Abstract
Steroid receptors exist and function in multiple compartments of cells in most organs. Although the functions and nature of some of these receptors is being defined, important aspects of receptor localization and signaling to physiology and pathophysiology have been identified. In particular, extranuclear sex steroid receptors have been found in many normal cells and in epithelial tumors, where they enact signal transduction that impacts both nongenomic and genomic functions. Here, I focus on the progress made in understanding the roles of extranuclear estrogen receptors (ER) in physiology and pathophysiology. Extranuclear ER serve as a model to selectively intervene with novel receptor reagents to prevent or limit disease progression. Recent novel mouse models and membrane ER-selective agonists also provide a better understanding of receptor pool cross-talk that results in the overall integrative actions of sex steroids.
Keywords: membrane estrogen receptor, signal transduction, steroid receptors
more than half a century ago, the eminent endocrinologist Hans Selye first described rapid actions of steroid hormones in mammals (58). Rapid actions of glucocorticoids in vascular models were not consistent with the emerging field of gene regulation by nuclear steroid receptors. Because of technological limitations, the mediators of these rapid actions of glucocorticoids were not defined in that period. In contrast, in the 1960s and 1970s, Pietras and Szego (48, 49), along with Szego and Davis (59), described rapid actions of estrogen, including calcium flux and cAMP generation that occurred in seconds after exposure of cells to this steroid. These authors provided evidence of estrogen binding to a presumed receptor, possibly at the cell surface, but the nature of this probable binding protein was unknown. These observations were consistent with the later description and isolation of a membrane protein that served as a receptor for plant brassinosteroids, bona fide steroids that mediate flowering and fertility. The brassinosteroid receptor was found to be a plasma membrane tyrosine kinase (64), establishing that the ancient and conserved function of steroids from plants to humans results from actions at the membrane. These rapid signals seemed unlikely to emanate from nuclear receptors, whose transcriptional function was defined as being much slower.
Today, we know that the distribution of steroid receptors in vertebrates favors nuclear localization. Increased genome complexity occurs as one moves up the phylogenetic order and probably provides the impetus for evolution to create nuclear pools of steroid receptors. This allows binding of these receptors to promoters and especially multiple steroid response elements at enhancer DNA throughout the genome of many cells, regulating gene expression that is necessary for normal development and function. Nuclear steroid receptors also serve as a model to understand the detailed mechanisms of transcription (10), resulting in most research in this area being focused on the nuclear receptor pool.
More recently, there has been increased interest in defining the nature and functions of extranuclear receptors. It is now appreciated that steroid hormone action results from the integrative actions and cross-talk between receptor pools within cells. In this review of current understanding, I detail key developments in the area of extranuclear estrogen receptor (ER) that serves as a model to investigate all steroid hormone(s) action.
Nature of Extranuclear ER
Using antibodies to classical “nuclear” ERα, a protein with comparable features was identified in the vicinity of the plasma membrane by immunohistochemistry (34). Furthermore, the ability of estrogen to bind to a protein at the membrane was decreased significantly by antisense oligonucleotides to classical ERα (29). When ERα-null cells were transfected with a single plasmid encoding classical ERα, both nuclear and membrane-localized ER were produced, and the cells responded to ligand [17β-estradiol (E2)] with rapid signal transduction (54). These results suggested that the membrane ERα is the classical nuclear receptor protein localized to this additional cellular domain. Rapid signaling by E2 at the membrane was consistent with the rapid actions of numerous steroids acting at often-undefined receptors in a variety of cells (23). Subsequent work showed the presence of endogenous membrane and nuclear ERα in human breast cancer cells that were identical by mass spectrometry analysis of subcellular proteins isolated from estradiol affinity columns (37). Additionally, expression of just the ligand-binding domain (E domain) of ERα resulted in membrane localization and rapid signal transduction in response to estrogen. The important functional consequences of engagement of membrane-localized ER by estrogenic compounds included protection against developing metabolic bone disease (18). These results suggested that important actions of the sex steroid might originate from signaling by a plasma membrane-localized ERα pool, often collaborating with liganded nuclear steroid receptors (38).
Additional Membrane-Localized ER
There is evidence in some organs that endogenous ERβ also localizes to the plasma membrane (47). ERβ was first described in 1996 and is the second receptor that mediates some actions of estrogen in various organs (19, 28). Many studies have indicated that truncated forms of ERα or alternative steroid-binding proteins are found in a variety of organs. ERα that is 46 (22) or 36 kDa (63) in size has been reported at the membrane, especially in breast cancer cell lines (63). ERα of 46 and 36 kDa are products of alternative promoter usage that generates the splice isoforms, and the truncated receptors have been demonstrated in various mammalian organs (13, 63). However, confirmation of the presence of 46-kDa receptors at the membrane of normal organs is not well established. Rather, abundant 66-kDa, full-length ERα at the cell membrane has been identified by many investigators in multiple animal and cell models, and the existing evidence suggests that this form of the receptor mediates most of the rapid actions of estrogen (15). Regarding the 36-kDa ERα, there is no conclusive evidence from the required multiple laboratories that this receptor performs important functions underlying normal or abnormal physiology. Furthermore, the modest abundance of this endogenous receptor at the membrane compared with ERα of 66 kDa in any organs/cells suggests a limited role.
Most recently, it has been reported that an orphan G-protein-coupled receptor, GPR30, serves as an ER and mediates E2 signaling from the membrane (12) or the endoplasmic reticulum (56). There are few publications supporting the idea that estrogen binds to GPR30, also designated as GPER1. Saturation binding by H3-E2 of endogenous membrane GPR30 in classical ERα negative breast cancer cells showed <300 disintegrations/min specifically bound (61), often a level of noise in receptor binding studies. In numerous ERα-null cells that express endogenous GPR30/GPER1, neither others nor we have seen appreciable specific binding by or signaling in response to E2 (32, 37). Furthermore, several laboratories have created GPR30-knockout (KO) mice (16, 33) and report few phenotypes in response to a variety of stresses or under basal conditions and no overlap with the profound phenotypes of ERα- or ERβ-KO mice. However, in some cell types, especially cancer cells, there may be collaboration between GPR30 and membrane ERα as part of a larger signalsome at the membrane, thus transmitting downstream rapid signals (3). In opposition to this idea, some investigators have not seen a functional linkage between membrane-localized ERα and GPR30 in breast cancer and other cell types (24, 60). At present, the endogenous ligand for GPR30 has not been identified, and it remains to be determined whether GPR30 and membrane ERα meaningfully collaborate in vivo.
Trafficking to and Functioning of ER at the Membrane
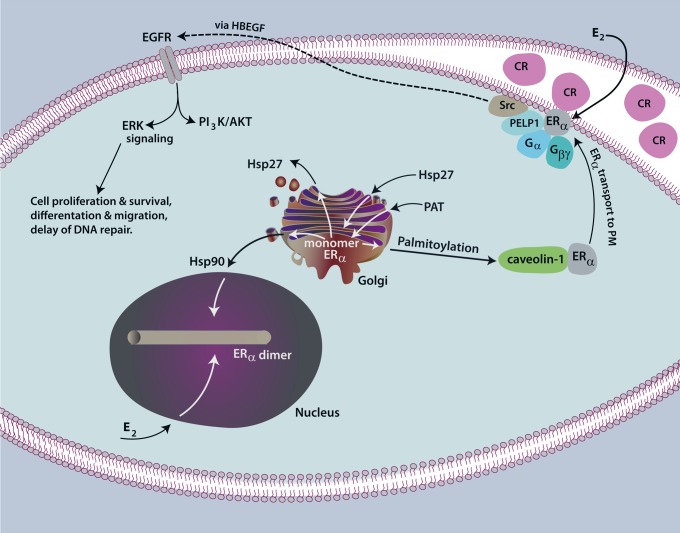
Attachment of palmitic acid to an internal cysteine residue promotes ER trafficking to the membrane (Fig. 1) (1). Palmitoylation of ERα at cysteine 451 (mouse)/447 (human) is required for ∼5% of total cellular receptors to localize to this cellular domain (1, 39). Palmitoylation at C451 occurs from the enzymatic actions of the palmitoyl acyltransferase proteins (PATs) DHHC7 and -21, resulting in disulfide bond linkage of the palmitic fatty acid with ERα (40). Palmitoylation also requires additional residues as part of a nine-amino acid motif that promotes the physical association of the PATs with ERα (39). Heat shock protein 27 is also important for promoting palmitoylation, probably by opening up the ERα monomeric structure to allow the DHHC PATs to gain access to the palmitoylation motif (50). Importantly, all of these aspects are highly conserved for the membrane localization of both progesterone and androgen receptor proteins, as we defined earlier (39, 40, 50). ERβ also undergoes palmitoylation and subsequent membrane localization in some cell types, enacting rapid signals in cardiomyocytes and cardiac fibroblasts, in vitro and in vivo (41, 42).
Fig. 1.
Localization of estrogen receptor (ER) at the plasma membrane. The ERα monomer is palmitolyated by DHHC7 and DHHC21 palmitoylacyltransferase (PAT) enzymes in Golgi, causing the physical asociation of ERα with caveolin-1 protein in cytoplasm. Heat shock protein 27 (Hsp27) binds to a 9-amino acid palmitoylation motif in the ligand-binding domain of ERα, probably opening the receptor structure for subsequent PAT binding and action. Upon binding of caveolin-1, caveolin-1 transports ERα to caveolae rafts (CR) in the plasma membrane. Here, caveolin-1 serves both as a structural coat protein of the caveolae and as a scaffold for ERα, linker proteins (PELP1), and numerous signaling molecules. In this confined area, membrane ERα dimerizes in response to estrogen and then activates various Gα and Gβγ proteins for selective signaling. It is not established whether endogenous ERα span the plasma membrane. In hormone-responsive breast cancer cells, membrane-initiated signaling by ERα liberates heparin-bound epidermal growth factor (HBEGF), a ligand for epidermal growth factor receptor (EGFR) family heterodimers, ultimately activating MEK/ERK, phosphatidylinositol 3-kinase (PI3K)/Akt, and other signaling cascades that affect cellular actions. Effector kinases phosphorylate proteins to alter their activity (non-genomic actions), and this signaling also conditions the chromatin environment to promote nuclear ERα actions impacting transcription (genomic actions). Nuclear-bound ERα is chaperoned by Hsp90, is not palmitoylated, and upon E2 binding, dimerizes in the nucleus to modulate gene expression.
The process of palmitoylation occurs only on ER monomers, and therefore, E2-induced receptor dimerization limits the available receptors that can undergo this posttranslational modification and traffic to the membrane (40). In breast cancer cells expressing endogenous ERα, nonreducing gel electrophoresis showed that ∼85% of receptors at the membrane are monomers in the absence of E2. However, dimerization of membrane ERα occurs within seconds when cells are exposed to E2, and this structural change is required for rapid signaling (51) as well as for transcriptional functions of nuclear ER. Rapid signaling by both ERα and ERβ first results in the activation of discrete Gα and Gβγ proteins (20, 54). Linkage of ER to the activation of specific G protein subunits occurs in a cell- and context-related fashion to control the downstream signal transduction pathways that provide the cellular response to various stimuli. Rapid depalmitoylation also occurs (14), perhaps to regulate the length and type of signal transduction, but this function has not been established in many in vitro or any in vivo models.
Palmitoylation results in the interaction of ER with the caveolin-1 protein that serves as the transporter of the steroid receptor to caveolae rafts within the cell membrane (1). Both ER isoforms have been found to be highly enriched in freshly isolated caveolae rafts (20, 52) and probably initiate signal transduction from this location. ER have also been found in early endosomes, but the precise nature of these organelles and the receptor functions that may occur here are unclear. Caveolin-1 also serves as a scaffold protein to bind and tether many signal molecules to the confined space in the vicinity of the membrane caveolae rafts. Scaffolding enhances the physical interactions of ER and Gα and Gβγ subunits that promote E2/ER activation of proximal signals in seconds, such as calcium flux, cAMP generation, and tyrosine kinase activation (e.g. Src, Ras) as examples. Signaling to the transactivation of growth factor receptors in cancer cells (53) results in activation of MEK/ERK and phosphatidylinositol 3-kinase (PI3K)/Akt pathways, impacting cell fate, proliferation, migration, and many other processes (53, 57). Rapid signaling in nontransformed cells impacts physiological processes, including the nongenomic regulation of protein function (e.g., from phosphorylation of kinases and other enzymes) and the regulation of transcription by acting in concert with nuclear ER (genomic impact from signaling).
Metabolic Effects of Membrane ER Signaling
Recent studies suggest that estrogen regulates glucose metabolism, at least partly from membrane ER signaling, both in normal and transformed cells (31, 65). A recent review details the extensive effects of estrogen in various aspects of normal organ metabolism (25). Breast cancer is a highly glycolytic malignancy, deriving ATP, cyclic nucleotides, NADPH, and phospholipids from the metabolism of glucose intermediates, while also maintaining glutathione in a reduced state to thwart oxidative stress. When glucose is plentiful and available to the tumor cells, estrogen signals through Akt to promote glycolysis (31). However, inadequate blood flow and therefore availability of nutrients such as glucose are known to occur for tumors in vivo and are inadequate to maintain glycolysis in breast tumors. We modeled this by decreasing the amount of glucose available to cultured breast cancer epithelial cells. In this setting, estrogen promotes a metabolic shift away from glycolysis by activating AMPK only under reduced glucose conditions (31). AMPK phosphorylates a key enzyme, pyruvate dehydrogenase, increasing its function to process the glucose metabolite pyruvate into acetyl-CoA. Acetyl-CoA enters the Krebs cycle as substrate for citrate formation and to produce electrons for the oxidative phosphorylation cycle. The latter produces increased ATP, and the former promotes reductive oxidation of fatty acids, thereby promoting survival of the cancer epithelial cells (31). Membrane ERα and ERβ also have important roles in promoting insulin sensitivity and insulin synthesis and secretion from the β-cells of the pancreas (4, 65). Interestingly, high glucose and heightened insulin signaling promote aggressive development of breast cancer in mouse models (11, 30), suggesting a possible important function by which estrogen/ER stimulates the development of this malignancy.
To better understand the impact of rapid signaling for cell function, we developed a mouse that expresses the ligand binding (E domain) of ERα, which is targeted exclusively to the membrane of all organs but lacks nuclear or other ERα receptor pools (35). Cells isolated from various organs of this membrane ERα-only mouse (MOER) were found to respond to E2 with rapid signaling through various kinase pathways, cyclic nucleotide generation, and calcium flux. Importantly, the rapid responses were comparable with those from E2 acting in WT mouse cells, indicating that only the membrane receptor pool is required for most signal transduction.
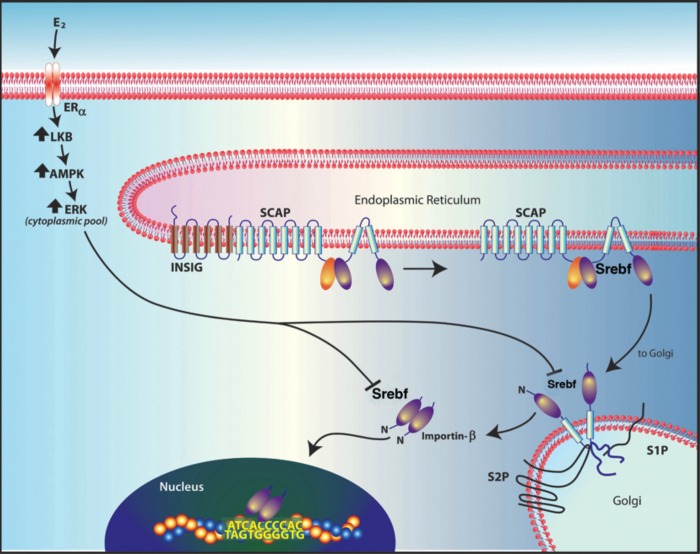
To determine whether membrane ERα signaling alone could induce gene transcription, we injected ovariectomized WT, MOER homozygous, and ERαKO mice with 4,4′,4′′-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT), an ERα agonist, and carried out DNA microarrays from liver RNA. Compared with mice injected with oil as a control, most genes that were significantly regulated by PPT were seen only in the WT mouse livers, indicating that the nuclear ERα pool is required for most messenger RNA regulation. However, 30 mRNAs were comparably suppressed by PPT in livers of WT and MOER mice, but the suppression was absent in ERα-KO, and these mRNAs were mainly the key transcripts for cholesterol, triglyceride, and fatty acid synthesis (36). Messenger RNA (mRNA) regulation correlated with comparable suppression by PPT of all lipid content in the livers of the WT and MOER female mice, which was not seen in ERα-KO mice. Further studies indicated that membrane ERα signaling to AMPK activation caused a phosphorylation of the key transcription factor (TF) sterol regulatory element-binding protein-1 (SREBP-1). This phosphorylation prevented the processing of SREBP-1 to a truncated protein that translocates to the nucleus, where the TF acts in trans to stimulate genes that are important for lipid synthesis. Specifically, AMPK-induced phosphorylation of the TF prevented the ability of the Golgi-located processing proteases S1P and S2P from physically associating with SREBP-1, therefore preventing processing (36). Thus, we described a mechanism by which estrogen signaling exclusively from membrane receptors represses some mRNAs and produces a metabolic phenotype, one that does not involve nuclear ER actions (Fig. 2).
Fig. 2.
ERα signaling from the membrane suppresses the expression of genes involved in lipid synthesis. Estrogen-triggered signaling in liver through the ERα receptor at the membrane activates a kinase cascade, including protein kinase A/liver kinase B (LKB) signaling to AMP kinase (AMPK) activation. AMPK phosphorylates sterol regulatory element-binding protein 1 (SREBF-1), thereby preventing its NH2-terminal cleavage by S1 or S2 proteases in Golgi. Failure of SREBF-1 processing prevents nuclear translocation of the transcription factor. Therefore, SREBF-1 is sequestered in cytoplasm and cannot augment the expression of mRNAs involved in lipid synthesis.
Requirement of Membrane ERα for Normal Organ Development and Function
The importance of steroid hormones to the development and functions of many organs is well established. There is growing evidence that some actions of steroid hormones in adult animals arise from membrane ER signaling; however, it is not clear whether extranuclear ER contribute to normal organogenesis. To investigate this potential function of membrane-localized ERα, we developed a cystine 451 alanine ERα-knock-in mouse by homologous recombination, replacing the endogenous esr1 gene in ES cells. This mutation prevents palmitoylation and hence, membrane trafficking by the receptor. The resulting mice show selective loss only of membrane-localized ERα but demonstrate abundant nuclear steroid receptors since palmitoylation does not impact nuclear ERα trafficking (46). The cells from homozygous nuclear ERα-only (NOER) mice fail to respond to E2 with the ERK and PI3K/Akt signaling that is seen in wild-type (WT) mouse cells. As a result, E2 stimulation of mRNAs that are regulated by both the mentioned rapid signaling and nuclear ERα binding to an estrogen response element in the gene promoter is significantly decreased (46).
We investigated various organ phenotypes and found the female homozygous NOER mice to be completely infertile due to a lack of ovulation, very low progesterone levels, and abnormal development of both the uterus and ovaries. Key developmental genes were stimulated by estrogen only in WT and heterozygous NOER mice (46). In the ovary, the lack of corpora lutea in homozygous NOER mice explained the very low serum progesterone levels (8). The mammary glands of only the homozygous NOER mice were poorly developed in part due to very low progesterone receptor-β expression that is important to ductal side branching (6). Additional phenotypes were also seen, and all of these abnormalities were significantly reversed in the heterozygous NOER female mice that were comparable with WT mice. Thus, membrane and nuclear ERα pools are required for the normal development and function of many organs mainly from coordinated regulation of key developmental genes.
What other mechanisms underlie membrane and nuclear ER collaboration for the regulation of key genes? Additional effects of membrane ERα signaling include transcription factor upregulation and recruitment, nuclear receptor phosphorylation that enhances transcription (7), and phosphorylation of coactivators such as the SRC family that then transit to the nucleus, where they bind to gene promoters and enhancers (66). Recent work has shown that p38α activation presumably by membrane ERα signaling results in this kinase acting in the nucleus to phosphorylate nuclear ERα and thereby promote interaction of the receptor with the Skp2 protein (5). This E3 ubiquitin ligase ubiquitinates nuclear ER, promoting its degradation, and presumably promotes the cycling of nuclear ER on/off gene promoters/enhancers that is necessary for gene transcription (55).
Impact of Membrane ER for Protection Against Cardiovascular Diseases
Increasingly, it has been shown that membrane ERα and ERβ play important roles in the prevention of cardiovascular diseases. Several forms of acute endothelial cell injury in arteries are mitigated by E2 action and are comparably prevented by the estrogen dendrimeric compound, which binds only to membrane ER (9). Rapid signaling by estrogen also prevents aspects of atherosclerosis in cells from animal models (62). Several groups have shown that ERβ mediates the ability of estrogen to prevent cardiac hypertrophy that results from Gqα signaling by angiotensin II (Ang II) (2), genetic hypertension (17), or pulmonary hypertension (27). We have shown that membrane ERβ signaling prevents the activation of calcineurin by Ang II or endothelin-1 (43). Calcineurin is a phosphatase that dephosphorylates the important family of transcription factors, i.e., the NFAT family. As a result, these transcription factors are retained in the cytoplasm of cardiomyocytes and cannot participate in activating the nuclear hypertrophic gene program that is downstream of various stimuli in vivo (26). Using a novel ERβ agonist, we showed prevention of Ang II-induced hypertension, cardiac hypertrophy, and cardiac fibrosis in WT but not ERβ-KO mice (42). The antifibrotic properties of ERβ were particularly impressive and resulted from activation of cAMP and PKA in cardiac fibroblasts that blocked TGFβ production and signaling to Smad phosphorylation. As a result, the Smad transcription factors were retained in cytoplasm, and collagen and other important genes that contribute to the fibrosis phenotype were not stimulated by Ang II or TGFβ (44). These mechanisms indicate a novel function by which ER prevent cardiac hypertrophy and fibrosis and also lipid synthesis in the liver (36, 41, 44). These ER actions stem exclusively from membrane receptor signaling to the posttranslational modification of TF proteins, sequestering them in cytoplasm to prevent target gene transactivation.
Several important mechanisms for ERβ prevention of cardiac disease were identified recently in our studies. These include prevention of Ang II-stimulated Akt activation through ERβ preserving Inpp5f phosphatase function (42). As a result, GSK-3β remains active to phosphorylate and suppress the activity of a key transcription factor, GATA4, resulting in inhibition of hypertrophic genes. Additionally, we found that membrane ERβ signals to the repression of histone deacetlyase (HDAC) 2 production and activity that is stimulated by Ang II, thus suppressing this prohypertrophic enzyme (45). At the same time, membrane ERβ signals to the increased production and nuclear localization of HDACs 4 and 5 that are antihypertrophic, resulting in repression of hypertrophic genes such as β-myosin heavy chain. Thus, membrane ERβ is the first described endogenous molecule that contributes to maintaining the default state in the heart of antihypertrophy by modulating both class I and II HDACs.
Perspectives
Investigators in the area of extranuclear steroid receptors have described rapid signaling impacting a variety of steroid hormone actions in many organs (15). This includes both sex steroid and non-sex steroid receptors (21). In most situations, collaboration between extranuclear and nuclear receptor pools is necessary for normal organ functions. However, we have described three in vivo models where signaling from the membrane ER alone is sufficient to prevent heart or liver pathology and may be applicable to other situations. It is likely that when mammals adjust rapidly to external stimuli, it is signaling from membrane receptors to the posttranslational modification of proteins such as enzymes that provide the mechanisms for rapid adaptation. This could include adjustments of blood pressure to changing position or stress, metabolic adaptations to ensure adequate glucose availability and optimal utilization, ion exchange in cells that modulate muscle contraction, and unfortunately, the ability of some cancers to adapt to cytotoxic therapies. This begs the larger question as to why ER are so widely expressed, including cardiovascular, bone, liver, and other organ systems where there is no obvious connection to enhancing fertility. The widespread distribution of receptors in most cells of the body likely reflects the additional actions of estrogen in younger mammals, including women, to ensure their overall health and, therefore, their physical ability to bear and raise their offspring. Considering that evolution and nature are highly geared toward reproduction and survival of the species, this concept is tenable. Extranuclear steroid receptors may then provide the organizing signals to ensure proper cell and organ function.
GRANTS
This work is supported by grants from the Veterans Administration Merit Review Program and National Institutes Of Health (CA100366) and represents the superb efforts of Ali Pedram and Mahnaz Razandi, my long-term colleagues in the laboratory.
AUTHOR CONTRIBUTIONS
E.R.L. prepared figures; E.R.L. drafted manuscript; E.R.L. edited and revised manuscript; E.R.L. approved final version of manuscript.
REFERENCES
- 1. Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell 16: 231–237, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 95: 10140–10145, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, ò S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67: 1859–1866, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Alonso-Magdalena P, Ropero AB, Garcia-Arévalo M, Soriano S, Quesada I, Muhammed SJ, Salehi A, Gustafsson JA, Nadal A. Antidiabetic actions of an estrogen receptor β selective agonist. Diabetes 62: 2015–2025, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS. Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor α turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol 32: 1928–1943, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brisken C, O'Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Carr BR, Sadler RK, Rochelle DB, Stalmach MA, MacDonald PC, Simpson ER. Plasma lipoprotein regulation of progesterone biosynthesis by human corpus luteum tissue in organ culture. J Clin Endocrinol Metab 52: 875–881, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249: 1266–1272, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes 59: 686–693, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, gpr30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Flouriot G, Griffin C, Kennealy MR, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-alpha gene are generated by alternative splicing and promoter usage. Mol Endocrinol 12: 1939–1954, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Galluzzo P, Ascenzi P, Bulzomi P, Marino M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology 149: 2567–2575, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev 28: 726–741, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150: 1722–1730, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, Poser-Klein CV, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ER lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized SHR. Cardiovasc Res 77: 774–778, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kousteni S, Chen JR, Beollido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298: 843–846, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with G α i and G βγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol 21: 1370–1380, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Levin ER. Minireview: Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25: 377–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100: 4807–4812, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4: 46–56, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22: 2116–2127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nadadur RD, Umar S, Wong G, Eghbali M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J Appl Physiol 113: 149–158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology 140: 3805–3814, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, Kurshan N, Mejia W, Santopietro S, Yakar S, Wood TL, LeRoith D. Insulin-mediated acceleration of breast cancer development and progression in a non-obese model of type 2 diabetes. Cancer Res 70: 741–751, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Mahony F, Pedram A, Razandi M, Harvey BH, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol 26: 2058–2070, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149: 4846–4856, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80: 34–41, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded ligand binding. FASEB J 9: 404–410, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Pedram A, Razandi M, Kim JK, O'Mahony F, Lee E, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 284: 3488–3495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedram A, Razandi M, O'Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal 6: ra36, 2013 [DOI] [PubMed] [Google Scholar]
- 37. Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20: 1996–2009, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem 277: 50768–50775, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282: 22278–22288, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pedram A, Razandi M, Deschenes R, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell 23: 188–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology 149: 3361–3369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pedram A, Razandi M, Korach K, Narayanan R, Dalton J, Levin ER. ERβ selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinology 154: 4352–4364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedram A, Razandi R, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280: 26339–26348, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor beta prevents cardiac fibrosis. Mol Endocrinol 24: 2152–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pedram A, Razandi M, Narayanan R, Dalton J, McKinsey A, Levin ER. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol Biol Cell 24: 3805–3818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-Localized Estrogen Receptor α Is Required for Normal Organ Development and Function. Dev Cell 29: 482–490, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pietras RJ, Márquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 70: 372–381, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature 253: 357–359, 1975 [DOI] [PubMed] [Google Scholar]
- 49. Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265: 69–72, 1977 [DOI] [PubMed] [Google Scholar]
- 50. Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol 30: 3249–3261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and function as dimers. Mol Endocrinol 18: 2854–2865, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16: 100–115, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Razandi M, Pedram A, Parks S, Levin ER. Proximal events in ER signaling from the plasma membrane. J Biol Chem 278: 2701–2712, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol 13: 307–319, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Reid G, Hubner MR, Metvier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11: 695–707, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol 24: 2114–2125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Selye H. Stress and the general adaptation syndrome. Br J Med 1: 1383–1392, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA 58: 1711–1718, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem 285: 10477–10486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Ueda K, Lu Q, Baur W, Aronovitz MJ, Karas RH. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 33: 1837–1843, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103: 9063–9068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA 107: 13057–13062, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. York B, Yu C, Sagen JV, Liu Z, Nikolai BC, Wu RC, Finegold M, Xu J, O'Malley BW. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci USA 107: 11122–11127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]