Abstract
Although obesity is a major clinical risk factor for lymphedema, the mechanisms that regulate this effect remain unknown. Recent reports have demonstrated that obesity is associated with acquired lymphatic dysfunction. The purpose of this study was to determine how obesity-induced lymphatic dysfunction modulates the pathological effects of lymphatic injury in a mouse model. We used a diet-induced model of obesity in adult male C57BL/6J mice in which experimental animals were fed a high-fat diet and control animals were fed a normal chow diet for 8–10 wk. We then surgically ablated the superficial and deep lymphatics of the midportion of the tail. Six weeks postoperatively, we analyzed changes in lymphatic function, adipose deposition, inflammation, and fibrosis. We also compared responses to acute inflammatory stimuli in obese and lean mice. Compared with lean control mice, obese mice had baseline decreased lymphatic function. Lymphedema in obese mice further impaired lymphatic function and resulted in increased subcutaneous adipose deposition, increased CD45+ and CD4+ cell inflammation (P < 0.01), and increased fibrosis, but caused no change in the number of lymphatic vessels. Interestingly, obese mice had a significantly increased acute inflammatory reaction to croton oil application. In conclusion, obese mice have impaired lymphatic function at baseline that is amplified by lymphatic injury. This effect is associated with increased chronic inflammation, fibrosis, and adipose deposition. These findings suggest that obese patients are at higher risk for lymphedema due to impaired baseline lymphatic clearance and an increased propensity for inflammation in response to injury.
Keywords: lymphedema, obesity, inflammation, fibrosis
lymphedema is a common complication of cancer treatment that results in the accumulation of protein-rich interstitial fluid, fibrosis, and adipose deposition (3, 4, 6). Epidemiological studies have identified a number of predisposing conditions for developing lymphedema, including obesity, radiation therapy, and postoperative infections (13, 17, 27). Although the incidence of lymphedema varies depending on surgical sites and other factors, most studies have shown that obese patients have a two- to eightfold increased risk compared with individuals with normal weight (2, 8, 12, 21, 22). Other studies have shown that even postoperative weight gain alone increases the risk of developing lymphedema in patients who were of preoperatively normal weight (18) and that treatments designed to promote weight loss improve the symptomatology (24).
The cellular mechanisms regulating the interaction between obesity and lymphedema remain poorly understood. Recent studies have shown that obesity impairs lymphatic function in both mice and humans (1, 29). For example, our group recently reported that obese mice had decreased ability to transport interstitial fluid in capillary lymphatics and decreased trafficking of inflammatory cells to regional lymph nodes (29). Other studies have shown that obese patients have decreased clearance of macromolecules from fat depots compared with normal control patients (1) and that severely obese individuals with a very high body mass index (body mass index > 59) spontaneously develop lower extremity lymphedema (10). The relationship between lymphatics and obesity appears to be bidirectional; mice with isolated lymphatic defects develop adult onset obesity even on a normal chow diet (11). Furthermore, even minor injury to the lymphatic system in mice activates adipose differentiation genes and leads to adipose tissue hypertrophy and proliferation (3). Thus, although it is clear that obesity and lymphatic function are related, it remains unknown how obesity increases the risk of lymphedema. This gap in our knowledge is important since a better understanding of the pathological processes differentiating lymphedema development in obese and lean individuals may provide clinical insights into the development of targeted treatment options for lymphedema.
In the present study, we analyzed the effects of diet-induced obesity (DIO) on lymphatic physiology and the development of lymphedema using a mouse model. Here, we show that adult male C57BL/6J mice fed a high-fat diet become obese and develop diminished lymphatic fluid transport capacity at baseline compared with lean littermates fed a normal chow diet. More importantly, we show that obese mice have a more severe tail lymphedema phenotype with increased adipose deposition, fibrosis, and inflammation. Interestingly, using a topical irritant model, we found that obese mice have a significantly increased propensity to tissue inflammation compared with lean mice, with markedly increased infiltration of macrophages and neutrophils. Taken together, our results suggest that obesity decreases lymphatic function and increases the propensity for inflammation at baseline and that these effects are amplified by lymphatic injury, leading to a more severe lymphedema phenotype.
MATERIALS AND METHODS
DIO.
All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Memorial Sloan Kettering Cancer Center. Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in a temperature- and light-controlled environment. To establish DIO, adult male mice were maintained on a high-fat diet (60% kcal from fat, W.F Fisher & Son) beginning at 6 wk of age ad libitum for 8–10 wk. Age-matched control male wild-type mice were maintained on a normal chow diet (13% kcal from fat; Purina PicoLab Rodent Diet 20, W.F Fisher & Son) for the same period of time (19, 29). At the conclusion of the experiment, animals were weighed using a digital scale (Sartorius, Bradford, MA).
Mouse tail model of lymphedema.
Obese and lean control animals underwent lymphatic injury using a well-described mouse tail model of lymphedema in which the superficial and deep lymphatic system of the tail was excised through a 2-mm circumferential skin excision in the midportion of the tail (5, 9, 23, 26). Our group and others have previously shown that this model results in sustained lymphedema of the distal tail, severe impairment in lymphatic function, and histological features of clinical lymphedema (e.g., chronic inflammation, adipose deposition, and fibrosis) for at least 10 wk postoperatively (5, 9, 23, 26). A separate set of obese and lean animals (n = 8–10 animals/group) had no tail surgery and served as our baseline controls. Animals were euthanized 6 wk postoperatively and analyzed as outlined below.
Tail volumes, lymphoscintigraphy, and histological analysis.
Tail volume calculations were analyzed using multiple digital caliper tail circumference measurements distal to the zone of lymphatic injury and the truncated cone formula as previously described (9). Lymphoscintigraphy was also performed as previously described to quantify lymphatic flow to the sacral lymph nodes by injecting 50 μl of filtered technetium (99mTc) sulfur colloid into the distal tail (6). Decay-adjusted uptake was recorded in the sacral lymph nodes using an X-SPECT camera (Gamma Medica, Northridge, CA), and region of interest analysis was performed using ASIPro software (CTI Molecular Imaging, Knoxville, TN) (9).
For histological and immunohistochemical analysis, tail sections were harvested, briefly fixed in 4% ice-cold paraformaldehyde (Sigma-Aldrich, St. Louis, MO), decalcified using 5% EDTA (Santa Cruz Biotechnology, Santa Cruz, CA) for 72 h, and paraffin embedded. Abdominal sections were harvested from obese and control mice using 5-mm punch biopsy kits (Fray Products, Buffalo, NY), fixed in 4% paraformaldehyde (Affymetrix, Cleveland, OH), and subsequently paraffin embedded. Hematoxylin and eosin-stained sections were prepared using standard techniques, and subcutaneous tissue thickness analysis was performed in histological cross-sections located 1.5 cm distal to the surgical site by blinded reviewers (n = 6–8 cross-sections/group). The distance from the basal layer of the epidermis to the deep fascia was analyzed in 4 standardized regions/section.
Immunohistochemical staining was performed according to our established techniques (5). Paraffin-embedded tissues were briefly rehydrated, and antigen unmasking was performed using boiling sodium citrate (Sigma-Aldrich). Endogenous peroxidase activity was quenched, and nonspecific binding was blocked with 2% BSA-20% animal serum. Tissues were incubated with primary antibody overnight at 4°C, and antibody staining was visualized using horseradish peroxidase-conjugated secondary antibodies developed with diaminobenzamine complex (Vector, Burlingame, CA). Primary antibodies used for immunohistochemical stains included lymphatic vessel endothelial hyaluronan receptor (LYVE)-1, CD45, and CD4 (all from R&D, Minneapolis, MN). All secondary antibodies were obtained from Vector Laboratories. Sections were analyzed using bright-field microscopy, and regions of interest were scanned using a Mirax slide scanner (Zeiss, Munich, Germany). Cell counts were performed on high-powered sections, with a minimum of 4–6 animals/group and 4–5 high-powered fields (HPFs)/animal by two blinded reviewers.
Fibrosis was assessed using our previously published methods to quantify Sirius red staining (Polysciences, Warrington, PA) and collagen type I immunohistochemistry (4). Briefly, the Sirius red scar index was calculated using polarized light microscopy to determine the ratio of orange/red to yellow/green birefringence and was analyzed using Metamorph Offline software. Collagen type I immunohistochemistry was performed using an antibody to mouse collagen type I (Abcam, Cambridge, MA) and quantified as a ratio of the area of positively stained dermis within a fixed threshold to total tissue area.
Croton oil-induced inflammation and flow cytometry.
To determine if obese mice have an increased propensity for inflammation, we used a well-described croton oil assay (7). Our preliminary experiments were performed using the mouse tail; however, we found that in these instances, croton oil caused only minimal inflammation due to the relative thickness of the tail skin. Subsequently, we performed these experiments by applying either croton oil or PBS (control) to the shaved abdominal skin, as previously described by Bao et al. (7). A total of 150 μl of 2% croton oil (Sigma-Aldrich) were dissolved in 70% acetone (Fisher Scientific, Pittsburgh, PA) and applied to a 2 × 2-cm abdominal region. Twelve hours after the application of croton oil, tissue sections were harvested for histological analysis and flow cytometry.
Flow cytometry was performed on a standardized 2 × 2-cm portion of abdominal skin as previously described (33). Briefly, abdominal skin was stripped from the underlying tissues and digested at 37°C for 30 min in PBS with collagenase D (0.2 mg/ml), DNAse I (0.1 mg/ml), and dispase (0.4 mg/ml, all from Roche Diagnostics, Indianapolis, IN). Tissue digests were then filtered through a 70-μm filter (BD Falcon, Franklin Lakes, NJ) and resuspended as a single cell suspension in PBS-2% FCS-sodium azide solution for flow cytometry analysis. Cells were blocked at 4°C with Fc block (CD16/CD32, eBioscience, San Diego, CA) to block endogenous Fc receptors. Single stains were performed on UltraComp eBeads (eBioscience, San Diego, CA) for optimization of cytometer settings. Cell suspensions isolated from peripheral tissues were then stained using fluorophore-conjugated antibodies for the following cell surface markers: CD45 (BioLegend, San Diego, CA), F4/80, CD11b, and lymphocyte antigen 6G (all from eBioscience, San Diego, CA). Cell suspensions were analyzed using a Fortessa 2 flow cytometer (BD Biosciences, San Jose, CA) with BD FACSDiva software, and subsequent data analysis was performed using FlowJo software (Tree Star, Ashland, OR). Cell populations were analyzed and defined using the cell surface markers listed above using 5 animals·group−1·experiment−1.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA) software. Student's t-test was used to compare differences between two groups. Analysis between multiple time points (lymphoscintigraphy) was performed using two-way ANOVA with post hoc tests to compare individual groups. Descriptive analysis and graphic methods were used to analyze and summarize results. Data are presented as means ± SD unless otherwise noted, with P < 0.05 considered significant.
RESULTS
Obese mice have increased adipose deposition after lymphatic injury.
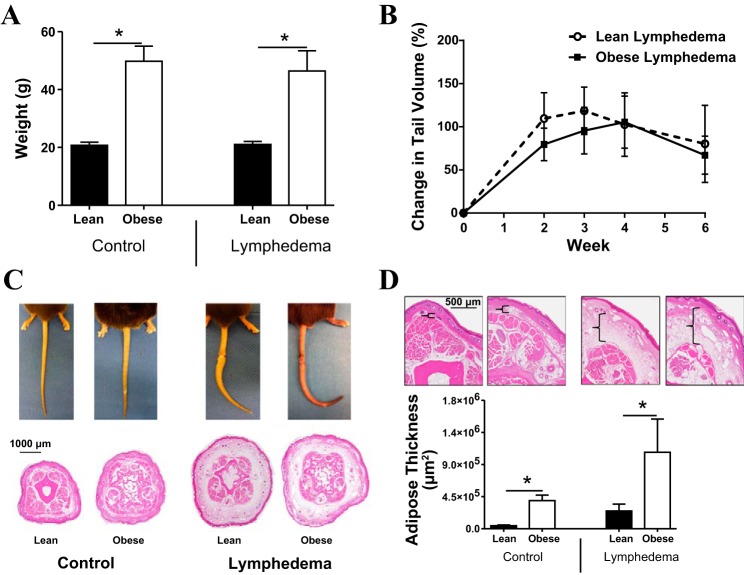
As expected, feeding male C57BL/6J mice a high-fat diet for 8–10 wk resulted in a significant (2.3-fold) increase in body weight compared with lean mice (P < 0.0001; Fig. 1A). However, lymphedema did not further cause systemic changes in body weight. Interestingly, we found no significant difference in tail volume measurements when we compared lean and obese mice that had undergone tail lymphatic excision (Fig. 1B). However, histological analysis of the tails demonstrated a significant increase in adipose deposition in obese mice both at baseline and after the induction of lymphedema (both P < 0.01; Fig. 1, C and D).
Fig. 1.
Obese mice have increased adipose deposition after lymphatic injury. A: body weight measurements in control (i.e., nonoperated) and lymphedematous mice fed either a high-fat diet (HFD; obese mice) or a normal chow diet (lean mice). *P < 0.05. B: tail volume measurements in lean or obese mice for 6 wk after tail lymphatic ablation. C: representative gross photographs (top) and representative cross-sectional histological sections (×2.5 magnification) of the distal tail in control and lymphedematous animals 6 wk after lymphatic ablation. D: quantification of adipose deposition in control and lymphedematous animals 6 wk after surgery. *P < 0.05. Brackets (top) demonstrate adipose tissue thickness in representative hematoxylin and eosin-stained cross-sectional sections shown at ×10 magnification.
Obesity with or without lymphatic injury decreases lymph node 99mTc uptake.
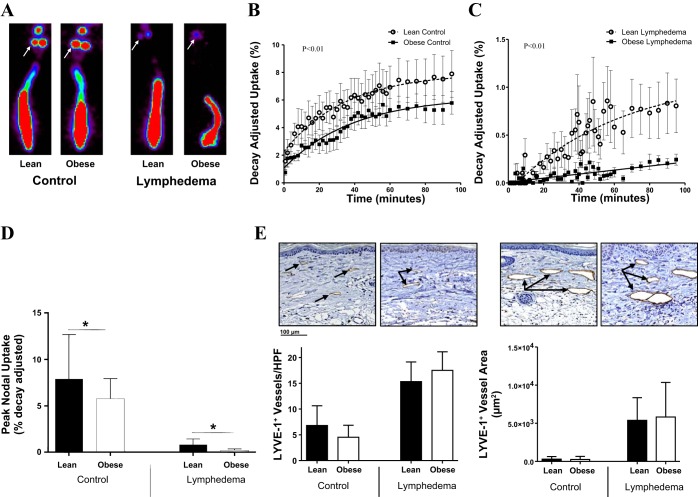
Consistent with our previous studies comparing lymph node uptake in the upper extremity of obese and lean mice, we found that uptake of 99mTc-labeled sulfur colloid by the sacral lymph nodes after distal tail injection was significantly decreased in obese mice compared with lean mice at baseline (Fig. 2, A–C) (29). Obese mice had decreased peak nodal uptake and decreased decay-adjusted uptake compared with control mice (P < 0.01; Fig. 2, B–D). Similarly, we found that, although lymph node uptake was significantly decreased in mice with lymphedema, obese mice had significantly worse function (4 times less uptake) than lean mice treated in the same manner (P < 0.01; Fig. 2, B and C). However, we did not find significant differences between groups in the number or area of LYVE+ dermal lymphatics in tail skin 1.5 cm distal to the zone of lymphatic injury (Fig. 2E).
Fig. 2.
Obesity with or without lymphatic injury decreases lymph node 99mTc uptake. A: representative heat maps of control and lymphedematous mouse tails injected with 99mTc for lymphoscintigraphy. White arrows point to sacral lymph nodes. B and C: quantification of decay-adjusted 99mTc uptake by sacral lymph nodes in control (B) and lymphedematous (C) mice. D: quantification of peak nodal uptake in control and lymphedematous animals 6 wk postoperatively. *P < 0.05. E: representative photomicrographs (×20 magnification) and quantification of lymphatic vessel endothelial hyaluronan receptor (LYVE)-1+ vessels (left graph) and LYVE-1+ vessel area (right graph) in control and lymphedematous tail sections 6 wk postoperatively.
Obese mice have an exaggerated inflammatory response after lymphatic injury.
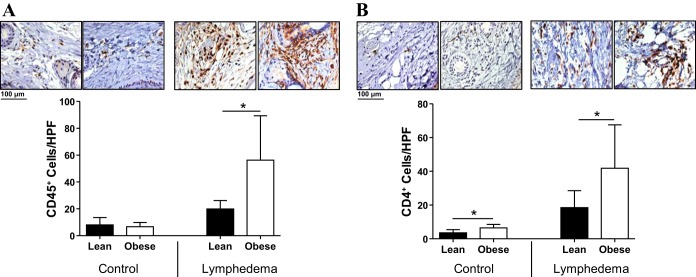
Previous studies have shown that visceral fat T cell inflammation is a crucial regulator of pathological responses to obesity, including metabolic syndrome and cancer (19, 30). In addition, our laboratory and others have shown that T cells negatively regulate lymphangiogenesis and lymphatic function and that CD4+ cell inflammation plays a crucial role in the pathogenesis of lymphedema (16, 31). Therefore, to analyze the effects of obesity on inflammation in general and CD4+ cell inflammation in particular, we performed immunohistochemical staining on mouse tail tissues in both control (i.e., nonoperated) and lymphedema models. Interestingly, we found that obese mice did not have significant changes in the number of CD45+ cells/HPF and only modest (although significant) increases in the number of CD4+ cells/HPF in tail subcutaneous tissues at baseline (P < 0.01 for CD4+ cells; Fig. 3, A and B). However, analysis of tissue sections from lymphedematous tails demonstrated a marked increase in CD45+ cell (3-fold) and CD4+ cell (2.5-fold) inflammation in obese mice compared with lean control mice (P < 0.01 for both; Fig. 3, A and B).
Fig. 3.
Obese mice have an exaggerated inflammatory response after lymphatic injury. A and B: representative photomicrographs (×20 magnification) and quantification of CD45+ (A) and CD4+ (B) cells per high-powered field (HPF) in control and lymphedematous mouse tail sections 6 wk after surgery. *P < 0.05.
Obese mice have increased fibrosis after lymphatic injury.
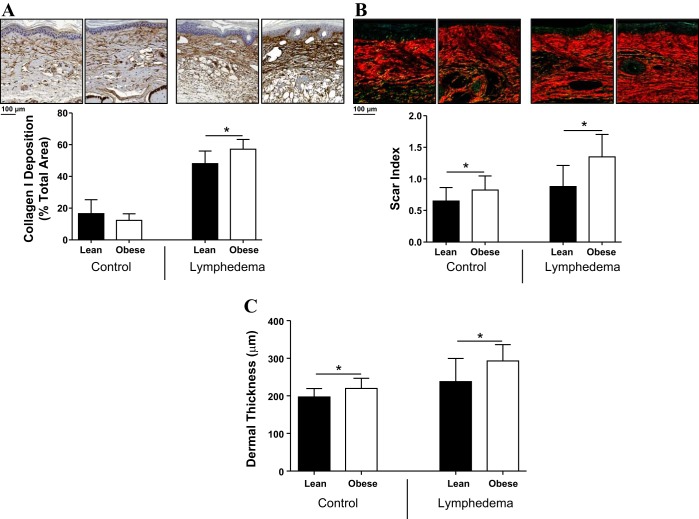
Fibrosis and hyperkeratosis are histological hallmarks of lymphedema (4, 5, 9, 20, 25). In addition, our group has previously shown that fibrosis is a critical regulator of lymphatic regeneration and lymphatic function and that this response is dependent on CD4+ cell inflammation (4, 33). Therefore, to analyze the effects of obesity on soft tissue fibrosis, we analyzed collagen deposition using immunohistochemistry and Sirius red staining as we have previously reported (6). Consistent with our analysis of CD4+ cells, we found that obese mice have significantly increased dermal fibrosis after lymphatic injury, as reflected by increased collagen deposition and increased scar index (P < 0.01; Fig. 4, A and B). Obese mice also demonstrated increased dermal thickness consistent with hyperkeratosis and a heightened response to lymphedema (P < 0.01; Fig. 4C).
Fig. 4.
Obese mice have increased fibrosis after lymphatic injury. A: representative photomicrographs (×20 magnification) and quantification of collagen type I deposition in control and lymphedematous animals 6 wk postoperatively. *P < 0.05. B: representative photomicrographs (×20 magnification) and quantification of scar index (Sirius red staining) in control and lymphedematous animals 6 wk postoperatively. *P < 0.05. C: quantification of dermal thickness in control and lymphedematous animals 6 wk postoperatively. *P < 0.05.
Obese mice have a heightened acute inflammatory response to noxious stimuli.
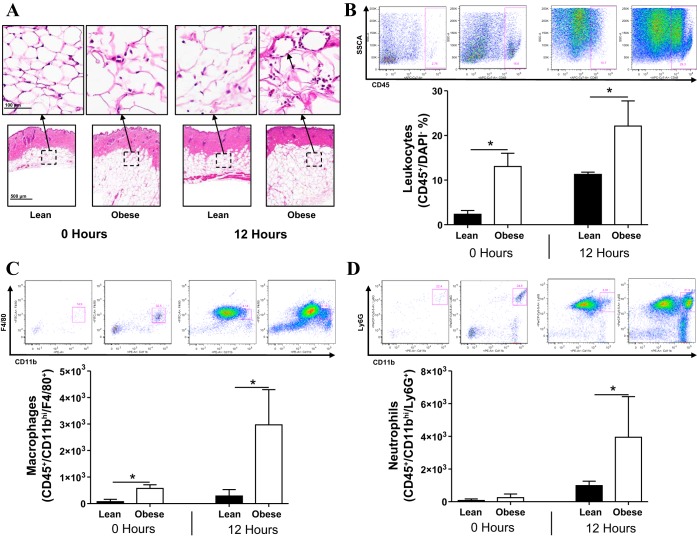
Given that we found that obese mice had an exaggerated chronic inflammatory response after lymphatic injury, we next sought to determine if obesity also increased the potential for acute inflammation. This is important since we have previously shown that lymphatic injury activates innate immune responses and endogenous danger signals very rapidly (32). To accomplish this goal, we applied croton oil, a well-established irritant dermatitis model, to investigate differences in acute inflammation between obese and lean mice. Consistent with our previous reports, we found that obese mice had increased numbers of macrophages and CD45+ leukocytes in their subcutaneous fat at baseline (Fig. 5A) (29). Before the application of croton oil, obese mice had crown-like structures composed of macrophages engulfing necrotic adipocytes. This effect was markedly increased 12 h after exposure to croton oil, resulting in a massive influx of macrophages and monocytes. These findings were confirmed with flow cytometry, which demonstrated a massive increase in the number of CD45+ cells, macrophages, and neutrophils in the subcutaneous tissues of obese mice 12 h after exposure to croton oil (P < 0.01 for CD45+ cells and P < 0.05 for macrophages and neutrophils; Fig. 5, B–D).
Fig. 5.
Obese mice have a heightened acute inflammatory response to noxious stimuli. A: representative low-power (bottom; ×5 magnification) and high-power (top; ×40 magnification) histological hematoxyalin and eosin-stained sections of lean and obese mice abdominal skin before (0 h) and 12 h after croton oil activation. The short black arrow points to a crown-like structure. B–D: flow plots and quantification of leukocytes (B), macrophages (C), and neutrophils (D) from the abdominal skin of lean and obese mice before (0 h) and 12 h after croton oil application. *P < 0.05.
DISCUSSION
The goal of the present study was to identify the cellular and molecular mechanisms by which obesity increases the risk of clinical lymphedema. To accomplish this goal, we chose to use a diet-induced model of obesity rather than a genetic model since we have previously shown that this model is useful for studying lymphatic function and, more importantly, because DIO mice do not have significant immune or metabolic abnormalities that are known to exist in other obesity models such as ob/ob or db/db mice (29). Consistent with our previous report, we found that exposure to a high-fat diet rapidly resulted in significant weight gain in adult male C57BL/6J mice (29). We additionally found that DIO mice had only minor metabolic derangements (not shown). Interestingly, DIO mice had impaired lymphatic transport even before lymphatic injury, and this response was significantly amplified 6 wk after lymphatic ablation in the tail. This finding suggests that obese patients may be at higher risk for developing lymphedema because they have a decreased lymphatic reserve before surgery. This concept is clinically supported by previous studies demonstrating a wide range of baseline lymphatic transport capacities and, more importantly, by the fact that patients who have a diminished capacity at baseline are at higher risk for developing lymphedema (28). Our findings are also supported by Angrim et al. (1), who reported that obese patients have decreased clearance of interstitial macromolecules compared with lean patients, suggesting that obesity decreases baseline lymphatic function. Similarly, Greene et al. (10) demonstrated a correlation between body mass index and the development of lower extremity lymphedema by demonstrating that severely obese patients (body mass index > 59) spontaneously developed lower extremity lymphedema and lymphatic dysfunction. The relationship between obesity and lymphatics is likely bidirectional, since previous studies have shown that mice with genetic lymphatic defects secondary to a heterozygous inactivating mutation of the prospero homeobox 1 gene become obese as adults, even when maintained on a normal diet. Thus, it is possible that obesity sets up a cycle of pathology with obesity causing lymphatic dysfunction, which, in turn, adds to the pathology of obesity and increases adipose deposition (11).
We have previously shown that CD4+ cell-mediated inflammatory reactions play a crucial role in the regulation of lymphatic function. For example, we have reported that lymphatic injury results in massive chronic CD4+ cell inflammation and that inhibition of this response (using either transgenic animals deficient in CD4+ cells or by depletion of these cells using neutralizing antibodies) significantly decreases the severity of lymphedema (6, 33). Moreover, we have shown that the severity of clinical lymphedema correlates with the degree of the CD4+ cell inflammatory response in subcutaneous and dermal tissues (6). Consistent with these findings, we found that obese mice had a significantly increased chronic inflammatory response after lymphatic injury. In fact, obese mice not only had increased tissue CD4+ cell inflammation at baseline but also a much more potent inflammatory response 6 wk after lymphatic ablation. This is important as we have previously shown that CD4+ cells regulate tissue fibrosis and contribute to lymphatic regeneration (6, 33). Consistent with this concept, we found that obese mice had significantly increased tissue fibrosis and adipose deposition compared with their lean littermates. Taken together, these findings suggest that in addition to baseline lymphatic defects, obese patients may be at higher risk for developing lymphedema due to heightened immune responses that promote fibrosis and further impair lymphatic function. Thus, antifibrotic treatment or strategies designed to decrease CD4+ cell inflammation after lymphatic injury may be a potentially attractive strategy for the prevention of lymphedema in obese patients.
Interestingly, we did not find significant differences in the number of lymphatic vessels or vessel area when we compared obese and lean mice either before injury or 6 wk after lymphatic ablation. This was somewhat surprising since we and others have previously shown that T cells in general secrete a number of antilymphangiogenic cytokines, including interferon-γ and transforming growth factor-β1 (16, 31). However, it is likely that the cytokine milieu in obese mice may be different (i.e., increased expression of lymphangiogenic cytokines), thereby resulting in a minimal net difference in the overall lymphangiogenic response in tissues. It is also possible that at the 6-wk time point, changes that had occurred in the lymphatic vessels may have resolved. Either way, the regulation of lymphatic endothelial cell proliferation and differentiation in obesity is an important area and will be the topic of future studies.
Consistent with our previous report, we found that obese mice had increased subcutaneous adipose tissue inflammation at baseline (29). More importantly, we found that obese mice had more profound acute inflammatory responses compared with lean mice when exposed to a topical irritant. These differences in acute responses may be related to impaired lymphatic function in obese mice since previous studies have shown that lymphatics play a critical role in clearing inflammation. For example, Huggenberger et al. (15) demonstrated that systemic treatment of K-14 VEGF-A transgenic mice with VEGF receptor-3-neutralizing antibodies decreased lymphangiogenesis and resulted in markedly increased inflammation and edema formation. In a separate study (14), these authors directly showed that increased lymphatic drainage in the skin of K-14 VEGF-C or VEGF-D transgenic mice resulted in decreased inflammation and edema formation in response to a variety of inflammatory stimuli, including ultraviolet radiation and oxalazone treatment. Taken together, these findings suggest that impaired lymphatic function in obese mice predisposes these animals to a more profound acute and chronic inflammatory responses and that this effect, in turn, leads to further impaired lymphatic function and more significant lymphedema. This hypothesis is supported by our previous studies demonstrating that impaired lymphatic function in obesity is due at least in part to T and B cell inflammation (29) as well as previous studies demonstrating antilymphangiogenic roles for T cells during wound healing and in inflammatory lymphangiogenesis models (6, 16, 31).
In conclusion, we have shown that obesity increases inflammation, fibrosis, and adipose deposition in response to lymphatic injury, thereby providing a rationale for epidemiological studies demonstrating an increased risk of lymphedema in obese patients. These results are important because they begin to identify the cellular and molecular mechanisms regulating the pathology of lymphedema and suggest that targeted anti-inflammatory or antifibrotic approaches may be useful in preventing the development of lymphedema in obese patients. In addition, the present study provides further observational evidence that the pathology of lymphedema is related to inflammatory reactions secondary to lymphatic stasis.
GRANTS
This work was supported by NIH Grants R01-HL111130-01 (to B. J. Mehrara) and 5-T32-CA009501-25 (to D. A. Cuzzone).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.L.S. and B.J.M. conception and design of research; I.L.S., J.S.T., D.A.C., S.G., N.J.A., J.C.G., and W.J.J. performed experiments; I.L.S., J.S.T., D.A.C., S.G., N.J.A., J.C.G., W.J.J., and B.J.M. analyzed data; I.L.S., J.S.T., D.A.C., S.G., N.J.A., J.C.G., W.J.J., and B.J.M. interpreted results of experiments; I.L.S. and B.J.M. prepared figures; I.L.S. drafted manuscript; I.L.S., J.S.T., D.A.C., S.G., N.J.A., J.C.G., W.J.J., and B.J.M. approved final version of manuscript; J.S.T., D.A.C., S.G., N.J.A., J.C.G., W.J.J., and B.J.M. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Mesruh Turkekul, Sho Fujisawa, and Yevgeniy Romin of the Molecular Cytology Core at Memorial Sloan Kettering Cancer Center for assistance with both histology and tissue imaging [National Institutes of Health (NIH) Core Grant P30-CA-008748]. Additionally, the authors thank Pat Zanzonico and Valerie Longo of the Small-Animal Imaging Core Facility at Memorial Sloan Kettering Cancer Center for the assistance with lymphoscintigraphy (NIH Shared Instrumentation Grant 1-S10-RR028889-01, which provided funding support for the purchase of the NanoSPECT/CT Plus, is gratefully acknowledged).
REFERENCES
- 1.Arngrim N, Simonsen L, Holst JJ, Bulow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 37: 748–750, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Arrault M, Vignes S. [Risk factors for developing upper limb lymphedema after breast cancer treatment]. Bull Cancer 93: 1001–1006, 2006 [PubMed] [Google Scholar]
- 3.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg 129: 838–847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg 124: 438–450, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202–3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27: 1114–1126, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao L, Yao X, Xu J, Guo X, Liu H, Kurihara H. Effects of Pithecellobium clypearia Benth extract and its main components on inflammation and allergy. Fitoterapia 80: 349–353, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM 98: 2343–2008, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 366: 2136–2017, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37: 1072–2081, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 16: 48–54, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Watroba NL, Rezaishiraz H, Giese W, Hurd T, Fassl KA, Edge SB. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Ann Surg Oncol 11: 573–580, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117: 4667–4678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med 207: 2255–2269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34: 96–107, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Mak SS, Yeo W, Lee YM, Mo KF, Tse KY, Tse SM, Ho FP, Kwan WH. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res 57: 416–425, 2008 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 26: 5213–5219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Olszewski WL, Jamal S, Manokaran G, Lukomska B, Kubicka U. Skin changes in filarial and non-filarial lymphoedema of the lower extremities. Trop Med Parasitol 44: 40–44, 1993 [PubMed] [Google Scholar]
- 21.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer 19: 853–857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 72: 161–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer 110: 1868–1874, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Suami H, Pan WR, Taylor GI. Changes in the lymph structure of the upper limb after axillary dissection: radiographic and anatomical study in a human cadaver. Plast Reconstr Surg 120: 982–991, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R, Rockson SG. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med 3: e254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ververs JM, Roumen RM, Vingerhoets AJ, Vreugdenhil G, Coebergh JW, Crommelin MA, Luiten EJ, Repelaer van Driel OJ, Schijven M, Wissing JC, Voogd AC. Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancer. Eur J Cancer 37: 991–999, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Weissleder H, Weissleder R. Lymphedema: evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 167: 729–735, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLOS ONE 8: e70703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115: 1029–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Zampell JC, Avraham T, Yoder N, Fort N, Yan A, Weitman ES, Mehrara BJ. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am J Physiol Cell Physiol 302: C392–C404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen S, Rockson SG, Mehrara BJ. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol 300: C1107–C1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLOS ONE 7: e49940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]