Abstract
Cardiac ischemia and angina pectoris are commonly experienced during exertion in a cold environment. In the current study we tested the hypotheses that oropharyngeal afferent blockade (i.e., local anesthesia of the upper airway with lidocaine) as well as systemic β-adrenergic receptor blockade (i.e., intravenous propranolol) would improve the balance between myocardial oxygen supply and demand in response to the combined stimulus of cold air inhalation (−15 to −30°C) and isometric handgrip exercise (Cold + Grip). Young healthy subjects underwent Cold + Grip following lidocaine, propranolol, and control (no drug). Heart rate, blood pressure, and coronary blood flow velocity (CBV, from Doppler echocardiography) were continuously measured. Rate-pressure product (RPP) was calculated, and changes from baseline were compared between treatments. The change in RPP at the end of Cold + Grip was not different between lidocaine (2,441 ± 376) and control conditions (3,159 ± 626); CBV responses were also not different between treatments. With propranolol, heart rate (8 ± 1 vs. 14 ± 3 beats/min) and RPP responses to Cold + Grip were significantly attenuated. However, at peak exercise propranolol also resulted in a smaller ΔCBV (1.4 ± 0.8 vs. 5.3 ± 1.4 cm/s, P = 0.035), such that the relationship between coronary flow and cardiac metabolism was impaired under propranolol (0.43 ± 0.37 vs. 2.1 ± 0.63 arbitrary units). These data suggest that cold air breathing and isometric exercise significantly influence efferent control of coronary blood flow. Additionally, β-adrenergic vasodilation may play a significant role in coronary regulation during exercise.
Keywords: lidocaine, oropharynx, propranolol, vascular resistance, blood pressure, heart rate
angina pectoris and acute myocardial infarction can be triggered by physical exertion in a cold environment. Indeed, numerous studies indicate that snow shoveling (13, 40, 66), downhill skiing (10, 46, 77), and deer hunting in winter (30) are associated with cardiovascular morbidity and mortality. The reason(s) why cold temperature has a negative effect on the human heart is not entirely clear. One concept is that surges in sympathetic nerve activity can restrain metabolic coronary vasodilation, thereby impairing the balance between myocardial oxygen supply and demand (2, 3, 38, 44); this scenario could potentially provoke symptoms of angina during exposure to cold temperatures. It has been established that coronary vasodilator responses to various stimuli (e.g., adenosine infusion, handgrip exercise, cold pressor test) have both diagnostic and prognostic value (14, 31, 34, 53, 65, 73, 75). If coronary vasodilation in response to exercise is restrained by sympathetic vasoconstriction (because of cold air inhalation), this could be a mechanistic link explaining why exertion in the cold is associated with poor outcomes. By using transthoracic Doppler echocardiography to measure coronary blood flow velocity (CBV) along with beat-by-beat measures of heart rate (HR) and arterial blood pressure (BP), our laboratory has begun to study the balance between myocardial oxygen supply and demand in vivo (22–24, 56, 58).
Because the mouth and face are most likely to be exposed to cold ambient conditions in daily life, we believe that studying how cold air inhalation influences coronary blood flow is clinically valuable. We recently established that cold air inhalation (−15 to −25°C) impairs the coronary supply-to-demand ratio compared with inhalation of neutral temperature air (20 to 25°C) in healthy subjects (60, 61). This impairment was also noted when cold air breathing was combined with isometric handgrip exercise, a laboratory intervention known to raise myocardial oxygen demand as well as sympathetic nerve activity (8, 19, 68). In our previous studies, we observed that rate-pressure product (RPP) increased to a greater extent in response to the combined stimulus of cold air inhalation and isometric handgrip (Cold + Grip) compared with handgrip under neutral air conditions. This augmented RPP response should signal metabolic vasodilation, but we consistently observed less coronary hyperemia compared with control (i.e., neutral air) conditions (60, 61). Taken together, it appears that cold air inhalation impairs metabolic coronary vasodilation when experienced alone or in combination with isometric work.
The purpose of the current study was to determine the effect of autonomic blockade on the coronary blood flow responses to Cold + Grip. In experiment 1, we tested the hypothesis that oropharyngeal afferent blockade (i.e., local anesthesia of the upper airway with 4% topical lidocaine) would attenuate the rise in RPP and enhance the CBV response to the Cold + Grip stimulus compared with control conditions (Cold + Grip without lidocaine). In experiment 2, we tested the hypothesis that systemic β-adrenergic receptor blockade (i.e., intravenous propranolol) would attenuate the rise in RPP in response to the Cold + Grip protocol. The current studies in healthy humans provide several unexpected findings and support the concept that vascular β-adrenergic receptors play an important role in coronary regulation during times of increased RPP.
METHODS
Design and subjects.
The overall study used a within-subjects, repeated-measures design whereby physiological parameters were continuously measured during baseline, 5 min of cold air inhalation at rest, and 2 min of isometric handgrip while continuing to breathe cold air (Fig. 1, timeline). The comparison for experiment 1 was between lidocaine and control (no lidocaine); the comparison for experiment 2 was between propranolol and control (no propranolol). All study protocols were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. All volunteers provided written informed consent.
Fig. 1.
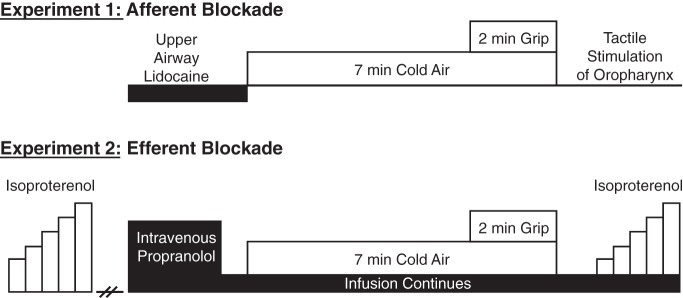
Experimental timeline. For both experiments, control trials (without drug) were performed on separate days. For experiment 1, the absence of the gag reflex following tactile stimulation of the oropharynx confirmed that afferent blockade was effective. For experiment 2, the absence of tachycardia in response to intravenous isoproterenol confirmed that efferent blockade was effective. Please see main text for details.
Thirteen young subjects (7 men and 6 women, 26 ± 1 yr, 1.77 ± 0.04 m, 77.3 ± 5.4 kg, and 24.7 ± 1.1 kg/m2) participated in experiment 1. Nine young subjects (4 men and 5 women, 27 ± 1 yr, 1.79 ± 0.05 m, 78.4 ± 4.7 kg, and 24.4 ± 1.2 kg/m2) participated in experiment 2. Three subjects participated in both experiments 1 and 2. We did not specifically control for menstrual cycle phase in young women. The sample size for experiment 2 was determined after the first six subjects had completed testing. Specifically, we determined that if the difference in the CBV-to-RPP ratio between propranolol and control conditions was 1.7 arbitrary units (AU) and had a standard deviation of 1.5 AU, we would be able to reject the null hypothesis with probability (power) of 0.92 and a type 1 error of 0.05.
All subjects had supine resting BPs below 120/80 mmHg and were nonasthmatic, nonobese, nonsmokers, not taking any prescription or vasoactive medication, and were in good health as determined by history and physical examination. All subjects reported being physically active, but none were competitive athletes. Subjects refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory following an overnight fast.
Physiological and perceptual measurements used in both experiments.
All imaging protocols were conducted in the supine or left lateral position in a dimly lit thermoneutral laboratory (22–25°C) in the morning hours (7:30 am to 12:00 pm). Upon arrival at the laboratory, subjects were outfitted with a three-lead EKG (Cardiocap/5, GE Healthcare) to monitor HR, a finger BP cuff (Finometer, FMS), and a pneumotrace to monitor respiratory movement. Before echocardiography imaging, resting BPs were obtained in triplicate by automated oscillometry of the left brachial artery (Philips Sure Signs VS3) after 15 min of quiet rest. The average baseline brachial artery pressures [systolic BP, diastolic BP, and mean arterial BP (MAP)] were used to adjust the Finometer values during offline analysis. For example, if brachial MAP was 90 mmHg at baseline and the Finometer value for MAP was 85 mmHg, then 5 mmHg was added to all Finometer values in subsequent minutes. By doing this, we ensured that RPP and coronary vascular resistance (CVR) were calculated with the brachial systolic BP and MAP values, respectively. Arterial oxygen saturation was monitored by pulse oximetry on the earlobe (Respiratory Gas Monitor 5250, Ohmeda). All of the variables listed above were collected at 200 Hz by a PowerLab (ADInstruments).
Rating of thermal sensation of the body (where 1 = cold and 7 = hot) (20), perception of mouth coldness (where 0 = neutral or no sensation of cold and 11 = unbearably cold) (27), perception of mouth pain (where 0 = no pain and 10 = unbearable pain) (64), and perceived exertion in the hand and forearm (where 6 = very, very light and 20 = maximal exertion) (7) were also quantified.
Basic pulmonary function was determined with a MiniSpir device (Medical International Research) in both the seated and left lateral positions. The variables of interest included forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC).
CBV, an index of myocardial O2 supply, was obtained from the adjusted apical four-chamber view using a GE Vivid 7 echocardiography system. The specific procedures used in our laboratory have been previously described (23–25, 58). Briefly, a variable frequency phased-array transducer (7S) was positioned to explore the left ventricular apex. The imaging depth was set at 5 cm, and the focal zones were set at ∼2 to 3 cm. Color flow mapping was used, and the two-dimensional gain was adjusted to obtain the best blood flow signal of the left anterior descending coronary artery. Once this was obtained, a 2.0-mm sample volume was placed over the color signal, and CBV was recorded at end expiration. The transducer was held still throughout the protocol, and care was taken to obtain at least one three-beat clip during the last 10 s of each minute. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv 3.0 to obtain peak diastolic CBV, as previously described (60, 61). Because of the limited spatial resolution and small vessel size, we did not attempt to measure the diameter of the left anterior descending coronary artery. However, our laboratory has documented that the percent increase in peak diastolic CBV measured via transthoracic Doppler echocardiography is similar to the percent increase in peak diastolic CBV measured by an intracoronary Doppler guidewire (57). Other studies have demonstrated strong correlations between CBV and coronary blood flow in response to vasodilator stimuli (51, 69).
Cold air inhalation plus handgrip protocol.
A custom system was used to deliver cold air to the subjects, as previously described (60). Briefly, a closed loop of copper coil was placed in an insulated container that was filled with liquid nitrogen. Compressed medical air was attached to the distal end of the copper coil system, and subjects were instructed to breathe normally through a Hans Rudolph 2700B mouthpiece. Breathing rate and air temperature were measured in real time using thermistors (TC-2000, Sable Systems) situated in the mouthpiece. Because breathing cold air is a novel laboratory stimulus, at least two familiarization trials always occurred during an initial visit to the laboratory. During the familiarization trial, pulmonary function was determined in the seated upright position before and after cold air breathing. Additionally, maximum voluntary contraction (MVC) of the hand and forearm was also obtained using a handgrip dynamometer. Thirty percent of the MVC was calculated and used in subsequent trials; this workload is known to raise sympathetic nerve activity (52) and RPP (i.e., needed to elicit metabolic coronary vasodilation). For both experiments 1 and 2, the protocol always included 3 min of baseline, 5 min of cold air inhalation and rest, and 2 min of 30% isometric handgrip while continuing to breathe cold air. Contrary to our previous publications (60, 61) in which inspired air temperature was the independent variable (cold vs. neutral), in the current report, drug was the independent variable (lidocaine vs. no lidocaine; propranolol vs. no propranolol).
Experiment 1: effect of lidocaine.
The procedures for upper airway anesthesia have been previously described for our laboratory (63). Briefly, participants were seated on an examination table and received 6 ml of 4% topical lidocaine via nebulizer. Compressed medical air was supplied at 4–8 l/min, and subjects breathed until the entire solution was gone (∼15 min). Subjects were then situated in the left lateral position and breathed an additional 2 ml of nebulized lidocaine while coronary baseline measurements were obtained. Throughout lidocaine administration, suction was allowed ad libitum (Allegiance K86 Medi-Vac Yankauer Suction). Cold air inhalation then occurred for a total of 7 min; the last 2 min also included isometric handgrip at 30% MVC (Fig. 1). Within 15 s of the end of exercise, the gag reflex was tested. If the gag reflex was absent at this time point, we considered the upper airway anesthesia to be effective. Subjects were not allowed to leave the laboratory until the gag reflex returned (typically 20–40 min). In four subjects, the gag reflex was present at the end of exercise, so all their data were excluded (i.e., afferent blockade ineffective in these people, so n = 9 in results). Control trials (without lidocaine) were performed on a separate day and were conducted in a counterbalanced fashion.
Experiment 2: effect of propranolol.
For experiment 2, subjects dressed in a high-density, tube-lined suit (Med-Eng Systems, Ottawa, ON, Canada) that covered the entire body except for the feet, hands, and head. Neutral water (34–35°C) was perfused through the suit to maintain mean skin temperature at a constant level. Two intravenous catheters were placed (one in a left antecubital vein and one in a right antecubital vein). Following baseline measurements in the supine posture, an intravenous infusion of isoproterenol, a nonselective β-adrenergic agonist, occurred in the left arm (Fig. 1). This infusion was based on previous human experiments (5, 70, 72) and began at a rate of 0.5 μg/min for 1 min and increased by 0.5 μg/min each minute until HR increased by 25–30 beats/min. After a 30-min washout period, a loading dose of propranolol was infused in the right arm over 15 min (0.25 mg/kg at a rate of 4 ml/min), followed by a maintenance infusion (0.006 mg·kg−1·min−1 at a rate of 1.45 ml/min) for the remainder of the study (11, 71). Pulmonary function was measured in the left lateral position before the start of propranolol infusion and after the loading dose of propranolol to ensure that β-adrenergic receptor blockade did not influence lung function (42). Baseline measurements were obtained, and then Cold + Grip occurred as described above. At the end of the study, the same duration and volume of isoproterenol was again infused into the left arm while the maintenance dose of propranolol continued in the right arm. Quantifying the tachycardia in response to isoproterenolol before and after propranolol allowed us to determine the effectiveness of our systemic β-adrenergic blockade. Because of the high dose of propranolol given, orthostatic vital signs were recorded before the participants were released from the laboratory. Control trials (without propranolol) were performed on a separate day and were conducted in a counterbalanced fashion.
Data collection and statistical analysis.
All variables were continuously measured and were analyzed off-line. An average of the last 15 s of each minute is presented. RPP (the product of HR and systolic BP) was used as a noninvasive index of myocardial oxygen demand (28). Both CBV and the ratio of ΔCBV to ΔRPP (i.e., the slope of the relationship between coronary flow and cardiac metabolism at peak exercise) were used as indexes of myocardial oxygen supply (22, 25). We also calculated CVR (the quotient of MAP and CBV) in an effort to compare with a previous study (59). For all variables, a two-treatment (drug, control) by nine-time point repeated-measures ANOVA was conducted using the raw physiological parameters. Paired t-tests were used when a significant drug by time interaction was found. Planned comparisons were also used to compare physiological parameters at baseline and at the end of exercise (i.e., when it was expected that treatment effects would be most prominent). Changes from baseline (Δ) were also compared between treatments.
RESULTS
Experiment 1: effect of lidocaine.
Lidocaine inhalation was generally well tolerated, but several subjects reported that it was difficult to talk following lidocaine treatment. At baseline before cold air breathing, CBV (22.7 ± 2.7 vs. 21.8 ± 2.7 cm/s, P = 0.621) and CVR (3.6 ± 0.3 vs. 3.7 ± 0.4 mmHg·cm−1·s−1, P = 0.592) were not altered by lidocaine compared with control conditions. In response to Cold + Grip, lidocaine did not have an effect on any of the measured variables compared with control (Fig. 2). The change in CBV at the end of exercise was not different between lidocaine (6.6 ± 2.1 cm/s) and control (4.1 ± 1.6 cm/s, P = 0.441). In a similar way, the change in CVR at the end of exercise was not different between lidocaine (−0.21 ± 0.21 mmHg·cm−1·s−1) and control (0.19 ± 0.33 mmHg·cm−1·s−1, P = 0.354). Thus upper airway afferent blockade (i.e., at a level that blocked the gag reflex) did not influence the hemodynamic and coronary responses to Cold + Grip. Thermal and perceptual variables are presented in Table 1.
Fig. 2.
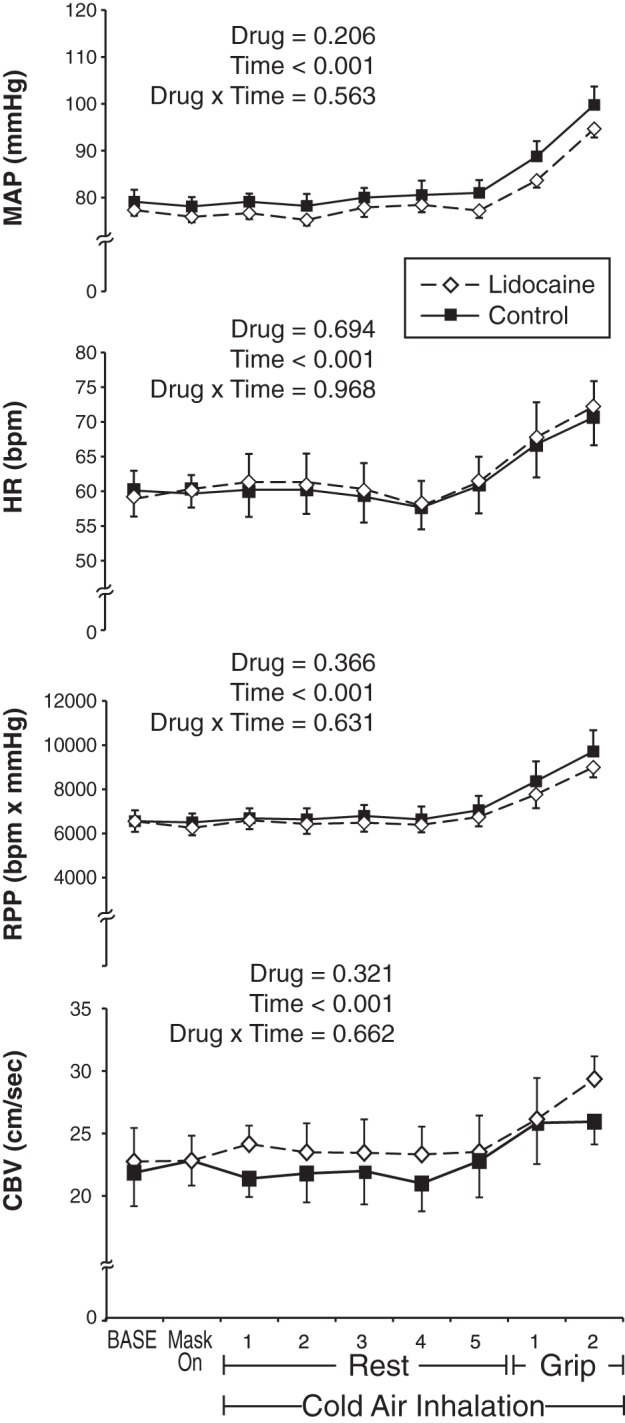
Hemodynamic and coronary response to experiment 1 (n = 9). Lidocaine trials (dashed line with white diamonds) and control trials (solid line with black squares) were performed on separate days. In response to the Cold + Grip protocol, lidocaine had no effect on mean arterial pressure (MAP), heart rate [HR, in bpm (beats/min)], rate-pressure product (RPP), or coronary blood flow velocity (CBV). Data are means ± SE.
Table 1.
Thermal and perceptual response to the Cold + Grip protocol
|
Experiment 1 |
Experiment 2 |
||||
|---|---|---|---|---|---|
| Lidocaine | Control | Propranolol | Control | ||
| Tair | °C | −22 ± 5 | −24 ± 3 | −19 ± 4 | −20 ± 3 |
| Tmouth | °C | 14 ± 3 | 13 ± 3 | 18 ± 2 | 17 ± 2 |
| TS body | AU | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
| TS mouth | AU | 2 ± 1 | 2 ± 1 | 3 ± 1 | 2 ± 1 |
| Pain mouth | AU | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Workload | kg | 12 ± 1 | 12 ± 1 | 13 ± 1 | 12 ± 1 |
| RPE | AU | 14 ± 1 | 15 ± 1 | 16 ± 1* | 15 ± 1 |
Nine subjects completed experiment 1 and eight subjects completed experiment 2. Temperatures are average values taken during the exercise part of the protocol. All perceptual variables were obtained by verbal report immediately after the protocol ended. Tair, air temperature; Tmouth, mouth temperature; TS, thermal sensation; RPE, rating of perceived exertion (where 13 = somewhat hard, 15 = hard, and 17 = very hard). Data are means ± SE
P < 0.05 compared with control.
Experiment 2: effect of propranolol.
The average dose of propranolol received was 51 ± 3 mg. Consistent with our previous report, which gave the same dose of propranolol in different subjects (62), the tachycardia in response to intravenous isoproterenol was completely blocked by intravenous propranolol (ΔHR pre = 29 ± 3 beats/min vs. post 1 ± 1 beats/min). Thus β-adrenergic blockade was effective. Pulmonary function was reduced in the left lateral position compared with the seated position (FEV1 from 3.75 ± 0.21 to 3.45 ± 0.17 liters and FVC from 4.93 ± 0.28 to 4.59 ± 0.23 liters, P < 0.001). However, there was no further reduction in FEV1 (to 3.41 ± 0.16 liters, P = 0.137) or FVC (to 4.51 ± 0.20 liters, P = 0.265) following the infusion of propranolol. Thus propranolol did not significantly influence lung function in these young healthy subjects. Importantly, orthostatic vital signs were clinically acceptable at the completion of the study (i.e., appropriate tachycardia upon sitting and standing, no hypotension, and no dizziness).
Technical difficulty in coronary imaging resulted in one young woman being excluded from analysis. As depicted in Fig. 3, the Cold + Grip protocol significantly raised all variables across time. For the variable MAP, there was no main effect for drug or drug by time interaction. For both HR and RPP, there was a significant drug by time interaction such that propranolol lowered these variables at baseline and also attenuated the reflex increase in response to the Cold + Grip protocol. Indeed, ΔHR at peak exercise was blunted by propranolol (8 ± 1 beats/min) compared with control (14 ± 3 beats/min, P = 0.025), and a similar effect was observed for RPP (2,393 ± 376 vs. 3,357 ± 660 beats·min−1·mmHg, P = 0.049). Moreover, the ΔHR (0 ± 1 vs. 5 ± 1 beats/min, P = 0.006) and ΔRPP (114 ± 128 vs. 895 ± 160 beats·min−1·mmHg, P < 0.001) in response to 5 min of cold air at rest (before exercise) were also significantly attenuated by propranolol compared with control; this effect was observed in 9 of the 10 subjects studied.
Fig. 3.
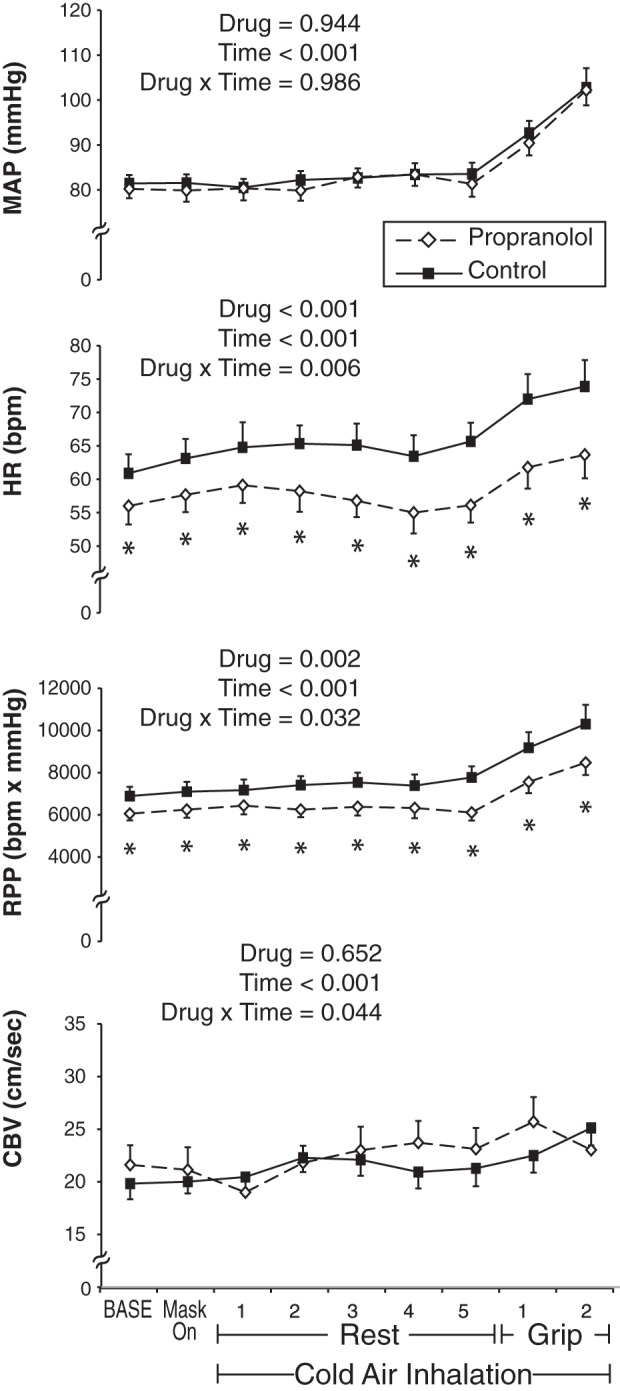
Hemodynamic and coronary response to experiment 2 (n = 8). Propranolol trials (dashed line with white diamonds) and control trials (solid line with black squares) were performed on separate days. In response to the Cold + Grip protocol, propranolol had no effect on MAP but lowered the HR and RPP response compared with control. Propranolol also impaired the coronary vasodilator response at peak exercise (i.e., the change in CBV was significantly less with propranolol than control). Data are means ± SE. *Difference between treatments at the specific time point.
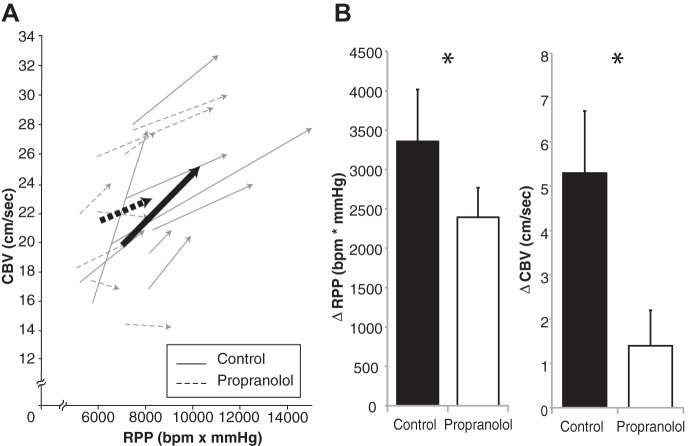
As shown in Fig. 3, CBV revealed a drug × time interaction. While none of the specific time points were different between treatments, the ΔCBV was significantly impaired (i.e., less coronary hyperemia) under propranolol (1.4 ± 0.8 cm/s) compared with control (5.3 ± 1.4 cm/s, P = 0.035, Fig. 4). The derived quantities ΔCVR (propranolol, 1.3 ± 0.41 vs. control, 0.16 ± 0.38 mmHg·cm−1·s−1, P = 0.015) and ΔCBV/ΔRPP (propranolol, 0.43 ± 0.37 vs. control, 2.1 ± 0.63 AU, P = 0.041) were also impaired under β-adrenergic receptor blockade. Individual data plotting CBV and RPP are displayed in Fig. 4. In total, the relationship between coronary flow and cardiac metabolism was impaired under propranolol such that less hyperemia occurred in response to the Cold + Grip protocol (i.e., a stimulus that raises myocardial O2 demand as well as sympathetic tone). Thermal and perceptual variables are presented in Table 1. It is interesting to note that rating of perceived exertion of the hand and forearm was significantly higher with propranolol (P = 0.028).
Fig. 4.
A: individual coronary O2 demand (RPP, x-axis) and coronary O2 supply (CBV, y-axis) are displayed for experiment 2. The arrows point from baseline to the peak of handgrip exercise; bold lines represent group averages. Note that the slopes of the control trials are significantly more steep (more coronary hyperemia) than the slopes of the propranolol trials (2.1 ± 0.63 vs. 0.43 ± 0.37 arbitrary units, P = 0.041). B: mean changes in RPP and CBV in response to Cold + Grip protocol. *Difference between treatments.
DISCUSSION
In the current study we hypothesized that local afferent blockade of the oropharynx (experiment 1) and systemic efferent blockade of β-adrenergic receptors (experiment 2) would improve the balance between myocardial oxygen supply (CBV) and demand (RPP) during the combined stimulus of cold air inhalation and isometric handgrip. The current data do not support these hypotheses. Nevertheless, herein we provide two key findings. First, lidocaine administration in the oropharynx did not attenuate the rise in RPP in response to the Cold + Grip protocol, and it also did not facilitate a larger coronary hyperemic response. Second, intravenous propranolol attenuated the HR and RPP response to the Cold + Grip protocol but actually impaired coronary hyperemia (presumably due to blockade of vascular β2-receptors in the coronary microcirculation). These data are novel on a physiological level and also may benefit patients with cardiovascular disease who undergo exertion in a cold environment.
We previously reported that cold air inhalation attenuated the coronary hyperemia in response to a 2-min bout of isometric handgrip compared with neutral air despite a significantly greater rise in RPP (i.e., a situation that should elicit a larger metabolic coronary vasodilation) (60, 61). These acute laboratory studies were consistent with previous experiments that used cold air inhalation alone (15, 33, 35, 47, 49, 67) or in combination with dynamic exercise in a whole body environmental chamber (9, 18, 43, 48, 50, 54). Additionally, the clinical observation that angina pectoris, arrhythmia, and sudden cardiac death are more prevalent following a heavy snowstorm (26, 32, 39, 74) suggests that synergistic effects between cold temperature and vigorous exertion exist. Based on a recent publication from our group (63), it is clear that tactile stimulation of the oropharynx raises both sympathetic nerve activity and myocardial oxygen demand; this effect can be blocked by pretreating the upper airway with 4% lidocaine (i.e., local afferent blockade). In the current study, we used this same anesthetic procedure to evaluate how cold stimulation influences CBV and RPP. The data in Fig. 2 indicate that afferent blockade had no effect on the balance between myocardial oxygen supply and demand during Cold + Grip. This was contrary to our hypothesis. It should be noted that breathing lidocaine through a nebulizer anesthetizes the oropharynx, the back of the tongue, and possibly the larynx in some subjects but not the teeth, gums, or bronchioles (12, 45, 76). The possibility that the teeth and/or lower airway contribute to sympathetic reflex pathways remains to be prospectively tested.
In experiment 2, we tested the hypothesis that intravenous propranolol would attenuate the rise in RPP in response to the Cold + Grip protocol. We chose propranolol (as opposed to an α-blocker such as phentolamine) because our previous studies have shown that the HR response to Cold + Grip was augmented, whereas the MAP response was similar to handgrip under neutral air breathing (60, 61). Additionally, propranolol is commonly used in patients with cardiovascular disease (i.e., the people most at risk for adverse events in the winter months) (78). Consistent with our hypothesis, the HR and RPP response to both cold air inhalation at rest and also the Cold + Grip protocol were significantly attenuated by propranolol (Fig. 3). This might indicate that β-blockers are useful for people who undergo exertion in the cold because the metabolic demand of the heart would be lower. However, when considering the coronary blood flow data, we found that propranolol actually impaired myocardial oxygen supply. Indeed, both CBV and the CBV-to-RPP ratio were impaired under propranolol, indicating a smaller coronary hyperemia when normalizing for the metabolic stimulus (Fig. 4). While this was an unexpected finding, it suggests that under normal conditions (i.e., no drug), young healthy people experience a significant amount of β-adrenergic coronary vasodilation in response to the Cold + Grip stimulus (i.e., a physiological stimulus that raises myocardial metabolism as well as sympathetic nerve activity). We speculate this is also true for exercise under neutral air conditions, but the current study was not designed to test this concept directly. In general, our findings are consistent with previous coronary studies in exercising dogs (29, 55) and pigs (16, 17, 21). Under nonselective β-adrenergic receptor blockade, α-adrenergic constriction is unchecked and circulating catecholamines elicit a net vasoconstriction (36, 41).
When comparing the data from experiment 2 with those of previous studies, there are several factors that must be considered. First, we used local cooling of the upper airway combined with isometric handgrip in the supine posture, whereas dynamic exercise such as snow shoveling is conducted outdoors in the upright position. Second, the changes in HR and RPP due to propranolol were small in magnitude and may have limited clinical significance. However, higher exercise intensities (i.e., greater sympathetic activation) conducted in a cold environment would likely magnify the effects of propranolol rather than diminish them. Third, vagal withdrawal and baroreflex buffering also occur when undergoing isometric handgrip at 30% MVC so the net effect of propranolol on coronary blood flow is an integrated response of several different inputs. Fourth, propranolol is a nonselective β-blocker and using an intravenous route of administration results in the entire body (both heart and blood vessels) being β-blocked. In this study, we chose propranolol to ensure a complete blockade of the β-adrenergic receptors. Had we chosen a different β-blocker we may not have observed the surprising finding that the CBV-to-RPP ratio was impaired at peak exercise (Fig. 3, bottom, and Fig. 4). Fifth, our subjects were young and did not have other cardiovascular disease risk factors. Young subjects presumably have higher coronary β-adrenergic vasodilatory capacity compared with older people or patients with overt cardiovascular disease (although this speculation is not known for certain). From a clinical perspective, young subjects typically do not experience chest pain when shoveling snow. Sixth, we did not use a systemic α-blocker in this study and it should be noted that systemic propranolol not only impairs β-adrenergic coronary vasodilation but also enhances α-adrenergic coronary vasoconstriction. Despite these six caveats listed above, we are the first to evaluate coronary responses to Cold + Grip under β-adrenergic receptor blockade. We found that myocardial oxygen demand was lowered by propranolol, but myocardial oxygen supply was also impaired. Thus, in addition to its intended effects on HR, propranolol also had physiological effects on the coronary blood vessels. The interaction between metabolic coronary vasodilation (due to a rise in RPP) and β1- or β2-adrenergic coronary vasodilation warrants further study in humans.
Conclusions.
Both epidemiological and clinical evidence indicate that adverse cardiovascular events are most common in the cold winter months compared with any other time of year (1, 6, 18a). It is also clear that acutely stressful situations (e.g., exposure to cold and exercise) are linked with adverse cardiovascular events (13, 40, 66) and that heightened sympathetic tone can impair coronary vasodilation (2, 3, 37, 38, 44). In the current study we used transthoracic Doppler echocardiography along with pharmacological blockade to understand how cold air inhalation and isometric exercise influence the balance between myocardial oxygen supply and demand. In experiment 1, we determined that afferent blockade of the oropharynx had no effect on RPP and CBV in response to the Cold + Grip protocol. In experiment 2, we found that intravenous propranolol lowered the HR and RPP responses to both cold air inhalation at rest and also the Cold + Grip protocol but also impaired coronary blood flow at peak exercise. These data in young healthy people support the concept that cold air breathing and isometric exercise significantly influence efferent control of coronary blood flow. Additionally, the data suggest that β-adrenergic vasodilation plays a significant role in coronary regulation during times of increased myocardial oxygen demand.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-096570 (to L. I. Sinoway) and UL1-TR-000127 (to L. I. Sinoway) and a Wilderness Medical Society research-in-training grant (to M. D. Muller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D.M., P.M.M., U.A.L., and L.I.S. conception and design of research; M.D.M., Z.G., P.M.M., and U.A.L. performed experiments; M.D.M. analyzed data; M.D.M., Z.G., P.M.M., U.A.L., and L.I.S. interpreted results of experiments; M.D.M. prepared figures; M.D.M. drafted manuscript; M.D.M., Z.G., P.M.M., U.A.L., and L.I.S. edited and revised manuscript; M.D.M., Z.G., P.M.M., U.A.L., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for nursing support provided by Cheryl Blaha, Jessica Mast, and Todd Nicklas; the technical assistance of Josh Oman, Matt Heffernan, and Hardikkumar Patel; and engineering support provided by Dr. Michael Herr. Gratitude is also extended to Anne Muller for preparing the graphics for this study. Finally, we acknowledge the administrative guidance of Kris Gray and Jen Stoner.
REFERENCES
- 1.Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health 72: 261–265, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbato E. Role of adrenergic receptors in human coronary vasomotion. Heart 95: 603–608, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Baumgart D, Haude M, Gorge G, Liu F, Ge J, Grosse-Eggebrecht C, Erbel R, Heusch G. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation 99: 2090–2097, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab 86: 4440–4444, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ 341: c3823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exec 14: 377–381, 1982 [PubMed] [Google Scholar]
- 8.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation 70: 18–24, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Brown CF, Oldridge NB. Exercise-induced angina in the cold. Med Sci Sports Exec 17: 607–612, 1985 [PubMed] [Google Scholar]
- 10.Burtscher M, Pachinger O, Mittleman MA, Ulmer H. Prior myocardial infarction is the major risk factor associated with sudden cardiac death during downhill skiing. Int J Sports Med 21: 613–615, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Acute beta-adrenergic blockade increases aortic wave reflection in young men and women: differing mechanisms between sexes. Hypertension 59: 145–150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest 71: 346–348, 1977 [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury PS, Franklin BA, Boura JA, Dragovic LJ, Kanluen S, Spitz W, Hodak J, O'Neill WW. Sudden cardiac death after manual or automated snow removal. Am J Cardiol 92: 833–835, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Cortigiani L, Rigo F, Gherardi S, Bovenzi F, Molinaro S, Picano E, Sicari R. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc Imaging 5: 1079–1085, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Dodds PA, Bellamy CM, Muirhead RA, Perry RA. Vasoconstrictor peptides and cold intolerance in patients with stable angina pectoris. Br Heart J 73: 25–31, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res 82: 1312–1322, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Epstein SE, Stampfer M, Beiser GD, Goldstein RE, Braunwald E. Effects of a reduction in environmental temperature on the circulatory response to exercise in man. Implications concerning angina pectoris. N Engl J Med 280: 7–11, 1969 [DOI] [PubMed] [Google Scholar]
- 18a.The Eurowinter Group. Cold exposure, and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet 349: 1341–1346, 1997 [PubMed] [Google Scholar]
- 19.Ferrara N, Vigorito C, Leosco D, Giordano A, Abete P, Longobardi G, Rengo F. Regional left ventricular mechanical function during isometric exercise in patients with coronary artery disease: correlation with regional coronary blood flow changes. J Am Coll Cardiol 12: 1215–1221, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Gagge AP, Stolwijk JA, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 2: 209–229, 1969 [DOI] [PubMed] [Google Scholar]
- 21.Gao F, de Beer VJ, Hoekstra M, Xiao C, Duncker DJ, Merkus D. Both β1- and β2-adrenoceptors contribute to feedforward coronary resistance vessel dilation during exercise. Am J Physiol Heart Circ Physiol 298: H921–H929, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Gao Z, Muller MD, Sinoway LI, Leuenberger UA. Intravenous phentolamine abolishes coronary vasoconstriction in response to mild central hypovolemia. J Appl Physiol 116: 216–221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Novick M, Muller MD, Williams RJ, Spilk S, Leuenberger UA, Sinoway LI. Exercise and diet-induced weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents. Eur J Appl Physiol 113: 519–528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol 112: 483–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass RI, Zack MM., Jr Increase in deaths from ischaemic heart-disease after blizzards. Lancet 1: 485–487, 1979 [DOI] [PubMed] [Google Scholar]
- 27.Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57: 549–556, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Haapaniemi S, Franklin BA, Wegner JH, Hamar S, Gordon S, Timmis GC, O'Neill WW. Electrocardiographic responses to deer hunting activities in men with and without coronary artery disease. Am J Cardiol 100: 175–179, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Hammoudeh AJ, Haft JI. Coronary-plaque rupture in acute coronary syndromes triggered by snow shoveling. N Engl J Med 335: 2001, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Hattenhaur M, Neill WA. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation 51: 1053–1058, 1975 [DOI] [PubMed] [Google Scholar]
- 34.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol 56: 1657–1665, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Heindl S, Struck J, Wellhoner P, Sayk F, Dodt C. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol 142: 69–80, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation 81: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Heusch G. The paradox of alpha-adrenergic coronary vasoconstriction revisited. J Mol Cell Cardiol 51: 16–23, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. α-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101: 689–694, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Yamamoto T, Takaku K, Tsutsui N, Sasagawa M, Hirono S, Suzuki T, Kodama M. Acute heart failure syndrome associated with snow shoveling. Int Heart J 53: 394–395, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Janardhanan R, Henry Z, Hur DJ, Lin CM, Lopez D, Reagan PM, Rudnick SR, Koshko TJ, Keeley EC. The snow-shoveler's ST elevation myocardial infarction. Am J Cardiol 106: 596–600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsson G. Influence of metoprolol and propranolol on hemodynamic effects induced by adrenaline and physical work. Acta Pharmacol Toxicol (Copenh) 36: 59–68, 1975 [DOI] [PubMed] [Google Scholar]
- 42.Johnsson G, Svedmyr N, Thiringer G. Effects of intravenous propranolol and metoprolol and their interaction with isoprenaline on pulmonary function, heart rate and blood pressure in asthmatics. Eur J Clin Pharmacol 8: 175–180, 1975 [DOI] [PubMed] [Google Scholar]
- 43.Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation 79: 1015–1020, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann PA, Rimoldi O, Gnecchi-Ruscone T, Bonser RS, Luscher TF, Camici PG. Systemic inhibition of nitric oxide synthase unmasks neural constraint of maximal myocardial blood flow in humans. Circulation 110: 1431–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Kirkpatrick MB, Sanders RV, Bass JB., Jr Physiologic effects and serum lidocaine concentrations after inhalation of lidocaine from a compressed gas-powered jet nebulizer. Am Rev Respir Dis 136: 447–449, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Klug G, Schenk S, Dorler J, Mayr A, Haubner BJ, Alber H, Schachinger V, Pachinger O, Metzler B. Occurrence of acute myocardial infarction in winter tourists: data from a retrospective questionnaire. Clin Res Cardiol 100: 669–674, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Lassvik C, Areskog NH. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J 43: 661–667, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassvik CT, Areskog NH. Angina in cold environment. Reactions to exercise. Br Heart J 42: 396–401, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leon DF, Amidi M, Leonard JJ. Left heart work and temperature responses to cold exposure in man. Am J Cardiol 26: 38–45, 1970 [DOI] [PubMed] [Google Scholar]
- 50.Marchant B, Donaldson G, Mridha K, Scarborough M, Timmis AD. Mechanisms of cold intolerance in patients with angina. J Am Coll Cardiol 23: 630–636, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Marcus M, Wright C, Doty D, Eastham C, Laughlin D, Krumm P, Fastenow C, Brody M. Measurements of coronary velocity and reactive hyperemia in the coronary circulation of humans. Circ Res 49: 877–891, 1981 [DOI] [PubMed] [Google Scholar]
- 52.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985 [DOI] [PubMed] [Google Scholar]
- 53.Meimoun P, Tribouilloy C. Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: a magic tool for the real world. Eur J Echocardiogr 9: 449–457, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Meyer P, Guiraud T, Curnier D, Juneau M, Gayda M, Nozza A, Nigam A. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol 26: e50–e53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyashiro JK, Feigl EO. Feedforward control of coronary blood flow via coronary beta-receptor stimulation. Circ Res 73: 252–263, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Momen A, Gao Z, Cohen A, Khan T, Leuenberger UA, Sinoway LI. Coronary vasoconstrictor responses are attenuated in young women as compared with age-matched men. J Physiol 588: 4007–4016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Momen A, Kozak M, Leuenberger UA, Ettinger S, Blaha C, Mascarenhas V, Lendel V, Herr MD, Sinoway LI. Transthoracic Doppler echocardiography to noninvasively assess coronary vasoconstrictor and dilator responses in humans. Am J Physiol Heart Circ Physiol 298: H524–H529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monahan KD, Feehan RP, Sinoway LI, Gao Z. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: Effect of ageing. J Physiol 591: 2937–2947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111: 1694–1702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, Sinoway LI. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol 302: H1737–H1746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller MD, Gao Z, Patel HM, Heffernan MJ, Leuenberger UA, Sinoway LI. β-Adrenergic blockade enhances coronary vasoconstrictor response to forehead cooling. Am J Physiol Heart Circ Physiol 306: H910–H917, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller MD, Mast JL, Cui J, Heffernan MJ, McQuillan PM, Sinoway LI. Tactile stimulation of the oropharynx elicits sympathoexcitation in conscious humans. J Appl Physiol 115: 71–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller MD, Muller SM, Ryan EJ, Bellar DM, Kim CH, Glickman EL. Pain and thermal sensation in the cold: the effect of interval versus continuous exercise. Eur J Appl Physiol 111: 979–987, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 124: 2215–2224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nichols RB, McIntyre WF, Chan S, Scogstad-Stubbs D, Hopman WM, Baranchuk A. Snow-shoveling and the risk of acute coronary syndromes. Clin Res Cardiol 101: 11–15, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Petersen CL, Hansen A, Frandsen E, Strange S, Jonassen O, Nielsen JR, Dige-Petersen H, Hesse B. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am Heart J 128: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Quinones MA, Gaasch WH, Waisser E, Thiel HG, Alexander JK. An analysis of the left ventricular response to isometric exercise. Am Heart J 88: 29–36, 1974 [DOI] [PubMed] [Google Scholar]
- 69.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 33: 1469–1475, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol 588: 2961–2972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson MM, Bell C, Peelor FF, 3rd, Miller BF. β-Adrenergic receptor blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in humans. Am J Physiol Regul Integr Comp Physiol 301: R327–R334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson MM, Richards JC, Hickey MS, Moore DR, Phillips SM, Bell C, Miller BF. Acute β-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol 298: R25–R33, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Spitalnic SJ, Jagminas L, Cox J. An association between snowfall and ED presentation of cardiac arrest. Am J Emerg Med 14: 572–573, 1996 [DOI] [PubMed] [Google Scholar]
- 75.Voci P, Testa G, Plaustro G, Caretta Q. Coronary Doppler intensity changes during handgrip: a new method to detect coronary vasomotor tone in coronary artery disease. J Am Coll Cardiol 34: 428–434, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Vuckovic DD, Rooney SM, Goldiner PL, O'Sullivan D. Aerosol anesthesia of the airway using a small disposable nebulizer. Anesth Analg 59: 803–804, 1980 [PubMed] [Google Scholar]
- 77.Windsor JS, Firth PG, Grocott MP, Rodway GW, Montgomery HE. Mountain mortality: a review of deaths that occur during recreational activities in the mountains. Postgrad Med J 85: 316–321, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Wolfson S, Heinle RA, Herman MV, Kemp HG, Sullivan JM, Gorlin R. Propranolol and angina pectoris. Am J Cardiol 18: 345–353, 1966 [DOI] [PubMed] [Google Scholar]