Abstract
During pathological hypertrophy, peroxisome proliferator-activated receptor coactivator 1α (PGC-1α) is repressed in concert with reduced mitochondrial oxidative capacity and fatty acid oxidation (FAO). We therefore sought to determine if maintaining or increasing PGC-1α levels in the context of pressure overload hypertrophy (POH) would preserve mitochondrial function and prevent contractile dysfunction. Pathological cardiac hypertrophy was induced using 4 wk of transverse aortic constriction (TAC) in mice overexpressing the human PGC-1α genomic locus via a bacterial artificial chromosome (TG) and nontransgenic controls (Cont). PGC-1α levels were increased by 40% in TG mice and were sustained following TAC. Although TAC-induced repression of FAO genes and oxidative phosphorylation (oxphos) genes was prevented in TG mice, mitochondrial function and ATP synthesis were equivalently impaired in Cont and TG mice after TAC. Contractile function was also equally impaired in Cont and TG mice following TAC, as demonstrated by decreased +dP/dt and ejection fraction and increased left ventricular developed pressure and end diastolic pressure. Conversely, capillary density was preserved, in concert with increased VEGF expression, while apoptosis and fibrosis were reduced in TG relative to Cont mice after TAC. Hence, sustaining physiological levels of PGC-1α expression following POH, while preserving myocardial vascularity, does not prevent mitochondrial and contractile dysfunction.—Pereira, R. O., Wende, A. R., Crum, A., Hunter, D., Olsen, C. D., Rawlings, T., Riehle, C., Ward, W. F., Abel, E. D. Maintaining PGC-1α expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function.
Keywords: mitochondria, heart failure
Cardiac muscle requires continuous energy supply to sustain its critical contractile activity. Although the heart utilizes multiple metabolic substrates, fatty acid oxidation (FAO) represents the major source of ATP generation (up to 70%) in normal hearts. Fatty acids (FAs) undergo β-oxidation in mitochondria to generate reducing equivalents and acetyl CoA that enters the tricarboxylic acid (TCA) cycle and generates additional reducing equivalents to fuel the electron transport chain and ATP synthesis. Mitochondrial bioenergetics therefore plays a central role in the regulation of cardiac contractility (1). Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is an inducible integrator of transcriptional circuits that regulate mitochondrial biogenesis and function. PGC-1α expression is induced by physiological stimuli that increase ATP demand and stimulate mitochondrial FAO, such as cold exposure, food withdrawal, and exercise (2). In the heart, PGC-1α regulates the expression of genes encoding oxidative phosphorylation (oxphos) subunits and FA metabolism proteins (3, 4). Thus, PGC-1α plays a central role in the regulation of fuel selection, mitochondrial ATP generating capacity, and energy production in the heart.
In response to exercise training, PGC-1α is activated concomitantly with enhanced mitochondrial function. Thus, as the heart hypertrophies in response to exercise training, there is a proportionate increase in mitochondrial function that sustains oxidative capacity (2). In contrast, during pathological hypertrophy, as occurs in hypertension, aortic stenosis, or postinfarction heart failure, many energetic abnormalities occur in the cardiomyocytes, such as decreased FA metabolism, increased reliance on glucose, decreased ATP content, decreased expression of oxidative enzymes, and reduced mitochondrial enzymatic and oxidative capacity (5). In many animal models of heart failure, these bioenergetic abnormalities in the failing heart develop concomitantly with repression of PGC-1α expression. A commonly used model to induce pathological hypertrophy in rodents is the induction of left ventricle (LV) pressure overload hypertrophy (POH) by surgical transverse aortic constriction (TAC) of the transverse aorta between the brachiocephalic trunk and the left carotid artery. In this model, POH and heart failure are associated with transcriptional repression of the genes encoding PGC-1α and its target binding partner, peroxisome proliferator-activated receptor α (PPARα), resulting in decreased expression of genes involved in FAO (6–8). As such, decreased expression or activity of the PGC-1α/PPARα transcriptional cascade has been proposed as an important molecular mechanism for energy starvation of the failing myocardium (9).
Studies using PGC-1α loss of function in cardiomyocytes suggest that PGC-1α expression is required for maintaining basal mitochondrial function, and its deficiency leads to modest age-dependent contractile dysfunction in nonstressed hearts and accelerated heart failure in response to TAC (10–12). Conversely, high-level cardiomyocyte-specific overexpression of PGC-1α leads to uncontrolled mitochondrial biogenesis, ultimately causing loss of sarcomeric structure and dilated cardiomyopathy (4). However, whether induction of PGC-1α in the physiological range could have beneficial effects under conditions of POH has not been determined. In the present study, we utilized transgenic (TG) mice that ubiquitously overexpress a bacterial artificial chromosome (BAC) harboring the human PGC-1α genomic locus to achieve a physiological increase in PGC-1α protein in the heart and subjected them to TAC. Our data suggest that a modest increase in PGC-1α expression will not prevent mitochondrial and contractile dysfunction after 4 wk of TAC but adverse remodeling is attenuated on the basis of preserved myocardial vascularity.
MATERIALS AND METHODS
Animals
Mice overexpressing PGC-1α ubiquitously were generated and previously characterized (13). Briefly, a BAC clone (CTD2238L2) containing endogenous human PGC-1α gene and its regulatory sequences was selected. Then, purified PGC-1α BAC DNA was injected into the protonucleus of the fertilized eggs that were implanted into the uterus of pseudopregnant female mice. PGC-1α TG mice were generated on the C57Bl6-DBA mixed background and were backcrossed to C57Bl6 ≥4 times. Littermates lacking the BAC were used as controls (Cont). The animals were housed at 22°C with a 12 h light-dark cycle with free access to water and standard chow (Harlan Teklad Diet 8656, 3.8 kcal/g of gross energy; Harlan Laboratories, Indianapolis, IN, USA) that contained 65% carbohydrate (corn and soybean meal), 24.5% protein (soy based), 4.4% fat (soybean oil), and 3.4% fiber and was supplemented with vitamins and minerals. Experiments were performed using exclusively male mice. All mouse experiments were approved by the Institutional Animal Care and Use Committee of the University of Utah and the Carver College of Medicine of the University of Iowa.
TAC surgery
Mice at 6 wk of age were anesthetized (single intraperitoneal injection of 400 mg chloral hydrate/kg body weight) and placed in the supine position on a heating pad (37°C). A topical depilatory agent was applied to the chest, and the area was cleaned. Following horizontal skin incision ∼ 0.5–1 cm in length at the level of the suprasternal notch, a 2- to 3-mm longitudinal cut was made in the proximal portion of the sternum. A 30 G clip was placed between the innominate artery and the left common carotid artery. After banding, the chest and overlying skin were closed (7). Animals were euthanized 4 wk after surgery.
Mouse characteristics
At 4 wk after TAC, mice were euthanized and hearts and lungs excised and weighed. Tibiae were also removed and their lengths measured.
Mitochondrial function
LV muscle fibers were dissected from freshly excised hearts and permeabilized with saponin. Mitochondrial respiration and ATP synthesis rates were measured using the following substrates: palmitoyl-carnitine (PC; 20 μM) combined with malate (5 mM) or pyruvate (10 mM) combined with malate (5 mM) or succinate (5 mM) in the presence of rotenone (10 mM), as described previously (14). The respiratory rates of cardiac fibers were measured using an oxygen sensor probe (Ocean Optics, Dunedin, FL, USA). ATP production was determined by a bioluminescence assay based on the luciferin/luciferase reaction with the ATP assay kit (Promega, Madison, WI, USA).
Electron microscopy
Hearts were freshly excised and immediately washed in ice-cold saline. Samples were collected from LV myocardium and processed as described previously (15). Mitochondrial volume density and number were analyzed by stereology in a blinded fashion using the point-counting method (15).
3′-Hydroxyacyl-CoA dehydrogenase (HADH)
HADH activity was determined spectrophotometrically using whole heart homogenates as described previously (16).
Isolated working heart
Cardiac substrate metabolism, cardiac power, oxygen consumption, cardiac output, and cardiac efficiency were measured in isolated working hearts obtained after 4 wk of TAC, as described previously (17). Hearts were perfused with 5 mM glucose and 0.4 mM palmitate. Throughout the 60 min perfusion, measurements of flow and pressure (Millar pressure catheter; Millar Instruments, Houston, TX, USA) were obtained every 20 min. With the use of a fiberoptic oxygen sensor (Ocean Optics), the oxygen content of freshly oxygenated buffer (arterial partial pressure of oxygen [PaO2]) and oxygen concentration in pulmonary artery effluent, collected using a capillary tube (venous partial pressure of oxygen [PvO2]) were measured. Palmitate oxidation was measured in one set of hearts, and glycolytic flux and glucose oxidation rates were measured simultaneously in a second set of hearts. Glucose oxidation was assessed by measuring 14CO2 released by the metabolism of [U-14C] glucose (specific activity 296 MBq/mol). The amount of 3H2O released from the metabolism of exogenous [5-3H] glucose (specific activity 177 Mbq/mol) was used to determine glycolytic flux.
Hemodynamic studies
At 4 wk after TAC, cardiac catheterizations were performed as described previously (18). Mice at 6 wk of age were anesthetized (single intraperitoneal injection of 400 mg chloral hydrate/kg body weight) and placed in the supine position on a heating pad (37°C). A Millar Mikro-Tip catheter (1.0F; Millar Instruments) was then inserted into the LV via the right carotid artery, and hemodynamic measurements were obtained using LabChart7 Pro software (ADInstruments, Colorado Springs, CO, USA). Heart rate, LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and the maximal rates of LV pressure change (+dP/dt and −dP/dt) were recorded and analyzed as described previously (19).
Transthoracic echocardiography
Mice were anesthetized with isoflurane and placed in the supine position on a heating pad (37°C). Next, the chest hair was removed with a topical depilatory agent. Two-dimensional guided M-mode images were taken in short- and long-axis projections using a 13 MHz linear probe (Vivid FiVe, GE Medical Systems, Milwaukee, WI, USA). LV dimensions and wall thickness were measured in ≤3 beats from each projection and were then averaged. Fractional shortening (%), ejection fraction (%), stroke volume (μl), and heart rate (beats/min) were calculated as described previously (19).
Histology
Myocardial fragments were stained by Masson's trichrome stain for visualization of fibrotic tissue and terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) stain for visualization of apoptotic cells. Endothelin-1 immunostaining was performed for visualization of blood vessels. Light and fluorescence microscopy were performed using an Olympus TH4-100 inverted microscope that was connected to an Olympus Microfire Digital Camera (Olympus, New York, NY, USA). For quantification of fibrotic tissue, pictures from each heart section (thickness of 3 μm) were taken systematically to ensure that the entire extent of the tissue section had been covered. Five different hearts were used per group, so a total of 20 sections were utilized in the study. The micrographs were then analyzed using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The percentage of fibrotic tissue in each micrograph was measured and averaged per heart. The average value for each heart was then utilized for statistical analysis. Quantification of TUNEL-positive cells was performed in 5 different micrographs from each heart section. A total of 25 micrographs from 5 hearts/group were quantified using the Image-Pro Plus software. The ratios between TUNEL-positive nuclei over DAPI-stained nuclei were generated for each section, and the values were averaged per heart. The averages were then utilized for statistical analysis. The vascularization index (vessels/mm2) was determined in endothelin-1 immunostained sections of the LV of mice from all groups. The number of capillaries per area was determined in 50 random fields per group, following the forbidden line principle (20).
Western blot analysis
For immunoblotting analysis, ∼50 mg of frozen tissue was homogenized in 200 μl lysis buffer (containing 50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 100 mM sodium fluoride 100 μM sodium vanadate, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) using the Tissue Lyser II (Qiagen, Valencia, CA, USA). Tissue lysates were resolved on SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Primary and secondary antibodies used are summarized in Table 1. IRDye 800CW anti-mouse (LiCor, Lincoln, NE, USA) and Alexa Fluor anti-rabbit 680 (Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies, and fluorescence was quantified using the LiCor Odyssey imager.
Table 1.
Primary and secondary antibodies
| Antigen | Supplier | ∼Size (kDa) | 2° |
|---|---|---|---|
| Phospho AKT (Ser473) | Cell Signaling (Danvers, MA, USA) | 60 | Rabbit |
| Akt | Cell Signaling | 60 | Mouse |
| Hif-1α | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | 120 | Rabbit |
| MnSod | BD Biosciences (San Jose, CA, USA) | 25 | Mouse |
| Complex I | Molecular Probes (Eugene, OR, USA) | 15.5 | Mouse |
| Complex II | Molecular Probes | 31.6 | Mouse |
| Complex IV | Molecular Probes | 19.6 | Mouse |
| Complex V | Molecular Probes | 59.8 | Mouse |
| VDAC | ABR Affinity BioReagents (Rockford, IL, USA) | 32 | Rabbit |
| VEGF | Santa Cruz Biotechnology | 21 | Mouse |
| Catalase | Sigma-Aldrich (St. Louis, MO, USA) | 60 | Mouse |
| PGC-1α | Santa Cruz Biotechnology | 91 | Rabbit |
| GAPDH | Cell Signaling | 37 | Rabbit |
RNA extraction and quantitative RT-PCR
Total RNA was extracted from hearts with TRIzol reagent (Invitrogen) and glycogen (Qiagen), as described previously and transcript levels normalized to 16S (15). Total RNA (∼ 3 μg) was reverse transcribed (SuperScript III Reverse Transcriptase Kit; Invitrogen). The resulting cDNA, Platinum TaqDNA polymerase (Invitrogen), primers, and SYBR-green (Invitrogen) fluorescent dye were transferred to a 384-well plate in triplicate and real-time polymerase chain reaction was performed with an ABI Prism 7900HT instrument (Applied Biosystems, Foster City, CA, USA). The primer sequences are listed in Table 2.
Table 2.
Primer sequences
| Gene | Sequence of forward and reverse primers, 5′→3′ | Accession number |
|---|---|---|
| Actin, α 1, skeletal muscle (ACTA1) | CCTGTATGCCAACAACGTCA | XM_134551 |
| CTCGTCGTACTCCTGCTTGG | ||
| Cytochrome c oxidase subunit IV isoform 1 (Cox4i1) | CGCTGAAGGAGAAGGAGAAG | NM_009941 |
| GCAGTGAAGCCAATGAAGAA | ||
| Cytochrome c oxidase, subunit Vb (Cox5b) | TGGAGGTGGTGTCCCTACTG | M_009942 |
| CTCTTGTTGCTGATGGATGG | ||
| Estrogen-related receptor α (ESRRA) | GGAGGACGGCAGAAGTACAA | NC_000085 |
| CAGGTTCAACAACCAGCAGA | ||
| Glutathione peroxidase 1 (Gpx1) | GACTGGTGGTGCTCGGTT | NM_008160 |
| TCACCATTCACTTCGCACTT | ||
| Glutathione peroxidase 4 (Gpx4) | GCAGGAGCCAGGAAGTAATCAAGA | NM_008162 |
| GCATCGTCCCCATTTACACA | ||
| Hydroxyacyl-CoA dehydrogenase-α subunit (HADHα) | TCAGGAGGGCTCAAAGAATAA | XM_131963 |
| GAAAGCCAAGCCCAAAGAC | ||
| Medium chain acetyl-coenzyme A dehydrogenase (MCAD) | ACTGACGCCGTTCAGATTTT | NM_007382 |
| GCTTAGTTACACGAGGGTGATG | ||
| NADH dehydrogenase (ubiquinone) 1 α subcomplex 9 (Ndufa9) | ATCCCTTACCCTTTGCCACT | NM_025358 |
| CCGTAGCACCTCAATGGACT | ||
| NADH dehydrogenase (ubiquinone) flavoprotein 1 (Ndufv1) | TGTGAGACCGTGCTAATGGA | NM_133666 |
| CATCTCCCTTCACAAATCGG | ||
| Natriuretic peptide precursor type A (NPPA) | ATGGGCTCCTTCTCCATCA | K02781 |
| CCTGCTTCCTCAGTCTGCTC | ||
| Natriuretic peptide precursor type B (NPPB) | GGATCTCCTGAAGGTGCTGT | D16497 |
| CCTGCTTCCTCAGTCTGCTC | ||
| Peroxisome proliferator activated receptor γ coactivator 1 α (PGC-1α) | GTAAATCTGCGGGATGATGG | NM_008904 |
| AGCAGGGTCAAAATCGTCTG | ||
| Peroxisome proliferator activated receptor γ coactivator 1 β (PGC-1β) | TGAGGTGTTCGGTGAGATTG | NM_133249 |
| CCATAGCTCAGGTGGAAGGA | ||
| Ribosomal subunit 16S (16S) | TGCTGGTGTGGATATTCGGG | NC_017501 |
| CCTTGAGATGGGCTTATCGG | ||
| Solute carrier family 27 (fatty acid transporter), member 1 (FATP1) | CCATCTTCCTGCGTCTTCTG | NM_011977.3 |
| GTGTCAGGCTCCCAGGTCTC | ||
| Superoxide dismutase 2 (Sod2) | ACAACTCAGGTCGCTCTTCA | NM_013671 |
| GAACCTTGGACTCCCACAGA | ||
| Transcription factor A, mitochondrial (TFAm) | CAAAAAGACCTCGTTCAGCA | NM_009360 |
| CTTCAGCCATCTGCTCTTCC | ||
| Uncoupling protein 2 (UCP2) | TCTCCTGAAAGCCAACCTCA | NM_011671.4 |
| CTACGTTCCAGGATCCCAAG | ||
| Uncoupling protein 3 (UCP3) | TTTGGAGCTGGCTTCTGTG | NM_009464.3 |
| AAGGCCCTCTTCAGTTGCTC |
Statistical analysis
Data are presented as means ± sem. RT-PCR, and Western blot results are presented as fold change vs. control sham surgery (Cont sham) group. A probability value of P ≤ 0.05 was considered significantly different. Significant differences were determined by 2-way ANOVA to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. Data that were not normally distributed were log transformed for normalization. Statistical calculations were performed using the GraphPad Prism Software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Mouse model
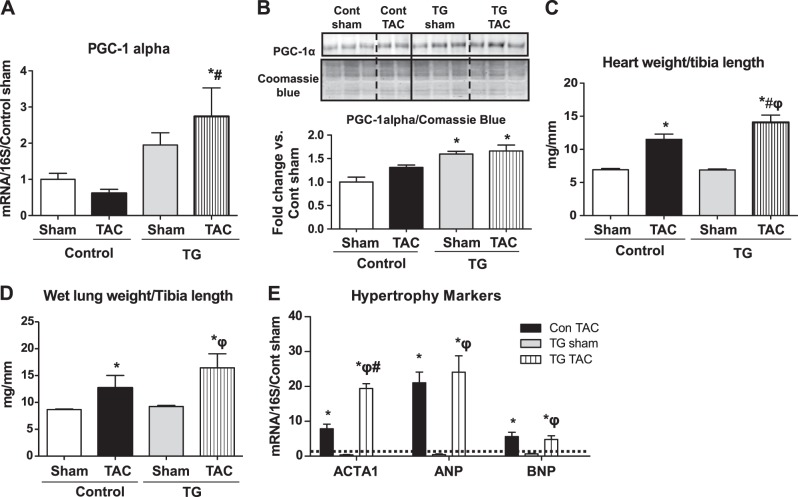
PGC-1α TG mice exhibited a 2-fold increase in PGC-1α mRNA expression in the heart relative to Cont (Fig. 1A) and a 40% increase in PGC-1α protein levels (Fig. 1B). PGC-1α mRNA expression was significantly increased in PGC-1α TG mice relative to Cont after TAC (Fig. 1A). PGC-1α protein levels were unchanged in Cont and TG mice, relative to sham surgery mice of the same genotype following TAC (Fig. 1B). The modest increase in PGC-1α protein content was sustained in TG mice after TAC. Heart weight to tibia length ratios were increased after TAC and were further increased in TG mice (Fig. 1C). Lung weight to tibia length ratios (Fig. 1D) were equivalently increased in Cont and TG mice following TAC, indicating similar degrees of pulmonary congestion. Similarly, mRNA expression of the hypertrophy markers atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) was equivalently increased following TAC in both genotypes, while ACTA1 was increased to a greater extent in TG mice (Fig. 1E).
Figure 1.
Characterization of mouse model and the hypertrophic response to TAC. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) mRNA levels of PGC-1α in Cont and TG mice 4 wk after TAC (P<0.05 for genotype). B) PGC-1α protein content in whole heart homogenates normalized to Coomassie blue staining (P<0.05 for genotype). C) Heart weight normalized to tibia length in Cont and TG hearts 4 wk after TAC (P<0.05 for treatment and genotype). D) Lung weight normalized to tibia length in Cont and TG hearts 4 wk after TAC (P<0.05 for treatment). E) mRNA expression of hypertrophy markers (P < 0.05 for treatment). Dashed line represents Cont sham. Data were normalized by 16S and are shown as fold change vs. Cont sham. Data are expressed as mean ± sem fold change; n = 6 mice/group. *P < 0.05 vs. Cont sham; #P < 0.05 vs. Cont TAC; φP < 0.05 vs. TG sham.
Mitochondrial adaptations after TAC
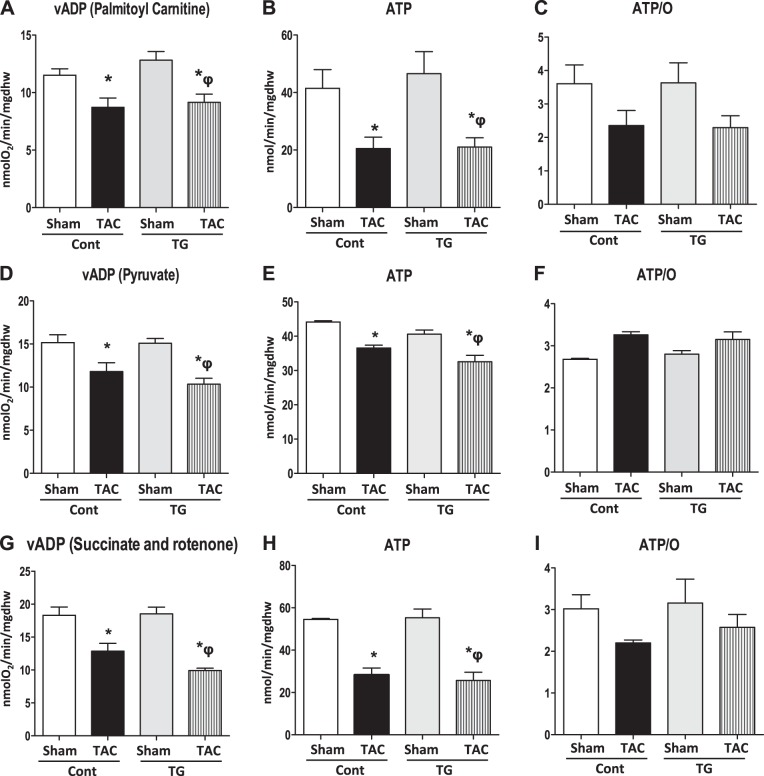
Mitochondrial function was assessed in Cont and TG mice 4 wk after TAC or sham surgery in saponin-permeabilized cardiac fibers. PC-, pyruvate-, or succinate (in the presence of rotenone)-supported maximal ADP-stimulated mitochondrial oxygen consumption (VADP; Fig. 2A, D, G) was equivalently reduced in Cont and TG mice following TAC. Likewise, ATP synthesis was equivalently reduced in control and TG mice after TAC, for all substrates utilized (Fig. 2B, E, H). ATP/O ratios were unchanged between groups (Figs. 2C, F, I). Mitochondrial morphology was unchanged between groups (Supplemental Fig. S1A), but mitochondrial number was significantly decreased in Cont TAC mice relative to sham, while no change was observed in TG mice (Supplemental Fig. S1B). Mitochondria were larger in Cont mice after TAC relative to sham surgery but not in TG mice (Supplemental Fig. S1C). No changes in volume density were observed between groups (Supplemental Fig. S1D). Consistent with the VADP data, HADH activity was equivalently reduced in Cont and TG mice following TAC (Supplemental Fig. S1E). Expression of FAO genes (Supplemental Fig. S2A) and oxphos genes (Supplemental Fig. S2B) was significantly reduced after TAC in Cont mice but was preserved in TG mice. Preserved expression of these genes did not equate with maintenance of mitochondrial function in TG mice after TAC. mRNA expression of PGC-1β was reduced in all groups relative to Cont sham but was slightly increased in TG mice after TAC, relative to TG sham mice (Supplemental Fig. S2A). The protein content of mitochondrial complexes I, II, IV, and V was unchanged in hearts of Cont and TG mice, regardless of surgery (Supplemental Fig. S3).
Figure 2.
Mitochondrial function in Cont and TG mice 4 wk after TAC. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when a significant interaction occurred. Mitochondrial respiration, ATP synthesis rates, and ATP/O ratios were measured in saponin-permeabilized cardiac fibers. A) PC-supported VADP (P<0.05 for treatment). B) PC-supported ATP synthesis rates (P<0.05 for treatment). C) ATP/O ratios when palmitoyl-carnitine was used as substrate. D) Pyruvate-supported VADP (P<0.05 for treatment). E) Pyruvate-supported ATP synthesis rates (P<0.05 for genotype and treatment). F) ATP/O ratios when pyruvate was used as substrate. G) Succinate (and rotenone)-supported VADP (P<0.05 for treatment). H) Succinate (and rotenone)-supported ATP synthesis rates (P<0.05 for treatment). I) ATP/O ratios when succinate (in the presence of rotenone) was used as substrate. Data are expressed as means ± sem; n = 5–7 mice/group. *P < 0.05 vs. Cont sham; φP < 0.05 vs. TG sham.
Substrate metabolism and cardiac efficiency in TG hearts following TAC
Substrate metabolism was determined in isolated working hearts, 4 wk after sham or TAC surgery. After TAC, glycolysis rates increased in Cont hearts, but no changes were observed in TG mice (Fig. 3A). There was a trend for higher glucose oxidation (GLOX) in TG sham mice, which did not reach statistical significance. After TAC, there was a significant reduction in GLOX in TG hearts but not in controls, resulting in similar rates of GLOX in mice of both genotypes after TAC (Fig. 3B). Palmitate oxidation (POX) was increased in sham TG relative to Cont sham. TAC decreased POX in Cont mice by ∼60%. Although POX was also reduced by TAC in TG mice, it remained higher than Cont TAC at levels that were equivalent to those of Cont sham mice (Fig. 3C). Cardiac power was equivalently reduced in Cont and TG mice (Fig. 3D). However, oxygen consumption (MVo2) was only reduced in Cont mice following TAC (Fig. 3E). Cardiac output was increased in TG sham mice relative to Cont shams but declined in both genotypes following TAC (Fig. 3F). Cardiac efficiency declined only in TG mice after TAC relative to controls (Fig. 3G).
Figure 3.
Cardiac substrate metabolism in isolated working hearts. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) Glycolysis (P<0.05 for genotype and interaction). B) Glucose oxidation (P<0.05 for genotype and interaction). C) Palmitate oxidation (P<0.05 for genotype and treatment). D) Cardiac power (P<0.05 for treatment). E) Oxygen consumption (P<0.05 for treatment). F) Cardiac output (P<0.05 for genotype and treatment). G) Cardiac efficiency (P<0.05 for genotype and treatment). Data are expressed as means ± sem; n = 6 mice/group. *P < 0.05 vs. Cont sham; #P < 0.05 vs. Cont TAC; φP < 0.05 vs. TG sham.
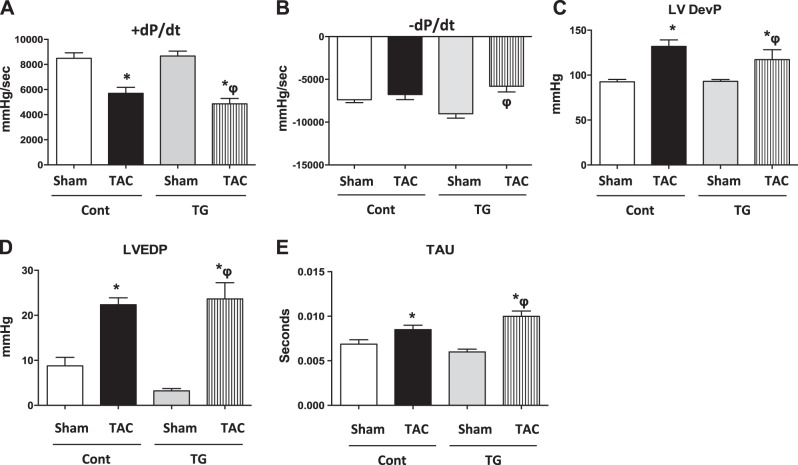
PGC-1α overexpression does not prevent contractile dysfunction
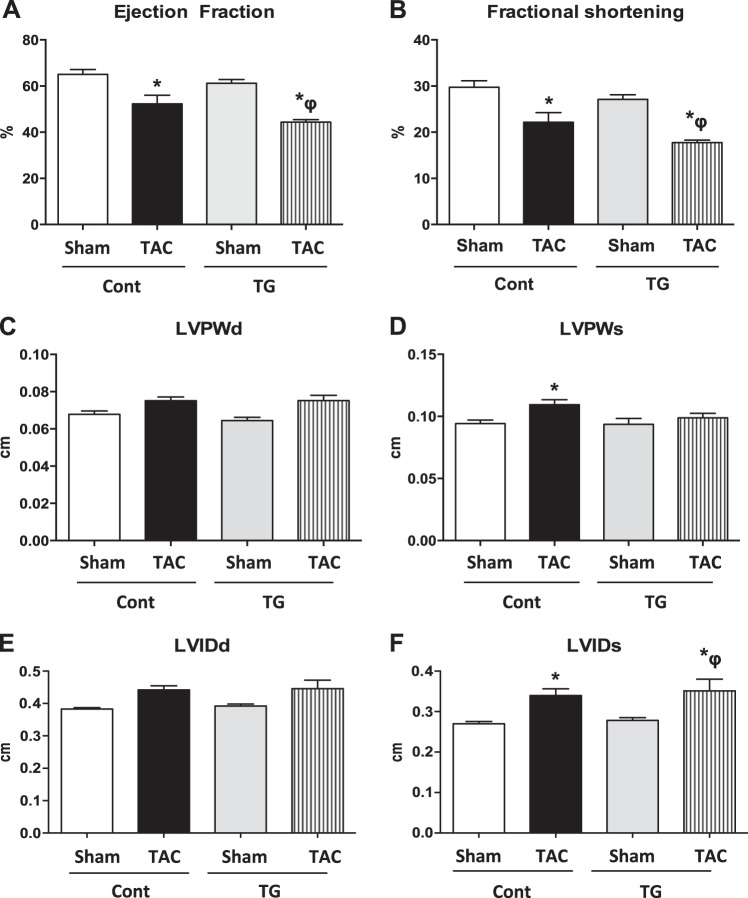
Cardiac contractile function was measured by LV catheterization and echocardiography in control and TG mice 4 wk after TAC. LV catheterization revealed similar decrements in peak rates of ventricular contraction (+dP/dt; Fig. 4A). −dP/dt was significantly reduced after TAC in TG mice (Fig. 4B). LV developed pressure (LVDevP; Fig. 4C), LVEDP (Fig. 4D), and isovolumic relaxation constant (Tau; Fig. 4E) were equivalently increased in Cont and TG mice following TAC. Ejection fraction (Fig. 5A) and fractional shortening (Fig. 5B) were similarly decreased in Cont and TG mice after TAC. LV wall dimensions were significantly increased in Cont TAC relative to sham surgery mice in systole but not in diastole. No changes were observed in TG mice regardless of treatment (Fig. 5C, D). The LV internal diameter was equivalently increased in Cont and TG mice following TAC relative to sham surgery hearts in systole, but not in diastole (Fig. 5E, F).
Figure 4.
LV catheterization. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) +dP/dt (rate of rise of LV pressure; P<0.05 for treatment). B) −dP/dt (rate of fall of LV pressure; P<0.05 for treatment and interaction). C) LVDevP (P<0.05 for treatment). D) LVEDP (P<0.05 for treatment). E) Tau (isovolumic relaxation constant; P < 0.05 for treatment). Data are expressed as means ± sem; n = 6 mice/group. *P < 0.05 vs. Cont sham; φP < 0.05 vs. TG sham.
Figure 5.
Echocardiography. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) Ejection fraction (%; P<0.05 for treatment). B) Fractional shortening (%; P<0.05 for treatment). C) LV posterior wall in diastole (LVPWd). D) LV posterior wall in systole (LVPWs; P<0.05 for treatment). E) LV internal diameter in diastole (LVIDd). F) LV internal diameter in systole (LVIDs; P<0.05 for treatment). Data are expressed as means ± sem; n = 5 mice/group. *P < 0.05 vs. Cont sham; φP < 0.05 vs. TG sham.
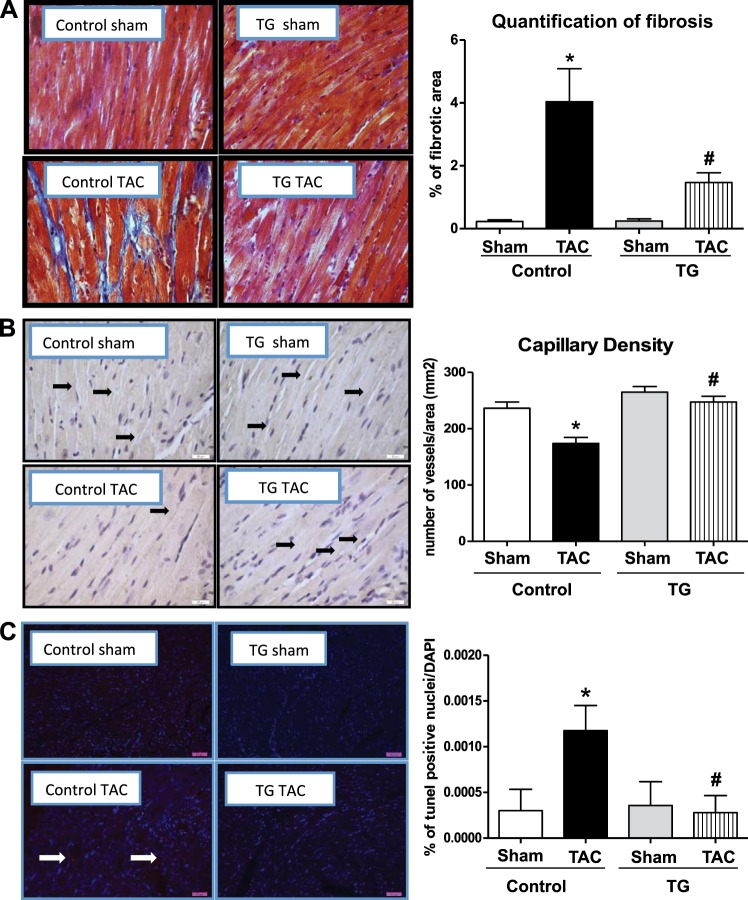
PGC-1α overexpression attenuates pathological remodeling after TAC
Pressure overload hypertrophy induces cardiac fibrosis, which was attenuated in TG mice (Fig. 6A). Capillary density was reduced in Cont TAC mice, as demonstrated by stereological analysis of heart sections immunostained for endothelin-1 (Fig. 6B) but was maintained in TG mice. Apoptosis was increased in Cont TAC mice, as evidenced by an increased percentage of TUNEL-positive cells but was unchanged in TG mice (Fig. 6C). To further investigate the mechanisms by which PGC-1α overexpression confers cardioprotection, we analyzed various signaling pathways that could modulate vascularity in response to hemodynamic stress. The hypoxia-inducible factor 1α (HIF-1α) protein content was unchanged in Cont and TG mice regardless of surgery (Fig. 7A). PGC-1α has been shown to induce vascular endothelial growth factor (VEGF) through activation of estrogen-related receptor α (ERRα) (21). The VEGF protein levels were significantly increased in TG mice after TAC, relative to all groups (Fig. 7B). ERRα mRNA expression was reduced in Cont mice after TAC but was preserved in TG mice (Fig. 7C). Expression levels of genes involved in antioxidant defense, namely SOD2 and GPX1, were equivalently increased in Cont TAC and TG sham mice relative to Cont sham but were reduced in TG TAC mice relative to Cont TAC and TG sham mice respectively (Supplemental Fig. S4A). The protein levels of catalase and manganese superoxide dismutase (MnSOD) were unchanged between groups, regardless of surgery (Supplemental Fig. S4B, C). Protein kinase B (Akt) was similarly activated in response to TAC in both Cont and TG hearts (Supplemental Fig. S4D).
Figure 6.
Histological analysis of fibrosis, vascularity, and apoptosis. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) Masson's trichrome staining of heart sections and quantification of percentage of fibrotic area (P<0.05 for genotype, treatment, and their interaction). B) Endothelin 1 staining and quantification of vascularization (P<0.05 for genotype, treatment and their interaction). C) Quantification of percentage of TUNEL-positive nuclei normalized to DAPI staining (P < 0.05 for genotype, treatment and their interaction). Data are expressed as means ± sem; n = 5 mice/group. *P < 0.05 vs. Cont sham; #P < 0.05 vs. Cont TAC.
Figure 7.
VEGF is increased in TG mice after TAC. Two-way ANOVA was performed to analyze differences by treatment and genotype, including a Tukey's post hoc analysis when significant interaction occurred. A) Representative Western blot for Hif-1α and densitometric quantification of multiple blots. B) Representative Western blot for VEGF and densitometric quantification of multiple blots (P<0.05 for genotype and interaction). C) mRNA expression of ERRα (P<0.05 for genotype, treatment, and their interaction). Data are expressed as mean ± sem fold change; n = 6 mice/group. *P < 0.05 vs. Cont sham; #P < 0.05 vs. Cont TAC; φP < 0.05 vs. TG sham.
DISCUSSION
Cardiac hypertrophy may be characterized as physiological or pathological. Endurance exercise training leads to physiological hypertrophy, which is accompanied by decreased resting and submaximal heart rate, and increased end-diastolic dimension, leading to improved ventricular function and increased resistance of the heart to ischemic insults (5). These adaptations coincide with increased PGC-1α expression and preserved or enhanced mitochondrial function (2). Thus, in physiological cardiac hypertrophy, mitochondrial function proportionately increases to sustain oxidative capacity. In contrast, during pathological hypertrophy as occurs in hypertension, many energetic abnormalities occur in cardiomyocytes, such as decreased FA metabolism, increased reliance on glucose, decreased ATP and phosphocreatine (PCr) content, decreased expression of oxidative enzymes, and reduced mitochondrial oxidative capacity (22). Pathological hypertrophy has also been associated with repression of PGC-1α gene expression, which suggests that decreased expression of the PGC-1α/PPARα transcriptional cascade could represent an important molecular mechanism for energy starvation in the failing myocardium (9). In the present study, we hypothesized that a modest increase in PGC-1α expression that maintains PGC-1α in the physiological range would prevent or retard the progression to heart failure in a model of POH. Here we show that maintaining PGC-1α expression in the physiological range in POH may preferentially signal toward angiogenic pathways but is not sufficient to prevent POH-induced mitochondrial or contractile dysfunction.
Although it is well described that pathological hypertrophy is accompanied by reduced PGC-1α expression (6, 7), a recent study reported no decrease of PGC-1α mRNA levels following POH in rodents (23). Our data revealed a nonsignificant reduction in PGC-1α mRNA levels in Cont TAC mice relative to sham surgery mice, but PGC-1α mRNA levels were significantly greater in TG mice relative to Cont after TAC. Protein content of PGC-1α was unchanged between sham surgery and TAC mice irrespective of genotype but remainedincreased in TG mice. Similarly, recent studies showed no decrease in PGC-1α protein levels in samples from human failing hearts (24, 25). Thus, mitochondrial dysfunction that characterizes the failing heart or that follows TAC might be mediated via mechanisms that are independent of PGC-1α. This conclusion is further supported by our observations that despite preserved PGC-1α protein levels, Cont mice exhibited reduced expression of FAO and oxphos genes and impaired mitochondrial function and ATP synthesis following TAC. Moreover, increased expression of PGC-1α in TG mice did not prevent the decline in mitochondrial function following TAC. Interestingly, PGC-1β mRNA levels were reduced in TG mice relative to Cont sham mice. Mouse models with deletion of PGC-1α or PGC-1β (7, 10), respectively, suggest redundant or overlapping roles for PGC-1 proteins in the regulation of mitochondrial gene expression and biogenesis (26). Thus, mechanisms that are independent of PGC-1α likely exist that result in impaired mitochondrial function in response to POH. As such, physiological levels of PGC-1α overexpression might not be sufficient to increase mitochondrial biogenesis or preserve its function in the context of POH.
PGC-1α overexpression prevented the increase in glycolysis generally observed after TAC and attenuated the fall in palmitate oxidation post-TAC. The increased palmitate oxidation in TG mice may reflect allosteric mechanisms, given that palmitoyl carnitine supported mitochondrial function and activity levels of the β-oxidation enzyme HADH were reduced. Despite increased FA metabolism, ex vivo contractile function was equivalently impaired in Cont and TG mice following TAC. Indeed, in vivo measurements of LV contractility revealed similar decreases in the ejection fraction and increases in LVEDP in Cont and TG mice after TAC, indicating that the metabolic adaptations did not offset mitochondrial dysfunction, which may have contributed to contractile dysfunction. In addition, the increase in FA utilization in PGC-1α TG after TAC was associated with increased oxygen consumption and decreased cardiac efficiency, which represents an additional mechanism that could impair myocardial performance in PGC-1α TG hearts after TAC.
Of interest, pathological remodeling was attenuated following TAC in TG mice relative to controls. Thus, we examined potential mechanisms that are independent of mitochondrial metabolism and focused on antioxidant mechanisms and angiogenesis, both of which are regulated by PGC-1α (21, 27). At the level of mRNA, transcripts encoding SOD2 and GPX1 were induced in sham PGC-1α transgenic mice and also in control mice following TAC. Unexpectedly, expression levels of these genes were repressed following TAC in PGC-1α TG mice. Notably, despite these changes in mRNA, protein levels of MnSOD were unchanged, as were levels of catalase. Thus, it is likely that the protective effects conferred by PGC-1α overexpression occurred independently of the activation of antioxidant genes. It is noteworthy that vascularization was preserved in TG mice. We, therefore, investigated the roles of VEGF and Hif-1α respectively. VEGF protein was markedly induced in PGC-1α TG mice following TAC. This occurred independently of any changes in Hif-1α content. PGC-1α can induce VEGF expression via ERRα activation, independently of HIF signaling (21). Moreover, PGC-1α was recently shown to be essential for increasing vascularity in pregnancy-related cardiac hypertrophy, with its absence precipitating peripartum cardiomyopathy (28). Overexpression of PGC-1α sustained ERRα mRNA expression following TAC, which contrasts with the repression that was observed in Cont hearts following TAC. These data suggest that PGC-1α might attenuate pathological remodeling during POH, via mechanisms that may include ERRα-mediated induction of VEGF, leading to preserved vascularization, decreased apoptosis, and, consequently, reduced fibrosis.
Our study has limitations. We utilized a mouse model in which PGC-1α is overexpressed in all cell types (13). Thus, the angiogenic mechanism that we propose could be secondary to increased PGC-1α expression in noncardiomyocytes, such as endothelial cells. Moreover, subtle changes in systemic metabolism have been described in these mice (13). Whether these systemic metabolic changes could influence the cardiac phenotypes observed in this study is unclear. To resolve these issues, future studies in mice with inducible overexpression of PGC-1α in cardiomyocytes in which PGC-1α can be titrated to a physiological range will be required to specifically address these questions in the context of POH, without possible confounding effects due to altered systemic metabolism. Nevertheless, our study suggests that a modest PGC-1α overexpression, within the physiological range, is not sufficient to preserve mitochondrial or contractile function during POH stress, although it might retard the loss of vascularity that represents an important mechanism that contributes to adverse LV remodeling in the context of POH.
In summary, the present study demonstrates divergent mechanisms by which maintaining PGC-1α in the heart in the physiological range may modulate the cardiac adaptations to POH. Specifically, we provide evidence that whereas the mitochondrial adaptations to POH might be largely independent of PGC-1α, the therapeutic value for PGC-1α induction might lie in its role for inducing or maintaining VEGF expression, which might retard the loss of vascularity that characterizes the transition from compensated cardiac hypertrophy to heart failure. Future studies will need to determine the cell-type compartment that mediates this effect and the optimal level of PGC-1α that needs to be achieved to sufficiently support myocardial vascularity to reverse or prevent ventricular dysfunction in response to pressure overload.
Supplementary Material
Acknowledgments
The authors thank Jeffrey Lei, Sylvia Hu, and Li Wang for important technical help during the course of these studies.
These studies were supported by U.S. National Institutes of Health (NIH) grants RO1-DK-092065 and U01-HL-087947 to E.D.A., who is an established investigator of the American Heart Association (AHA), and by grant PO1-HD-038129. R.O.P. was supported by a postdoctoral fellowship from the AHA Western Affiliates and NIH grant 5T32-HL-007576. A.R.W. was supported by an advanced postdoctoral fellowship from the Juvenile Diabetes Research Foundation and a postdoctoral fellowship from the AHA Western Affiliates. C.R. was supported by a postdoctoral fellowship from the German Research Foundation (DFG).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BAC
- bacterial artificial chromosome
- Cont
- control
- Cont sham
- control sham surgery
- dP/dt
- rate of left ventricle pressure change
- ERRα
- estrogen-related receptor α
- FA
- fatty acid
- FAO
- fatty acid oxidation
- HADH
- 3′-Hydroxyacyl-CoA dehydrogenase
- Hif-1α
- hypoxia-inducible factor 1α
- LV
- left ventricle
- LVEDP
- left ventricle end-diastolic pressure
- MnSOD
- manganese superoxide dismutase
- oxphos
- oxidative phosphorylation
- PC
- palmitoyl-carnitine
- PCr
- phosphocreatine
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator 1α
- PGC-1β
- peroxisome proliferator-activated receptor γ coactivator 1β
- POH
- pressure overload hypertrophy
- PPARα
- peroxisome proliferator-activated receptor α
- TAC
- transverse aortic constriction
- TCA
- tricarboxylic acid
- TG
- transgenic
- TG sham
- transgenic sham surgery
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick-end labeling
- VEGF
- vascular endothelial growth factor
REFERENCES
- 1. Rimbaud S., Garnier A., Ventura-Clapier R. (2009) Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol. Rep. 61, 131–138 [DOI] [PubMed] [Google Scholar]
- 2. Huss J. M., Kelly D. P. (2005) Mitochondrial energy metabolism in heart failure: a question of balance. J. Clin. Invest. 115, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huss J. M., Torra I. P., Staels B., Giguere V., Kelly D. P. (2004) Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol. 24, 9079–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lehman J. J., Barger P. M., Kovacs A., Saffitz J. E., Medeiros D. M., Kelly D. P. (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abel E. D., Doenst T. (2011) Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc. Res. 90, 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arany Z., Novikov M., Chin S., Ma Y., Rosenzweig A., Spiegelman B. M. (2006) Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl. Acad. Sci. U. S. A. 103, 10086–10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riehle C., Wende A. R., Zaha V. G., Pires K. M., Wayment B., Olsen C., Bugger H., Buchanan J., Wang X., Moreira A. B., Doenst T., Medina-Gomez G., Litwin S. E., Lelliott C. J., Vidal-Puig A., Abel E. D. (2011) PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ. Res 109, 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huss J. M., Imahashi K., Dufour C. R., Weinheimer C. J., Courtois M., Kovacs A., Giguere V., Murphy E., Kelly D. P. (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 6, 25–37 [DOI] [PubMed] [Google Scholar]
- 9. Riehle C., Abel E. D. (2012) PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 22, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., Rybkin I. I., Shelton J. M., Manieri M., Cinti S., Schoen F. J., Bassel-Duby R., Rosenzweig A., Ingwall J. S., Spiegelman B. M. (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271 [DOI] [PubMed] [Google Scholar]
- 11. Lehman J. J., Boudina S., Banke N. H., Sambandam N., Han X., Young D. M., Leone T. C., Gross R. W., Lewandowski E. D., Abel E. D., Kelly D. P. (2008) The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am. J. Physiol. Heart Circ. Physiol. 295, H185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leone T. C., Lehman J. J., Finck B. N., Schaeffer P. J., Wende A. R., Boudina S., Courtois M., Wozniak D. F., Sambandam N., Bernal-Mizrachi C., Chen Z., Holloszy J. O., Medeiros D. M., Schmidt R. E., Saffitz J. E., Abel E. D., Semenkovich C. F., Kelly D. P. (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang H., Balas B., Tantiwong P., Dube J., Goodpaster B. H., O'Doherty R. M., DeFronzo R. A., Richardson A., Musi N., Ward W. F. (2009) Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 296, E945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boudina S., Sena S., O'Neill B. T., Tathireddy P., Young M. E., Abel E. D. (2005) Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112, 2686–2695 [DOI] [PubMed] [Google Scholar]
- 15. Boudina S., Sena S., Theobald H., Sheng X., Wright J. J., Hu X. X., Aziz S., Johnson J. I., Bugger H., Zaha V. G., Abel E. D. (2007) Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56, 2457–2466 [DOI] [PubMed] [Google Scholar]
- 16. Riehle C., Wende A. R., Sena S., Pires K. M., Pereira R. O., Zhu Y., Bugger H., Frank D., Bevins J., Chen D., Perry C. N., Dong X. C., Valdez S., Rech M., Sheng X., Weimer B. C., Gottlieb R. A., White M. F., Abel E. D. (2013) Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J. Clin. Invest. 123, 5319–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazumder P. K., O'Neill B. T., Roberts M. W., Buchanan J., Yun U. J., Cooksey R. C., Boudina S., Abel E. D. (2004) Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53, 2366–2374 [DOI] [PubMed] [Google Scholar]
- 18. Pereira R. O., Wende A. R., Olsen C., Soto J., Rawlings T., Zhu Y., Anderson S. M., Abel E. D. (2013) Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J. Am. Heart Assoc. 2, e000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sena S., Rasmussen I. R., Wende A. R., McQueen A. P., Theobald H. A., Wilde N., Pereira R. O., Litwin S. E., Berger J. P., Abel E. D. (2007) Cardiac hypertrophy caused by peroxisome proliferator- activated receptor-gamma agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology 148, 6047–6053 [DOI] [PubMed] [Google Scholar]
- 20. Gundersen H. J., Osterby R. (1977) Glomerular size and structure in diabetes mellitus. II. Late abnormalities. Diabetologia 13, 43–48 [DOI] [PubMed] [Google Scholar]
- 21. Arany Z., Foo S. Y., Ma Y., Ruas J. L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S. M., Baek K. H., Rosenzweig A., Spiegelman B. M. (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451, 1008–1012 [DOI] [PubMed] [Google Scholar]
- 22. Mettauer B., Zoll J., Garnier A., Ventura-Clapier R. (2006) Heart failure: a model of cardiac and skeletal muscle energetic failure. Pflügers Archiv. 452, 653–666 [DOI] [PubMed] [Google Scholar]
- 23. Hu X., Xu X., Huang Y., Fassett J., Flagg T. P., Zhang Y., Nichols C. G., Bache R. J., Chen Y. (2008) Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ. Res. 103, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu X., Xu X., Lu Z., Zhang P., Fassett J., Zhang Y., Xin Y., Hall J. L., Viollet B., Bache R. J., Huang Y., Chen Y. (2011) AMP activated protein kinase-alpha2 regulates expression of estrogen-related receptor-alpha, a metabolic transcription factor related to heart failure development. Hypertension 58, 696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karamanlidis G., Nascimben L., Couper G. S., Shekar P. S., del Monte F., Tian R. (2010) Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 106, 1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patten I. S., Arany Z. (2012) PGC-1 coactivators in the cardiovascular system. Trends Endocrinol. Metab. 23, 90–97 [DOI] [PubMed] [Google Scholar]
- 27. Lu Z., Xu X., Hu X., Fassett J., Zhu G., Tao Y., Li J., Huang Y., Zhang P., Zhao B., Chen Y. (2010) PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid. Redox Signal. 13, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patten I. S., Rana S., Shahul S., Rowe G. C., Jang C., Liu L., Hacker M. R., Rhee J. S., Mitchell J., Mahmood F., Hess P., Farrell C., Koulisis N., Khankin E. V., Burke S. D., Tudorache I., Bauersachs J., del Monte F., Hilfiker-Kleiner D., Karumanchi S. A., Arany Z. (2012) Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485, 333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.