Abstract
Exposure to poor maternal nutrition around the time of conception results in an early prepartum activation of the fetal pituitary-adrenal axis and in increased adrenal growth and stress response after birth associated with epigenetic changes in a differentially methylated region (DMR) of adrenal IGF2/H19. We have determined the effects of maternal undernutrition during the periconceptional period (PCUN: 70% of control intake from 60 days before until 6 days after conception) and early preimplantation period (PIUN: 70% of control intake for 6 days after conception) on fetal plasma ACTH and cortisol concentrations and fetal adrenal ACTHR, StAR, 3βHSD, CYP11B, CYP17, TGFβ1, IGF1, IGF1R, IGF2, and IGF2R mRNA expression and the methylation level of sites within the DMRs of IGF2/H19 and IGF2R in the adrenal of twin and singleton fetuses at 136–138 days gestation. Being a twin resulted in a delayed prepartum increase in fetal ACTH and in a lower cortisol response to CRH in the control but not PCUN and PIUN groups. PCUN, but not PIUN, resulted in an increase in adrenal weight and CYP17 expression in singletons, a decrease in adrenal IGF2 expression in singletons, and an increase in adrenal IGF2R expression in both twins and singletons. IGF2/H19 and IGF2R DMR methylation levels and ACTHR expression were lower in the twin adrenal. Thus, exposure of the oocyte and embryo to maternal undernutrition or to the environment of a twin pregnancy have differential effects on epigenetic and other factors that regulate fetal adrenal growth and IGF2 and IGF2R expression.
Keywords: adrenal, epigenetic, periconceptional, twin, fetus
there is evidence that early exposure of the embryo or fetus to a range of environmental stressors, including maternal undernutrition, restriction of placental growth, or excess glucocorticoids, alters the development of the hypothalamo-pituitary-adrenal (HPA) axis and the stress responsiveness of the offspring for life (4, 7, 13, 23, 31). Developmental programming of the HPA axis after exposure to signals of early adversity may represent an evolutionary response that prepares an individual to face a lifetime of continuing adversity (33). When this prediction fails, however, as occurs when there is a mismatch between a poor prenatal and an abundant postnatal nutritional environment, then the individual is at risk of hypercortisolism, hypertension, and metabolic disease (18, 22). While exposure to maternal undernutrition early in life programs longer-term changes in the HPA axis, it is not clear which developmental windows of exposure are important and what adaptive responses occur within the pituitary-adrenal axis that may be important in the longer-term programming of this axis.
In the sheep, it is well established that prepartum activation of the HPA axis and an increase in fetal plasma cortisol concentrations are essential for the normal timing of parturition and the successful transition from intrauterine to extrauterine life (term = 150 ± 3 days of gestation) (5, 15, 16, 32). There is a significant increase in the mRNA expression of the key steroidogenic enzyme cytochrome P-450 17α-hydroxylase (CYP17) in the fetal adrenal during late gestation (21, 24), which is preceded by a decrease in the expression of the putative inhibitor of adrenal steroidogenesis, transforming growth factor-β1 (TGFβ1) (6).
Previous reports highlight the importance of both embryo number and the periconceptional environment in determining the timing and magnitude of the increase in fetal plasma ACTH and cortisol in the sheep during late gestation (2, 8, 19, 28). The prepartum activation of the fetal HPA axis is delayed in twin compared with singleton sheep fetuses, and this difference in timing has its origins as early as 55 days gestation (8, 17). Interestingly, a 30% reduction in maternal nutrition during the periconceptional period (from 60 days before until 6 days after conception) resulted in an earlier activation of the HPA axis in twin fetuses, whereas a more severe restriction of maternal nutrition imposed during the periconceptional and up to 30 days of pregnancy resulted in a premature activation of adrenal steroidogenesis in singleton pregnancies (2, 3, 8). Thus, embryo number and the embryonic nutritional environment each impacts on the timing of the prepartum activation of the adrenal.
A mounting body of evidence suggests that phenotypic effects invoked by the nutritional environment during gestation occur via epigenetic modifications to gene expression (12, 14). These epigenetic changes have been best characterized by changes in the level of cytosine (DNA), and we have recently shown that they can occur when undernutrition is restricted to the periconceptional period (34). This indicates that nutrition can affect the setting of epigenetic states at a time when the nutritional demands of the embryo are negligible.
Genes normally regulated by DNA methylation include imprinted genes, where allele-specific methylation of imprinting control regions within or adjacent to the gene allows transmission of the parental identity of each allele through the germline and creates parent-of-origin-specific patterns of gene expression. Two imprinted genes important for fetal organ growth and development are IGF2 and its clearance receptor IGF2R. IGF2 is generally expressed from the paternal allele and IGF2R from the maternal allele; in each case, expression is linked to methylation of a CpG island on the same allele, known as the differentially methylated region (DMR) (11). IGF2 is maximally expressed in the fetal sheep adrenal during early gestation, where it is present in adrenocortical steroidogenic cells (10); IGF2R is also expressed in the sheep adrenal from early gestation (6). IGF2 and IGF2R have both been implicated in the regulation of adrenal growth and steroidogenesis in the sheep (17, 26). Maternal undernutrition during the periconceptional period results in decreased adrenal IGF2 mRNA and reduced DNA methylation in the proximal CTCF binding site of the IGF2/H19 DMR, and this was associated with an increased adrenal weight in 4-mo-old offspring (34). It is not known, however, whether exposure of the embryo to maternal undernutrition in the early preimplantation period alone is sufficient to result in a premature activation of the fetal pituitary-adrenal axis. It is also not known whether there are differences in the epigenetic programming of adrenal IGF2/H19 or IGF2R between twins and singletons. In this study, we therefore investigated the effects of maternal undernutrition during the periconceptional period, from 60 days before until 6 days after conception (PCUN), and during the preimplantation period alone (PIUN), i.e., for the first 6 days after conception, on fetal plasma ACTH and cortisol concentrations during basal conditions and after corticotropin-releasing hormone (CRH) stimulation in twin and singleton fetuses in late gestation. Moreover, we determined the effects of PCUN and PIUN on fetal adrenal growth and the adrenal expression of ACTH receptor (ACTHR), steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3βHSD), steroid 11β-hydroxylase (CYP11B), CYP17, TGFβ1, IGF1, IGF2, IGF1R, and IGF2R mRNA as well as the protein abundance of IGF1R and IGF2R in twin and singleton pregnancies at 136–138 days gestation i.e., just prior to the prepartum cortisol surge. Finally, we investigated the effects of embryo number and/or periconceptional undernutrition (PCUN) and preimplantation undernutrition (PIUN) on the methylation level of specific sites within the DMR in IGF2/H19 and IGF2R DMR in the fetal sheep adrenal.
MATERIALS AND METHODS
All procedures were approved by The University of Adelaide Animal Ethics Committee.
Nutritional management.
Sixty-three South Australian Merino ewes were used in this study. Ewes were fed a diet that consisted of lucerne chaff and pellets containing cereal hay, lucerne hay, barley, oats, almond shells, lupins, oat bran, lime, and molasses (Johnsons & Sons Pty., South Australia, Australia). Eighty percent of the total energy requirements was obtained from the lucerne chaff and 20% of the energy requirements from the pellet mixture. The lucerne chaff provided 8.3 MJ/kg metabolizable energy and 193 g/kg crude protein and contained 85% dry matter, and the pellets provided 8.0 MJ/kg metabolizable energy and 110 g/kg crude protein and contained 90% dry matter. All ewes received 100% of nutritional requirements to provide sufficient energy for the maintenance of a nonpregnant ewe as defined by the Agricultural and Food Research Council in 1993 (1). At the end of an acclimatization period, ewes were randomly assigned to one of three feeding regimens: 1) control (C) group (n = 21): ewes received 100% of the metabolizable energy requirements (MER), from ∼60 days prior to mating until 6 days after mating; 2) periconceptional undernutrition (PCUN) group (n = 21): ewes received 70% of the control allowance from ∼60 days prior to mating until 6 days after mating; and 3) preimplantation undernutrition (PIUN) group (n = 21): ewes received 70% of the control allowance for 6 days after mating only.
All of the dietary components were reduced by an equal amount in the restricted diet. From day 7 of pregnancy, all ewes were fed a control diet (100% of nutritional requirements). Ewes were weighed approximately every week after commencing the feeding regimen until post mortem at day 136–138 of pregnancy. Pregnancy and fetal number were estimated by ultrasound between 40 and 80 days of gestation and the nutritional intake for the ewes adjusted for gestational age and fetal number.
Ewes were released in a group with rams of proven fertility and the occurrence of mating (day 0) was confirmed by the presence of a crayon mark on the ewe's rump. Pregnancy was confirmed by ultrasound between 40 and 80 days of gestation and 48 ewes were pregnant.
Animals and surgery.
Surgery was performed in the pregnant ewes (C: n = 17; PCUN: n = 18; PIUN: n = 13) under general anesthesia between 105 and 110 days gestation, as previously described (8). In brief, vascular catheters were inserted into a fetal carotid artery and jugular vein, a maternal jugular vein, and the amniotic cavity. Vascular catheters were inserted into only one fetus in twin pregnancies. All catheters were filled with heparinized saline, and fetal catheters were exteriorized through an incision in the ewes' flanks. Ewes were housed in individual pens in animal holding rooms with a 12:12-h light-dark cycle and fed once daily at 1100.
Maternal health, fetal outcome, and post mortem.
Three pregnant ewes in the Control group and one pregnant ewe in the PCUN group failed to eat normally and were therefore excluded from the study. One singleton fetus in the Control group and one twin fetus of the PIUN group died after surgery, and two ewes in the PIUN group carried triplets, and these animals were therefore excluded from the study. Thirteen fetal sheep delivered or died prior to post mortem at 136–138 days gestation, and fetal plasma samples but not tissues were available from these fetuses.
Blood collection for radioimmunoassays.
Fetal arterial blood samples (3.5 ml) were collected three times per week at 0800–1100 [Control singleton (S): n = 7, Control twin (T): n = 6; PCUN S: n = 8, PCUN T: n = 4; PIUN S: n = 3, PIUN T: n = 6]. Blood samples for cortisol determination were collected in heparinized tubes (125 IU; Sarstedt Australia, Ingle Farm, SA, Australia). Blood samples for ACTH determination were collected in tubes coated in EDTA (Sarstedt Australia) containing aprotinin (1,000 KIU/ml; Sigma Chemicals, St. Louis, MO). All blood samples were centrifuged at 1,500 g, and plasma was separated into aliquots and stored at −20°C.
CRH challenge.
At 130–132 days gestation, CRH (1 μg bolus in 1 ml saline; Peninsula Laboratories, San Carlos, CA) was injected into the fetal jugular vein in 32 fetal sheep (Control S: n = 6, Control T: n = 6; PCUN S: n = 8, PCUN T: n = 4; PIUN S: n = 3, PIUN T: n = 5). Fetal arterial blood samples (2.5 ml) were collected at −30, −5, 10, 20, 40, 60, 120, and 240 min relative to CRH administration. All blood samples were centrifuged at 1,500 g, and plasma was separated into aliquots and stored at −20°C.
Adrenal collection.
Pregnant ewes (n = 35) were killed with an overdose of sodium pentobarbitone between 136 and 138 days gestation, and the uteroplacental unit was delivered by hysterotomy. Fetuses were weighed and fetal adrenals collected (Control S: n = 6, Control T: n = 9; PCUN S: n = 8, PCUN T: n = 8; PIUN S: n = 3, PIUN T: n = 11) and weighed, and samples were snap-frozen in liquid nitrogen prior to storage at −70°C.
ACTH and cortisol radioimmunoassay.
Immunoreactive ACTH concentrations in fetal sheep plasma were measured using a DiaSorin ACTH radioimmunoassay kit (DiaSorin S.p.A., Vercelli, Italy) previously validated for sheep plasma (30). The sensitivity of the assay was 4.5 pg/ml, and the intra- and interassay coefficients of variation (CVs) were <10% and 13.9%, respectively.
Plasma cortisol concentrations were measured as previously described (30). The sensitivity of the assay was 0.2 nmol/l. The intra- and interassay CVs were <5% and 13.8% respectively.
Isolation of RNA and reverse transcription PCR.
Total RNA was isolated from fetal adrenals (Control S: n = 6, Control T: n = 9; PCUN S: n = 8, PCUN T: n = 8; PIUN S: n = 3, PIUN T: n = 10) using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) and treated with DNase 1 (Ambion, Austin, TX) to minimize genomic DNA contamination (17). Each sample was purified using the RNeasy Mini Kit (QIAGEN, Basel, Switzerland), and RNA was quantified by spectrophotometric measurements at 260 and 280 nm. Total RNA (3 μg) was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen Life Technologies) and random oligohexamers (100 μM). Nonamplification controls (NACs) containing no Superscript III reverse transcriptase for each sample were also synthesized.
Quantitative real-time PCR.
The relative abundance of IGF1, IGF2, IGF1R, IGF2R, CYP17, ACTHR, StAR, 3βHSD, CYP11B, and TGFβ1 mRNA transcripts in the fetal adrenal was measured by quantitative real-time PCR (qRT-PCR) using the SYBR Green system in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Each qRT-PCR well contained 5 μl of SYBR Green Master Mix (Applied Biosystems), 1 μl each of forward and reverse primer (GeneWorks, SA, Australia) for the appropriate gene (17), sterile water (2 μl), and 50 ng/μl cDNA (1 μl) to give a total volume of 10 μl. Each amplicon was sequenced to ensure the authenticity of the DNA product, and qRT-PCR melt curve analysis was performed to demonstrate amplicon homogeneity. Controls for each primer set containing no cDNA and NACs from each sample were also included.
Three replicates of cDNA from each sample of fetal adrenal were performed for each gene on each plate, and each plate was repeated twice. Amplification efficiencies were determined, from the slope of a plot of CT (defined as the cycle number at which the fluorescence generated within a reaction crossed a set threshold line) against the log of the cDNA template concentration (ranging from 1 to 100 ng/μl). The abundance of each transcript relative to the abundance of the reference gene, the acidic ribosomal protein P0 (RpP0), was calculated using Q Gene analysis software (20).
Methylation analysis.
DNA methylation within the DMRs of IGF2/H19 and IGF2R were analyzed by combined bisulfite restriction assay (COBRA) (Control S: n = 6, Control T: n = 8; PCUN S: n = 7, PCUN T: n = 8; PIUN S: n = 3, PIUN T: n = 9) (34). Approximately 2 μg of DNA from individual adrenals was subjected to bisulfite conversion (Epitect; Qiagen, Basel, Switzerland). PCR was performed on 100 ng of bisulfite-converted DNA using primers and conditions that amplified methylated and unmethylated templates with no bias. For IGF2/H19, we investigated three amplicons covering three proximal CTCF binding sites within the IGF2/H19 DMR. For IGF2R, we investigated the amplicon of a 148-bp fragment derived from the intron 2 DMR. COBRA was performed using restriction endonucleases that cleave only those amplicons derived from methylated templates. IGF2/H19 and IGF2R amplicons were digested with either NruI, HinfI or MluI (New England Biolabs, Ipswich, MA), for 2 h at 37°C. Digests were resolved on a 2.5% high-resolution agarose gel, and the intensity of uncut and cut fragments were quantified using Fujifilm FLA-5100 (Minatoku, Tokyo, Japan). Percentage of methylation was calculated by measuring the ratio of cut to uncut PCR product.
Western blotting.
The protein abundance of IGF1R and IGF2R was determined using Western blotting, as described in detail elsewhere (37). Briefly, adrenal samples (50 mg) (Control S: n = 4, Control T: n = 4; PCUN S: n = 4, PCUN T: n = 4; PIUN S: n = 3, PIUN T: n = 5) were sonicated in extraction buffer [50 mmol/l Tris (pH 8), 150 mmol/l sodium chloride, 1% HP-40, 1 mmol/l sodium orthovanadate, 30 mmol/l sodium fluoride, 10 mmol/l sodium pyrophosphate, 10 mmol/l EDTA, and a protease inhibitor cocktail] and centrifuged at 12,400 rpm at 4°C for 14 min to remove lipid and insoluble material. Protein content of the extracts was determined as previously described (38). Equal volumes of protein (20 μg) were subjected to SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membrane (Merck Millipore, Billerica, MA), blocked, and then incubated with primary antisera raised against IGF1R from Cell Signaling Technology (Danvers, MA) and IGF2R from BD Transduction Laboratories (Franklin Lakes, NJ).
Membranes were washed and bound antibody was detected using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents according to the manufacturer's instructions (Thermo Fisher Scientific, Australia). AlphaEaseFC software (Alpha Innotech, San Leandro, CA) was used to quantify the density of specific bands. To monitor the linearity of the density measurements, 10 and 20 μg of the same protein sample was loaded onto each gel to confirm that the chemiluminescent signal changed in a linear manner for all experiments. Prior to Western blotting analysis, samples (20 μg protein) were subjected to SDS-PAGE and gels stained with Coomassie Brilliant Blue (Thermo Fisher Scientific), and there were no differences in abundance of the major proteins present in samples between the different experimental groups.
Statistical analysis.
Data are presented as means ± SE. Hormonal data were log transformed where required, to normalize data variance for parametric analysis. When a significant interaction between major factors was identified by analysis of variance (ANOVA), the data were split on the basis of the interacting factor and reanalyzed. Duncan's new multiple range test was used post-ANOVA to identify significant differences between mean values. A probability of 5% (P < 0.05) was taken as the level of significance for all analyses.
The effects of PCUN and PIUN on ewe weight and the change in ewe weight were determined using a one-way ANOVA using STATA 10 for Windows (StataCorpLp, College Station, TX). A two-way repeated-measures ANOVA (mixed model) was used to determine the effects of time and nutritional treatment on maternal weight change in the periconceptional period.
The effects of maternal nutritional treatment and fetal number on the gestational age profile of the fetal plasma ACTH and cortisol concentrations were determined using a multifactorial ANOVA with repeated measures. Specified factors for the ANOVA included maternal nutritional treatment (C, PCUN, or PIUN), fetal number (singletons or twins), and time windows (119–122, 123–126, 127–130, 131–134, and 135–138 days gestation) as specified factors.
The effects of maternal nutritional treatment and fetal number on the plasma ACTH and cortisol responses to CRH were compared using a multifactorial ANOVA with repeated measures. Specified factors for the ANOVA included maternal nutritional treatment (C, PCUN, or PIUN), fetal number and time relative to CRH administration (−30, −5, 10, 20, 40, 60, 120, and 240 min). Additionally, the postinfusion area under the curve (AUC) for ACTH and cortisol was calculated using the trapezoidal rule. The effects of maternal nutritional treatment and fetal number on ACTH and cortisol AUC were determined using a two-way ANOVA.
The effects of maternal nutritional treatment and fetal number on the fetal adrenal weight, expressed as an absolute weight or relative to body weight, the relative expression of IGF1, IGF1R, IGF2, IGF2R, CYP17, ACTHR, StAR, 3βHSD, CYP11B, and TGFβ1 mRNA and the methylation (%) levels for IGF2/H19 and IGF2R in the fetal adrenal were determined using a two-way ANOVA. When there was a significant effect of nutritional treatment in the absence of any interaction between the effects of treatment and fetal number, adrenal weight and relative gene expression data from singleton and twin animals were generally pooled for presentation. Relationships between adrenal IGF2R and CYP17 mRNA expression were assessed by linear regression using Sigma Plot 10.0 (SPSS, Chicago, IL). A probability level of 5% (P < 0.05) was taken as significant.
RESULTS
Effects of PCUN and PIUN on maternal weight.
The weights of the nonpregnant ewes assigned to the Control (60.1 ± 2.1 kg), PCUN (60.5 ± 1.9 kg), and PIUN (59.5 ± 2.5 kg) groups were not significantly different before the start of the feeding regimen. Ewes in the PCUN group lost significantly more weight (−2.7 ± 0.3 kg, n = 18) than the Control (2.3 ± 0.5 kg, n = 17) and PIUN ewes (0.8 ± 0.7 kg, n = 11) during the periconceptional period between 60 days before mating and 6 days after mating (P < 0.001). The weight change in the PIUN group compared with the Control group during the period between the starting of feeding regimen and 6 days after conception was also significant (P < 0.001).
Plasma ACTH and cortisol concentrations during late gestation.
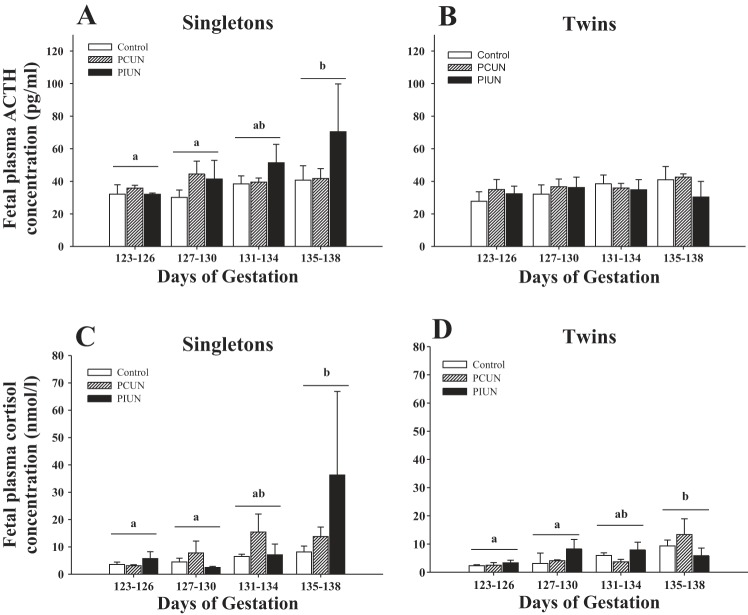
There was a significant interaction between the effects of maternal nutritional treatment, fetal number, and gestational age on fetal plasma ACTH and cortisol concentrations. In singletons in all nutritional groups, plasma ACTH and cortisol concentrations were significantly higher (P < 0.01 and P < 0.02, respectively) at 135–138 days gestation than those of either 123–126 or 127–130 days gestation. There was no effect of maternal PCUN or PIUN on plasma ACTH or cortisol concentrations in singletons during late gestation (Fig. 1).
Fig. 1.
Fetal plasma ACTH and cortisol concentrations in singleton and twin fetal sheep during late gestation. Fetal plasma ACTH concentrations significantly increased (P < 0.01) with gestational age in singletons (A) but not twins (B). There was no effect of periconceptional undernutrition (PCUN) or preimplantation undernutrition (PIUN) on fetal plasma ACTH concentration in either singletons or twins. Fetal plasma cortisol concentrations significantly increased with gestational age in both singleton (C) and twin (D) fetuses (P < 0.02 and P < 0.05, respectively). There was no effect of PCUN or PIUN on fetal plasma cortisol concentrations in either singletons or twins. Different letters (a, b) denote mean values that are significantly different within the singleton and twin groups.
In contrast to the singletons, plasma ACTH concentrations in twins in all nutritional groups did not increase after 135 days compared with earlier in gestation (Fig. 1). There was, however, a significant increase (P < 0.05) in fetal plasma cortisol concentrations in twins between 126 and 138 days gestation (Fig. 1). There was no independent effect of maternal PCUN or PIUN on fetal plasma ACTH and cortisol concentrations in twin fetuses.
ACTH and cortisol responses to CRH stimulation.
Fetal plasma ACTH concentrations increased (P < 0.0001) in all nutritional treatment groups at 10–240 min after CRH (Fig. 2). There was no significant effect of PCUN or PIUN on the fetal ACTH response to CRH. The ACTH response to CRH expressed as the area under the curve (AUC) was significantly smaller (P < 0.05), however, in twin fetuses compared with singletons independent of nutritional treatment (Fig. 2).
Fig. 2.
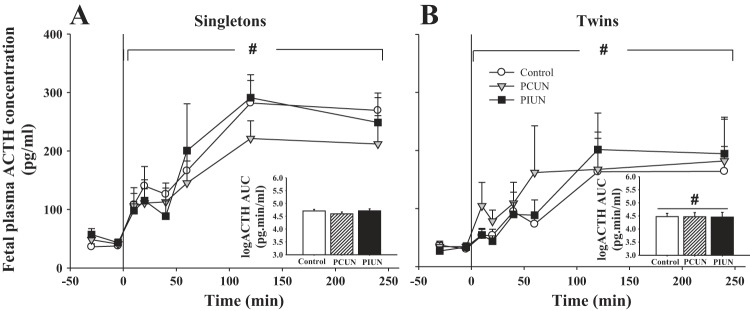
Fetal plasma ACTH concentrations in response to CRH in singleton and twin fetal sheep. Plasma ACTH concentrations increased (P < 0.0001) in response to CRH (1 μg bolus) in singleton (A) and twin (B) fetuses in all nutritional groups. CRH injected at time 0. #Significant increase in plasma ACTH concentrations vs. preinfusion values. Area under the curve (AUC) for the ACTH response post-CRH, shown as inset histograms, was significantly lower (P < 0.05) in twins (B) than in singletons (A) independently of nutritional treatment, as denoted by #.
There was a significant increase (P < 0.0001) in plasma cortisol concentrations at 10–240 min after administration of CRH in both singleton and twin fetuses, and the profile of this response with time was the same in all nutritional groups (Fig. 3).
Fig. 3.
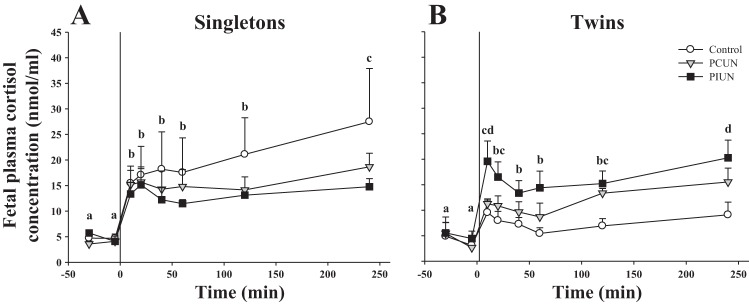
Fetal plasma cortisol concentration in response to CRH in singleton and twin fetal sheep in late gestation. Plasma cortisol concentrations increased (P < 0.0001) in response to CRH (1 μg bolus) in singleton (A) and twin (B) fetuses in all nutritional groups. CRH injected at time 0. #Significant increase in plasma cortisol concentration vs. preinfusion values.
In the Control group, the cortisol AUC after CRH was significantly smaller in twin fetuses (P < 0.05) than in singletons (Fig. 4). In contrast, in the PCUN and PIUN groups, the cortisol AUC after CRH administration was not smaller in twins compared with singletons (Fig. 4).
Fig. 4.
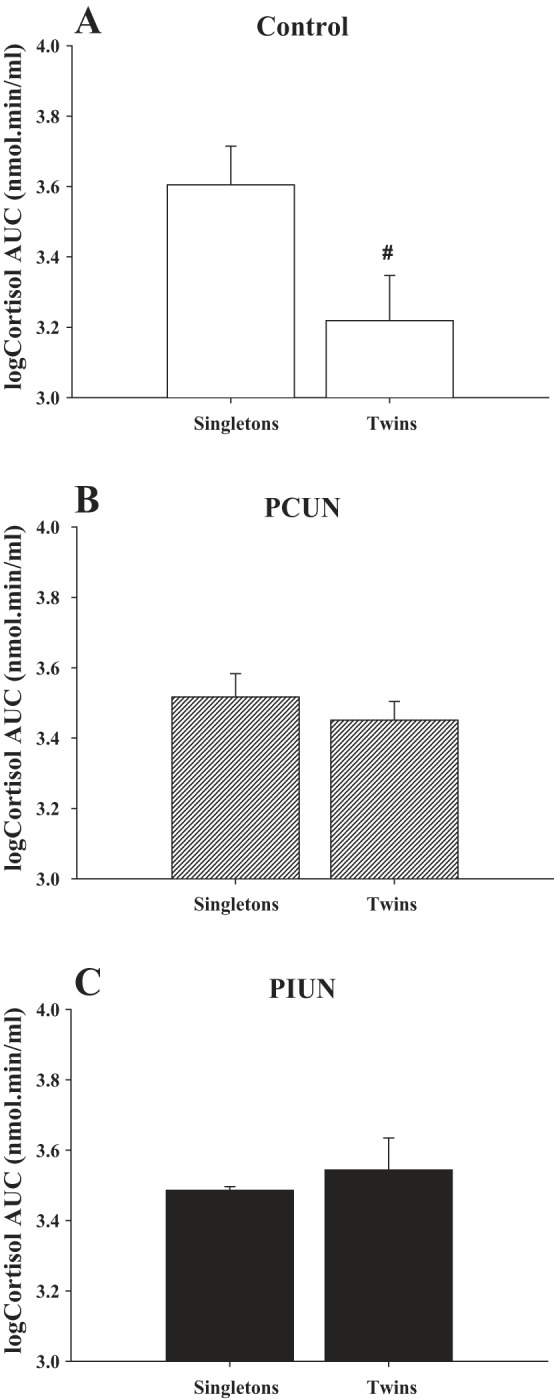
Cortisol response denoted as area under the curve (AUC) after CRH in Control, PCUN, and PIUN groups. The AUC of the cortisol response post-CRH was significantly lower (P < 0.0001) in twins than in singletons in the Control group (A), as denoted by #. There was no difference, however, in cortisol AUC post-CRH between twin and singleton fetuses in the PCUN (B) and PIUN (C) groups.
Fetal adrenal weight.
Total adrenal weight was significantly greater (P < 0.02) in the PCUN (S: 0.42 ± 0.02 g; T: 0.47 ± 0.04 g) but not PIUN fetuses (S: 0.41 ± 0.69 g; T: 0.41 ± 0.01 g) compared with Controls (S: 0.35 ± 0.02 g; T: 0.39 ± 0.02 g) in both singletons and twin pregnancies at 136–138 days gestation. Relative adrenal weight was significantly greater (P < 0.05) in twin (0.099 ± 0.004 g/kg, n = 26) compared with singleton (0.087 ± 0.005 g/kg, n = 17) fetuses, independent of maternal nutritional treatment.
Adrenal ACTHR, IGF1, IGF1R, IGF2, StAR, 3βHSD, CYP11B, CYP17, TGFβ1, and IGF2R mRNA expression.
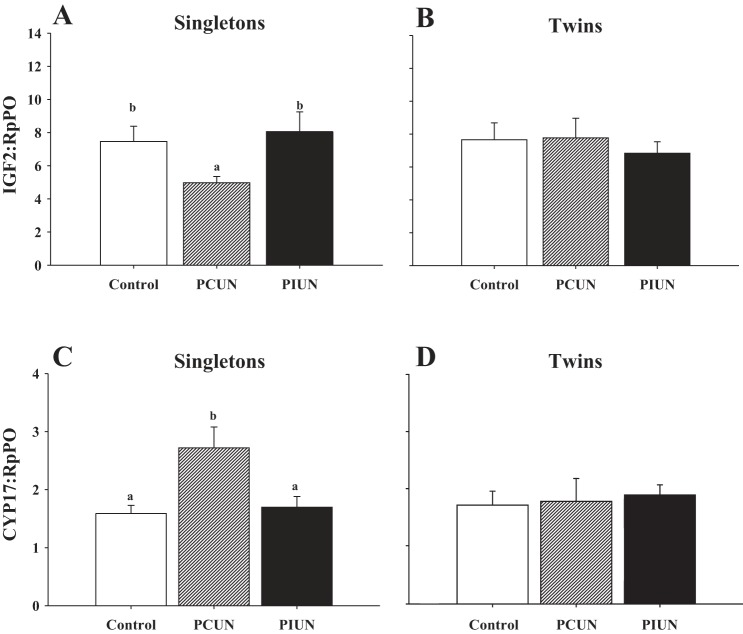
There was no effect of maternal nutritional treatment or fetal number on adrenal IGF1, IGF1R, StAR, 3βHSD, and CYP11B mRNA expression at 136–138 days gestation (Table 1). Adrenal ACTHR mRNA expression was significantly lower (P < 0.05) in twin than in singleton fetuses, but there was no effect of maternal nutritional treatment on adrenal ACTHR expression. In singleton fetuses, adrenal IGF2 mRNA expression in the PCUN group was significantly lower (P < 0.03) compared with the Control and PIUN groups, whereas there was no effect of either PCUN or PIUN on adrenal IGF2 mRNA expression in twin fetuses (Fig. 5).
Table 1.
Effect of fetal number and PCUN on the expression of IGF1, IGF1R, ACTHR, StAR, 3βHSD, and CYP11B mRNA relative to RpPO in the fetal adrenal
| IGF1:RpPO | IGF1R:RpPO | ACTHR:RpPO | StAR:RpPO | 3βHSD:RpPO | CYP11B:RpPO | |
|---|---|---|---|---|---|---|
| Singleton | ||||||
| Control | 0.022 ± 0.002 | 0.15 ± 0.02 | 0.50 ± 0.03 | 5.3 ± 0.4 | 27.0 ± 1.9 | 5.9 ± 0.6 |
| PCUN | 0.019 ± 0.003 | 0.12 ± 0.01 | 0.50 ± 0.04 | 4.7 ± 0.3 | 28.6 ± 3.1 | 6.2 ± 0.8 |
| PIUN | 0.023 ± 0.007 | 0.10 ± 0.02 | 0.40 ± 0.03 | 5.5 ± 0.8 | 25.9 ± 4.4 | 6.1 ± 0.1 |
| Twin | ||||||
| Control | 0.022 ± 0.002 | 0.15 ± 0.03 | 0.36 ± 0.03* | 4.8 ± 0.5 | 22.5 ± 2.7 | 5.1 ± 0.7 |
| PCUN | 0.028 ± 0.003 | 0.16 ± 0.03 | 0.43 ± 0.03* | 4.4 ± 0.8 | 24.5 ± 3.2 | 5.9 ± 0.7 |
| PIUN | 0.028 ± 0.002 | 0.18 ± 0.02 | 0.38 ± 0.03* | 5.3 ± 0.5 | 22.5 ± 1.7 | 6.1 ± 0.3 |
Values are means ± SE. PCUN, periconceptional nutrition; PIUN, preimplantation undernutrition.
Significant difference overall between singleton and twin fetuses (*P < 0.05).
Fig. 5.
IGF2 and CYP17 mRNA expression relative to RpPO in Control, PCUN, and PIUN groups. In singletons, there was a significant decrease (P < 0.03) in adrenal IGF2 mRNA expression (A) and a significant increase (P < 0.03) in CYP17 mRNA expression (C) in the PCUN group compared with the Control or the PIUN group. In twins, there was no effect of maternal nutritional treatment on adrenal IGF2 (B) or CYP17 (D) mRNA expression. Different letters (a, b) denote mean values that are significantly different within the singleton or twin groups.
In singleton but not twin fetuses, adrenal CYP17 mRNA expression in the PCUN group was significantly higher (P < 0.03) than in the Control and PIUN groups (Fig. 5). IGF2R mRNA expression in the fetal adrenal was significantly increased (P < 0.03) in the PCUN group compared with the Control and PIUN groups, and this occurred in singletons and twins (Fig. 6). There was no effect of either maternal nutritional treatment or fetal number on adrenal TGFβ1 mRNA expression at 136–138 days gestation.
Fig. 6.
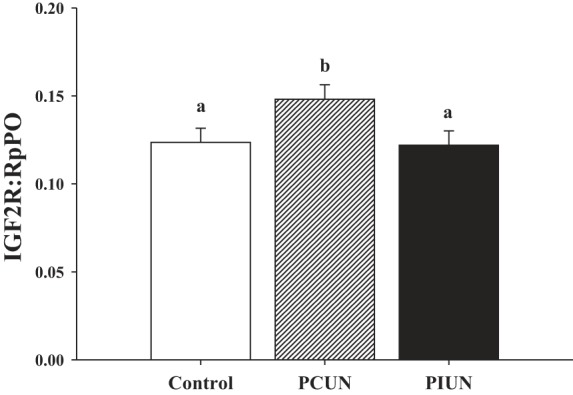
IGF2R mRNA expression relative to RpPO in Control, PCUN, and PIUN groups. There was no difference in adrenal IGF2R mRNA responses to PCUN and PIUN between twins and singletons. Adrenal IGF2R expression was significantly higher (P < 0.03) in the PCUN group (n = 16) vs. Control (n = 15) or PIUN (n = 13) groups. Different letters (a, b) denote mean values that are significantly different from each other.
In singletons and twins, there was a direct relationship between fetal adrenal IGF2R (x) and CYP17 (y) mRNA expression in the Control (y = 13.9x − 0.06; r = 0.71, n = 15, P < 0.004) and PCUN (y = 18.9x − 0.55; r = 0.56, n = 15, P < 0.04) groups. This relationship was not present, however, in the PIUN group (y = 1.8x + 0.87; r = 0.05, n = 14).
Adrenal IGF1R and IGF2R protein abundance.
Adrenal IGF1R abundance was significantly decreased (P < 0.05) in the PCUN group compared with the Control group, and this occurred independently of fetal number (Fig. 7). There was no effect of either maternal nutritional treatment or fetal number on adrenal IGF2R abundance at 136–138 days gestation.
Fig. 7.
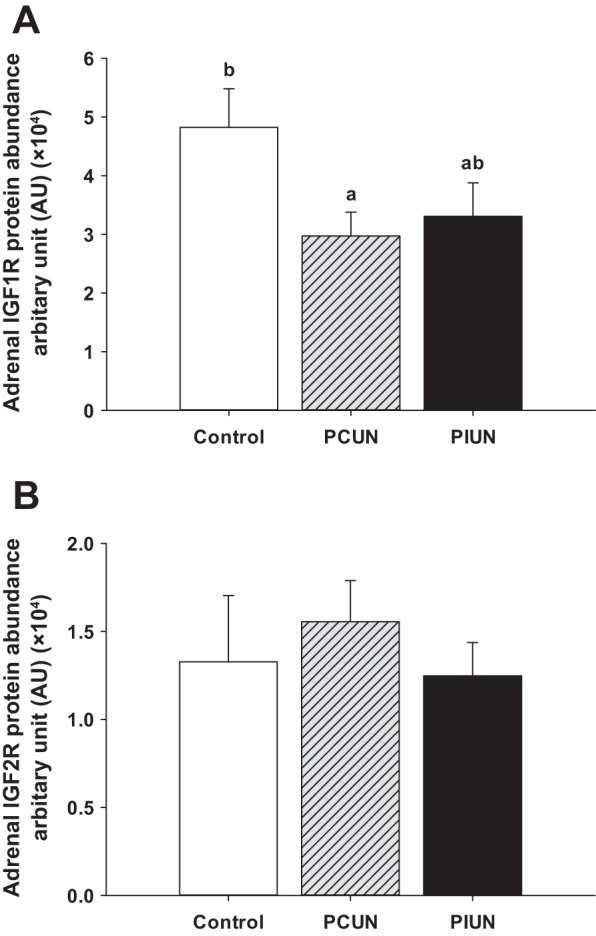
Adrenal IGF1R and IGF2R abundance in Control, PCUN, and PIUN groups. Adrenal IGF1R abundance was significantly decreased (P < 0.05) in the PCUN group compared with the Control group. There was no difference in adrenal IGF2R abundance between the treatment groups in twins and singletons. Different letters (a, b) denote mean values that are significantly different from each other.
Adrenal IGF2/H19 and IGF2R DMR methylation.
The level of methylation in the fourth CTCF binding region of the IGF2/H19 DMR was significantly lower (P < 0.05) in twin adrenals compared with singletons (Table 2). Similarly, the level of methylation in the IGF2R DMR was also significantly lower (P < 0.01) in twin adrenals compared with singletons (Table 2). There was no effect, however, of either PCUN or PIUN on the methylation level of the second, third, and fourth CTCF binding regions in the IGF2/H19 DMR or of the IGF2R DMR in either twin or singleton adrenals.
Table 2.
Percentage of methylation of the IGF2/H19 DMR and IGF2R DMR in adrenals of Control, PCUN, and PIUN fetuses during late gestation
| IGF2/H19 2nd CTCF Region | IGF2/H19 3rd CTCF Region | IGF2/H19 4th CTCF Region | IGF2R intron 2 DMR | |
|---|---|---|---|---|
| Singleton | ||||
| Control | 20.5 ± 1.8 | 47.1 ± 14.9 | 22.8 ± 1.3 | 32.0 ± 2.0 |
| PCUN | 23.3 ± 1.6 | 59.6 ± 14.5 | 21.6 ± 1.2 | 31.5 ± 0.8 |
| PIUN | 21.3 ± 1.9 | 58.3 ± 26.0 | 24.7 ± 1.5 | 32.8 ± 0.7 |
| Twin | ||||
| Control | 23.8 ± 2.3 | 62.3 ± 10.3 | 21.6 ± 0.5* | 29.3 ± 1.1** |
| PCUN | 22.8 ± 0.7 | 47.1 ± 14.3 | 19.7 ± 1.4* | 29.6 ± 0.5** |
| PIUN | 22.5 ± 2.7 | 47.2 ± 11.3 | 21.1 ± 0.5* | 29.3 ± 0.6** |
Values are means ± SE. DMR, differentially methylated region.
Significant difference overall between singleton and twin fetuses (*P < 0.05, **P < 0.01).
DISCUSSION
We have shown that there is a differential effect of maternal undernutrition imposed during the periconceptional and early preimplantation periods on fetal adrenal growth, adrenal IGF2 and CYP17 expression, and adrenal IGF1R abundance. We have also shown that ACTHR mRNA expression and the methylation level in specific loci in the IGF2/H19 and IGF2R DMRs are also lower in the adrenal of twin compared with singleton fetuses in late gestation.
Epigenetic changes in IGF2/H19 and IGF2R in the adrenal of the twin fetus.
As reported in previous studies (8, 9), we have found evidence of a blunting of pituitary-adrenal activation in the twin fetus in late gestation compared with the singleton fetus. In the present study, plasma ACTH concentrations did not increase after 123 days gestation in twins in contrast to singletons. Although there was an increase in basal cortisol concentrations in both twins and singletons after 135 days gestation, the fetal ACTH and cortisol responses to CRH were each lower in control twins compared with control singletons. In contrast, however, the relative adrenal weight was higher in twin fetuses compared with singletons. We have previously shown that at 55 days gestation the fetal adrenal is smaller, rather than larger, in the twin compared with the singleton and that the adrenal expressions of IGF1, IGF2, IGF1R, and IGF2R mRNA are each lower in the twin fetus (17). We found evidence that, in control singletons at 55 days gestation, adrenal IGF2R may be important in determining both the intra-adrenal bioavailability of IGF2 and adrenal size. In twins, however, where adrenal IGF2 expression is low at 55 days gestation, adrenal growth appeared to be predominantly regulated by IGF1 rather than IGF2 (17, 26, 27). One possibility is that the small but significant decreases in methylation in IGF2/H19 present in the twin adrenal in late gestation represent epigenetic changes induced in early gestation to mitigate against the impact of a reduction in placental substrate supply on the pituitary-adrenal axis in later gestation. The decrease in the methylation of the IGF2R DMR may in turn be an early response to the epigenetic change in IGF2/H19. It is possible that the difference in relative methylation levels of these parentally imprinted genes between twins and singletons may have a greater impact on gene expression in early gestation, when intra-adrenal growth factors may have a more dominant role in the regulation of adrenal growth. Interestingly, whereas the methylation levels of adrenal IGF2/H19 and IGF2R DMR were lower in twins at 136–138 days gestation, adrenal IGF2 and IGF2R mRNA expression were not different between twins and singletons at this stage of gestation. Factors associated with an increase in gestational age and the decrease in placental substrate supply characteristic of a twin pregnancy may act in late gestation to maintain adrenal IGF2 and IGF2R mRNA expression and to stimulate adrenal growth. Interestingly, in the present study we have shown that the adrenal expression of the ACTHR was lower in the twin than in the singleton fetus. An alternate possibility, therefore, is that in the twin an adrenal growth factor, other than ACTH1–39, stimulates adrenal growth through a non-IGF2-dependent pathway in late gestation. One potential candidate is N-proopiomelanocortin (N-POMC) (1–77), which has been shown to be present in high amounts in the fetal circulation (29) and to stimulate fetal adrenal growth but not an increase in circulating cortisol between 136 and 138 days gestation (25).
Impact of maternal undernutrition during PCUN or early PIUN period on adrenal growth and steroidogenic capacity.
SINGLETONS.
In the singleton pregnancy, maternal nutrient restriction during either the PCUN or the PIUN period did not affect the timing or magnitude of the gestational increase in plasma ACTH and cortisol concentrations or the pituitary and adrenal responsiveness to exogenous CRH stimulation. There was no effect of maternal nutritional treatment on adrenal IGF1, IGF1R, StAR, 3βHSD, and CYP11B mRNA expression in singleton fetuses. Fetal adrenal weight and adrenal CYP17 mRNA expression were each higher, however, in the PCUN, but not PIUN, fetuses compared with controls at 136–138 days gestation. This highlights that PCUN results in an increase in fetal adrenal growth and specific steroidogenic capacity in advance of any changes in circulating cortisol concentrations. Furthermore, it appears that maternal undernutrition imposed during the first week after conception alone is not sufficient to program an increase in adrenal growth and steroidogenic capacity in the late-gestation fetus. Interestingly, although adrenal weight and CYP17 expression were increased in the PCUN group, adrenal IGF2 expression was lower and IGF2R mRNA expression was higher in the adrenal of the PCUN fetus compared with controls. As discussed above, although there are positive relationships between adrenal growth and IGF2 expression in early gestation (17), the role of IGFs in the control of adrenal growth in late gestation is less clear. In a recent study, we also found that when either normally or overnourished ewes were exposed to periconceptional undernutrition, there was an increase in the weight of the adrenals in the postnatal lamb at 4 mo after birth and that this was associated with a decrease in adrenal IGF2 mRNA expression (34). These consistent findings of the impact of maternal PCUN on adrenal growth and IGF2 expression suggest that either IGF2 acts as an inhibitory growth factor in the perinatal adrenal or that adrenal IGF2 expression and bioavailability are suppressed after exposure to PCUN as a result of an increase in adrenal growth stimulated by other intra-adrenal trophic factor/s that are upregulated in the PCUN group.
Interestingly, we have found that, in the postnatal lamb, in contrast to the late-gestation fetus, the decrease in adrenal IGF2 expression after exposure to PCUN was associated with a significant decrease in the level of methylation in the proximal CTCF binding site in the DMR region of the IGF2/H19 gene (34).
TWINS.
In the current study, as previously reported (8), there was a delay in the prepartum increase in fetal plasma ACTH concentration in twins compared with singletons. The fetal ACTH and cortisol responses to CRH stimulation were also lower in control twins than in control singletons. In contrast to our previous study (8), however, maternal PCUN did not advance the timing of the prepartum ACTH rise in twins. One explanation of the difference between these two studies may be the use of different ACTH radioimmunoassays in each study, which measure different molecular weight forms of circulating ACTH-containing peptides in the fetal circulation. Interestingly, although the fetal ACTH response to CRH was lower, the cortisol response to CRH was the same in PCUN twins compared with PCUN singletons in the present study. Thus, PCUN may act to increase the pituitary output of ACTH containing peptides other than ACTH1–39 or, alternatively, increase the adrenal steroidogenic response to ACTH1–39 in the twin adrenal.
As in the PCUN singleton, fetal adrenal weight was increased in the PCUN twin compared with the control twin. Interestingly, in the PCUN twin this occurred in the absence of a decrease in adrenal IGF2 mRNA expression, although adrenal IGF2R mRNA expression was increased in the PCUN twin compared with controls. It may be, therefore, that any decrease in IGF2 bioavailability within the fetal adrenal as a consequence of either the decrease in IGF2 expression (PCUN singleton) or increased IGF2R expression (PCUN singleton and twin) may result in an increase in adrenal growth.
To summarize, we have shown that the methylation level in specific loci in the IGF2/H19 and IGF2R DMRs is lower in the adrenal of twin compared with singleton fetuses. We speculate that the decreased methylation levels in these parentally imprinted genes represent an epigenetic change induced early in a twin embryo as an adaptation designed to mitigate against the effects of the potential decrease in fetal substrate supply on the adrenal in later gestation. Interestingly, the expression of the ACTH receptor was also lower in the adrenal of the twin compared with that of the singleton fetus, which may explain, in part, the delay in the prepartum cortisol increase in twins compared with singletons. We have also found that exposure to maternal undernutrition during the periconceptional period had a greater effect on adrenal growth and IGF2, IGF2R, and CYP17 mRNA expression than exposure to undernutrition in the early preimplantation period alone. Changes in IGF2 and IGF2R expression occurred in the absence of changes in the methylation levels in the DMRs of the IGF2/H19 and IGF2R genes. The present study highlights that embryo number and the “nutritional memory” of the oocyte (or the matching of the nutritional environments of the oocyte and embryo) are each important in determining the longer-term consequences for adrenal growth and responsiveness to stimulation in later life.
GRANTS
This study was supported by funding from the Australian Research Council (I. C. McMillen and C. T. Roberts) and the National Health and Medical Research Council of Australia (I. C. McMillen). J. L. Morrison was supported by the Heart Foundation (South Australian Cardiovascular Research Network).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.W.-W., S.Z., S.M.M., D.O.K., S.K.W., C.T.R., and I.C.M. conception and design of research; O.W.-W., S.Z., S.M.M., D.O.K., S.K.W., C.M.S., J.E.C., J.L.M., C.T.R., and I.C.M. performed experiments; O.W.-W., S.Z., S.M.M., C.M.S., J.E.C., and I.C.M. analyzed data; O.W.-W., S.Z., S.M.M., D.O.K., S.K.W., C.M.S., J.E.C., J.L.M., C.T.R., and I.C.M. interpreted results of experiments; O.W.-W. prepared figures; O.W.-W. and I.C.M. drafted manuscript; O.W.-W., S.Z., S.M.M., D.O.K., S.K.W., C.M.S., J.E.C., J.L.M., C.T.R., and I.C.M. edited and revised manuscript; O.W.-W., S.Z., S.M.M., D.O.K., S.K.W., C.M.S., J.E.C., J.L.M., C.T.R., and I.C.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the research assistance provided by Anne Jurisevic, Laura O'Carroll, and Andrew Snell during the course of this study.
REFERENCES
- 1.Agricultural and Food Research Council. Energy and Protein Requirements of Ruminants. An Advisory Manual Prepared by the AFRC Technical Commitee on Responses to Nutrients. Wallingford, UK: CAB International, 1993 [Google Scholar]
- 2. Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JR, Harding JE. A periconceptional nutritional origin for noninfectious preterm birth. Science 300: 606, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JRG. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology 145: 4278–4285, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Butler TG, Schwartz J, McMillen IC. Differential effects of the early and late intrauterine environment on corticotrophic cell development. J Clin Invest 110: 783–791, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Challis JRG, Bloomfield FH, Bocking AD, Casciani V, Chisaka H, Connor K, Dong X, Gluckman P, Harding JE, Johnstone J, Li W, Lye S, Okamura K, Premyslova M. Fetal signals and parturition. J Obstet Gynaecol Res 31: 492–499, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Coulter CL, Salkeld MD, McMillen IC. Adrenal TGF(beta)1 mRNA levels fall during late gestation and are not regulated by cortisol in the sheep fetus. Mol Cell Endocrinol 206: 85–91, 2003 [DOI] [PubMed] [Google Scholar]
- 7. de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 117: 1058–1067, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biol Reprod 66: 1562–1569, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gardner DS, Jamall E, Fletcher AJW, Fowden AL, Giussani DA. Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses. J Physiol 557: 1021–1032, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han VK, Lu F, Bassett N, Yang KP, Delhanty PJ, Challis JR. Insulin-like growth factor-II (IGF-II) messenger ribonucleic acid is expressed in steroidogenic cells of the developing ovine adrenal gland: evidence of an autocrine/paracrine role for IGF-II. Endocrinology 131: 3100–3109, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Ideraabdullah FY, Vigneau S, Bartolomei MS. Genomic imprinting mechanisms in mammals. Mutat Res 647: 77–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet 8: 253–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 64: 412–418, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Li CCY, Maloney CA, Cropley JE, Suter CM. Epigenetic programming by maternal nutrition: shaping future generations. Epigenomics 2: 539–549, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994 [DOI] [PubMed] [Google Scholar]
- 16. MacLaughlin SM, McMillen IC. Impact of periconceptional undernutrition on the development of the hypothalamo-pituitary-adrenal axis: does the timing of parturition start at conception? Curr Drug Targets 8: 880–887, 2007 [DOI] [PubMed] [Google Scholar]
- 17. MacLaughlin SM, Walker SK, Kleemann DO, Sibbons JP, Tosh DN, Gentili S, Coulter CL, McMillen IC. Impact of periconceptional undernutrition on adrenal growth and adrenal insulin-like growth factor and steroidogenic enzyme expression in the sheep fetus during early pregnancy. Endocrinology 148: 1911–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 18. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005 [DOI] [PubMed] [Google Scholar]
- 19. McMillen IC, Schwartz J, Coulter CL, Edwards LJ. Early embryonic environment, the fetal pituitary-adrenal axis and the timing of parturition. Endocr Res 30: 845–850, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1379, 2002 [PubMed] [Google Scholar]
- 21. Myers DA, McDonald TJ, Nathanielsz PW. Effect of placement of dexamethasone adjacent to the ovine fetal paraventricular nucleus on adrenocortical steroid hydroxylase messenger ribonucleic acid. Endocrinology 131: 1329–1335, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Phillips DIW. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med 261: 453–460, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Phillips DIW, Walker BR, Reynolds RM, Flanagan DEH, Wood PJ, Osmond C, Barker DJP, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35: 1301–1306, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Phillips ID, Ross JT, Owens JA, Young IR, McMillen IC. The peptide ACTH(1–39), adrenal growth and steroidogenesis in the sheep fetus after disconnection of the hypothalamus and pituitary. J Physiol 491: 871–879, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross JT, Bennett HPJ, James S, McMillen IC. Infusion of N-proopiomelanocortin-(1–77) increases adrenal weight and messenger ribonucleic acid levels of cytochrome P450 17α-hydroxylase in the sheep fetus during late gestation. Endocrinology 141: 2153–2158, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Ross JT, McMillen IC, Lok F, Thiel AG, Owens JA, Coulter CL. Intrafetal insulin-like growth factor-I infusion stimulates adrenal growth but not steroidogenesis in the sheep fetus during late gestation. Endocrinology 148: 5424–5432, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Ross JT, Phillips ID, Simonetta G, Owens JA, Robinson JS, McMillen IC. Differential effects of placental restriction on IGF-II, ACTH receptor and steroidogenic enzyme mRNA levels in the foetal sheep adrenal. J Neuroendocrinol 12: 79–85, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Rumball CWH, Oliver MH, Thorstensen EB, Jaquiery AL, Husted SM, Harding JE, Bloomfield FH. Effects of twinning and periconceptional undernutrition on late-gestation hypothalamic-pituitary-adrenal axis function in ovine pregnancy. Endocrinology 149: 1163–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Saphier PW, Glynn BP, Woods RJ, Shepherd DA, Jeacock MK, Lowry PJ. Elevated levels of N-terminal pro-opiomelanocortin peptides in fetal sheep plasma may contribute to fetal adrenal gland development and the pre-parturient cortisol surge. Endocrinology 133: 1459–1461, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Warnes KE, McMillen IC, Robinson JS, Coulter CL. Metyrapone infusion stimulates adrenal growth without activating the cell cycle or the IGF system in the late gestation fetal sheep. Endocr Res 30: 535–539, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci 7: 847–854, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomorey S, Lye SJ, Gibb W, Challis JRG. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol Reprod 64: 1019–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Worthman CM, Kuzara J. Life history and the early origins of health differentials. Am J Hum Biol 17: 95–112, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, Molloy L, Kleemann D, Walker SK, Muhlhausler BS, Morrison JL, McMillen IC. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J 24: 2772–2782, 2010 [DOI] [PubMed] [Google Scholar]