Abstract
The mechanisms underlying developmental programming are poorly understood but may be associated with adaptations by the fetus in response to changes in the maternal environment during pregnancy. We hypothesized that maternal nutrient restriction during pregnancy alters vasodilator responses in fetal coronary arteries. Pregnant ewes were fed a control [100% U.S. National Research Council (NRC)] or nutrient-restricted (60% NRC) diet from days 50 to 130 of gestation (term = 145 days); fetal tissues were collected at day 130. In coronary arteries isolated from control fetal lambs, relaxation to bradykinin was unaffected by nitro-l-arginine (NLA). Iberiotoxin or contraction with KCl abolished the NLA-resistant response to bradykinin. In fetal coronary arteries from nutrient-restricted ewes, relaxation to bradykinin was fully suppressed by NLA. Large-conductance, calcium-activated potassium channel (BKCa) currents did not differ in coronary smooth muscle cells from control and nutrient-restricted animals. The BKCa openers, BMS 191011 and NS1619, and 14,15-epoxyeicosatrienoic acid [a putative endothelium-derived hyperpolarizing factor (EDHF)] each caused fetal coronary artery relaxation and BKCa current activation that was unaffected by maternal nutrient restriction. Expression of BKCa-channel subunits did not differ in fetal coronary arteries from control or undernourished ewes. The results indicate that maternal undernutrition during pregnancy results in loss of the EDHF-like pathway in fetal coronary arteries in response to bradykinin, an effect that cannot be explained by a decreased number or activity of BKCa channels or by decreased sensitivity to mediators that activate BKCa channels in vascular smooth muscle cells. Under these conditions, bradykinin-induced relaxation is completely dependent on nitric oxide, which may represent an adaptive response to compensate for the absence of the EDHF-like pathway.
Keywords: maternal nutrient restriction, fetal coronary artery, nitric oxide, endothelium-derived hyperpolarizing factor, BKCa channels
human epidemiological and experimental animal studies provide convincing support for the concept of developmental programming, which suggests that developmental insults or stressors [i.e., maternal undernutrition and exposure to stress-related hormones (e.g., cortisol)] during pregnancy result in reprogramming or adaptation of the fetal physiology in a manner that increases the risk of developing disease in subsequent years of life (42). Developmentally compromised infants have an increased risk of health complications, not just as infants but throughout their lifespan, including a range of metabolic, neurological, behavioral, reproductive, and cardiovascular disorders (1, 4, 30, 41).
Historically, the concept of developmental programming was based on epidemiological studies that focused exclusively on individuals with low birth weight. Subsequent studies in humans and animals now increasingly suggest that developmental programming can occur independently of birth weight (4, 41), such that individual organ systems or physiologic processes are adversely affected by one or more developmental insults or stressors, resulting in offspring phenotypes similar to those associated with low birth weight. These phenotypes include poor growth, increased adiposity, poor glucose tolerance, and dislipidemia (4, 16, 18, 50).
Endothelial cells lining the arterial lumen produce numerous vasoactive mediators that regulate the tone of the underlying vascular smooth muscle (13), thereby adjusting blood supply to the target organ to meet changes in metabolic needs. These mediators include both vasodilators [e.g., nitric oxide (NO) and endothelium-derived hyperpolarizing factor (EDHF)] and vasoconstrictors (e.g., endothelin). Several studies using a variety of animal species and experimental paradigms demonstrate that maternal undernutrition during pregnancy leads to endothelial dysfunction in the offspring and that these impairments contribute to the development of cardiovascular disease (3, 29, 41). Mechanisms underlying this endothelial dysfunction and the programming of cardiovascular disease are poorly understood, but emerging evidence suggests that a poor nutrient supply at critical periods of early development leads to permanent alterations in vascular structure or function. Impairment in both vasoconstriction and vasorelaxation responses has been reported. For example, in humans, low birth weight is associated with impaired endothelium-dependent relaxation in infants (32), children (33), and young adults (27). Similarly, maternal undernutrition leads to impaired endothelium-dependent and -independent relaxation of sheep femoral arteries (38) and rat mesenteric arteries (6, 48) and aortae (40) in the offspring. Moreover, in rats, maternal undernutrition causes alterations in vasoconstrictor responses in femoral and carotid arteries of offspring (39, 53).
The effects of maternal undernutrition during pregnancy on fetal coronary arteries are poorly understood at present. The aim of the present study was to determine the effect of maternal nutrient restriction on fetal coronary arterial function using a well-established mid- to late-gestational sheep model of maternal undernutrition. Since the endogenous endothelium-dependent vasodilator, bradykinin, is capable of activating several endothelial vasorelaxant signaling pathways (i.e., NO, prostacyclin, and EDHF) in arteries (14, 37, 47), bradykinin was used as a pharmacological tool to assess the effects of maternal undernutrition on endothelial function. We specifically tested the hypothesis that maternal undernutrition during the last two-thirds of pregnancy impairs endothelium-dependent vasorelaxation and large-conductance, calcium-activated potassium channel (BKCa) function in fetal coronary artery smooth muscle cells in late pregnancy.
MATERIALS AND METHODS
Materials
The following drugs or chemicals were used: acetylcholine, bradykinin, DL-DTT, glyburide, indomethacin, nitro-l-arginine (NLA), NS1619, serotonin, sodium nitroprusside, and soybean trypsin inhibitor (Sigma Chemical, St Louis, MO); BMS 191011 and iberiotoxin (Tocris, Ellisville, MO); collagenase, elastase, and papain (Worthington Biochemical, Lakewood, NJ); and 14,15-epoxyeicosatrienoic acid (EET) and 9,11-dideoxy-11α,9α-epoxymethano-PGF2α (U46619; Cayman Chemical, Ann Arbor, MI). All other chemicals were purchased from Sigma Chemical, unless stated otherwise. Drug solutions were prepared daily, kept on ice, and protected from light until used. All drugs were dissolved initially in double-distilled water, with the exception of aprikalim (70% ethanol), indomethacin (0.1 mM; sodium carbonate solution), glyburide (0.1 N; NaOH), BMS 191011, and NS1619 (DMSO), before further dilution in distilled water. Drugs were added to the organ chambers in volumes not greater than 0.2 ml. Drug concentrations are reported as the final molar concentration in the organ chamber. The composition of the physiological salt solution was as follows (in mM): NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, and glucose 11.1.
Methods
Animals and experimental design.
This study was approved by the North Dakota State University (NDSU) Animal Care and Use Committee and was designed as described previously (11, 28). Briefly, nulliparous Western whiteface ewes, carrying singleton pregnancies, were transported to the NDSU Animal Nutrition and Physiology Center and fed to meet nutrient requirements [U.S. National Research Council (NRC), 2007] during early gestation. At day 28 of gestation, ewes were moved from the pasture into a temperature-controlled (14°C) facility with a 12:12 light:dark cycle with lights on at 0700 and off at 1900 for the remainder of the study. On day 45 of gestation, ewes were acclimated to a pelleted diet in a stepwise manner and provided trace mineral salt blocks and ad libitum access to water. Dietary treatments began on day 50 of gestation and consisted of a control [100% of NRC (2007) recommendations] or nutrient-restricted [60% of NRC (2007) recommendations] diet until day 130 of gestation (term = 145 days). Nutritional requirements were based on NRC (37a) recommendations for a 60-kg body wt pregnant ewe lamb during mid to late gestation. Feed offered to individual ewes was adjusted based on weekly body weight and body-weight change to achieve the desired average daily gain (11, 28).
Tissue collection.
Ewes were killed on day 130 of gestation. The uterus was removed, and the gravid uterine horn was dissected. Fetuses of either sex (n = 7 control and 9 nutrient-restricted males; n = 7 control and 6 nutrient-restricted females) were removed, weighed, and exsanguinated. Fetal hearts were removed at dissection, weighed, and further processed. The fetal heart left- and right-ventricle thickness at top, mid, and base positions and overall average thickness of ventricles were recorded using digital calipers. Subsequently, the left-circumflex coronary artery and the anterior interventricular coronary artery were isolated and placed into cold physiological salt solution.
Organ chamber studies.
Left-circumflex coronary arteries were cleaned of adherent fat and connective tissue and cut into rings (3–4 mm in length), with care being taken to avoid damaging the vascular endothelium. Four to eight arterial rings were prepared from each heart. In some rings, the endothelium was removed by gently rubbing the intimal surface with fine forceps. Coronary arterial rings were suspended in water-jacketed organ chambers filled with 25 ml physiological salt solution, as described previously (46, 49, 51). The organ chamber solution was aerated with a mixture of 95% O2/5% CO2, and the temperature was maintained at 37°C throughout the experiment. Each ring was suspended by means of two fine stainless-steel wire clips passed through the lumen; one clip was anchored inside of the organ chamber and the other connected to a force transducer (Model FT03; Grass Instrument, Warwick, RI). Isometric tension was measured and recorded on a Grass polygraph. The tissues were stretched progressively to the optimal point of their length-tension relationship, using KCl (20 mM) to generate a standard contractile response. Optimal resting tension was ∼1.5 g in rings from both control and nutrient-restricted animals (P > 0.05). After this procedure, the preparations were equilibrated at their optimal length for at least 30 min before further exposure to any vasoactive substances. Removal of the endothelium was confirmed in endothelium-denuded arterial rings by the absence of relaxation to the endothelium-dependent vasodilator, bradykinin (10−7 M).
Relaxation of fetal coronary arteries was studied in rings contracted with the thromboxane A2 mimetic, U46619 (3 × 10−9 M). After the U46619-induced contraction had reached a stable plateau, relaxation responses to increasing concentrations of bradykinin (10−10-10−6 M), sodium nitroprusside (10−9-10−5 M), aprikalim (10−9-10−5 M), NS1619 (10−8-10−5 M), BMS 191011 (10−9-10−5 M), or 14,15-EET (10−6 M) were obtained. A single concentration of 14,15-EET was used, since in preliminary studies, this concentration produced the most consistent and reproducible relaxations in isolated fetal coronary arteries; cumulative addition of 14,15-EET (10−7-10−5 M) failed to produce reproducible responses on a consistent basis in these preparations. Relaxation responses to bradykinin (10−10-10−6 M) were also obtained in rings contracted with KCl (60 mM). In some experiments, the preparations were incubated with indomethacin (10−5 M), NLA (3 × 10−5 M), iberiotoxin (10−7 M), or glyburide (10−6 M) for 30 min before contracting the tissue with U46619. These inhibitors remained in contact with the tissues throughout the remainder of the experiment. Contractile responses to increasing concentrations of acetylcholine (10−9-10−5 M) and serotonin (10−9-10−5 M) were also determined.
Vascular smooth muscle cell isolation.
Anterior interventricular fetal coronary arteries were cut into small pieces in low-calcium Tyrodes's solution of the following composition (in mM): 145 NaCl, 4 KCl, 0.05 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH = 7.4, adjusted with NaOH) at 4°C. Tissue pieces were incubated for 15 min with gentle shaking at 37°C in 1 ml of the low-calcium Tyrodes's solution containing 1.5 mg/ml papain (14 U/mg) and 1 mg/ml DTT, followed by incubation for 15 min at 37°C in 2 ml of the low-calcium Tyrodes's solution containing 2 mg/ml collagenase (196 U/ml), 0.5 mg/ml elastase (90 U/ml), and 1 mg/ml soybean trypsin inhibitor (10,000 U/ml). The enzyme solutions were removed by centrifugation at 500 g for 1–2 min, followed by removal of the supernatant with a pipette. Fresh low-calcium Tyrodes's solution (5 ml) was then added to the tissue pieces. Single vascular smooth muscle cells were released by gently triturating with a 5-ml glass pipette and collected in the supernatant. The cells in the supernatant were centrifuged at 500 g for 5 min, resuspended in fresh low-calcium Tyrodes's solution, and stored at 4°C. Cells were used within 5–8 h of isolation.
Electrophysiology studies.
Freshly isolated vascular smooth muscle cells were placed in a small recording chamber and were perfused constantly with extracellular solution, as described previously (26). The extracellular solution for recording BKCa current amplitude contained (in mM): 145 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, and 10 glucose (pH = 7.4, adjusted with NaOH). The internal pipette solution was composed of (in mM): 145 potassium aspartate, 5 NaCl, 1 CaCl2, 2.2 EGTA, 10 HEPES, and 7.5 glucose (pH = 7.4, adjusted with KOH). Drugs were dissolved in extracellular solution as 10 mM stocks and diluted to the desired concentrations in extracellular solution.
The whole-cell voltage-clamp technique was used for recording BKCa current. Micropipettes (2–5 MΩ resistance) were made from capillary tubing (World Precision Instruments, Sarasota, FL) using a programmable puller (P-80/PC; Sutter Instrument, Novato, CA). Series resistance and capacitance compensation were adjusted maximally using a voltage-clamp amplifier (Axopatch 200A; Axon Instrument, Union City, CA), as described previously (26). Data were digitized at 2 kHz and filtered at 1 kHz.
To allow for equilibration of the pipette solution with the cell interior, all recordings were initiated ∼5 min after establishing the whole-cell configuration. Most experiments were performed within 15–20 min of gaining access, during which time, the macroscopic whole-cell BKCa current amplitude remained stable. The whole-cell BKCa current was elicited from a holding potential of −60 mV in response to successive voltage pulses of 200 ms duration, increasing in 10-mV increments from −70 mV to +60 mV in the absence or presence of NS1619 (10−5 M) or 14,15-EET (10−6 M). The BKCa amplitude was recorded before (control), during, and after (wash) the administration of a particular drug. Current-voltage plots were determined for each drug tested.
Quantitative real-time RT-PCR.
Quantitative real-time RT-PCR was performed as reported previously (24). Briefly, snap-frozen fetal coronary arteries were homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH), according to the manufacturer's specifications. The quality and quantity of total cellular RNA (tcRNA) were determined via capillary electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE). Real-time RT-PCR reagents, probes, and primers were purchased from and used as recommended by Applied Biosystems (Foster City, CA). For each sample, 30 ng tcRNA was reverse transcribed in triplicate 20-μl reactions using random hexamers. An ABI PRISM 7000 was used for the detection of sequences amplified at 60°C, typically for 40 or 45 cycles (Applied Biosystems). Expression of the BKCa α (GenBank accession number: EU419755) and β (GenBank accession number: JF499670) subunits was normalized to the expression of 18S in a multiplex reaction using the human 18S predeveloped assay reagent from Applied Biosystems, which is primer limited and contains a VIC-labeled probe. The predeveloped assay reagent was adjusted further using one-fourth of the normal amount so that it would not interfere with amplification of the FAM-labeled gene of interest. The multiplex reaction was also used to generate standard curves for quantification of 18S and the gene of interest based on dilutions of the cDNA generated from tcRNA, collected from pooled tissues on day 130 of pregnancy.
Data analysis.
Relaxation responses are expressed as a percentage of the initial tension induced by U46619, and contractile responses are expressed as a percentage of the maximal response evoked by KCl (60 mM). For each vasodilator or vasoconstrictor, both the maximal percent response and the EC50 were determined. The EC50 values were converted to the negative logarithms and expressed as −log molar EC50. Membrane capacitance was calculated by integrating capacitive currents generated by a 10-mV hyperpolarizing pulse after electronic cancellation of the pipette-patch capacitance. Peak current amplitudes were measured as the mean current during the last 100 ms of voltage steps and expressed in picoamperes/picofarad (pA/pF) to normalize for differences in the cell membrane area between isolated vascular smooth muscle cells. Data were analyzed using pCLAMP software (Axon Instrument). Expression of the BKCa α- and β-subunit mRNA was normalized to the expression of 18S mRNA. All results are expressed as mean ± SE, and n refers to the number of animals from which blood vessels were taken. Values were compared by Student's t-test for paired or unpaired observations or by ANOVA to determine significance between groups as appropriate. Values were considered to be significantly different when P < 0.05.
RESULTS
Fetal Weight and Heart Development Parameters at Day 130 of Gestation
At day 130 of gestation, neither fetal body weight nor fetal heart weight differed significantly between the control and nutrient-restricted ewes (P > 0.05; Table 1). Similarly, maternal nutrient restriction had no effect on fetal left and right ventricular-wall thickness, with the exception of right-ventricle base thickness, which was increased in fetuses from the nutrient-restricted group compared with controls (P = 0.04; Table 1).
Table 1.
Fetal weight and heart development parameters at day 130 of gestation
| Mean ± SE |
|||
|---|---|---|---|
| Control Diet, 100% | Restricted Diet, 60% | P Values | |
| Fetal weight, g | 3,383 ± 214 | 3,212 ± 122 | 0.48 |
| Heart weight, g | 23.9 ± 1.1 | 22.2 ± 0.8 | 0.23 |
| Heart weight/fetal weight, g/kg | 7.1 ± 0.3 | 6.9 ± 0.3 | 0.63 |
| Average left-ventricle thickness, mm | 5.1 ± 0.2 | 4.6 ± 0.2 | 0.09 |
| Left-ventricle thickness, mm | |||
| Top | 6.0 ± 0.4 | 5.2 ± 0.3 | 0.11 |
| Mid | 5.6 ± 0.2 | 5.2 ± 0.3 | 0.33 |
| Base | 3.7 ± 0.3 | 3.5 ± 0.4 | 0.73 |
| Average right-ventricle thickness, mm | 4.2 ± 0.1 | 4.4 ± 0.1 | 0.24 |
| Right-ventricle thickness, mm | |||
| Top | 4.5 ± 0.2 | 4.6 ± 0.2 | 0.46 |
| Mid | 4.8 ± 0.2 | 4.3 ± 0.2 | 0.25 |
| Base | 3.3 ± 0.2 | 5.1 ± 0.2 | 0.04 |
Pharmacologic Studies
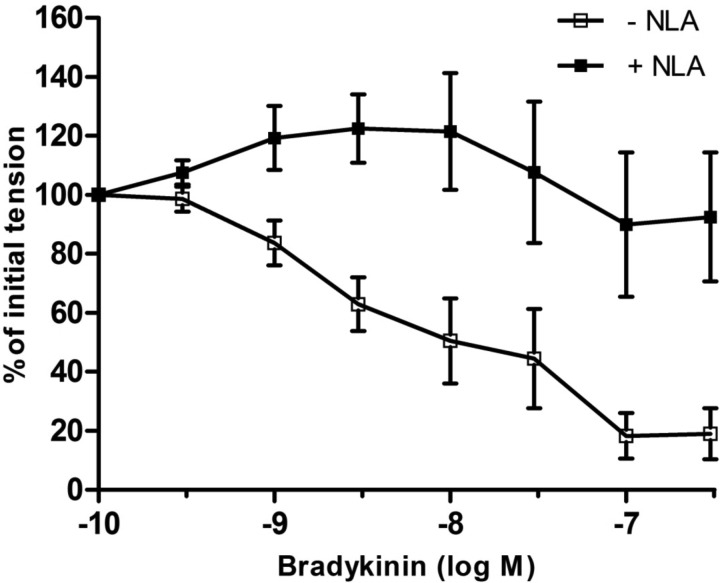
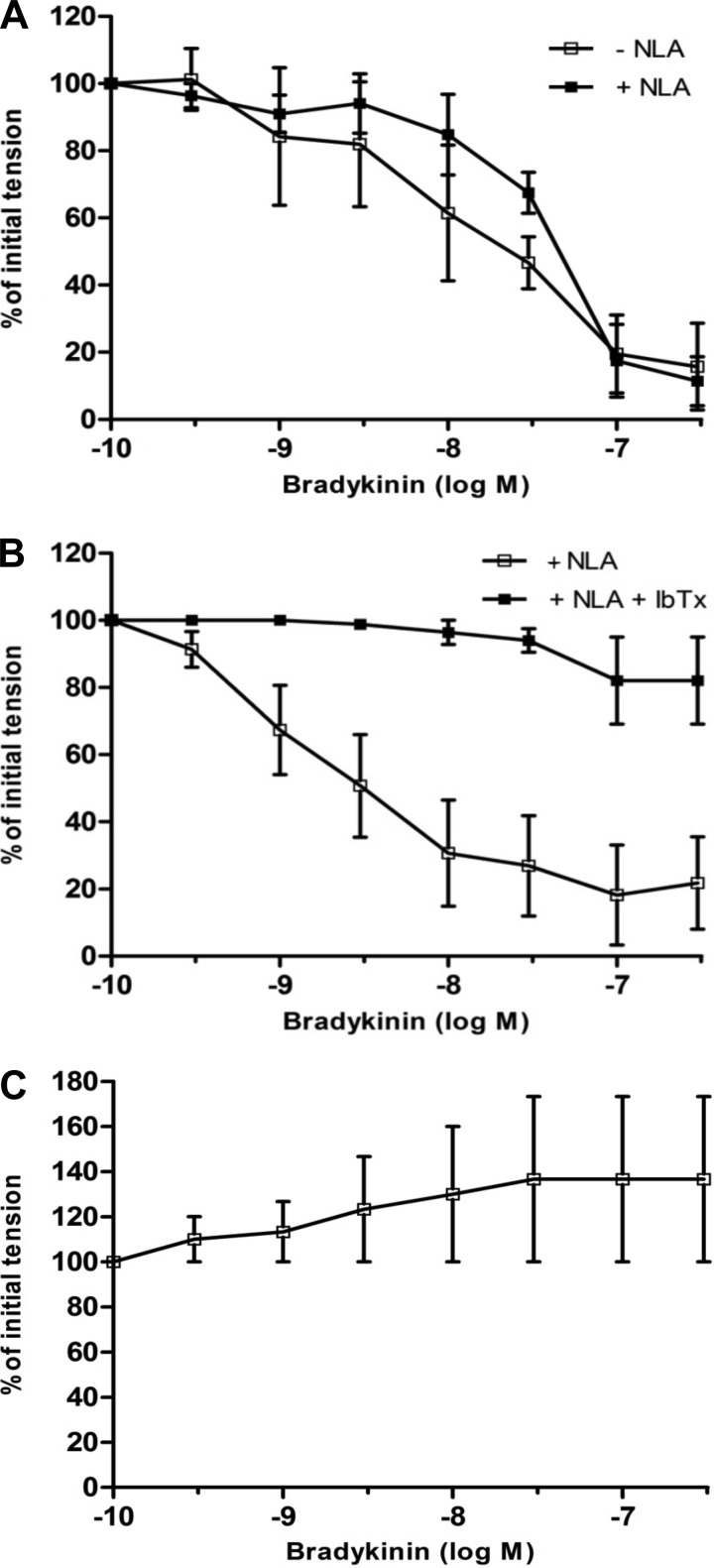
The endothelium-dependent vasodilator, bradykinin (10−10–10−6 M) (19, 34), produced concentration-dependent relaxations that were similar in fetal coronary artery rings from control and nutrient-restricted ewes (Fig. 1 and Table 2; P > 0.05). In nutrient-restricted ewes, bradykinin-induced relaxation of fetal coronary arteries was fully suppressed by the NO synthase (NOS) inhibitor NLA (3 × 10−5 M) and converted to a contractile response (Fig. 2). In contrast, NLA had no effect on bradykinin-induced relaxation in control fetal coronary artery rings (Fig. 3A). The cyclooxygenase inhibitor, indomethacin (35), had no effect on bradykinin-induced relaxation of fetal coronary arteries from control or nutrient-restricted ewes treated with or without NLA (data not shown). The NLA-resistant response to bradykinin in control coronary artery rings was unaffected by the selective ATP-sensitive potassium (KATP) channel blocker, glyburide (44), but was nearly abolished in the presence of iberiotoxin (10−7 M), a selective BKCa channel blocker (20) (Fig. 3B), or by contracting the rings with a maximal depolarizing concentration of KCl (60 mM) instead of U46619 (Fig. 3C).
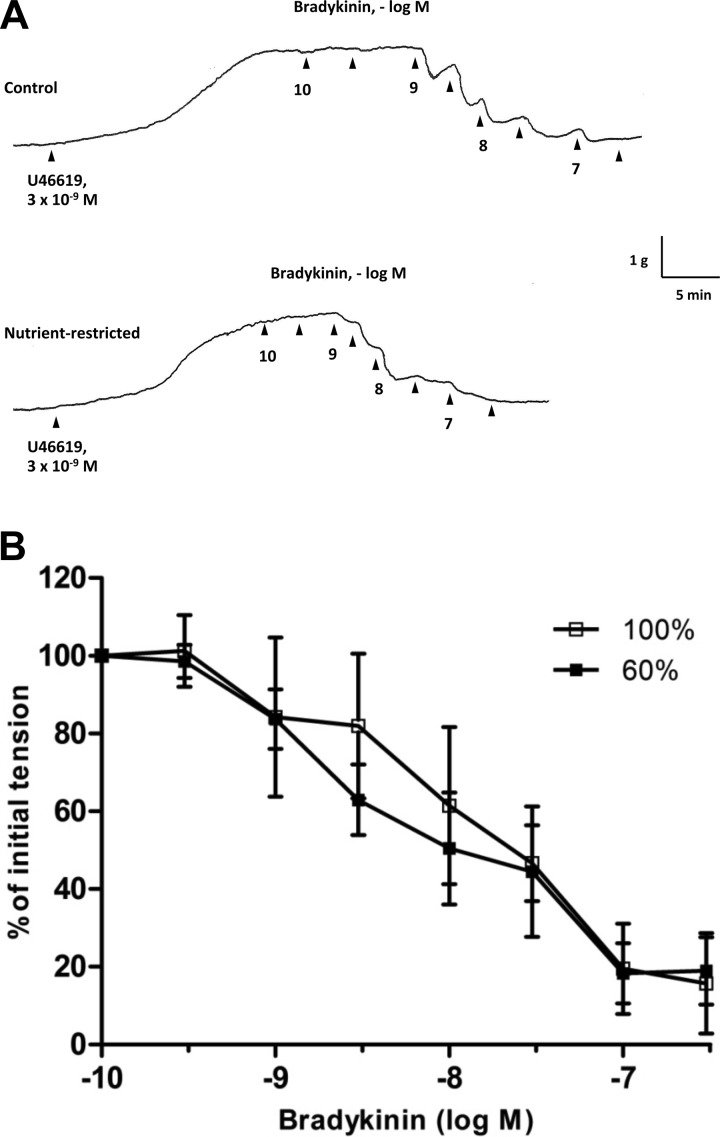
Fig. 1.
A: isometric tension recordings demonstrating responsiveness of isolated rings of fetal coronary artery to bradykinin. Arterial rings were contracted with 9,11-dideoxy-11α,9α-epoxymethano-PGF2α (U46619), followed by cumulative addition of bradykinin at the time points marked by arrowheads. B: log concentration-response curves for bradykinin in producing relaxations of isolated fetal coronary arteries from ewes fed a control (100%) or nutrient-restricted (60%) diet during the last ⅔ of pregnancy. Data are expressed as a percentage of the initial increase in tension induced by U46619 (3 × 10−9 M), which averaged 2.22 ± 0.3 and 2.38 ± 0.2 g in fetal coronary arteries from ewes fed a control and nutrient-restricted diet, respectively (P > 0.05). Each point represents the mean ± SE; n = 6.
Table 2.
pD2 and Emax values for various vasodilators and vasoconstrictors in fetal coronary arteries at day 130 of gestation
| pD2 |
Emax, % Relaxation* |
||||
|---|---|---|---|---|---|
| Control Diet, 100% | Restricted Diet, 60% | Control Diet, 100% | Restricted Diet, 60% | n | |
| Vasodilators | |||||
| Bradykinin | 8.06 ± 0.3 | 8.22 ± 0.3 | 86 ± 10 | 84 ± 8 | 6 |
| Sodium nitroprusside | 7.89 ± 0.2 | 8.03 ± 0.1 | 99 ± 1 | 99 ± 1 | 4 |
| Aprikalim | 6.44 ± 0.2 | 6.35 ± 0.2 | 97 ± 3 | 98 ± 2 | 4 |
| NS1619 | 5.86 ± 0.5 | 5.36 ± 0.5 | 100 ± 0 | 100 ± 0 | 5 |
| BMS 191011 | 5.76 ± 0.4 | 5.51 ± 0.3 | 94 ± 6 | 100 ± 0 | 6 |
| pD2 |
Emax, % Contraction† |
||||
|---|---|---|---|---|---|
| Control Diet, 100% | Restricted Diet, 60% | Control Diet, 100% | Restricted Diet, 60% | n | |
| Vasoconstrictors | |||||
| Acetylcholine | 5.92 ± 0.1 | 5.74 ± 0.2 | 92 ± 3 | 92 ± 5 | 5 |
| Serotonin | 5.74 ± 0.2 | 5.34 ± 0.1 | 90 ± 2 | 91 ± 4 | 5 |
pD2, −log molar EC50; Emax, maximal percent response.
Data are expressed as percent relaxation of coronary artery rings contracted with 9,11-dideoxy-11α,9α-epoxymethano-PGF2α (3 × 10−9 M). Contractions averaged 2.42 ± 0.2 and 2.34 ± 0.2 g in rings from control and nutrient-restricted animals, respectively (P > 0.05). †Data are expressed as a percent of the contractile response to KCl (60 mM), which averaged 1.82 ± 0.3 and 1.48 ± 0.3 g in coronary arteries from control and nutrient-restricted animals, respectively (P > 0.05).
Fig. 2.
Effect of nitro-l-arginine (NLA; 3 × 10−5 M) on bradykinin-induced relaxation of isolated fetal coronary arteries from ewes fed a nutrient-restricted (60%) diet during the last ⅔ of pregnancy. Data are expressed as a percentage of the initial increase in tension induced by U46619 (3 × 10−9 M), which averaged 2.38 ± 0.2 and 2.50 ± 0.3 g in coronary arteries in the absence and presence of NLA, respectively (P > 0.05). Each point represents the mean ± SE; n = 6.
Fig. 3.
Effect of (A) NLA (3 × 10−5 M) or (B) NLA (3 × 10−5 M) + iberiotoxin (IbTx; 10−7 M) on bradykinin-induced relaxation of isolated fetal coronary arteries contracted with U46619 (3 × 10−9 M) from ewes fed a control diet (100%) during the last ⅔ of pregnancy. C: cumulative concentration-response curve for bradykinin [in the presence of NLA (3 × 10−5 M)] in isolated control fetal coronary arteries contracted with KCl (60 mM). Data are expressed as a percentage of the initial increase in tension induced by U46619 (3 × 10−9 M), which averaged 2.26 ± 0.5 (A and B), or KCl (60 mM), which averaged 2.06 ± 0.3 (C). Each point represents the mean ± SE; n = 5–6.
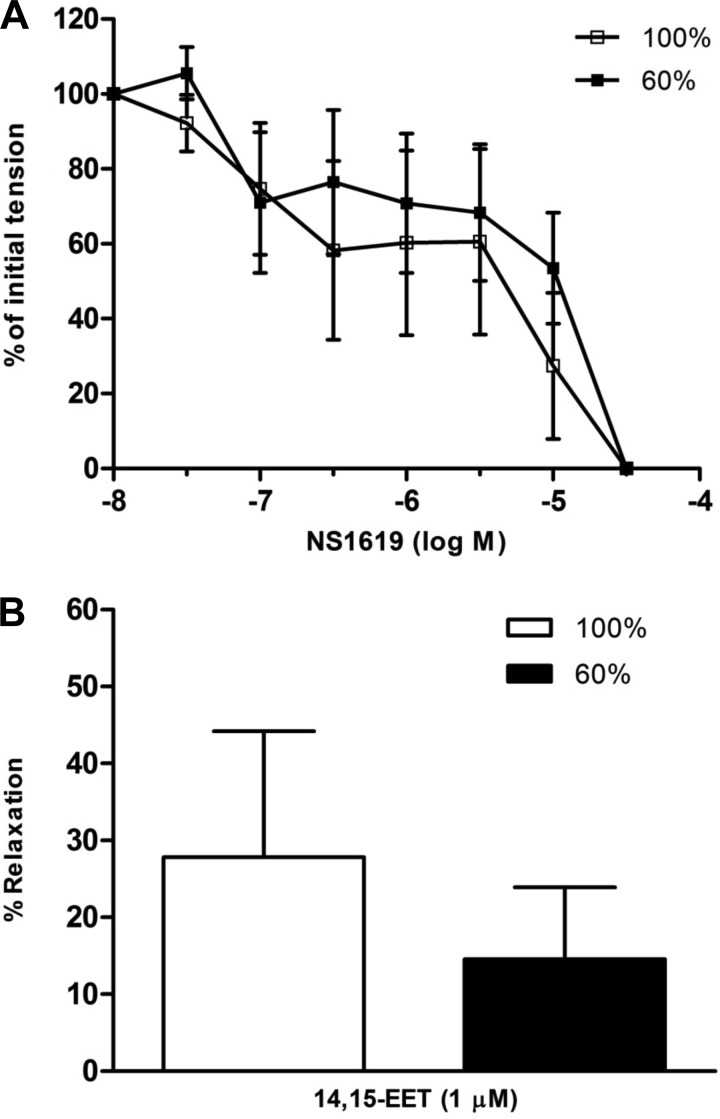
Sodium nitroprusside (10−9-10−5 M), an exogenous NO donor (25), also caused relaxation of fetal coronary arteries that did not differ between tissues taken from control and nutrient-restricted ewes (Table 2). Similarly, maternal nutrient restriction had no effect on relaxation responses to the KATP channel opener (2) aprikalim (10−9-10−5 M; Table 2), the BKCa channel openers (22, 43) NS1619 and BMS 191011 (10−8-10−5 M; Fig. 4A and Table 2), or 14,15-EET (10−6 M; Fig. 4B), a putative EDHF in coronary arteries (5, 9, 10, 21). Vasoconstrictor responses to acetylcholine and serotonin in fetal coronary arteries were unaltered by maternal nutrient status (Table 2; P > 0.05).
Fig. 4.
Relaxation responses to (A) NS1619 (10−8-10−5 M) or (B) 14,15-epoxyeicosatrienoic acid (EET; 10−6 M) in isolated fetal coronary arteries from ewes fed a control (100%) or nutrient-restricted (60%) diet during the last ⅔ of pregnancy. Data are expressed as a percentage of the initial increase in tension induced by U46619 (3 × 10−9 M), which averaged 2.49 ± 0.3 and 2.20 ± 0.3 g in fetal coronary artery rings from ewes fed a control and nutrient-restricted diet, respectively (P > 0.05). Each point represents the mean ± SE; n = 4–5.
Electrophysiology Studies
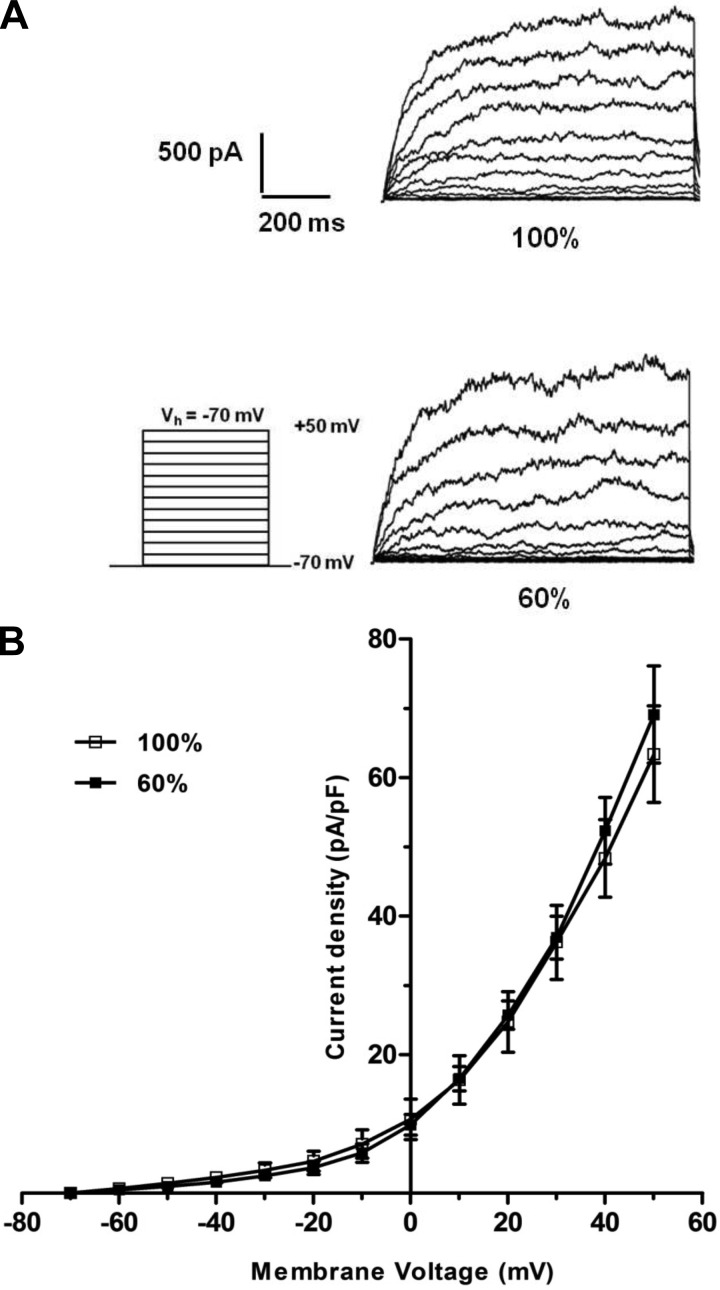
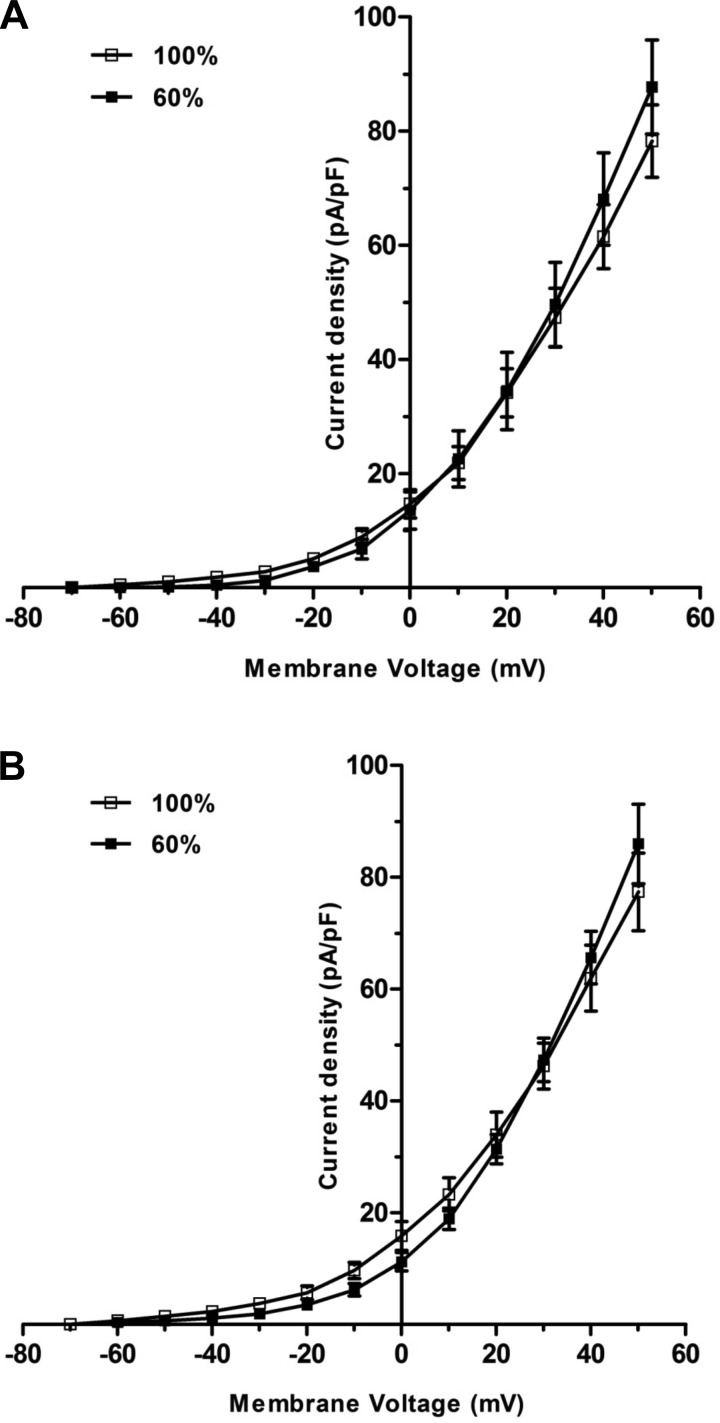
Whole-cell BKCa currents were nearly identical in fetal coronary artery smooth muscle cells from control and nutrient-restricted ewes (peak current density = 46 ± 5 vs. 39 ± 6 pA/pF; n = 6; P > 0.05; Fig. 5). Similarly, the whole-cell BKCa currents obtained in the presence of NS1619 (10−5 M) or 14,15-EET (10−6 M) did not differ significantly between fetal coronary smooth muscle cells from control and nutrient-restricted ewes (Fig. 6; P > 0.05).
Fig. 5.
Representative recordings (A) or the current-voltage (I/V) relationship curve (B) for the whole-cell large-conductance calcium-activated potassium channel (BKCa) currents induced by successive voltage pulses of 200 ms duration, increasing in 10-mV increments from −70 mV to +60 mV, in freshly isolated fetal coronary smooth muscle cells from ewes fed a control (100%) or nutrient-restricted (60%) diet during the last ⅔ of pregnancy. Each point represents the mean ± SE; n = 6. Vh, holding potential; pA/pF, picoamperes/picofarad.
Fig. 6.
The I/V relationship curves for whole-cell BKCa currents obtained in the presence of (A) NS1619 (10−5 M) or (B) 14,15-EET (10−6 M) in freshly isolated fetal coronary smooth muscle cells from ewes fed a control (100%) or nutrient-restricted diet (60%) during the last ⅔ of pregnancy. Each point represents the mean ± SE; n = 6.
Quantitative RT-PCR
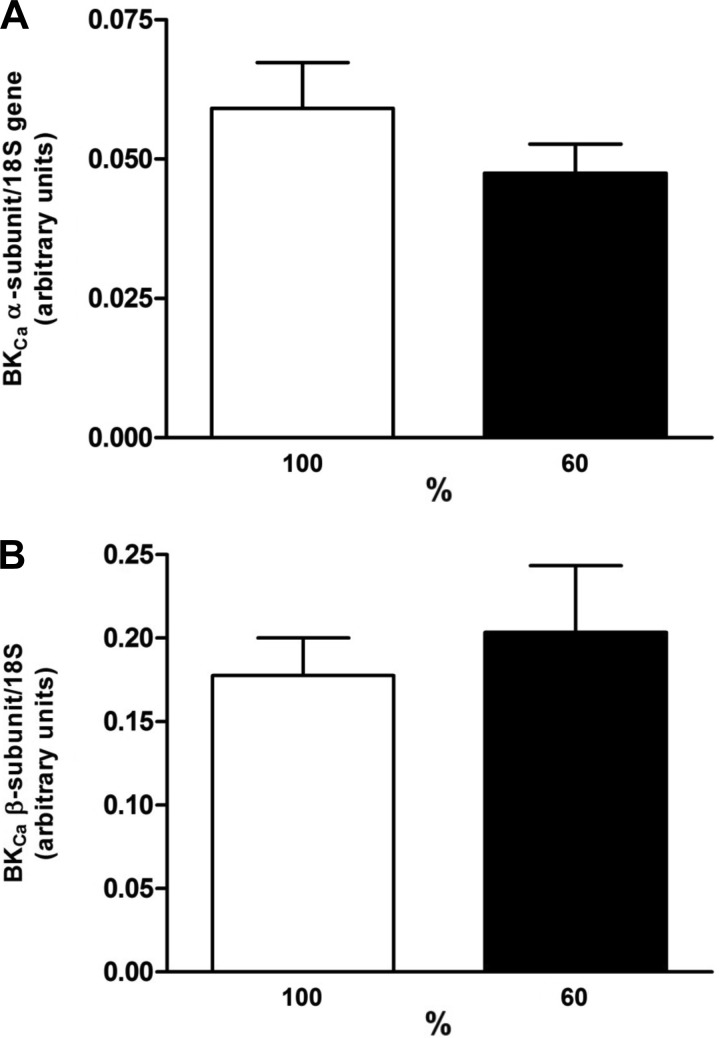
Quantitative RT-PCR identified the expression of BKCa α and β subunits in fetal coronary arteries, which did not differ between the control and nutrient-restricted ewes (Fig. 7; P > 0.05).
Fig. 7.
Expression of mRNA for BKCa α (A) and β (B) subunits in fetal coronary arteries from ewes fed a control (100%) or nutrient-restricted (60%) diet during the last ⅔ of pregnancy. The mRNA expression is normalized with respect to the total 18S mRNA expression in pooled tissues. Each point represents the mean ± SE; n = 7–8.
DISCUSSION
An increasing number of epidemiological studies in humans, corroborated by numerous controlled studies in animal models, provide compelling evidence for the concept of developmental programming. Such studies show a strong association between various stressors or insults to the developing fetus and the subsequent increased risk of developing a range of pathologies as adults, e.g., obesity, diabetes, and coronary artery disease (1, 4, 30, 41). With regard to the cardiovascular system, adverse maternal nutrition and/or low birth weight are associated with impaired endothelium-dependent relaxation and increased sensitivity to vasoconstrictors in several animal species, including humans, later in life (6, 7, 29, 32, 33, 38–40, 48, 53). Such adverse vascular changes are hallmark characteristics of endothelial dysfunction, which is a known risk factor for coronary artery disease (12, 17).
In the present study, we assessed the effect of maternal nutrient restriction during pregnancy on fetal coronary artery function. The major finding of this study is that although maternal undernutrition during pregnancy has no quantitative effect on endothelium-dependent relaxation of fetal coronary arteries in response to bradykinin, the underlying mechanisms mediating this response differ between arteries obtained from control and nutrient-restricted animals. In fetal coronary arteries from control animals, bradykinin-induced relaxation is resistant to inhibitors of endothelial NOS (eNOS) and cyclooxygenase, suggesting that the response is mediated primarily via a pathway that is independent of NO or prostacyclin. By contrast, bradykinin-induced relaxation of fetal coronary arteries from nutrient-restricted animals is abolished completely by inhibition of eNOS, suggesting that the response is mediated solely by the release of NO from the endothelium under these conditions. These data are consistent with the notion that qualitatively different mechanisms underlie endothelium-dependent relaxation of fetal coronary arteries, depending on maternal nutritional status during pregnancy.
Bradykinin evokes endothelium-dependent relaxation by activating multiple vasodilator pathways. Two such pathways include the release of NO and prostacyclin from endothelial cells (23, 35). A third pathway, which is resistant to inhibitors of eNOS and cyclooxygenase, is associated with endothelium-dependent hyperpolarization of vascular smooth muscle cells and is generally attributed to the release of an EDHF or in some blood vessels, the direct transfer of endothelial cell hyperpolarization to vascular smooth muscle cells via gap junctions (8, 9, 13).
The present findings indicate that at least two pathways are present in fetal coronary arteries; i.e., endothelium-derived NO mediates bradykinin-induced relaxation in fetal coronary arteries from nutrient-restricted ewes, whereas the response in control arteries is resistant to eNOS inhibition and thus unlikely to involve NO. The mechanism underlying this NLA-resistant response in coronary arteries from well-nourished animals is not clear but appears to involve activation of BKCa channels, since it was abolished by the potent and selective BKCa channel blocker, iberiotoxin (20), whereas the selective KATP channel blocker, glyburide, was without effect. Moreover, the NLA-resistant relaxation did not occur in arteries contracted by a high external concentration of K+, which markedly reduces or eliminates the driving force for efflux of K+ through membrane K channels and is consistent with a mechanism that is sensitive to changes in membrane potential (8). That iberiotoxin abolishes the response to bradykinin diminishes the likelihood of a role for gap junctions, since endothelial cells do not express BKCa channels, and BKCa channels do not typically play a role in the electrotonic transfer of endothelial hyperpolarization via gap junctions. These characteristics (i.e., NO and prostacyclin independent, inhibition by K channel blockade, inhibition by elevated external K+ concentrations) are typical of the classic EDHF pathway (8, 13); however, since membrane potential was not measured in the present study, we cannot state definitively that hyperpolarization of the coronary smooth muscle occurred. Given these similarities and limitations, the data are most consistent with an “EDHF-like” pathway mediating the response to bradykinin in control coronary arteries.
In coronary arteries of fully developed animals, bradykinin causes the release of NO and EDHF (14, 37, 52). The EDHF pathway is attributed to the release of endothelium-derived EETs (e.g., 14,15-EET) that activate BKCa channels in coronary vascular smooth muscle to cause relaxation (8–10). In the present study, we found that 14,15-EET also causes vasorelaxation and activates BKCa channels in smooth muscle cells from fetal coronary arteries. Maternal nutrient restriction had no effect on these responses to 14,15-EET nor did it affect fetal coronary artery relaxations or the increase in whole-cell BKCa currents induced by the BKCa channel openers, BMS 191011 (43) or NS1619 (22). Moreover, maternal nutrient restriction did not alter the expression of BKCa α and β subunits in fetal coronary arteries. Thus the absence of the EDHF-like response to bradykinin in undernourished animals cannot be explained by a decreased number or activity of BKCa channels or by a decreased sensitivity to mediators that activate BKCa channels in vascular smooth muscle cells. These results point to the possibility that the lack of an EDHF-like response to bradykinin is the result of a marked reduction in the synthesis or release of a putative EDHF, whether it is an EET or another yet-to-be-identified mediator. In addition, alterations in bradykinin signaling (e.g., receptor number or subtype, receptor coupling, intracellular signaling mechanisms) or enzymatic degradation could also play a role in the effect of maternal nutrient restriction.
Several studies have investigated the effect of different developmental insults or stressors on fetal vascular reactivity. In these studies, there appeared to be specific alterations in fetal vascular reactivity dependent on animal species, stressor, and/or vascular bed. For example, in a sheep placental embolization model, sodium nitroprusside-induced relaxation of fetal coronary arteries was unaltered (7), which is consistent with the findings from the present study and suggests that maternal nutrient restriction has no adverse effect on the smooth muscle guanylyl cyclase/cGMP signaling pathway. In contrast, sodium nitroprusside-induced relaxation was blunted in the femoral artery of nutrient-restricted fetal sheep (38), and NO-mediated relaxation of fetal coronary arteries was potentiated by exposure to betamethasone during gestation (19). In humans, low birth weight is linked to impaired endothelium-dependent relaxation of brachial arteries later in life (27, 32). In the present study, there was no quantitative difference in bradykinin-induced, endothelium-dependent relaxation of fetal coronary arteries from control and nutrient-restricted ewes, which is in agreement with previous findings in sheep fetal coronary arteries from the placental embolization model (7); however, the underlying mechanism(s) involved in endothelium-dependent relaxation of fetal sheep coronary arteries under these experimental conditions were not elucidated. In this regard, the present study clearly demonstrates that different mechanisms mediate bradykinin-induced relaxation of fetal coronary arteries from animals fed a normal vs. nutrient-restricted diet during mid to late gestation. It is also worth noting that the observed functional differences occurred, independent of a reduction in fetal weight, inasmuch as the day 130 fetal weight did not differ between the control and nutrient-restricted animals. These observations are in agreement with the increasing number of studies suggesting that developmental programming can occur, independent of low birth weight (4, 41). For example, in humans and in animal models, offspring of mothers who experience nutrient restriction early in pregnancy but receive adequate nutrition later in pregnancy, resulting in normal birth weights, still exhibit many of the same phenotypes (e.g., poor growth, increased adiposity, poor glucose tolerance, and dyslipidemia) as offspring from mothers that are undernourished for the entire pregnancy (4, 16, 18, 50).
Redundancy in cellular signaling pathways is an evolutionarily conserved mechanism to ensure optimum physiologic performance with changing micro- and macroenvironments. Within the vascular wall, multiple vasodilatory signaling systems are present to maintain adequate tissue perfusion under stressful or pathologic conditions. For example, endothelium-dependent relaxations appear to be unaffected in arteries from animals with hypercholesterolemia, heart failure, and knockout of eNOS, but further investigation into the underlying mechanism of relaxation reveals that the NO-dependent component is markedly inhibited or abolished and that an EDHF pathway is upregulated, such that the relaxation response is not compromised (31, 36, 45). The results of the present study suggest that a similar type of compensatory response can also occur in fetal coronary arteries and add to our knowledge of how the developing fetus adapts to changes in its nutritional environment. As our understanding of developmental programming of the cardiovascular system continues to evolve, the present findings suggest several lines of inquiry for future investigation, including, for example, studies designed to determine the effects of undernutrition at different stages of pregnancy, whether the effects are observed in offspring, and what happens to the responses in adulthood. Such information may provide new insights into potential mechanisms linking exposure to developmental insults during pregnancy and the increased risk of cardiovascular disease later in life.
GRANTS
Support for this study was provided, in part, by grants from the National Institute of Child Health and Human Development (HD61532) and U.S. Department of Agriculture (USDA-NRI 2005-35206-15281 and NIFA-AFRI 2011-67012-30683).
DISCLOSURES
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.O.L., K.V., J.S.C., L.P.R., C.S., and S.T.O. conception and design of research; P.S., S.G., N.D., M.L.J., and A.M. performed experiments; P.S., S.G., M.L.J., A.M., J.S.C., L.P.R., C.S., and S.T.O. analyzed data; C.O.L., M.L.J., K.V., J.S.C., L.P.R., C.S., and S.T.O. interpreted results of experiments; P.S., S.G., and A.M. prepared figures; P.S. and S.T.O. drafted manuscript; C.O.L., K.V., J.S.C., L.P.R., C.S., and S.T.O. edited and revised manuscript; P.S., S.G., N.D., C.O.L., M.L.J., A.M., K.V., J.S.C., L.P.R., C.S., and S.T.O. approved final version of manuscript.
REFERENCES
- 1.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol 561: 355–377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwal KS. Modulation of potassium channels by organic molecules. Med Res Rev 12: 569–591, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab 13: 364–367, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The developmental origins of well-being. Philos Trans R Soc Lond B Biol Sci 359: 1359–1366, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauersachs J, Hecker M, Busse R. Display of the characteristics of endothelium-derived hyperpolarizing factor by a cytochrome P450-derived arachidonic acid metabolite in the coronary microcirculation. Br J Pharmacol 113: 1548–1553, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54: 83–90, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578: 871–881, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–80, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflügers Arch 459: 881–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Carlson DB, Reed JJ, Borowicz PP, Taylor JB, Reynolds LP, Neville TL, Redmer DA, Vonnahme KA, Caton JS. Effects of dietary selenium supply and timing of nutrient restriction during gestation on maternal growth and body composition of pregnant adolescent ewes. J Anim Sci 87: 669–680, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol 42: 1037–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: beyond nitric oxide and cyclic GMP. Circulation 92: 3337–3349, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Cowan CL, Cohen RA. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am J Physiol Heart Circ Physiol 261: H830–H835, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J Nutr Biochem 19: 409–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation 110: 1926–1932, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ford S, Hess B, Schwope M, Nijland M, Gilbert J, Vonnahme K, Means W, Han H, Nathanielsz P. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Zhou H, Raj JU. Antenatal betamethasone therapy potentiates nitric oxide-mediated relaxation of preterm ovine coronary arteries. Am J Physiol Heart Circ Physiol 270: H538–H544, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Giangiacomo KM, Garcia ML, McManus OB. Mechanism of iberiotoxin block of the large-conductance calcium-activated potassium channel from bovine aortic smooth muscle. Biochemistry 31: 6719–6727, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol 481: 407–414, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol 117: 119–129, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J 3: 31–36, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine 30: 333–342, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kowaluk EA, Seth P, Fung H. Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. J Pharmacol Exp Ther 262: 916–922, 1992 [PubMed] [Google Scholar]
- 26.Leamy AW, Shukla P, McAlexander MA, Carr MJ, Ghatta S. Curcumin ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci Lett 503: 157–162, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Leeson C, Kattenhorn M, Morley R, Lucas A, Deanfield J. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103: 1264–1268, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lemley CO, Meyer AM, Camacho LE, Neville TL, Newman DJ, Caton JS, Vonnahme KA. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 302: R454–R467, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev 81: 745–751, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Luther JS, Redmer DA, Reynolds LP, Wallace JM. Nutritional paradigms of ovine fetal growth restriction: implications for human pregnancy. Hum Fertil (Camb) 8: 179–187, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Malmsjö M, Bergdahl A, Zhao XH, Sun XY, Hedner T, Edvinsson L, Erlinge D. Enhanced acetylcholine and P2Y-receptor stimulated vascular EDHF-dilatation in congestive heart failure. Cardiovasc Res 43: 200–209, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Martin H, Gazelius B, Norman M. Impaired acetylcholine-induced vascular relaxation in low birth weight infants: implications for adult hypertension? Pediatr Res 47: 457–462, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation 102: 2739–2744, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Molnar J, Nijland MJ, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0.7, 075, and 08 gestation in sheep. Am J Physiol Regul Integr Comp Physiol 283: R561–R567, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Moncada S, Vane J. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 30: 293–331, 1978 [PubMed] [Google Scholar]
- 36.Najibi S, Cohen RA. Enhanced role of K+ channels in relaxations of hypercholesterolemic rabbit carotid artery to NO. Am J Physiol Heart Circ Physiol 269: H805–H811, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Nakashima M, Mombouli JV, Taylor AA, Vanhoutte PM. Endothelium-dependent hyperpolarization caused by bradykinin in human coronary arteries. J Clin Invest 92: 2867–2871, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.National Research Council Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academies Press, 2007 [Google Scholar]
- 38.Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol 183: 1301–1307, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ozaki T, Nishina H, Hanson M, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 42: 768–774, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Hammer CJ, Carlin KRM, Grazul-Bilska AT, Redmer DA. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J Anim Sci 88: E61–E72, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Reynolds LP, Caton JS. Role of the pre- and post-natal environment in developmental programming of health and productivity. Mol Cell Endocrinol 354: 54–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romine JL, Martin SW, Meanwell NA, Gribkoff VK, Boissard CG, Dworetzky SI, Natale J, Moon S, Ortiz A, Yeleswaram S, Pajor L, Gao Q, Starrett JE., Jr 3-[(5-Chloro-2-hydroxyphenyl)methyl]-5-[4-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2(3H)-one, BMS-191011: opener of large-conductance Ca(2+)-activated potassium (maxi-K) channels, identification, solubility, and SAR. J Med Chem 50: 528–542, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Schmid-Antomarchi H, de Weille J, Fosset M, Lazdunski M. The antidiabetic sulfonylurea glibenclamide is a potent blocker of the ATP-modulated K+ channel in insulin secreting cells. Biochem Biophys Res Commun 146: 21–25, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Shukla P, Sun C, O'Rourke ST. Melatonin inhibits nitric oxide signaling by increasing PDE5 phosphorylation in coronary arteries. Am J Physiol Heart Circ Physiol 303: H1418–H1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation 100: 1400–1405, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Tare M, Parkington HC, Bubb KJ, Wlodek ME. Uteroplacental insufficiency and lactational environment separately influence arterial stiffness and vascular function in adult male rats: novelty and significance. Hypertension 60: 378–386, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Tunstall RR, Shukla P, Grazul-Bilska A, Sun C, O'Rourke ST. MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J Pharmacol Exp Ther 336: 127–133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vonnahme K, Zhu M, Borowicz P, Geary T, Hess B, Reynolds L, Caton J, Means W, Ford S. Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J Anim Sci 85: 2464–2472, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Vonnahme KA, Lemley CO, Shukla P, O'Rourke ST. Placental programming: how the maternal environment can impact placental function. J Anim Sci 91: 2467–2480, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Weston AH, Félétou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol 145: 775–784, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288: R360–R367, 2005 [DOI] [PubMed] [Google Scholar]