frequencies recruiting several mechanisms. In nonmammalian vertebrates, electrical tuning of the receptor potential by voltage- and Ca2+-dependent K+ conductances is the foremost mechanism (8, 48, 66, 70, 151). However, because there is an upper limit to the speed with which the K+ channels can be gated, this mode of frequency analysis is restricted to frequencies below ∼1 kHz (269), and in mammals, with their much higher frequency range, another mechanism has evolved. In the mammalian cochlea, passive mechanical tuning of the basilar membrane is greatly amplified by the electromotility of the OHCs (9, 10, 27) mediated by the membrane protein prestin (51, 52, 276). The mechanical amplification attributable to OHC electromotility (the cochlear amplifier; Ref. 51) has often been referred to as an “active process” to contrast it with the tuning bequeathed by the passive mechanics of the basilar membrane, and also because it is susceptible to the metabolic state of the animal. In nonmammals, and also conceivably in mammals, a third mechanism, entailing tuned force generation by the stereociliary bundle, may also operate (108). The performance of the MT channels in vivo is critical for the operation of all three tuning mechanisms.
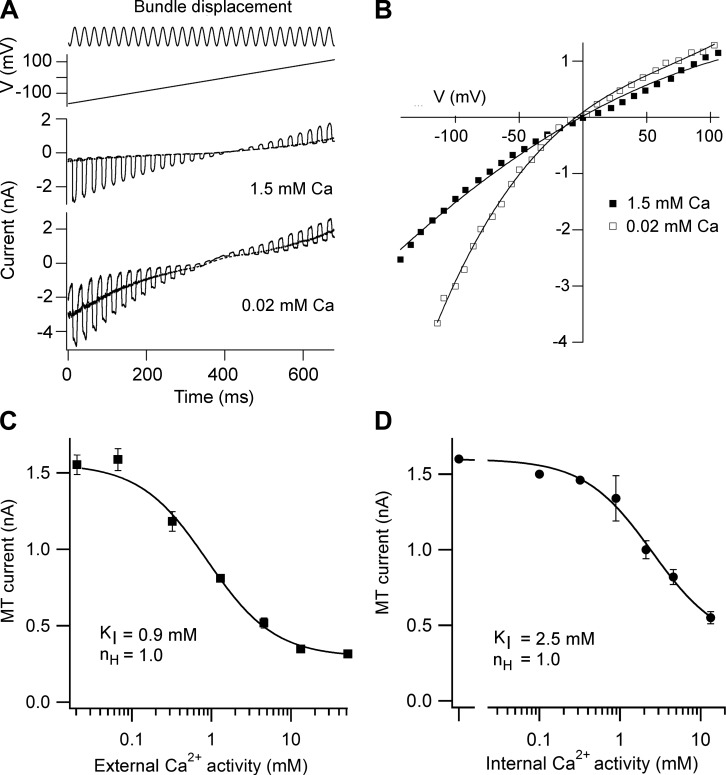
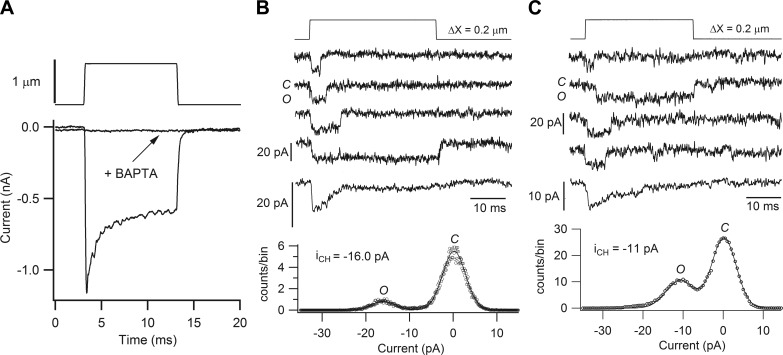
Two features of the mammalian cochlea will augment the MT current amplitudes in vivo relative to those measured in isolated preparations. First, an endolymphatic potential (EP) of ∼100 mV in rats (25) and mice (235) and up to 80 mV in guinea pigs (80) will increase the electrical driving force across ion channels in the stereociliary membrane facing the endolymph, thereby increasing the current for a given MT conductance. Second, the MT channels are blocked by millimolar external Ca2+ (42, 46, 207), a block that is relieved by the low Ca2+ concentration in the endolymph bathing the bundles. Like the EP, endolymphatic Ca2+ concentration varies with location (80). Normally, isolated preparations are studied when they are totally immersed in an artificial perilymph resembling interstitial fluid high in Na+ and Ca2+. The MT current increases by ∼60% when the external Ca2+ concentration is reduced from 1.5 mM to 20 μM, similar to that in mammalian endolymph (26, 110). The increase is confined to negative membrane potentials, with little change at positive potentials rendering the MT current-voltage relationship more strongly inwardly rectifying (Figure 6B). The half-blocking concentration (KI) for Ca2+, whether applied extracellularly or intracellularly (Figure 6, C and D) is 1–2 mM (129, 207). The similarity of KI for block by internal and external Ca2+ suggests a common binding site within the pore, and the result differs from block by other larger antagonists such as DHS and FM1–43, which require much higher concentrations from the cytoplasmic aspect than the extracellular side to block the MT channel. It should also be emphasized that there are (at least) two Ca2+-binding sites associated with the MT channel: one inside the channel that can block it and a second elsewhere that mediates adaptation. These are different binding sites that probably have distinct selectivities for the divalent ion.
FIGURE 6.
Rectification and Ca2+ block in MT channels of mouse OHCs. A: apical OHC in which hair bundles were deflected with a sinusoidal fluid jet stimulus (top) superimposed on a voltage ramp from −150 to +100 mV (middle), MT currents being recorded in an extracellular saline containing 1.5 mM Ca2+ (perilymph) or 0.02 mM in Ca2+ (endolymph). The continuous line through the MT currents is the current-voltage response in the absence of bundle stimulation; note that in the low Ca2+, the resting open probability of the channels (PO,r) is ∼0.5. B: current-voltage relationships for an apical OHC in 1.5 mM Ca2+ and 0.02 mM Ca2+. The effect of reducing external Ca2+ concentration is to remove block at negative membrane potentials and produce a more marked inwardly rectifying relationship. C: block of MT channel as a function of the extracellular Ca2+ concentration. Mean ± SE of the MT current at −84 mV is plotted against the Ca2+ activity; intracellular solution contains 142 mM CsCl and 1 mM EGTA. Points fitted with a Hill equation with half-blocking concentration, KI = 0.9 mM and Hill coefficient, nH = 1.0. D: block of MT channel as a function of intracellular Ca2+ activity. Mean ± SE of the MT current at −84 mV is plotted against the Ca2+ activity; extracellular solution contains 160 mM NaCl and 0.02 mM Ca2+. The lowest intracellular Ca2+ activity is equivalent to 1 mM EGTA. Points fitted with a Hill equation with KI = 2.5 mM and nH = 1.0.
Owing to the Ca2+ regulation of adaptation, a second consequence of exposure to low Ca2+ endolymph is an increase in the resting open probability of the MT channels. This is attributable to a shift of the I-X relationship in the negative direction, hence increasing the probability of the channel being open at the resting (nonstimulated) bundle position (PO,r). A high resting open probability is seen across vertebrate classes when the hair bundles are exposed to low Ca2+ concentration similar to endolymph; for example, PO,r = 0.28, turtle (65, 209); 0.28, chicken (246); and 0.46, rodents (20, 116). The increase in PO,r generally depends on the intracellular calcium buffer concentration, being larger with stronger buffering, which rapidly removes the Ca2+ from the internal face of the channel (209, 214). To estimate PO,r under conditions resembling those in vivo, it is necessary to make hair cell recordings with perforated-patch electrodes for which low-resistance electrical contact is made to the cell interior without eluting the Ca2+-binding proteins. The main mobile calcium buffers include calbindinD-28k, calretinin, and parvalbumin-β (also named oncomodulin in mammals), which are present in the soma and the hair bundle at up to millimolar concentrations (62, 92, 93, 182, 246, 273).
A high PO,r in vivo will maximize sensitivity and minimize distortion. For pure-tone sinusoidal stimuli, the MT current will be generated close to the point of maximum slope of the I-X relationship and will be approximately symmetrical about rest, increasing on one half-cycle and decreasing by an equal amount on the other half-cycle of the sinusoid. For low level stimuli, transduction is approximately linear, thereby minimizing distortion. But another more significant consequence is that, in the absence of stimulation, a standing inward current will flow through the partially open MT channels to depolarize the hair cell. This may be termed a “silent current” (116, 279) by analogy with the dark current in photoreceptors, and results in a resting potential of −40 to −45 mV well depolarized from the K+ equilibrium potential. In the turtle auditory papilla, this is essential to optimize electrical tuning by setting the membrane potential near that required for activating the voltage-sensitive Ca2+ and Ca2+-activated K+ channels (65). As a consequence, the quality factor of electrical tuning is maximal at rest. In mammalian OHCs, the standing current adjusts the membrane potential to be near the half-activation of the somatic electromotility underscored by prestin, thereby optimizing the somatic motor feedback (116). In both reptile and mammal, both the inward MT current in the stereocilia and the outward voltage-dependent K+ current in the basolateral membrane increase in amplitude along the tonotopic axis towards its high-frequency end (Refs. 8, 116; Figure 7), the current amplitudes being approximately matched to maintain a constant resting potential. In birds too, the MT current increases tonotopically reflecting increases in single-channel conductance and number of stereocilia per bundle (Ref. 146; Figure 8).
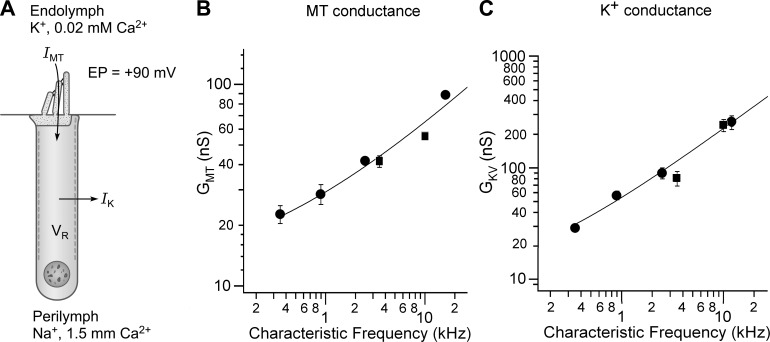
FIGURE 7.
Tonotopic variation in the MT conductance and K+ conductance in rodent OHCs. A: schematic of an OHC illustrating the two principal ionic currents that determine the resting potential. An inward current (IMT) flows through open MT channels, the electrical driving force resulting from the positive endolymphatic potential (EP = +90 mV) and the negative resting potential, −40 to −50 mV. The MT channels are partially open due to a low endolymph Ca2+ of 0.02 mM. B: maximum MT conductance (GMT) in endolymph Ca2+ as a function of the CF of the cochlear location: ●, gerbil; ■, rat. C: maximum voltage-dependent K+ conductance (GK) as a function of the CF of the cochlear location: ●, gerbil; ■, rat. The K+ current in hearing animals older than P12 is dominated by IK,n flowing through KCNQ4-containing channels in the OHC basolateral membrane. Measurements are from Johnson et al. (116), extrapolated to the in vivo condition including a body temperature of 37°C.
FIGURE 8.
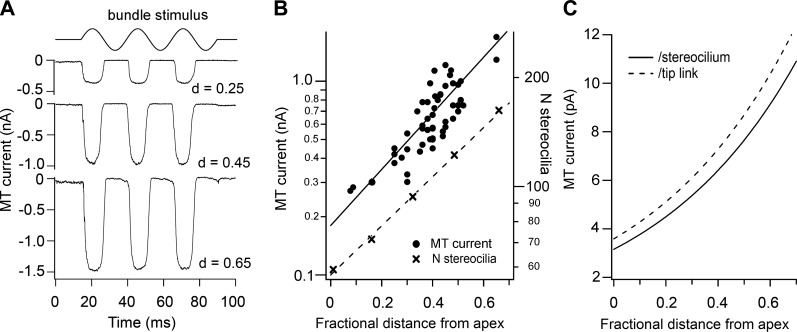
Tonotopic variation in the MT currents in hair cells of the chicken auditory papilla. A: MT currents in three short hair cells in response to fluid jet deflections of the hair bundle; holding potential is −84 mV. Sinusoidal driving voltage to the fluid-jet is shown at the top. Cell locations in the epithelium given beside traces as the fractional distance (d) from the apical end of the papilla (d = distance from the apex scaled by the total length of papilla, ∼3.6 mm). B: collected peak amplitudes of MT currents versus location for tall and short hair cells at 33°C. Line is exponential fit to all points. Tonotopic variation in the number of stereocilia per bundle (crosses referred to the right hand axis) replotted from the data of Tilney and Sauders (248). C: MT current per stereocilium (continuous line) derived from the data in B by dividing the fit to the experimental MT currents by the fit to the number of stereocilia per bundle. MT current per tip link (dashed line) derived by dividing the continuous line by 0.88, the approximate ratio of tip-links to stereocilia in a chicken hair bundle. [Modified from Tan et al. (246).]
A further outcome of the tonotopic gradient in conductances in OHCs is a systematic decrease in the membrane time constant (τm) along the cochlea so that the frequency of the “membrane filter” (1/2π τm) approximately matches the CF of the location, thus minimizing low-pass filtering of the receptor potential (116, 179). Thus it is expected that the receptor potential will faithfully reflect the MT current up to high frequencies, ensuring voltage-dependent gating of prestin on every cycle of the stimulus. Such cycle-by-cycle activation of prestin is a necessary requirement of this process to amplify the vibrations of the cochlear partition. Thus a 200-Hz acoustic stimulus is expected to elicit a 200-Hz modulation in membrane potential around the resting potential, as should a 20-kHz stimulus. In the mammalian cochlea, such behavior is confined to OHCs, whereas in IHCs there is no evidence for a tonotopic gradient in either the MT current or the membrane time constant, and the cell resting potential is more hyperpolarized at −55 mV (116, 137). The IHC membrane time constant is ∼0.25 ms, and the corner frequency of the equivalent low-pass filter is 600 Hz (116, 137, 186). Above this frequency, the sinusoidal component of an asymmetric receptor potential will be attenuated to produce a sustained depolarization known as the summating potential (186, 219). This transformation ensures that the signal (a steady depolarization) is transmitted by the synaptic machinery, which otherwise would filter out a high-frequency sinusoidal signal due to the time course of exocytosis and neurotransmitter release.
B. Hair Bundle Mechanics and Amplification
The MT channels are also implicated in amplification through their involvement in force generation by the stereociliary bundle. This property reflects reversibility in MT channel activation, an applied force opening the channels and channel gating generating a force output. The phenomena have emerged from study of hair bundle mechanics in both mammalian and nonmammalian hair cells. To characterize hair bundle mechanics, as with other mechanical systems, it is necessary to apply a force to the system and observe the resulting displacement. Calibrated force stimuli can be delivered to the tip of the hair bundle using a fine glass fiber, fabricated to be more flexible (i.e., have a smaller stiffness) than the bundle (49, 104, 105, 241). (The flexible fiber is equivalent to a spring, and its spring constant is determined by observing the bending produced by weights hung from the tip.) The deflection of the hair bundle is derived by projecting an image of the bundle onto a pair of photodiodes and monitoring the change in photocurrent as the shadow of the bundle, or the attached fiber, traverses the photodiodes (49). Such measurements have shown that the hair bundle does not behave like a simple spring: its mechanical stiffness is compound, with both a passive linear component and an active, nonlinear component (106, 204, 220, 256). The observation of a nonlinear component when first reported was surprising as it depended on the gating of the MT channels (106).
The passive stiffness arises from the parallel combination of the stiffness of the stereociliary pivots, the interciliary links (especially the tip links), and the MT channel gating springs (192). The pivot accrues from bending of the the densely packed bundle of actin filaments, cross-linked by TRIOBP, at the stereociliary rootlets (134). The presence of the cross-linking protein TRIOBP is essential for proper hair-bundle function, and when this protein is mutated, the stereocilia lack rootlets and become much easier to deflect and damage, leading to profound deafness (134). The passive stiffness also includes a contribution from the gating springs connected to active MT channels, and it therefore has a larger value in hair cells having more functional channels. Measurements on hair cells with large MT currents have yielded passive stiffness values of 1–5 mN/m in frog saccule (106), turtle auditory papilla (49, 204) and apical OHCs of mouse (78) and rat (21), all of which have bundles of similar height (4–6 μm). The passive stiffness increases in proportion to the number of stereocilia and inversely with the square of the bundle height (49, 104), so hair bundles of basal OHCs, composed of more and shorter stereocilia, will be substantially stiffer than those of apical OHCs (241). Destruction of the tip links, and hence loss of the gating springs, by treatment with low-calcium BAPTA, reduced the stiffness of frog hair bundle by ∼30% (113) and rat OHC bundles by 40% (21). That portion of the bundle stiffness contributed by the gating springs represents the fraction of work done in deflecting the bundle that is funnelled through the gating springs to open the channels. This 30–40% fraction implies that the stimulus energy is efficiently coupled to the MT channel.
The effect of channel gating is to generate a force in the same direction as the imposed displacement, the gate flipping open under tension from the tip link, which effectively reduces bundle stiffness. This component of bundle stiffness is therefore nonlinear and can be shown to exhibit a Gaussian dependence on PO, the open probability of the MT channels. The stiffness of the bundle decreases as the channels open, reaching a minimum at the point where PO is ∼0.5, and then increases again at larger open probabilities (106). The nonlinearity has been extensively documented in frog saccular hair cells, and it is abolished, producing an increase in bundle stiffness, by blocking the MT channels with dihydrostreptomycin (106). Although the nonlinearity is less prominent in mammalian cochlear hair cells, the stiffness of rat OHC bundles also increased significantly by blocking the MT channels with dihydrostreptomycin (21). The relative magnitudes of the passive and active components determine the extent of the nonlinearity. For some hair bundles, the stiffness is dominated by the active component to the extent that, over the central range of channel gating, the stiffness can become negative, a feature that has been linked to spontaneous oscillations of the bundle (167, 251). The more usual behavior is that the bundle stiffness remains positive over the entire operating range of transduction (106, 204, 220). Nevertheless, whenever there is a substantial nonlinear component, factors affecting the probability of opening of the MT channels, such as adaptation, will effectively alter the stiffness of the bundle, and may cause it to move (17, 203; Figure 9). This is often referred to as “active bundle motion,” by analogy with the active amplification process in the mammalian cochlea, because it is powered by energy supplied from the hair cell. Since adaptation is thought to be driven by influx of Ca2+ and its interaction with the MT channels, a source of energy for the active bundle motion may derive from the large gradient in Ca2+ concentration across the stereociliary membrane. Since the time constant of fast adaptation in auditory organs decreases inversely with CF, the time constant of active hair bundle motion will also vary tonotopically (Figure 9), and it is therefore conceivable that active mechanical feedback contributes to frequency selectivity.
FIGURE 9.
Active force generation in turtle auditory hair cells. A: one model of the MT channel fast adaptation. The channel is anchored to the tip link and to the cytoskeleton by springs, GS and GS', respectively. Bundle deflection causes force to be applied via the tip link. This extends the spring and opens the channel allowing influx of Ca2+ which binds to the channel and/or the cytoskeletal spring causing channel reclosure. Closing of the channel exerts force on the tip link which pulls the bundle back in the negative direction. B: a force step applied to a turtle auditory hair cell bundle with a flexible fiber elicits a positive bundle deflection (monitored by imaging the bundle on a photodiode pair) and an inward MT current. As the current adapts, the bundle moves back in the negative direction, with a parallel time course. C: the time constant of the hair bundle recoil decreases with hair cell location along the papilla towards the high frequency end. [Modified from Ricci et al. (203).]
Active hair bundle motion attributable to channel gating has been amply documented in isolated hair-cell epithelia in which the overlying gelatinous otolithic or tectorial membrane has been removed, but whether it is significant, or is employed to amplify and tune hair cell responses in an intact auditory end organ, is debatable. One immediate difference between the two types of hair-cell preparation is that the spontaneous bundle oscillations observed in isolated epithelia as a manifestation of the active process (167) are suppressed when a gelatinous membrane is left attached to the hair bundles (242). The best case for a functional role for active bundle movements has been made for the abneurally placed short hair cells of the avian cochlea, which by their position and meager afferent innervation resemble OHCs of the mammalian cochlea. It has been proposed that their function, similar to OHCs, is to augment the vibrations of the tectorial membrane, thereby modifying the input to the tall hair cells, the avian equivalent of the IHCs, which contact the majority of the afferent nerve fibers (160). Although tall avian hair cells possess electrical tuning (70, 246), this mechanism may not extend to the upper limit of bird hearing, 5–10 kHz (66, 269). Modeling the contribution of active bundle motion to frequency tuning (employing a scheme that involves Ca2+ binding to the MT channel) has suggested that, with the distribution of avian hair bundle morphologies, resonant frequencies from 0.05 to 5 kHz might be achievable (35, 244); the predicted range was partly dictated by altering the number of stereocilia, and hence number of MT channels per bundle (248). In the chicken, as in other vertebrates, the MT current increases along the tonotopic axis because of increases in both single-channel conductance and numbers of stereocilia (Figure 8), and the two factors together might in theory increase the speed of force generation at higher CFs (Figure 9). However, recent experimental measurements of active hair bundle movement in chick auditory hair cells have indicated that besides any processes driven by MT channel gating, there is an equal component attributable to voltage-dependent activation of prestin, as in the mammalian cochlea (22). At the very least, the two processes, hair bundle motility and prestin-driven somatic motility, might act synergistically, with the latter becoming dominant towards the higher frequencies.
It remains an open question as to whether force generated by MT channel gating and adaptation supplements the contribution of prestin to amplification and frequency analysis in mammalian cochlear hair cells (51, 108). There are indications that OHC bundle mechanics possess a nonlinearity linked to fast adaptation (21, 126, 220), and moreover manipulations that interfere with adaptation can alter the mechanics of the cochlear partition (29). In addition, OHC depolarization evokes hair bundle movements due both to prestin (115, 128) and to MT channel gating (128). Most of these studies have employed isolated hair-cell epithelia with unencumbered hair bundles. However, there is sparse evidence for a role of active hair bundle motility in vivo where the viscoelastic behavior is complicated by the OHC bundles being tightly attached to and loaded by the tectorial membrane, which is affixed to the sidewall of the cochlea (156) and contributes significant additional stiffness as well as mass. In contrast, both targeted deletion of prestin (152), and mutations that diminish its physiological activation (52), abolish OHC contractility and produce a 100- to 1,000-fold loss of auditory sensitivity in vivo. It is, however, difficult to isolate experimentally the contribution of the MT channels in vivo, since forward transduction is obligatory to generate the receptor potentials needed for activating prestin. This difficulty is illustrated by the recent attempt to eliminate active hair bundle motility in vivo by injecting Ca2+ into the endolymph. The ensuing loss of sensitivity and nonlinear compression in the basilar membrane vibrations were interpreted as due to Ca2+-dependent effects on MT channel adaptation (180). But these effects could have equally well resulted from diminished activation of prestin secondary to changes in OHC resting and receptor potentials. Thus elevating endolymphatic Ca2+ will both block the MT channels and reduce their resting open probability, causing a decrease in the receptor potential amplitude and a hyperpolarization of the OHC, respectively. At this stage, more experiments are needed to establish unequivocally whether active hair bundle motility has a significant role in the mammalian cochlea.
V. THE MECHANOTRANSDUCER CHANNEL PROPERTIES
A. MT Channel Pore Properties: Ion Selectivity and Block
In the absence of a molecular composition, measurements of ion conduction through the MT channel pore can provide some insight into the channel structure. The native MT channel is a nonselective cation channel with high permeability to Ca2+. Various groups have made partial measurements (40, 46), but the most complete study was performed on chicken vestibular hair cells (183) for which the ion selectivity was inferred from reversal potential measurements. The permeability sequence for monovalent cations is Li+ > Na+ > K+ > Rb+ > Cs+. The permeability varies inversely with the ionic radius and is equivalent to the Eisenman sequence XI (98), indicating the obstacle to permeation is not ion dehydration but strong interaction with negative charges in the selectivity filter of the pore. The MT channel in chicken vestibular hair cells is the only one for which a comprehensive permeability sequence has been obtained, but a sequence in turtle auditory hair cells derived from the relative amplitudes of MT currents in different external solutions is Cs+ > K+ > Na+ > Li+, the reverse order to the permeability sequence inferred from reversal potentials (64). The opposite order for the two sequences is inconsistent with a simple channel model occupied by a single ion (98), and requires binding and interaction of multiple ions within the pore. A corroborate finding is the anomalous mole fraction for frog hair cell channels exposed to mixtures of Ca2+ and Na+, which displays a nonmonotonic change in current with systematic increase in concentration of one of the components of the mixture (157). Similar properties, in terms of selectivity sequences and anomalous mole fraction, were found for anion permeation through glycine and GABA receptor channels and were modeled with two ion binding sites (24). Because MT channels in different end organs may have distinct subunit compositions and properties, it would be desirable to measure all three properties (permeability and conductance ratios and anomalous mole fraction) on a single cell type such as the mammalian OHC.
The MT channel of chicken vestibular hair cells also conducts divalent cations with a permeability sequence of Ca2+ > Sr2+ > Ba2+ > Mn2+ > Mg2+ (183), all being more permeable than K+ or Cs+, e.g., PCa/PCs = 4.7. Unlike monovalent cations, the sequence does not follow ionic size, and the impermeability of Mg is atypical. Since the influx of Ca2+ is important for MT channel adaptation, its permeability has been well studied in several cell types under various conditions (18, 40, 130, 157, 207), and in all cases, a high permeability for the divalent was found. In the mammalian cochlea, the permeability differs between OHCs and IHCs with PCa/PCs ∼4.5 and 6, respectively, implying differences in the pore region between these two cell types (18, 130). For the OHCs there are also changes in PCa/PCs with development (130). In extracellular solutions containing 1.5 mM Ca2+, the divalent ion carries ∼12–15% of the MT current (18). The high permeability to Ca2+ must be associated with stronger binding of the divalent ion in the pore, and as a consequence, Ca2+ lingers around in the pore and can block the current carried by monovalent ions, the half-blocking concentration (KI) being 1–2 mM (129, 208). The importance of this KI value is that in vivo, when the hair bundles are immersed in endolymph containing 0.02 mM Ca2+, channel block is fully relieved, thus maximizing the monovalent current carried by K+ while allowing influx of sufficient Ca2+ to mediate adaptation. Under such conditions, the channel's current-voltage relationship displays strong inward rectification, even with identical monovalent ions on each side (188), indicative of an asymmetric pore configuration. Such asymmetry is also evident in the permeation of large organic cations and blocking agents (64, 165, 257). In acting as a permeable blocker for the channel, Ca2+ resembles other larger positively charged antagonists such as dihydrostreptomycin and FM1–43.
The MT channel also exhibits substantial permeability to organic cations, even large ones such as choline, tetramethylammonium (TMA). and tetraethylammonium (TEA) ions which have permeabilities relative to Cs+ (PX/PCs) of 0.33, 0.20, and 0.17, respectively (183). Such high permeability to organic ions is unusual among ion channels (98) and suggests a large-diameter pore. More systematic measurements using a range of organic cations with different dimensions have estimated that the diameter of the narrowest part of the pore when it is accessed from the extracellular face is greater than 1.2 nm (64). In contrast, when organic cations were introduced into the intracellular solution to be used for carrying outward current at positive membrane potentials, the apparent diameter of the pore was only 0.6 nm (188). This again suggests an asymmetric pore, having an enlarged extracellular vestibule to funnel large cations into the pore, and a smaller intracellular face for which elongated organic cations must adopt an “end on” configuration to gain access to the pore. A similar model has emerged from quantifying and modeling the voltage dependence of blocking agents (165, 257).
Current flow through the MT channel is inhibited by inorganic polyvalent cations such as Gd3+, La3+, and Ca2+, all of which block other mechanosensitive channels, as well as an assortment of organic polycations (64; Table 1). The most efficacious nonpeptidergic blockers are FM1–43 and curare, with half-blocking concentrations (KI) of ∼2 μM when applied extracellularly. The highest affinity is achieved with three polycationic peptides all of which block at submicromolar concentrations. For example, HIV-TAT is a 12-residue peptide, of which 8 are basic arginines or lysines, and has a half-blocking concentration of ∼27 nM (56). Another class of basic peptide is GsMTx-4, which is a 34-residue peptide toxin from the tarantula spider Grammostola spatulata and has a half-blocking concentration of ∼0.6 μM (23). The interesting feaure of this peptide is that it is known also to block mechanosensitive (stretch-activated) channels in various tissues (243). A prominent feature of most blocking compounds is the voltage dependence of their action and, although the exact voltage sensitivity differs between the compounds, all are most effective at hyperpolarized membrane potentials where they may be expected to be swept into the pore from the outside. The voltage dependence occurs with most of the smaller molecules and even extends to the peptides.
Table 1.
Some blocking agents of the hair cell's mechanotransducer channel
| Blocking Agent | KI, μM | nH | References |
|---|---|---|---|
| Ca2+ | 1,000 | 1 | Ricci and Fettiplace (207); Kim et al. (129) |
| Gd3+ | 10 | 1.1 | Kimitsuki et al. (131) |
| La3+ | 4 | 0.7 | Farris et al. (64) |
| Diltiazem | 228 | 1.8 | Farris et al. (64) |
| Amiloride | 50 | Jørgensen and Ohmori (119) | |
| Amiloride | 53 | 1.7 | Rüsch et al. (218) |
| Amiloride | 24 | 2.2 | Ricci (202) |
| Benzamil | 6 | 1.6 | Rüsch et al. (218) |
| Dihydrostreptomycin | 8 (0.25 mM Ca2+) | 1 | Kroese et al. (136) (frog) |
| Dihydrostreptomycin | 44 (5 mM Ca2+) | Kroese et al. (136) (frog) | |
| Dihydrostreptomycin | 23 (2.5 mM Ca2+) | 0.9 | Kimitsuki and Ohmori (132) (chicken) |
| Dihydrostreptomycin | 14, 75‡ (2.8 mM Ca2+) | Ricci (202) (turtle) | |
| Dihydrostreptomycin | 7* (1.3 mM Ca2+) | 0.96 | Marcotti et al. (165) (mouse) |
| Quinine | 10 | 1.2 | Farris et al. (64) |
| Ruthenium red | 3.6 | 1.4 | Farris et al. (64) |
| Curare | 2.3 | 1.0 | Glowatzki et al. (82) |
| Curare | 6.3–16 | 2.1 | Farris et al. (64) |
| FM1-43 | 2.4† | 1.2*† | Gale et al. (75); Meyers et al. (171) |
| GsMTx-4§ | 0.65 | 0.92 | Beurg et al. (23) |
| d-JNK1, d-HIV-TAT§ | 0.01–0.07, 0.03 | Desmonds et al. (56) |
KI is the half-blocking concentration, and nH is the Hill coefficient.
Value at −84 mV is given. For some blockers, such as dihydrosteptomycin, KI depends on membrane potential and block is relieved at positive potentials.
KI and nH given for −104 mV.
KI values in low-frequency and high-frequency cells quoted.
Small peptides, 12–34 residues.
The voltage dependence of block has been quantified experimentally for the aminoglycoside antibiotic dihydrosteptomycin (DHS) (136, 165, 183), the fluorescent dye FM1–43 (75), and the diuretic amiloride (218), and each has been modeled using rate theory to deduce the energy profile along the pore (257). For the divalent DHS, a good description of the block was achieved with a single negatively charged binding site ∼80% of the way across the energy profile with energy barriers at the extracellular and intracellular extremities of the pore. The intracellular energy barrier is larger, preventing access of the blocking molecules from the cytoplasmic face, so the half-blocking concentration for DHS is at least 100-fold larger from the inside than from the outside (165, 257). When presented at a high intracellular concentration, DHS does block, but only at positive membrane potentials, indicating that the binding site can be accessed from the internal face of the pore, and supporting the notion that DHS is a permeant blocker. Channel block by DHS also depends on the Ca2+ concentration (Table 1), the affinity being higher in reduced external Ca2+ (Table 1) suggesting that DHS and Ca2+ compete for the same binding site. FM1–43, like DHS, is divalent but blocks with a Hill coefficient of ∼2 at intermediate membrane potentials, indicating two drug molecules are needed to block the channel (75), and suggesting that FM1–43 competes with Ca2+ for a minimum of two binding sites about halfway through the pore of the MT channel. However, it should be noted that the Hill coefficient for FM1–43 binding is voltage dependent and at hyperpolarized potentials of −104 mV, nH = 1.2 (75). Since FM1–43 is an elongated molecule with a maximum end-on diameter of ∼0.8 nm and a length of ∼2.2 nm, this sets a minimum diameter of ∼0.8 nm for the pore; moreover, the vestibule facing the extracellular side of the channel must be sufficiently large to accommodate the heads of at least two FM1–43 molecules (257). Block of MT channels by the diuretic amiloride and its derivatives, which have a Hill coefficient of ∼2 (218), could also be modeled with a negatively charged binding site within the pore at 0.55 and 0.45 of the way across the energy profile for amiloride and benzamil, respectively (257). It is not clear why the location of the binding site is different for the amiloride diuretics and for DHS, although the value refers to the distance across an energy profile and not a physical distance.
Similar to Ca2+, both FM1–43 and DHS are permeant blockers that can traverse the pore and accumulate within the hair cell, a property with significant implications. Since FM1–43 is fluorescent when bound to membranes, the lighting up of hair cells on exposure to external FM1–43 provides a good test of functional MT channels (75, 171); it is therefore a useful assay for transduction defects in mutants without recourse to single-cell recordings (e.g., Ref. 123). This assay assumes that in a hair cell under normal conditions, some of the MT channels are open at rest and also that there are no other routes by which FM1–43 enters the cytoplasm, ignoring the fact that it can accumulate via clathrin-coated pit endocytosis. In particular, in using FM1–43 as an assay for mechanotransduction, it is important to perform the appropriate controls to eliminate other access routes to the cytoplasm, such as endocytosis, which has been described for FM1–43 in cochlear hair cells (86). Intracellular accumulation of aminoglycoside antibiotics is important because it culminates in hair cell death and apoptosis (213) possibly by targeting the mitochondria. There has been considerable debate over whether DHS can enter the hair cells via other pathways, but the current consensus is that the predominant access route is via the MT channels (4, 264).
Several lines of evidence, including assays of pore size with organic cations, the asymmetry of inhibition by certain blocking compounds, and the inwardly rectifying current-voltage relationship, point to the MT channel being asymmetric between its external and cytoplasmic faces. The pore has been hypothesized as possessing a wide external vestibule, narrowing down to a conduction path that embodies the selectivity filter (Figure 10; Refs. 18, 188, 257). A specific model for the pore represents it energetically with one or two negative-binding sites, and a large intracellular energy barrier. It is instructive to compare models of the MT channel with the cyclic nucleotide-gated (CNG) channel, which has similar physiological properties, but also a known molecular structure (122), so it is possible to relate binding sites in the two types of channel. The CNG channel, underlying transduction in the photoreceptors and olfactory receptors, is a nonselective cation channel with a high permeability to Ca2+ (PCa/PNa = 6.5 for rods). Ca2+ is also a permeant blocker (KI = 0.3 mM in rods), whose antagonistic behavior is abolished by mutating a single glutamate in the pore region (E363 in rods; Refs. 122, 215). Recent structures of CNG-like channels (55) crystallized in K+ or in Ca2+ indicate multiple negative binding sites in the pore. Ca2+ binding in NaK2CNG-D is more complex, and the difference map reveals four major peaks along the ion conduction pathway: three at sites inside the filter and one within the funnel or vestibule just above the external entrance. If these features of the CNG channel resemble the MT channel, the arrangement is more complicated than the single site postulated in models of the MT channel pore based on the properties of blockers (257). It then becomes unclear which negative sites (in the vestibule or selectivity filter; see Figure 10) might be crucial for binding Ca2+ and/or cationic blockers and whether these sites are the same for the different antagonists. Significantly, many blockers such as curare and DHS are polyvalent and may interact with negative charges in both the vestibule and the pore (23).
FIGURE 10.
Hypothetical structure of the MT channel illustrating the ion conduction pathway. A: cross-section of a channel showing a large vestibule on the external aspect of the channel and a tight selectivity filter lined with negatively charged residues at the cytoplasmic end of the channel. It is proposed that in the low-conductance isoform, there are critical neutral residues in the vestibule. B: in the high-conductance isoform of the channel, these neutral residues are replaced with negatively charged residues that cause accumulation of ions in the vestibule, hence increasing channel conductance (18). C: one scheme for generating a range of single-channel conductances by mixing the low-conductance and high-conductance isoforms in A and B in a tetrameric channel, assuming properties are a linear function of subunit composition. D: in a second model, the MT channel comprises a pore-forming modulated by an accessory subunit with negatively charged residues to generate a high-conductance isoform as in B. E: a scheme using the second model for producing a range of unitary conductances, in which different numbers of accessory subunits, such as LHFPL5 or TMC1, associate with the channel to systematically vary its properties (see sect. VIB).
B. Location, Size, and Number of MT Channels
A milestone in understanding hair cell mechanotransduction was the surprising proposal that the MT channel is localized to the tops of the stereocilia (107) and might be activated by tension in the tip links (193). As argued by those authors, the latter hypothesis could account for the directional selectivity for bundle deflections towards its tallest edge, which would elevate tension in the tip links. The hypothesis was pursued by specific experiments showing that 1) hair bundle exposure to submicromolar extracellular Ca2+ severed the tip links and abolished MT currents (12, 46, 73); 2) the channel blocker DHS was more immediately effective when iontophoresed near the top rather than the bottom of the bundle (112); and 3) the first signs of Ca2+ influx following bundle stimulation occurred principally at the tops of the stereocilia (54, 158). Defining the site of Ca2+ influx through the Ca2+-permeable MT channels was based on filling frog saccular hair cells with a fluorescent Ca2+ indicator and monitoring the initial bloom of fluorescence with confocal or two-photon microscopy. This approach was subsequently applied to rat cochlear hair cells containing three stereociliary rows (19) and demonstrated that the initial Ca2+ response was confined to the two shorter rows, implying that the MT channels are present only at the lower ends of the tip links. This conclusion differed from the earlier supposition, where it was posited that channels occurred at both ends of the tip link (54). The more recent experiments (19) employed low-affinity Ca2+ indicators (Fluo4-FF) that would not be saturated by the Ca2+ influx, and monitored the fluorescence with a fast (1 kHz) swept-field confocal that could follow Ca2+ diffusion along the stereocilia. These two features helped distinguish the Ca2+ signals at the tops and bottoms of the stereocilia. The most significant factor though was the larger 0.5-μm diameter and more widely spaced stereocilia of IHCs compared with those in the frog bundle, making the signals easier to resolve at the tips of the IHC stereocilia. The asymmetry in channel location accords with the asymmetrical structure of the tip link, now known to be composed of twisted pairs of cadherin-23 molecules at the upper end and protocadherin-15 molecules at the lower end (124), and would raise the possibility that each protocadherin-15 monomer interacts with one of the MT channels.
The extended extracellular domains of the two adhesion molecules interact at their NH2 termini to form 150-nm-long filaments (73), the bond between them being stabilized by Ca2+ ions (124, 232). The integrity of the tip links persists in endolymphatic Ca2+, but if the Ca2+ concentration is reduced below 1 μM, buffered with EGTA or BAPTA, the tip links are disrupted along with the transient ankle links (12, 73, 84). Prolonged exposure to BAPTA also totally abolishes mechanotransduction, but after brief (20 s) treatment, single-MT channel currents are visible in whole cell recordings (18, 46, 129, 205; Figure 11); this provides a method to quantify the channels without the impossibly difficult task of cell-attached patch recordings from individual submicron stereocilia. Other approaches have found channels in untreated cells in which there was virtually no MT current (78, 183), or have drastically reduced the MT current by treatment with the peptide channel blocker GsMTx-4 (23). One method derived the amplitude from analysis of current fluctuations (101). All are in agreement that the MT channel has a large amplitude of 100 pS in the absence of block by extracellular Ca2+ (cf., voltage-dependent K+ channels or acetylcholine receptor channels are typically 30 pS or less), although unitary currents in vestibular hair cells are generally smaller than those in auditory hair cells (Table 2).
FIGURE 11.
Experimental records of single MT channels. A: method of isolating single-channel events by recording in whole cell configuration in which the majority of the tip links have been destroyed by exposure to BAPTA. B: single-channel events in an apical rat IHC during imposition of a displacement step to the hair bundle, the timing of which is given above. Four representative traces are shown illustrating evoked transitions from closed (C) to open (O) state. The ensemble average of 12 stimuli is shown below displaying fast adaptation. The amplitude histogram of the events indicates a single-channel current of 16 pA corresponding to a conductance of 190 pS. C: single-channel events in an OHC from the middle turn of a rat cochlea, the timing of the displacement step to the hair bundle being given above. The ensemble average also displays adaptation, and the amplitude histogram of the events indicates a single-channel current of 11 pA, equivalent to a conductance of 131 pS. In B and C, the holding potential was −84 mV, and measurements were made at room temperature. [B and C from Beurg et al. (18).]
Table 2.
Single MT channel conductance
| Hair Cell Preparation | Conductance, pS | Condition | References |
|---|---|---|---|
| Chicken vestibule | 50* | 30°C | Ohmori (183) |
| Frog saccule | 23† | Corrected to 25°C | Holton and Hudspeth (101) |
| Turtle auditory | 110 | 2.8 mM Ca2+ | Crawford et al. (46) |
| Turtle auditory | 80–160 | 2.8 mM Ca2+ | Ricci et al. (205) |
| Turtle auditory | 150–300 | 0.05 mM Ca2+ | Ricci et al. (205) |
| Rat OHC | 100–144 | Apex, middle | Beurg et al. (18) |
| Rat IHC | 180 | Apex, middle | Beurg et al. (18) |
| Mouse OHC | 112 | Cochlear apex | Géléoc et al. (78) |
| Mouse OHC | 80 | Middle | Xiong et al. (271) |
| Mouse OHC | 63–110 | Apex-base | Kim et al. (129) |
| Mouse IHC | 90–320 | 0.05 mM Ca2+ | Pan et al. (187) |
| Mouse IHC | 67 | 1.5 mM Ca2+ | Beurg et al. (23); Caberlotto (28) |
All measurements were made at a negative holding potential (approximately −80 mV) in millimolar external Ca2+ unless otherwise indicated, and at room temperature, except 30°C (*).
Conductance derived from noise analysis at 10°C and extrapolated to 25°C using a Q10° = 1.45.
Several conclusions can be drawn from the single-channel recordings: 1) the single events can be driven with hair bundle displacements of the same size and polarity as macrosocopic currents and can be blocked with DHS; 2) the single-channel amplitude is increased about twofold when extracellular Ca2+ concentration is switched from 1.5 mM to an endolymphatic Ca2+; and 3) the single-channel open probability determined from ensemble averages activates rapidly and displays adaptation. These three observations argue that the channels underlie the macroscopic MT currents (46, 129, 205; Figure 11D). A concern about the magnitude of the channel conductance is that if two channels are connected to each tip link, then severing the tip links with BAPTA should leave an even number of channels. In the end just two channels should remain, and hence, the conductance values may be twice that of a single-channel event. For prolonged recordings, it is occasionally possible to see multiple conductance levels, or with a fast recording speed to see flickering between these levels. But the strongest evidence derives from the observation that using two different methods to reduce the channel number, by BAPTA or by peptide block, yield similar conductance values (23).
For channels isolated in this way, an unexpected finding was that the unitary conductance varies along the auditory epithelium, being smallest at the low frequency end. This conclusion is true of all hair cells studied in turtle papilla (205), but is restricted to OHCs in the mammalian cochlea and no such tonotopic gradient is evident in IHCs (18, 23). Increases in unitary conductance and in number of stereocilia per bundle means that there will be a severalfold gradient in the maximum MT current from low to high frequencies (see also Figure 8 for the chicken). Two questions arise. First, what is the function of the gradient? Since there will be greater Ca2+ influx in larger conductance channels, this may account for the decrease in the fast adaptation time constant, seen in both the macroscopic current and at the single-channel level (205). A second question concerns the underlying mechanism of the variation. The simplest explanation is that MT channels in OHCs at the two ends of the cochlea are composed of different protein isoforms (Figure 10, A and B) or have accessory subunits (Figure 10, D and E; see sect. VIB) that modify the channel conductance. One specific hypothesis is that negative charges in the vestibule of one isoform (Figure 10B) concentrate the cations and increase the unitary conductance (18, 23).
With knowledge of the single-channel conductance and the amplitude of the macroscopic MT current at a given location, a lower bound on the number of channels per tip link can be derived. The maximum number of tip links was obtained from stereociliary counts in scanning electron micrographs (18), and the number of channels per tip link was calculated for turtle auditory hair cells (1.7, 205) and for rat cochlear OHCs (1.8, 18) and IHCs (2.4, 19). Within measurement error, these values suggest there are two channels per tip link, a surprisingly small number, but comprehensible if each channel makes direct connection to a component of the extracellular tip link: each of the two channels could connect to an intracellular domain of protocadherin-15 of which there are two at the lower end of the tip link.
VI. THE MOLECULES OF THE MECHANOTRANSDUCTION MACHINERY
A. Molecular Structure of the Transduction Apparatus: The Tip-Link Complex
The main experimental approach that has driven the search for the molecular underpinning of hair cell mechanotransduction over the last 15 years has been the exploration of inherited forms of deafness (210), and to date, close to 100 mutations have been identified (http://hereditaryhearingloss.org). The majority is linked solely to inner ear defects and is known as nonsyndromic, denoted either as DFNBn (autosomal recessive) or DFNAn (autosomal dominant). The largest syndromic group comprises those of Usher syndrome (210, 230), types I, II, and III, named for Charles Usher, who was one of the first to examine transmission of this recessive deafness and blindness disorder. Type I is the most severe and is linked to profound deafness at birth attributable to hair cell degeneration combined with progressive photoreceptor degeneration that begins during the first decade of life (230). Remarkably, five of the proteins mutated in Usher type I form part of the upper tip-link density (myosin VIIa, harmonin, and sans; Ref. 85) or the tip link itself (cadherin-23 and protocadherin-15; Ref. 124). This set of proteins also occurs in the calyceal microvilli linking the photoreceptor inner and outer segments in primates but not in mice (221), which may structurally reinforce the cilial link between inner and outer segments.
In mammalian cochlear hair cells, cadherin-23, the upper component of the tip link, is thought to be attached to the stereociliary actin cytoskeleton through its intracellular COOH terminus associating with harmonin-b, a PDZ-domain protein, and with sans and myosin VIIa (Figure 12; Ref. 85). Quantitative immunofluorescence was used to demonstrate that the ternary complex clusters at the upper tip-link density with an approximate stoichiometry of three harmonin-b molecules to one each of the two others. The simplest hypothesis is that myosin VIIa is the motor that maintains tension in the tip link (14, 85). There is no evidence in mammalian OHCs that this function is usurped by myosin Ic, as has been proposed for vestibular hair cells (100, 234). In support of its localization at the UTLD, mutations in harmonin result in a loss in sensitivity and slowing of adaptation in the MT currents (87, 173), although the effects are more subtle than might be expected.
FIGURE 12.

Schematic of the tip link and its attachments at the top of a shorter stereocilium and at the side wall of its taller neighbor. The prevailing view of the molecular composition of the transduction complex (see text) is illustrated, including the tip link (124), the upper tip-link densities (tripartite complex of myosin-7a, harmonin-b, and sans; Ref. 85) and the lower tip-link densities (myosin XVa, whirlin, and Eps8; Refs. 53, 16, 161) and CIB2 (Ca2+ and integrin binding protein isoform 2; Ref. 201). Many of the proteins are mutated in Usher syndrome. In addition, the MT channel is localized to the lower end of the tip link (19), with LHFPL5 (also known as TMHS, Ref. 271) as a possible coupling protein.
One of the more conspicuous achievements in recent hair-cell biology has been the structural identification of the tip-link complex. Cadherins are normally regarded as mediating cell-cell interactions, but in the tip link they join one part of the cell with another by means of large extracellular domains. A combination of evidence, including the absence of tip links in cadherin-23 (USH1D) mutants, immunolocalization of the protein to near the tops of the stereocilia, and the consistency of the size of the extracellular portion of the protein with the ∼150-μm tip-link length led to the conclusion that cadherin-23 was a component of the tip link (229, 231). Similar arguments were made for protocadherin-15 (USH1F; Ref. 2), culminating in the proposal that the tip link is a heterophilic complex of homodimers of cadherin-23 at its upper end and homodimers of protocadherin-15 at its lower end (124) joined at their NH2 termini. The extracellular region of each cadherin is assembled from multiple ectodomains (EC): 27 EC repeats for cadherin-23 and 11 EC repeats for the shorter protocadherin-15. In addition to binding to the EC subregions of each cadherin, Ca2+ is required for formation of the heterophilic complex; consequently, removal of extracellular Ca2+ is expected to sever the tip links as observed experimentally. Evidence for a functional interaction between cadherin-23 and protocadherin-15 was provided by showing that regeneration of tip links severed by low Ca2+ could be blocked by extracellular fragments of either protein (150). This scheme places the two strands of protocadherin-15 at the lower end of the tip link where they might convey force (directly or indirectly) to a pair of MT channels located at the tops of the stereocilia (19).
Thus protocadherin-15 becomes a central player in the transduction apparatus and might interact with the MT channel. It occurs in three isoform classes, differing in their cytoplasmic domains CD1, CD2, and CD3 (2, 267). Absence of protocadherin-15-CD1 or -CD3 had no effect on hair-bundle morphology or hearing, but mice lacking protocadherin CD2 were deaf (267). The most prominent abnormality was loss of links between the kinocilium and the tallest stereocilia and an associated abnormal polarization of the bundles, suggesting that the protocadherin-15-CD2 isoform is localized to the kinocilium and influences bundle orientation orchestrated by the planar cell polarity proteins. The kinociliary links, like the tip links, are oriented along the bundle's axis of symmetry and are also composed of cadherin-23 and protocadherin-15, but their polarization is opposite to that of the normal tip links, with protocadherin-15 being at the kinociliary extremity of the link and cadherin-23 facing the stereocilium (83, 267). Under some cirumstances, functional interciliary links can also be made from homophilic complexes of PCDH15. After tip-link destruction with BAPTA, the links regenerate at the tops of the stereocilia along with recovery of mechanotransduction over a period of 24 h (111, 275). During the initial phase of regeneration, shorter tip links comprising only PCDH15 appear first but are still able to mediate MT currents of normal amplitude (111). Only later do the mature hetereophilic CDH23/PCDH15 links materialize.
Usher syndrome has so far shed little light on the identity of the MT channel or of the composition of the lower tip-link density. The genes responsible for two Usher type I subtypes are unknown, and the gene for USH1J was recently identified as a Ca2+- and integrin-binding protein (CIB2) present in the stereocilia (201). A type II gene, USH2D, encodes whirlin, a PDZ protein present at the tops of the stereocilia that interacts with the myosin XVa (16, 53), also localized to the stereociliary tips, along with the actin-regulatory protein Eps8 (161). The three proteins, myosin XVa, whirlin, and Eps8, are thus proposed as components of a complex at the stereociliary tips and may be a major contributor to the lower tip-link density. Other proteins at the tips of the stereocilia that may enhance the lower tip-link density include Eps8L2 (74), twinfilin-2 (191), myosin IIIa (224), and espin 1, a long isoform of espin containing an NH2-terminal ankyrin repeat domain (222). The protein complex may have two important and interrelated roles: anchoring the MT channel to the actin cytoskeleton and regulating actin polymerization and elongation of the stereocilia actin core, thus being a major factor determining the stereociliary height and the staircase. For example, Eps8 and Eps8L2 collaborate to maintain the hair bundle staircase structure, which is lost in their absence (74); myosin IIIa transports espin 1 to the tips of the stereocilia and causes their elongation (222). In contrast, twinfilin-2 has an opposite action to reduce stereociliary length (191). The actin bundling protein fascin-2 is also concentrated towards the tips of the bundle's longest stereocilia; its expression increases during stereociliary elongation, and its mutation affects bundle height (226). Clearly, a large and disparate set of proteins is involved in regulating stereociliary length and staircase.
The lower tip-link density bears comparison with the postsynaptic density on dendritic spines (125), both of which may harbor multimolecular complexes associated with signal transduction. Synaptic transmission at the tip of the spine relies on AMPA receptors, ligand-gated ion channels opened by the binding of glutamate neurotransmitter. Mechanical transduction is mediated by MT channels at the tips of the stereocilia, which are of similar size and shape to the dendritic spines. In the two structures, Ca2+ signaling and homeostasis play a crucial role in functional maintenance, although stereocilia possess an actin-based internal core different from that of the spine. Nevertheless, in both cases, the transduction complex may have equivalent accessory proteins to regulate the insertion, localization, and properties of the ion channel. Examples are the transmembrane AMPA-receptor regulatory proteins (TARP) family of proteins in the dendritic spine which influence the expression, kinetics, and conductance of the AMPA receptors (239). A tetraspan protein similar to the TARPs is TMHS (tetraspan membrane protein of hair cell stereocilia, elsewhere referred to as LHFPL5), a four transmembrane-domain protein that exists in the stereocilia of IHCs and OHCs, and its gene-mutation underlies deafness in mice (155) and humans (225). LHFPL5 has been proposed to link PCDH15 to the channel complex, and its knockout or mutation in the hurry-scurry (hscy) mouse affects integrity of the tip link and also, significantly, reduces the MT channel unitary conductance (271).
B. Molecular Structure of the Transduction Apparatus: The Ion Channel
A number of channel-protein candidates have been advanced as being components of the MT-channel pore, but none has so far survived careful scrutiny (262). Several criteria must be satisfied to offer definitive evidence in support of a given candidate: 1) it must be present in the hair bundle as demonstrated by immunolabeling and/or exogenous expression of a GFP-labeled transcript; 2) mutation or knockout of the candidate gene should be associated with the absence of transduction and behavioral deafness, and the defect should be rescuable by exogenous introduction of the normal transcript; 3) expression of the protein in a heterologous cell system, such as HEK293 cells, should bestow a new ion channel with the appropriate pore properties (ion selectivity, blockers and unitary conductance), preferably gated by mechanical stimulation. Unfortunately, there are confounding circumstances for each of these criteria, including the nonspecificity of antibodies, the presence of multiple isoforms that can substitute for each other (e.g., TRPC channels, TMCs), and the requirement for other accessory proteins to secure heterologous expression. Problems with immunolabeling might arise because there are likely to be only a few channel proteins in the stereocilia and also the hair bundle glycocalyx is “sticky” causing nonspecific binding. Therefore, antibody specificity must be verified by its absence in the appropriate knockout. A good example is the antibody to the NH2 terminus of the nonselective cation channel HCN1, which labeled hair bundles suggesting it could be a stereociliary channel; however, this antibody also decorated hair bundles from HCN1 null mice, demonstrating the labeling was an artifact (102). The channel protein might have been missed because it is essential elsewhere in the body and its gene-knockout is embryonically lethal.
TRP channels, named for the transient receptor potential phenotype in Drosophila photoreceptors, are six transmembrane-domain proteins creating tetrameric channels redolent of voltage-gated K+ channels. Several members of the TRP channel superfamily have been put forward as candidates for the MT channel, based on observations that this heterogeneous family includes members with unusual mechanisms of activation including mechanoreception (189). This is exemplified by TRPN (NOMPC) which, on account of the reduction in the receptor current in the nompC mutant, was proposed as a component of the mechanotransduction apparatus in Drosophila bristle organs (266). TRPN is crucial for gentle touch in Drosophila larvae, and expression of TRPN in a heterologous system has shown that it forms a mechanosensitive cation channel (272). However, nompC is absent from the mammalian genome. Other members of the vertebrate TRP superfamily have been considered including TRPA1, TRPML3, and one or more isoform of TRPC. Immunolabeling localized the TRPA1 protein to the hair bundle, especially the stereociliary tips, and such labeling was lost when transduction was disrupted by severing of the tip links; furthermore, knockdown of the transcript attenuated the MT current (39). When expressed heterologously, it produced an ion channel with properties comparable to the MT channel (177), but with no evidence for mechanosensitivity. However, it was later discovered that Trpa1 null mice exhibited no defects in audition or balance (144). Moreover, immunolabeling of the hair bundle with the TRPA1 antibody persisted in the knockout, indicating it was an artifact. Why knockdown of Trpa1 reduced the MT current is still unclear. TRPA1 is most highly expressed in dorsal root ganglion nociceptors and may mediate sensations such as cold and itch (144).
The evidence for TRPML3, encoded by the mucolipin-3 gene, is more complicated. It is expressed in a number of tissues including the inner ear, and its gene mutation in the varitint-waddler mouse shows deafness and balance defects along with other problems (13). In cochlear hair cells of mutants, the hair bundles become disorganized and the MT currents are reduced (but not lost). The normal protein is localized to the hair cells including at the base of the hair bundle, but the latter localization is lost in the varitint-waddler (255). Recording in hair cells and in heterologous systems have demonstrated that TRPML3 forms an inwardly rectifying cation channel permeable to Ca2+, and the point mutation in the varitint-waddler renders it constitutively active, thus loading the cells with Ca2+, a byproduct thought to lead to hair cell degeneration (88, 178, 255). More recent work has shown that the TRPML3 channel is also localized to the endosomes and lysosomes, and because channel opening is pH sensitive, it may regulate the acidification of early endosomes and be important in the endocytic pathway (13, 88). As a postscript to this story, targeted knockout of the TRPML3 gene surprisingly displayed no auditory or vestibular defects (120).
Unlike the other two families, there was prior evidence for a role of the canonical transient receptor potential family TRPC in mechanotransduction, especially TRPC6 in the muscle membrane (189). Moreover, the founder member of the TRP superfamily, the transduction channels in Drosophila photoreceptors (to which the vertebrate TRPC channels are most closely related), were recently suggested to be mechanosensitive (95). However, there is debate over whether TRPC6, or other TRPC channels, is directly gated by force rather than indirectly via a G protein-coupled pathway (189). Trpc3 and Trpc6 transcripts are present in cochlear hair cells (197), and double knockouts of Trpc3 and Trpc6 (but neither alone) produced a moderate high-frequency hearing loss and reduction of MT currents in basal OHCs (197). TRPC3 immunolabeling occurred on the hair cell membrane, and the channels could be activated by depletion of cytoplasmic Ca2+, but a G protein-coupled receptor pathway was implicated (198). It seems unlikely that these proteins form the MT channel, although they may be involved in hair-cell Ca2+ homeostasis, disruption of which almost certainly impairs mechanotransduction. Confinement of the effects of knockouts to basal high-frequency OHCs may reflect the higher metabolic vulnerability of basal OHCs and their susceptibility to Ca2+ loading (20). These three TRP-channel examples emphasize how in searching for the hair-cell MT-channel protein, incomplete evidence can be deceptive and lead to false conclusions. Indeed, for all three TRP channels, knocking out the genes failed to produce profound deafness or complete abolition of MT currents.
The strongest evidence currently available is for a member of the transmembrane channel-like (TMC) protein family (123). TMC isoform-1, the founding member of the family, is a ∼760-amino acid protein predicted to contain multiple (6 or more) transmembrane domains (145). In contrast to those TRP channels described above, knockout of Tmc1 causes profound deafness (142, 236) due to complete loss of MT currents in mice after the first postnatal week (130). The TMC family has eight genes (143, 176), of which at least two (Tmc1 and Tmc2) occur in the mammalian cochlea (123) and four (TMC1, TMC2, TMC3 and TMC6) in the chicken basilar papilla (176). The dn and Beethoven mutations of the Tmc1 gene in mice are associated with hearing loss, absence of cochlear microphonics, and OHC degeneration (142, 162, 236, 263). There are also equivalent human nonsyndromic deafnesses, an autosomal dominant deafness, DFNA36, that is progressive and a recessive profound deafness DFNB7/11 linked to TMC1 mutations (142); there are over 30 mutations of the TMC1 gene associated with human deafness making it one of the more common nonsyndromic deafnesses (97). However, it has been unclear whether deafness stems from lack of MT channel function or is secondary to a developmental defect (162). Nevertheless, double knockouts of Tmc1 and Tmc2 were reported to abolish MT currents in mouse auditory and vestibular epithelia, a defect that could be rescued by transfection with either Tmc1 or Tmc2 message (123). Thus TMC1 satisfies the second criterion. While these results are suggestive, there are inconsistencies about whether the MT current vanishes in Tmc1 mutants. Complications arise because of the developmental changes that occur during the first two neonatal weeks prior to the onset of hearing in mice (162). For example, over this period there are changes in cellular morphology and ion channel complement and also downregulation of Tmc2 in the cochlea (123).
Although mRNA for Tmc1 and Tmc2 is found in cochlear and vestibular hair cells (123), the presence of the protein in the stereocilia is uncertain. For example, antibody labeling indicated that in avian hair cells, TMC2 protein was principally associated with the lateral membranes (176). In contrast, transfection of mouse hair cells with Tmc2-GFP showed localization to the tops of the stereocilia (123). With neither labeling technique is there evidence on the subcellular localization of TMC1 in mammals. Thus they do not fully satisfy the first criterion. Attempts to express TMCs in heterologous systems have also been ambiguous. When TMC1 from C. elegans was expressed in several mammalian cell lines, it generated a cation conductance that was activated by high extracellular Na+, although the channel was not mechanically sensitive (31); however, this result has not been replicated for vertebrate isoforms, and there is again no evidence for mechanosensitivity, thus not fulfilling the third criterion. Circumstantial support for a role in the ion conduction pathway has emerged from the observation that, in Tmc1 or Tmc2 gene knockouts, the Ca2+ permeability and the unitary conductance of the MT channel were altered (129, 130, 187). Taken at face value, these results support a role for the TMC proteins in forming the pore, a conclusion strongly reinforced by the finding that a point mutation, M412K, in theTmc1 Beethoven mutant conferred a reduced single MT channel conductance and Ca2+ permeability (187).
However, the waters have been muddied by the discovery that mechanically sensitive currents can still be evoked by bundle displacements in mice with mutations in both Tmc1 and Tmc2 (23, 129). The current in the double mutant was blocked by MT channel blockers, such as DHS and amiloride, but it was anomalous in being activated by large bundle deflections away from its tallest edge, and therefore opposite in stimulus polarity to that in the wild type. It should be noted that these anomalous currents were recorded with similar properties in Tmc1dn/dn Tmc2−/− and in Tmc1Δ/Δ Tmc2−/−, which are derived from two different Tmc1 mutants (23), implying they are not products of still functional (but truncated) TMC protein. MT currents responding to the opposite stimulus polarity have also been recorded in certain Pcdh15 and Cdh23 mutants in which the tip links are lost (3). In line with this finding, the MT current in the Tmc1/Tmc2 double mutants was unaffected by treatment with BAPTA to break the tip links, implying that the channels were not activated in a conventional manner by increased tip-link tension (129). Where the channels underlying the anomalous current are located in the bundle, and why they respond to reversed polarity displacements of the bundle remain to be determined. Nevertheless, their existence argues that the mechanosensitive current in the Tmc1/Tmc2 double mutants may flow through the MT channels, but these channels are mislocalized and not attached to the tip links. A corollary is that the TMC proteins are not pore-forming subunits of the MT channel but more likely accessory and/or trafficking proteins. Lack of correct targeting to the stereociliary tips, so preventing interaction with other components of the transduction complex (e.g., LHFPL5; Ref. 271), may account for the altered pore properties.
Thus the attributes of the TMC proteins resemble those of auxiliary proteins for other ion channels, for example, the TARP proteins (tetraspan membrane proteins weakly homologous to LHFPL5) and the cornichon (CNIH) proteins. The TARP and CNIH proteins may act synergistically to influence expression and gating properties of AMPA receptors at glutamatergic synapses on dendritic spines (239). For example, CNIH-2 association increases both the Ca2+ permeability and single-channel conductance of GluA1 receptors (37). It is generally thought that besides controlling membrane expression of the GluA1 channel, both TARP and CNIH proteins bind to the channel to modulate its properties. By analogy, the simplest hypothesis for the hair cell proteins is that LHFPL5 and TMC1 (and possibly TMC2) bind to the MT channel and in their presence, the channel conductance is increased. The implication is that the tonotopic gradient in MT channel conductance is conferred by TMC1 and possibly other subunits and does not stem directly from different isoforms of the pore-forming subunit (see Figure 10, C and E). Thus, in both the Tmc1 mutant and the Tmc1/Tmc2 double mutant, the single-channel conductance is similar at the apex and base (Figure 13; Ref. 129). Definitive evidence on the nature of the interaction awaits clarification of the channel's molecular identity. However, the current conjecture is that both TMC1 and LHFPL5 behave like auxiliary subunits for the MT channel and are able to alter its permeation properties. A third membrane protein, that has been studied less but may have a parallel role, is TMIE, the mutation of which is associated with deafness in humans and mice (174), and loss of transduction in zebrafish (79).
FIGURE 13.
Single mechantransducer channels in mouse cochlear hair cells. A: examples of single-channel currents recorded in an apical OHC for step hair-bundle deflections, stimulus monitor shown above; closed (C) and open (O) states are indicated beside traces. B: amplitude histogram of events like those in A, giving single-channel current of −5.2 pA at −84 mV holding potetnial. C: single-channel currents in a basal OHC for step hair-bundle deflections. D: amplitude histogram of events like those in C, giving single-channel current of −8.5 pA at −84 mV holding potetnial. E: mean single-channel currents (left axis) and conductance (right axis) ± SD, plotted against cochlear location, expressed as the fraction of the distance along the basilar membrane from the low-frequency apical end. The current is larger at the base for wild-type OHC but not for Tmc1−/− OHC in which the tonotopic gradient is virtually abolished. Number of cells: wild-type OHC apex, 6; middle, 5; base, 6; OHC from deafness mutant, Tmc1dn/dn: apex 17, middle 3, base 7. Mouse age range was P2 to P6.
C. The Mechanism of MT Channel Activation and the Role of the Membrane Lipids
The first mechanotransducer channel to be identified was the bacterial MscL channel, and its mechanical sensitivity was revealed after reconstituting the purified channel protein into a lipid bilayer (141). Such bilayers consisted of defined lipids and contained no other proteins, the unavoidable conclusion being that the MscL channel was mechanically sensitive by directly detecting the tension in the bilayer. The MscL channel is a homopentamer of 136 residue subunits, each forming two transmembrane domains (30). On the basis of X-ray diffraction studies, stretch of the bilayer membrane is thought to pull the transmembrane domains outwards and tilt them, so the MscL barrel expands and adopts a flatter conformation (237); the central pore region has been proposed to open like an iris, thereby creating a nanometer-sized channel having an ionic conductance of 3 nS. On this type of mechanism, it is natural to suppose that channel activation might be sensitive to the lipid composition of the bilayer. Possible factors include lipids that alter the thickness and fluidity of the membrane, which could affect the transition rate constants and stability of the open conformation, as well as the surface charge and membrane curvature (195, 278). Given these influences, stability may be accomplished by specific lipids congregating around the ion channel to optimize its function. Prime examples of this compartmentalization are the lipid domains consisting of cholesterol and sphingolipids that assemble with proteins into platforms tens of nanometers wide (154). Such platforms might be anchored to the cytoskeleton and act as a mechanism for focusing force stimuli onto mechanosensitive channels embedded in the lipid raft (5). Since the lipid platform itself could be attached to the cytoskeleton, this arrangement does not explicitly require the MT channel also to be anchored to the cytoskeleton.
There is evidence for lipid specialization towards the top of the hair bundle. Phosphatidylinositol 4,5-bisphosphate (PIP2) is enriched in the top half of the stereocilia, and PIP2 depletion with phenylarsine oxide or quercetin has been shown to reduce the amplitude and adaptation of MT currents in frog vestibular hair cells (99). Another membrane lipid, cholesterol, detected by the fluorescence of the cholesterol-binding antibiotic filipin, may be localized at the very tips of the stereocilia (274), although an earlier freeze-fracture study did not find any filipin-induced deformations at the stereociliary tips (69). In the latter study, the membrane at the tip of the stereocilium exhibited a number of large particles of ∼11.0 nm in diameter that could conceivably correspond to the MT channels. So what is the proximate stimulus for MT channel activation? Is it force between the extracellular and intracellular channel connections to the tip link and the cytoskeleton, respectively, or does the hair cell MT channel, like MscL, detect changes in the bilayer tension (36, 141)? These extremes are depicted in Figure 3. A related question pertains to the physical manifestation of the gating spring and the origin of its stiffness; this gating spring has been hypothesized as a means of coupling bundle displacement with force delivery to the MT channel (see sect. IIIA). The most obvious manifestation of the gating spring is the tip link itself, but calculations based on its structure have suggested that it is too stiff to account for the ∼1 mN/m associated with channel activation (121, 232). A second site is in the channel attachments to the cytoskeleton or to the surrounding membrane. The tip link is probably attached directly or indirectly to the MT channel. The stoichiometry of two PCDH15 molecules and a pair of MT channels is circumstantial evidence for mechanical coupling; furthermore, LHFPL5 might be a coupling protein since it regulates expression of PCDH15, and likely interacts with the channel whose ion conduction properties it can influence (271). The problem has been further addressed by modeling the extent to which the mechanical properties of membrane lipids can explain channel activation and kinetics (194). Indirect evidence supporting the involvement of membrane stretch is that in transmission electron micrographs, the plasma membrane at the top of the stereocilia, where the tip link inserts, is pulled away from the LTLD to produce a tentlike appearance (Figure 3A). The modeling concluded that the properties of the bilayer are sufficient to explain channel activation in frogs, but because of the larger gating stiffness in mammals, a cytoskeletal tether may also be required. However, the modeling did not consider the possibility that the membrane stiffness might be augmented by the presence of cholesterol in an organized membrane platform. This will be resolved only when the full protein composition of the channel and the LTLD has been established.
VII. CONCLUSIONS
Over the period during which hair cells have been studied physiologically, substantial progress was initially achieved in defining the characteristics of hair-cell mechanotransduction, including its force sensitivity, kinetics, Ca2+-dependent adaptation, ionic selectivity, and single-channel conductance. An important insight stemmed from discovering a nonlinearity in the hair bundle mechanics, attributable to force generated during channel gating. Consequently, channel reclosure during Ca2+-dependent fast adaptation was proposed to generate a force to oppose the stimulus, and this was taken as evidence that the bundle can perform work. Since the speed of adaptation changes along the cochlea, force generation is high-pass filtered with a corner frequency that may be matched to the CF of the appropriate cochlear location. However, further progress in elucidating the mechanism has been partly hindered by the inability to isolate the channel protein, and it was not until the advent of molecular genetics that the complexity of the transduction complex became apparent. Knowledge of the composition of the tip link, a helical dimer of CDH23 and PCDH15, and the protein complex at the upper attachment point including myosin VIIa, gleaned largely from studies of Usher syndrome, provided a starting point. Localization of the MT channel to the lower end of the tip link facilitates an unbiased search for the channel itself and its protein partners which might interact with PCDH15. Recent results from mouse mutants have suggested that the tonotopic variation in single MT-channel conductance may be at least partly due to gradients in accessory subunits such as LHFPL5 and TMC1, but more work is needed to clarify the basis of channel diversity. There are several unresolved questions about the MT channel function including the location of the Ca2+-binding site for fast adaptation and the mechanism of channel activation, whether it senses force delivered through accessory proteins or is gated indirectly by deformation of the lipid bilayer. In the latter case, what part does the lipid environment play in gating? It will also be important to provide definite evidence one way or the other on whether hair bundle motilty makes any contribution to amplification and tuning of the mammalian cochlea. More thorny questions pertain to how the channels are targeted to the stereociliary tips and how incorporation into the transduction complex and attachment to the tip links is regulated. It is hoped that the channel will be identified in the near future, which should enable many of these questions to be addressed in a rational manner.
GRANTS
Work in the authors' lab was supported by National Institute on Deafness and Other Communication Disorders Grant RO1 DC01362 (to R. Fettiplace).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
We thank Maryline Beurg for helpful comments, Carole Hackney for providing electron micrographs, and the anonymous reviewers for their many helpful comments.
Address for reprint requests and other correspondence: R. Fettiplace, 185 Medical Sciences Bldg., 1300 University Ave., Madison, WI 53706 (e-mail: fettiplace@wisc.edu).
REFERENCES
- 1.Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H. Developmental expression of the outer hair cell motor prestin in the mouse. J Membr Biol 215: 49–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26: 7022–7034, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One 6: e19183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One 6: e22347, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anishkin A, Kung C. Stiffened lipid platforms at molecular force foci. Proc Natl Acad Sci USA 110: 4886–4892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys 39: 111–137, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Arnadóttir J, O'Hagan R, Chen Y, Goodman MB, Chalfie M. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J Neurosci 31: 12695–12704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol 385: 207–242, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashmore JF. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol 388: 323–347, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashmore J. Cochlear outer hair cell motility. Physiol Rev 88: 173–210, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci USA 86: 2918–2922, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7: 985–994, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Atiba-Davies M, Noben-Trauth K. TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta 1772: 1028–1031, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bahloul A, Michel V, Hardelin JP, Nouaille S, Hoos S, Houdusse A, England P, Petit C. Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet 19: 3557–3565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci 20: RC116, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7: 148–156, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Benser ME, Marquis RE, Hudspeth AJ. Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus. J Neurosci 16: 5629–5643, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26: 10992–11000, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechano-transducer channels using high-speed calcium imaging. Nat Neurosci 12: 553–558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurg M, Nam JH, Chen Q, Fettiplace R. Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104: 18–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beurg M, Nam JH, Crawford AC, Fettiplace R. The effects of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94: 2639–2653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beurg M, Tan X, Fettiplace R. A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron 79: 69–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol 385: 243–286, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosher SK, Warren RL. A study of the electrochemistry and osmotic relationships of the cochlear fluids in the neonatal rat at the time of the development of the endocochlear potential. J Physiol 212: 739–761, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosher SK, Warren RL. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature 273: 377–378, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227: 194–196, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Caberlotto E, Michel V, de Monvel JB, Petit C. Coupling of the mechanotransduction machinery and F-actin polymerization in the cochlear hair bundles. Bioarchitecture 1: 169–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan DK, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci 8: 149–155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282: 2220–2226, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. Tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans Nature 494: 95–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Mahendrasingam S, Tickle JA, Furness DN, Hackney CM, Fettiplace R. The development, distribution and density of the PMCA2 calcium pump in rat cochlear hair cells. Eur J Neurosci 36: 2302–2310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Salvi R, Shero M. Cochlear frequency-place map in adult chickens: intracellular biocytin labeling. Hear Res 81: 130–136, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Cheung EL, Corey DP. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys J 90: 124–139, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choe Y, Magnasco MO, Hudspeth AJ. A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channels. Proc Natl Acad Sci USA 95: 15321–15326, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 510–521, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Coombs ID, Soto D, Zonouzi M, Renzi M, Shelley C, Farrant M, Cull-Candy SG. Cornichons modify channel properties of recombinant and glial AMPA receptors. J Neurosci 32: 9796–9804, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper NP, Rhode WS. Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: sharp tuning and nonlinearity in the absence of baseline position shifts. Hear Res 63: 163–190, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Géléoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432: 723–730, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281: 675–677, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Corey DP, Hudspeth AJ. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci 3: 942–961, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3: 962–976, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483: 176–181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol 419: 405–434, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford AC, Evans MG, Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol 434: 369–398, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford AC, Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol 306: 79–125, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol 312: 377–412, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364: 359–379, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dallos P. Organ of Corti kinematics. J Assoc Res Otolaryngol 4: 416–421, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dallos P. Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18: 370–376, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delprat B, Michel V, Goodyear R, Yamasaki Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson G, Hardelin JP, Petit C. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum Mol Genet 14: 401–410, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15: 1311–1321, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Derebe MG, Zeng W, Li Y, Alam A, Jiang Y. Structural studies of ion permeation and Ca2+ blockage of a bacterial channel mimicking the cyclic nucleotide-gated channel pore. Proc Natl Acad Sci USA 108: 592–597, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desmonds T, Kirkwood N, Goodyear R, Derudas M, Gale J, van Netten S, Richardson G, Kros C. Positional gradients and voltage dependence of block of the hair cell's mechano-electrical transducer channel by the d-HIVTAT and d-JNKi1 peptides. Assoc Res Otolaryngol Abstracts 37: PD64, 2014 [Google Scholar]
- 57.Dinklo T, Meulenberg CJ, van Netten SM. Frequency-dependent properties of a fluid jet stimulus: calibration, modeling, and application to cochlear hair cell bundles. J Assoc Res Otolaryngol 8: 167–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doll JC, Peng AW, Ricci AJ, Pruitt BL. Faster than the speed of hearing: nanomechanical force probes enable the electromechanical observation of cochlear hair cells. Nano Lett 12: 6107–6111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drescher MJ, Khan KM, Beisel KW, Karadaghy AA, Hatfield JS, Kim SY, Drescher AJ, Lasak JM, Barretto RL, Shakir AH, Drescher DG. Expression of adenylyl cyclase type I in cochlear inner hair cells. Brain Res Mol Brain Res 45: 325–330, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 21: 5066–5078, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci 7: 2821–2836, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edmonds B, Reyes R, Schwaller B, Roberts WM. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat Neurosci 3: 786–790, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J Am Audiol Soc 1: 179–184, 1976 [PubMed] [Google Scholar]
- 64.Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558: 769–792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farris HE, Wells GB, Ricci AJ. Steady-state adaptation of mechanotransduction modulates the resting potential of auditory hair cells, providing an assay for endolymph Ca2+. J Neurosci 26: 12526–12536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol 61: 809–834, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7: 19–29, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Flock A, Cheung HC. Actin filaments in sensory hairs of inner ear receptor cells. J Cell Biol 75: 339–343, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forge A, Davies S, Zajic G. Characteristics of the membrane of the stereocilia and cell apex in cochlear hair cells. J Neurocytol 17: 325–334, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Fuchs PA, Nagai T, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci 8: 2460–2467, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furness DN, Hackney CM. Cross-links between stereocilia in the guinea pig cochlea. Hear Res 18: 177–188, 1985 [DOI] [PubMed] [Google Scholar]
- 72.Furness DN, Hackney CM. The structure and composition of the stereociliary bundle of vertebrate hair cells. In: Vertebrate Hair Cells, edited by RA Eatock, RR Fay, AN Popper. New York: Springer, 2006, p. 95–153 [Google Scholar]
- 73.Furness DN, Katori Y, Nirmal Kumar B, Hackney CM. The dimensions and structural attachments of tip links in mammalian cochlear hair cells and the effects of exposure to different levels of extracellular calcium. Neuroscience 154: 10–21, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Furness DN, Johnson SL, Manor U, Rüttiger L, Tocchetti A, Offenhauser N, Olt J, Goodyear RJ, Vijayakumar S, Dai Y, Hackney CM, Franz C, Di Fiore PP, Masetto S, Jones SM, Knipper M, Holley MC, Richardson GP, Kachar B, Marcotti W. Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc Natl Acad Sci USA 110: 13898–13903, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci 21: 7013–7012, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.García JA, Yee AG, Gillespie PG, Corey DP. Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells. J Neurosci 18: 8637–8647, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geisler CD. A model of stereociliary tip-link stretches. Hear Res 65: 79–82, 1993 [DOI] [PubMed] [Google Scholar]
- 78.Géléoc GS, Lennan GW, Richardson GP, Kros CJ. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc R Soc Biol Sci 264: 611–621, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, Hudspeth AJ. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci USA 106: 21347–21352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gill SS, Salt AN. Quantitative differences in endolymphatic calcium and endocochlear potential between pigmented and albino guinea pigs. Hear Res 113: 191–197, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Gillespie PG, Cyr JL. Myosin-1c, the hair cell's adaptation motor. Annu Rev Physiol 66: 521–545, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Glowatzki E, Ruppersberg JP, Zenner HP, Rüsch A. Mechanically and ATP-induced currents of mouse outer hair cells are independent and differentially blocked by d-tubocurarine. Neuropharmacology 36: 1269–1275, 1997 [DOI] [PubMed] [Google Scholar]
- 83.Goodyear RJ, Forge A, Legan PK, Richardson GP. Asymmetric distribution of cadherin 23 and protocadherin 15 in the kinocilial links of avian sensory hair cells. J Comp Neurol 518: 4288–4297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol 485: 75–85, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci USA 108: 11476–11481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Griesinger CB, Richards CD, Ashmore JF. Apical endocytosis in outer hair cells of the mammalian cochlea. Eur J Neurosci 20: 41–50, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, Dumont RA, Hintermann E, Sczaniecka A, Schwander M, Williams D, Kachar B, Gillespie PG, Müller U. Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron 62: 375–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jörs S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci USA 104: 19583–19588, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guinan JJ., Jr How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res 292: 35–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hackney CM, Fettiplace R, Furness DN. The functional morphology of stereociliary bundles on turtle cochlear hair cells. Hear Res 69: 163–175, 1993 [DOI] [PubMed] [Google Scholar]
- 91.Hackney CM, Furness DN. The composition and role of cross links in mechanoelectrical transduction in vertebrate sensory hair cells. J Cell Sci 126: 1721–1731, 2013 [DOI] [PubMed] [Google Scholar]
- 92.Hackney CM, Mahendrasingam S, Jones EM, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J Neurosci 23: 4577–4589, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25: 7867–7875, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hacohen N, Assad JA, Smith WJ, Corey DP. Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J Neurosci 9: 3988–3997, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science 338: 260–263, 2012 [DOI] [PubMed] [Google Scholar]
- 96.He DZ, Jia S, Dallos P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 429: 766–770, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Hildebrand MS, Kahrizi K, Bromhead CJ, Shearer AE, Webster JA, Khodaei H, Abtahi R, Bazazzadegan N, Babanejad M, Nikzat N, Kimberling WJ, Stephan D, Huygen PL, Bahlo M, Smith RJ, Najmabadi H. Mutations in TMC1 are a common cause of DFNB7/11 hearing loss in the Iranian population. Ann Otol Rhinol Laryngol 119: 830–835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hille B. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 99.Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44: 309–320, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108: 371–381, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Holton T, Hudspeth AJ. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol 375: 195–227, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horwitz GC, Lelli A, Géléoc GS, Holt JR. HCN channels are not required for mechanotransduction in sensory hair cells of the mouse inner ear. PLoS One 5: e8627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Howard J. Mechanics of Motor Proteins. Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 104.Howard J, Ashmore JF. Stiffness of sensory hair bundles in the sacculus of the frog. Hear Res 23: 93–104, 1986 [DOI] [PubMed] [Google Scholar]
- 105.Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci USA 84: 3064–3068, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1: 189–199, 1988 [DOI] [PubMed] [Google Scholar]
- 107.Hudspeth AJ. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci 2: 1–10, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron 59: 530–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA 74: 2407–2411, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ikeda K, Kusakari J, Takasaka T, Saito Y. The Ca2+ activity of cochlear endolymph of the guinea pig and the effect of inhibitors. Hear Res 26: 117–125, 1987 [DOI] [PubMed] [Google Scholar]
- 111.Indzhykulian AA, Stepanyan R, Nelina A, Spinelli KJ, Ahmed ZM, Belyantseva IA, Friedman TB, Barr-Gillespie PG, Frolenkov GI. Molecular remodeling of tip links underlies mechanosensory regeneration in auditory hair cells. PLoS Biol 11: e1001583, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaramillo F, Hudspeth AJ. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron 7: 409–420, 1991 [DOI] [PubMed] [Google Scholar]
- 113.Jaramillo F, Hudspeth AJ. Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle. Proc Natl Acad Sci USA 90: 1330–1334, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jia S, Dallos P, He DZ. Mechanoelectric transduction of adult inner hair cells. J Neurosci 27: 1006–1014, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jia S, He DZ. Motility-associated hair-bundle motion in mammalian outer hair cells. Nat Neurosci 8: 1028–1034, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Johnson SL, Beurg M, Marcotti W, Fettiplace R. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70: 1143–1154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol 563: 177–191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones TA, Jones SM, Paggett KC. Emergence of hearing in the chicken embryo. J Neurophysiol 96: 128–141, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Jørgensen F, Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol 403: 577–588, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jörs S, Grimm C, Becker L, Heller S. Genetic inactivation of Trpml3 does not lead to hearing and vestibular impairment in mice. PLoS One 5: e14317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. High-resolution structure of hair-cell tip links. Proc Natl Acad Sci USA 97: 13336–13341, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Kawashima Y, Géléoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121: 4796–4809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449: 87–91, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Kennedy MB. Signal-processing machines at the postsynaptic density. Science 290: 750–754, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433: 880–883, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6: 832–836, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Depolarization of cochlear outer hair cells evokes active hair bundle motion by two mechanisms. J Neurosci 26: 2757–2766, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol 142: 493–505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim KX, Fettiplace R. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol 141: 141–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Komiyama S. Gadolinium blocks mechano-electric transducer current in chick cochlear hair cells. Hear Res 101: 75–80, 1996 [DOI] [PubMed] [Google Scholar]
- 132.Kimitsuki T, Ohmori H. Dihydrostreptomycin modifies adaptation and blocks the mechano-electric transducer in chick cochlear hair cells. Brain Res 624: 143–150, 1993 [DOI] [PubMed] [Google Scholar]
- 133.Kindt KS, Finch G, Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell 23: 329–341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA, 3rd, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 141: 786–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kozlov AS, Risler T, Hudspeth AJ. Coherent motion of stereocilia assures the concerted gating of hair-cell transduction channels. Nat Neurosci 10: 87–92, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kroese AB, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear Res 37: 203–217, 1989 [DOI] [PubMed] [Google Scholar]
- 137.Kros CJ, Crawford AC. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol 421: 263–291, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 5: 41–47, 2002 [DOI] [PubMed] [Google Scholar]
- 139.Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc R Soc Biol Sci 249: 185–193, 1992 [DOI] [PubMed] [Google Scholar]
- 140.Kumagami H, Beitz E, Wild K, Zenner HP, Ruppersberg JP, Schultz JE. Expression pattern of adenylyl cyclase isoforms in the inner ear of the rat by RT-PCR and immunochemical localization of calcineurin in the organ of Corti. Hear Res 132: 69–75, 1999 [DOI] [PubMed] [Google Scholar]
- 141.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Ann Rev Microbiol 64: 313–329, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PS, Deshmukh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Deininger PL, Hampton LL, Sullivan SL, Battey JF, Jr, Keats BJ, Wilcox ER, Friedman TB, Griffith AJ. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30: 277–284, 2002 [DOI] [PubMed] [Google Scholar]
- 143.Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics 82: 300–308, 2003 [DOI] [PubMed] [Google Scholar]
- 144.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Labay V, Weichert RM, Makishima T, Griffith AJ. Topology of transmembrane channel-like gene 1 protein. Biochemistry 49: 8592–8598, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lefèvre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin JP, Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development 135: 1427–1437, 2008 [DOI] [PubMed] [Google Scholar]
- 147.Legan PK, Lukashkina VA, Goodyear RJ, Kössi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 28: 273–285, 2000 [DOI] [PubMed] [Google Scholar]
- 148.Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem 272: 8791–8801, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Lelli A, Asai Y, Forge A, Holt JR, Géléoc GS. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophys 101: 2961–2973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lelli A, Kazmierczak P, Kawashima Y, Müller U, Holt JR. Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15. J Neurosci 30: 11259–11269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lewis RS, Hudspeth AJ. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature 304: 538–541, 1983 [DOI] [PubMed] [Google Scholar]
- 152.Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419: 300–304, 2002 [DOI] [PubMed] [Google Scholar]
- 153.Lim DJ. Functional structure of the organ of Corti: a review. Hear Res 22: 117–146, 1986 [DOI] [PubMed] [Google Scholar]
- 154.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science 327: 46–50, 2010 [DOI] [PubMed] [Google Scholar]
- 155.Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci USA 102: 7894–7899, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lukashkin AN, Legan PK, Weddell TD, Lukashkina VA, Goodyear RJ, Welstead LJ, Petit C, Russell IJ, Richardson GP. A mouse model for human deafness DFNB22 reveals that hearing impairment is due to a loss of inner hair cell stimulation. Proc Natl Acad Sci USA 109: 19351–19356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lumpkin EA, Marquis RE, Hudspeth AJ. The selectivity of the hair cell's mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci USA 94: 10997–11002, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lumpkin EA, Hudspeth AJ. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc Natl Acad Sci USA 92: 10297–10301, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McGee J, Goodyear RJ, McMillan DR, Stauffer EA, Holt JR, Locke KG, Birch DG, Legan PK, White PC, Walsh EJ, Richardson GP. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J Neurosci 26: 6543–6553, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manley GA, Köppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol 8: 468–474, 1998 [DOI] [PubMed] [Google Scholar]
- 161.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol 21: 167–172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol 574: 677–698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol 548: 383–400, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol 520: 653–660, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechanoelectrical transducer channels. J Physiol 567: 505–521, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Markin VS, Hudspeth AJ. Gating-spring models of mechanoelectrical transduction by hair cells of the internal ear. Annu Rev Biophys Biomol Struct 24: 59–83, 1995 [DOI] [PubMed] [Google Scholar]
- 167.Martin P, Bozovic D, Choe Y, Hudspeth AJ. Spontaneous oscillation by hair bundles of the bullfrog's sacculus. J Neurosci 23: 4533–4548, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin P, Mehta AD, Hudspeth AJ. Negative hair-bundle stiffness betrays a mechanism for mechanical amplification by the hair cell. Proc Natl Acad Sci USA 97: 12026–12031, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.May-Simera H, Kelley MW. Planar cell polarity in the inner ear. Curr Top Dev Biol 101: 111–140, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Mendelson M, Lowenstein WR. Mechanisms of receptor adaptation. Science 144: 554–555, 1964 [DOI] [PubMed] [Google Scholar]
- 171.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci 23: 4054–4065, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Michalski N, Michel V, Bahloul A, Lefèvre G, Barral J, Yagi H, Chardenoux S, Weil D, Martin P, Hardelin JP, Sato M, Petit C. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci 27: 6478–6488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Michalski N, Michel V, Caberlotto E, Lefèvre GM, van Aken AF, Tinevez JY, Bizard E, Houbron C, Weil D, Hardelin JP, Richardson GP, Kros CJ, Martin P, Petit C. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflügers Arch 459: 115–130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, Kohrman DC. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet 11: 1887–1898, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Müller M. Frequency representation in the rat cochlea. Hear Res 51: 247–254, 1991 [DOI] [PubMed] [Google Scholar]
- 176.Mutai H, Mann S, Heller S. Identification of chicken transmembrane channel-like (TMC) genes: expression analysis in the cochlea. Neuroscience 132: 1115–1122, 2005 [DOI] [PubMed] [Google Scholar]
- 177.Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, García-Añoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci USA 105: 353–358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nam JH, Fettiplace R. Optimal electrical properties of outer hair cells ensure cochlear amplification. PLoS One 7: e50572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nin F, Reichenbach T, Fisher JA, Hudspeth AJ. Contribution of active hair-bundle motility to nonlinear amplification in the mammalian cochlea. Proc Natl Acad Sci USA 109: 21076–21080, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nowotny M, Gummer AW. Vibration responses of the organ of Corti and the tectorial membrane to electrical stimulation. J Acoust Soc Am 130: 3852–3872, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Oberholtzer JC, Buettger C, Summers MC, Matschinsky FM. The 28-kDa calbindin-D is a major calcium-binding protein in the basilar papilla of the chick. Proc Natl Acad Sci USA 85: 3387–3390, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359: 189–217, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oliver D, Fakler B. Expression density and functional characteristics of the outer hair cell motor protein are regulated during postnatal development in rat. J Physiol 519: 791–800, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Overstreet EH, Temchin AN, Ruggero MA. Basilar membrane vibrations near the round window of the gerbil cochlea. J Assoc Res Otolaryngol 3: 351–361, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Palmer AR, Russell IJ. Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear Res 4: 1–15, 1986 [DOI] [PubMed] [Google Scholar]
- 187.Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79: 504–515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pan B, Waguespack J, Schnee ME, LeBlanc C, Ricci AJ. Permeation properties of the hair cell mechanotransducer channel provide insight into its molecular structure. J Neurophysiol 107: 2408–2420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflügers Arch 460: 571–581, 2010 [DOI] [PubMed] [Google Scholar]
- 190.Peng AW, Effertz T, Ricci AJ. Mammalian auditory hair cell mechanotransducer adaptation is independent of calcium entry. Neuron 80: 960–972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peng AW, Belyantseva IA, Hsu PD, Friedman TB, Heller S. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J Neurosci 29: 15083–15088, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pickles JO. A model for the mechanics of the stereociliar bundle on acousticolateral hair cells. Hear Res 68: 159–172, 1993 [DOI] [PubMed] [Google Scholar]
- 193.Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15, 103–112, 1984 [DOI] [PubMed] [Google Scholar]
- 194.Powers RJ, Roy S, Atilgan E, Brownell WE, Sun SX, Gillespie PG, Spector AA. Stereocilia membrane deformation: implications for the gating spring and mechano-transduction channel. Biophys J 102: 201–210, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Powl AM, Lee AG. Lipid effects on mechanosensitive channels. Curr Top Membranes 58: 151–178, 2007 [Google Scholar]
- 196.Pujol R, Lavigne-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea. In: Development of the Auditory System, edited by Rubel EW, Popper AN, Fay RR. New York: Springer-Verlag; 1998, p. 146–193 [Google Scholar]
- 197.Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, Raouf R, Gringhuis M, Sexton JE, Abramowitz J, Taylor R, Forge A, Ashmore J, Kirkwood N, Kros CJ, Richardson GP, Freichel M, Flockerzi V, Birnbaumer L, Wood JN. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2: 120068, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Raybould NP, Jagger DJ, Kanjhan R, Greenwood D, Laslo P, Hoya N, Soeller C, Cannell MB, Housley GD. TRPC-like conductance mediates restoration of intracellular Ca2+ in cochlear outer hair cells in the guinea pig and rat. J Physiol 579: 101–113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rhode W. Basilar membrane mechanics in the 6–9 kHz region of sensitive chinchilla cochleae. J Acoust Soc Am 121: 2792–2804, 2007 [DOI] [PubMed] [Google Scholar]
- 200.Rhode W, Cooper NP. Nonlinear mechanics in the apical turn of the chinchilla cochlea in vivo. Auditory Neurosci 3: 101–121, 1996 [Google Scholar]
- 201.Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, Hegde RS, Ali RA, Anwar S, Andrade-Elizondo PB, Sirmaci A, Parise LV, Basit S, Wali A, Ayub M, Ansar M, Ahmad W, Khan SN, Akram J, Tekin M, Riazuddin S, Cook T, Buschbeck EK, Frolenkov GI, Leal SM, Friedman TB, Ahmed ZM. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44: 1265–1271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ricci A. Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol 87: 1738–1748, 2002 [DOI] [PubMed] [Google Scholar]
- 203.Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 20: 7131–7142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ricci AJ, Crawford AC, Fettiplace R. Mechanisms of active hair bundle motion in auditory hair cells. J Neurosci 22: 44–52, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ricci AJ, Crawford AC, Fettiplace R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40: 983–990, 2003 [DOI] [PubMed] [Google Scholar]
- 206.Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 501: 111–124, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cellmechanotransducer channel and its relation to the composition of endolymph. J Physiol 506: 159–173, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R. The transduction-channel filter in auditory hair cells. J Neurosci 25: 7831–7839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18: 8261–8277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Richardson GP, de Monvel JB, Petit C. How the genetics of deafness illuminates auditory physiology. Ann Rev Physiol 73: 311–334, 2011 [DOI] [PubMed] [Google Scholar]
- 211.Richardson GP, Lukashkin AN, Russell IJ. The tectorial membrane: one slice of a complex cochlear sandwich. Curr Opin Otolaryngol Head Neck Surg 16: 458–464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Richter CP, Emadi G, Getnick G, Quesnel A, Dallos P. Tectorial membrane stiffness gradients. Biophys J 93: 2265–2276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg 15: 352–357, 2007 [DOI] [PubMed] [Google Scholar]
- 214.Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci 14: 3246–3262, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Root MJ, MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron 11: 459–466, 1993 [DOI] [PubMed] [Google Scholar]
- 216.Roth B, Bruns V. Postnatal development of the rat organ of Corti. II. Hair cell receptors and their supporting elements. Anat Embryol 185: 571–581, 1992 [DOI] [PubMed] [Google Scholar]
- 217.Ruggero MA, Rich NC, Recio A, Narayan SS, Robles L. Basilar-membrane responses to tones at the base of the chinchilla cochlea. J Acoust Soc Am 101: 2151–2163, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rüsch A, Kros CJ, Richardson GP. Block by amiloride and its derivatives of mechano-electrical transduction in outer hair cells of mouse cochlear cultures. J Physiol 474: 75–86, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Russell IJ, Cody AR, Richardson GP. The responses of inner and outer hair cells in the basal turn of the guinea-pig cochlea and in the mouse cochlea grown in vitro. Hear Res 22: 199–216, 1986 [DOI] [PubMed] [Google Scholar]
- 220.Russell IJ, Kössl M, Richardson GP. Nonlinear mechanical responses of mouse cochlear hair bundles. Proc R Soc Biol Sci 250: 217–227, 1992 [DOI] [PubMed] [Google Scholar]
- 221.Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I, Pepermans E, Estivalet A, Carette D, Aghaie A, Ebermann I, Lelli A, Iribarne M, Hardelin JP, Weil D, Sahel JA, El-Amraoui A, Petit C. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol 199: 381–399, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Salles FT, Merritt RC, Jr, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dosé AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol 11: 443–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Santos-Cortez RL, Lee K, Giese AP, Ansar M, Amin-Ud-Din M, Rehn K, Wang X, Aziz A, Chiu I, HussainAli R, Smith JD, University of Washington Center for Mendelian Genomics. Shendure J, Bamshad M, Nickerson DA, Ahmed ZM, Ahmad W, Riazuddin S, Leal SM. Adenylate cyclase 1 (ADCY1) mutations cause recessive hearing impairment in humans and defects in hair cell function and hearing in zebrafish. Hum Mol Genet February 18 [Epub ahead of print], 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schneider ME, Dosé AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B. A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci 26: 10243–10252, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, Friedman TB, Riazuddin S. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43: 634–640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, Johnson KR. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30: 9683–9694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shoelson B, Dimitriadis EK, Cai H, Kachar B, Chadwick RS. Evidence and implications of inhomogeneity in tectorial membrane elasticity. Biophys J 87: 2768–2777, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shotwell SL, Jacobs R, Hudspeth AJ. Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann NY Acad Sci 374: 1–10, 1981 [DOI] [PubMed] [Google Scholar]
- 229.Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Müller U. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428: 950–955, 2004 [DOI] [PubMed] [Google Scholar]
- 230.Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, Möller CG, Pelias MZ, Tranebjaerg L. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am J Med Genet 50: 32–38, 1994 [DOI] [PubMed] [Google Scholar]
- 231.Söllner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Müller U, Nicolson T, Tübingen 2000 Screen Consortium Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428: 955–959, 2004 [DOI] [PubMed] [Google Scholar]
- 232.Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492: 128–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stauffer EA, Holt JR. Sensory transduction and adaptation in inner and outer hair cells of the mouse auditory system. J Neurophysiol 98: 3360–3369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron 47: 541–553, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Steel KP, Barkway C. Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development 107: 453–463, 1989 [DOI] [PubMed] [Google Scholar]
- 236.Steel KP, Bock GR. The nature of inherited deafness in deafness mice. Nature 288: 159–161, 1980 [DOI] [PubMed] [Google Scholar]
- 237.Steinbacher S, Bass R, Strop P, Rees DC. Structure of the prokaryotic mechanosensitive channels MscL and MscS. Curr Top Membr 58: 1–24, 2007 [Google Scholar]
- 238.Stepanyan R, Frolenkov GI. Fast adaptation and Ca2+ sensitivity of the mechano-transducer require myosin-XVa in inner but not outer cochlear hair cells. J Neurosci 29: 4023–4034, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol 22: 488–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet 19: 390–394, 1998 [DOI] [PubMed] [Google Scholar]
- 241.Strelioff D, Flock A. Stiffness of sensory-cell hair bundles in the isolated guinea pig cochlea. Hear Res 15: 19–28, 1984 [DOI] [PubMed] [Google Scholar]
- 242.Strimbu CE, Fredrickson-Hemsing L, Bozovic D. Dynamic state and evoked motility in coupled hair bundles of the bullfrog sacculus. Hear Res 265: 38–45, 2010 [DOI] [PubMed] [Google Scholar]
- 243.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115: 583–598, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sul B, Iwasa KH. Effectiveness of hair bundle motility as the cochlear amplifier. Biophys J 97: 2653–2663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Szymko YM, Dimitri PS, Saunders JC. Stiffness of hair bundles in the chick cochlea. Hear Res 59: 241–249, 1992 [DOI] [PubMed] [Google Scholar]
- 246.Tan X, Beurg M, Hackney C, Mahendrasingam S, Fettiplace R. Electrical tuning and transduction in short hair cells of the chicken auditory papilla. J Neurophysiol 109: 2007–2020, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tanaka K, Smith CA. Structure of the avian tectorial membrane. Ann Otol 84: 287–296, 1975 [DOI] [PubMed] [Google Scholar]
- 248.Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol 96: 807–821, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tilney LG, Tilney MS. Functional organization of the cytoskeleton. Hear Res 22: 55–77, 1986 [DOI] [PubMed] [Google Scholar]
- 250.Tilney LG, Tilney MS, Cotanche DA. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J Cell Biol 106: 355–365, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tinevez JY, Jülicher F, Martin P. Unifying the various incarnations of active hair-bundle motility by the vertebrate hair cell. Biophys J 93: 4053–4067, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tsunozaki M, Bautista DM. Mammalian somatosensory mechanotransduction. Curr Opin Neurobiol 19: 362–369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tsuprun V, Santi P. Structure of outer hair cell stereocilia side and attachment links in the chinchilla cochlea. J Histochem Cytochem 50: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 254.Tsuprun V, Schachern PA, Cureoglu S, Paparella M. Structure of the stereocilia side links and morphology of auditory hair bundle in relation to noise exposure in the chinchilla. J Neurocytol 32: 1117–1128, 2003 [DOI] [PubMed] [Google Scholar]
- 255.Van Aken AF, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol 586: 5403–5418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Van Netten SM, Kros CJ. Gating energies and forces of the mammalian hair cell transducer channel and related hair bundle mechanics. Proc R Soc Biol Sci 267: 1915–1923, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Van Netten SM, Kros CJ. Insights into the pore of the hair cell transducer channel from experiments with permeant blockers. Curr Top Membr 59: 375–398, 2007 [DOI] [PubMed] [Google Scholar]
- 258.Vater M, Lenoir M. Ultrastructure of the horseshoe bat's organ of Corti. I. Scanning electron microscopy. J Comp Neurol 318: 367–379, 1992 [DOI] [PubMed] [Google Scholar]
- 259.Vater M, Meng J, Fox RC. Hearing organ evolution and specialization: early and late mammals. In: Evolution of the Vertebrate Auditory System, edited by Manley GA, Popper AN, Fay RR. New York: Springer-Verlag, 2004, p. 256–288 [Google Scholar]
- 260.Verpy E, Leibovici M, Michalski N, Goodyear RJ, Houdon C, Weil D, Richardson GP, Petit C. Stereocilin connects outer hair cell stereocilia to one another and to the tectorial membrane. J Comp Neurol 519: 194–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vollrath MA, Eatock RA. Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: comparison with frog saccular hair cells. J Neurophysiol 90: 2676–2689, 2003 [DOI] [PubMed] [Google Scholar]
- 262.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 30: 339–365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, Hrabé De Angelis M, Avraham KB, Steel KP. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet 30: 257–258, 2002 [DOI] [PubMed] [Google Scholar]
- 264.Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One 8: e54794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27: 13890–13902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science 287: 2229–2234, 2000 [DOI] [PubMed] [Google Scholar]
- 267.Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Müller U. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development 138: 1607–1617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 10.1038/nature13251 [Epub ahead of print], 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wu YC, Art JJ, Goodman MB, Fettiplace R. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol 63: 131–158, 1995 [DOI] [PubMed] [Google Scholar]
- 270.Wu YC, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J Neurophysiol 82: 2171–2181, 1999 [DOI] [PubMed] [Google Scholar]
- 271.Xiong W, Grillet N, Elledge HM, Wagner HF, Zhao B, Johnson KR, Kazmierczak P, Müller U. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151: 1283–1295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493: 221–225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang D, Thalmann I, Thalmann R, Simmons DD. Expression of alpha and beta parvalbumin is differentially regulated in the rat organ of corti during development. J Neurobiol 58: 479–492, 2004 [DOI] [PubMed] [Google Scholar]
- 274.Zhao H, Williams DE, Shin JB, Brügger B, Gillespie PG. Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci 32: 4600–4609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA 93: 15469–15474, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155, 2000 [DOI] [PubMed] [Google Scholar]
- 277.Zheng L, Sekerková G, Vranich K, Tilney LG, Mugnaini E, Bartles JR. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102: 377–385, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhong D, Blount P. Phosphatidylinositol is crucial for the mechanosensitivity of Mycobacterium tuberculosis MscL. Biochemistry 52: 5415–5420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zidanic M, Brownell WE. Fine structure of the intracochlear potential field. I. The silent current. Biophys J 57: 1253–1268, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]