Abstract
The ferritin core is composed of fine nanoparticulate Fe3+ oxohydroxide, and we have developed a synthetic mimetic, nanoparticulate Fe3+ polyoxohydroxide (nanoFe3+). The aim of this study was to determine how dietary iron derived in this fashion is absorbed in the duodenum. Following a 4 wk run-in on an Fe-deficient diet, mice with intestinal-specific disruption of the Fpn-1 gene (Fpn-KO), or littermate wild-type (WT) controls, were supplemented with Fe2+ sulfate (FeSO4), nanoFe3+, or no added Fe for a further 4 wk. A control group was Fe sufficient throughout. Direct intestinal absorption of nanoFe3+ was investigated using isolated duodenal loops. Our data show that FeSO4 and nanoFe3+ are equally bioavailable in WT mice, and at wk 8 the mean ± sem hemoglobin increase was 18 ± 7 g/L in the FeSO4 group and 30 ± 5 g/L in the nanoFe3+ group. Oral iron failed to be utilized by Fpn-KO mice and was retained in enterocytes, irrespective of the iron source. In summary, although nanoFe3+ is taken up directly by the duodenum its homeostasis is under the normal regulatory control of dietary iron absorption, namely via ferroportin-dependent efflux from enterocytes, and thus offers potential as a novel oral iron supplement.—Aslam, M. F., Frazer, D. M., Faria, N., Bruggraber, S. F. A., Wilkins, S. J., Mirciov, C., Powell, J. J., Anderson, G. J., Pereira, D. I. A. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice.
Keywords: nanoiron, basolateral export, iron homeostasis, hepcidin, knockout mice
Iron deficiency anemia (IDA) persists as the major nutritional deficiency disorder in the world, affecting >1 billion people (1). This places IDA in the World Health Organization's top 10 list of target diseases for cure and prevention (2–4). Current therapy for IDA involves supplementation with an oral iron preparation. First-generation oral iron agents are simple Fe2+ salts, which are cheap and well absorbed, but are associated with significant upper and lower gastrointestinal side effects, such as nausea, constipation, and abdominal discomfort (5, 6). Second-generation oral iron agents are soluble chelated forms of Fe2+ or Fe3+ and, while they reduce upper gastrointestinal side effects, they are expensive to manufacture and, chronically, they appear to retain their distal gastrointestinal adverse effects (7–9). Indeed, recent studies have consistently shown that soluble oral iron negatively affects the colonic flora, promoting the presence of potentially pathogenic bacteria at the expense of beneficial bacteria (10–13). Other studies have also raised serious concerns over “available” iron in the colon as a risk factor for inflammatory signaling and colorectal carcinogenesis (8, 12).
To help address these issues, we have developed a nanodispersed, ligand-modified material, nanoparticulate Fe3+ polyoxohydroxide (nanoFe3+). Analysis by high-contrast, high-resolution electron microscopy showed that nanoFe3+ has a very similar structure to the Fe3+ oxohydroxide core of ferritin (14); that is, it is destabilized 2-line ferrihydrite (15, 16). It also resembles the digestion product of dietary Fe3+ in the small bowel (17). NanoFe3+ has potential advantages as a novel oral iron supplement. It is absorbed whole into duodenal enterocytes and then readily broken down intralysosomally (17). Soluble iron that is released from the nanostructure in this fashion can be utilized systemically, being readily incorporated into hemoglobin (Hb) in both rats (14) and iron-deficient human volunteers (unpublished results). Notably, the nanoFe3+ preparation appears safe compared to soluble forms of iron, as it is not toxic to gut epithelial cells and does not influence the commensal flora negatively (unpublished results). Given the above, as well as current interest in nanoparticle handling by the gastrointestinal tract, a detailed understanding of the absorption and metabolism of nanoFe3+ is required.
Dietary nonheme iron in the intestinal lumen is, predominantly, in the Fe3+ form (18–20). Prior to intestinal uptake, Fe3+ is widely considered to undergo reduction to Fe2+, a reaction that is thought to be catalyzed by brush border ferrireductases, such as duodenal cytochrome b (Dcytb; refs. 21, 22), although luminal ascorbate may also be involved (23). The reduced iron is then transferred across the brush border membrane and into enterocytes by an Fe2+ transporter, namely solute carrier family 11, member 2 and also termed proton-coupled divalent metal ion transporter 1 (DMT-1; refs. 24, 25). Once inside the cell, iron can be stored within intracellular ferritin (26) or, if there are systemic requirements, it can be transported across the basolateral membrane through ferroportin-1 (Fpn; refs. 21, 27, 28). To date, Fpn is the only identified mammalian iron export protein (29). Intestine-specific Fpn-knockout (KO) mice develop severe systemic iron deficiency and show iron accumulation in enterocytes (30).
Intestinal iron efflux through Fpn is tightly regulated at the systemic level by hepcidin, a peptide “master regulator” of iron homeostasis that is encoded by the Hamp gene (31). It is produced predominantly by hepatocytes and secreted into the circulation (31), where it binds to cell surface Fpn, causing the iron export protein to be internalized and degraded (32). As such, hepcidin regulates intestinal iron absorption: hepcidin production is increased when the body is iron replete, such that dietary iron absorption is reduced, and is conversely decreased when the body is iron deplete, such that iron absorption is increased (33–36).
The aim of the current study was to investigate whether the absorption of iron from nanoFe3+ is mediated basolaterally by Fpn as it appears to be for soluble forms of iron that are acquired by the enterocyte. To address this, we made use of the intestine-specific Fpn-KO mouse. Our results show that systemic absorption of iron from nanoFe3+ is under normal Fpn-dependent iron homeostasis.
MATERIALS AND METHODS
Iron materials
Ferrous sulfate heptahydrate (FeSO4) was purchased from Sigma-Aldrich (Gillingham, Dorset, UK). Ferric citrate monohydrate was purchased from Sigma-Aldrich (Sydney, Australia). Ferric nitrilotriacetate chelate (FeNTA2) was produced by mixing an acidified solution of FeCl3 (10 mM) with an NTA solution to achieve a molar ratio of Fe:NTA of 1:2. The pH of the final solution was adjusted to 7.4 with NaOH. NanoFe3+ was prepared according to the protocol by Powell et al. (14). Briefly, an acidic concentrated stock solution of FeCl3 was added to a solution containing tartaric acid and adipic acid in 0.9% (w/v) of electrolyte (KCl) to achieve a molar ratio of Fe:tartaric acid:adipic acid in the final suspension of 2:1:1 and [Fe] = 40 mM. The initial pH of the mixture was always below 2.0 and the Fe was fully soluble. The pH was then slowly increased by dropwise addition of a concentrated solution of NaOH until ca. pH 7.4. The entire mixture was then oven-dried at 45°C for a minimum of 24 h.
Rodent diets
All diets were prepared by Specialty Feeds (Glenn Forest, WA, Australia) and supplied in a powdered form. Other than varying the amount and form of the iron added, the diets were equivalent and conformed to AIN-93G purified rodent diet (Supplemental Table S1 and ref. 37). The iron materials used to supplement the rodent diet were FeSO4, Fe3+ citrate, and nanoFe3+ as defined above. The total iron content of the test diets was determined by inductively coupled plasma optical emission spectrometry (ICP-OES; JY2000, Horiba-Jobin, Stanmore, UK) at 259.94 nm following digestion with concentrated nitric acid at 37°C for 4 d, followed by 16 h at 70°C, and then diluted (1:5) with UHP water. ICP-OES calibration was with sample-based standards (the sample matrix used was that of the Fe-deficient diet similarly digested and diluted) which were spiked with iron ranging from 0 to 18 μM, in a similar fashion to previous work (38).
Animals
Mice carrying the floxed Fpn (Fpnflox/flox) allele were bred with vil-Cre-ERT2 mice, which carry a tamoxifen-inducible, intestine-specific Cre recombinase gene (30). Mice with intestine-specific deletion of Fpn (here referred to as Fpn KO) were produced by injecting the resulting vil-Cre-ERT2/Fpnflox/flox mice subcutaneously with tamoxifen (0.075 mg/g body weight) once daily for 3 d starting at 28 d of age. Littermate wild-type (WT) control mice were also injected with tamoxifen 1×/d for 3 d starting at 28 d of age. Lack of Fpn expression in intestinal enterocytes taken from tamoxifen-injected mice was confirmed by Western blotting (Supplemental Fig. S1). This study was carried out in strict accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All animal procedures were approved by the Queensland Institute of Medical Research Berghofer Medical Research Institute Animal Ethics Committee (registration number A0192-609M). All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Feeding study design and tissue collection
WT and Fpn-KO mice were allocated to the different diet groups (n=4–8 mice/set) as outlined in Supplemental Fig. S2. All animals were housed individually and had unlimited access to food and deionized water throughout the study. Three-week-old mice were fed an iron-deficient diet for 4 wk (i.e., 0–4 wk=iron-depletion period) to induce iron deficiency. Administration of the test diets was to the two study groups and comprised the iron-deficient diet supplemented with ca. 20 mg iron/kgdiet as FeSO4 or nanoFe3+. This began at the end of week 4 and lasted a further 4 wk (i.e., 4–8 wk; Supplemental Fig. S2). Two further sets of mice (one Fpn KO and the other WT) remained on the iron deficient diet throughout the study (here named Fe-deficient group). Similarly, two control sets of animals (one Fpn KO and the other WT) were fed an iron sufficient diet, with ca. 35 mg iron/kgdiet as Fe3+ citrate monohydrate to conform with AIN-93G diet formulation (37), for the entire 8 wk study period (i.e., from 0 to 8 wk), and this is termed the Fe-sufficient group.
Blood samples were collected from each animal at the end of wk 4 (i.e., after iron depletion for the study groups and Fe-deficient group), wk 5 (1 wk into the iron repletion period for the study groups) and wk 6 (2 wk into the iron repletion period for the study groups) by piercing a facial vein. Approximately 25 μl of blood was collected into EDTA tubes at each time point. After the iron repletion period of the study groups (i.e., end of wk 8), the mice were anesthetized with a single intraperitoneal injection of xylazine (10 mg/kg) and ketamine (200 mg/kg) and euthanized by exsanguination, and blood, liver, spleen, and duodenal tissues were collected. Liver and spleen samples were diced prior to snap-freezing in liquid nitrogen. The proximal section of the small intestine (∼2 cm) was cut open lengthwise, washed in ice-cold PBS, and transferred into 5 ml of enterocyte-isolating solution consisting of 15 μM EDTA, protease inhibitor (Complete; Roche Molecular Biochemicals, Basel, Switzerland) and 5 μM phenylmethylsulfonyl fluoride (PMSF) in PBS. Samples were then gently inverted for 30 min at 4°C. The detached cells were centrifuged (1000 g, 4°C, 5 min) and washed twice with ice-cold PBS prior to snap-freezing in liquid nitrogen.
Measurement of iron absorption using duodenal loops
Duodenal loops were used to assess iron absorption from iron compounds directly introduced into the duodenum (pylorus to the ligament of Treitz; ref. 39). WT and Fpn-KO mice (8–12 wk old) were maintained on an iron-deficient diet for 1 wk prior to the intestinal loop procedure to ensure that the enterocytes were minimally loaded with dietary iron. Mice were anesthetized (200 mg/kg ketamine and 10 mg/kg xylazine), and a midline incision was made in the abdomen. After the duodenum was exposed, two ligatures were tied immediately after the pylorus, and a third was tied immediately before the ligament of Treitz. Special care was taken to prevent ligation of blood vessels. Two incisions were made in the duodenum, one proximal to the first ligature and the other distal to the third ligature. A cannula was inserted through the proximal incision, and the first ligature was tightened to hold the cannula in place. The exposed intestinal segment was flushed with ca. 5 ml of saline (prewarmed to 37°C) injected through the cannula. After flushing the exposed segment, the third ligature at the distal end of the duodenum was tightened. The loop was infused with 100 μl of solution containing 500 μM iron as either FeNTA2 (soluble iron control; n=7 WT and n=7 Fpn KO) or nanoFe3+ (n=10 WT and n=9 Fpn KO) in 125 mM NaCl, 3.5 mM KCl, and 16 mM HEPES, pH 7.5 (iron phase distribution in this solution confirmed that FeNTA2 was mainly soluble and nanoFe3+ was mainly nanoparticulate; Supplemental Fig. S3). Approximately 100 μl of saline was then infused through the cannula to flush any residual test solution into the intestinal lumen. The second ligature was tightened to seal the test solution inside the intestinal loop and the cannula removed. The abdomen was then covered with damp gauze, which was continually moistened with prewarmed saline to prevent drying. Blood was collected by heart puncture 30 min after infusion, and the animals were killed by cervical dislocation.
Analysis of blood parameters and tissue iron stores
The Hb concentration in the blood samples was determined with a Coulter Ac·T diff Analyzer (Beckman Coulter Australia, Sydney, NSW, Australia) according to the manufacturer's protocol. Histochemical staining for iron in paraffin-embedded duodenal, liver, and spleen sections was carried out with Perls' Prussian blue (40) using hematoxylin-eosin as a counterstain. Serum iron levels were determined colorimetrically using a ferrozine-based iron assay kit (Pointe Scientific Inc., Canton, MI, USA) following the manufacturer's instructions. Liver and spleen iron levels were also determined colorimetrically using a modification of the ferrozine-based assay described by Rebouche et al. (41). Briefly, liver and spleen samples were wrapped in foil and dried overnight in an oven at 110°C. Samples were mixed with a solution containing 3 M hydrochloric acid and 0.6 M trichloroacetic acid. Calibration standards were prepared in the range 2–100 μg Fe/ml. Tissues and standards were incubated at 65°C for 20 h, then vortexed and centrifuged (10,000 g, 5 min). Two parts of chromogen solution (0.508 mM ferrozine, 1.5 M sodium acetate, and 1.5% v/v thioglycolic acid in H2OUHP) were mixed with 1 part standard/tissue supernatant, and the absorbance was read at 595 nm following 30 min incubation at room temperature.
Analysis of gene expression
RNA was extracted from snap-frozen liver and duodenal tissue samples using TRIzol reagent (Invitrogen, Melbourne, VIC, Australia) as per the manufacturer's instructions. The RNA (500 ng) was then used for cDNA synthesis using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and an oligo-dT primer as per the manufacturer's instructions. Gene expression was determined by quantitative real-time polymerase chain reaction (rtPCR) as described previously (42).
The primers used were as follows: Hamp1, forward CCTGAGCAGCACCACCTATC, reverse TGCAACAGATACCACACTGGG; Slc11a2, forward TCATACCCATCCTCACGTTCAC, reverse GGTCAAATAAGCCACGCTAACC; HPRT, forward ATGATCAGTCAACGGGGGAC, reverse TTGGGGCTGTACTGCTTAAC.
Western blotting
Protein was extracted from isolated enterocytes, and expression of Fpn was assessed by Western blotting as described previously (43).
Statistical analysis
Unless otherwise stated, all values are expressed as means ± sd. Statistical differences between the Hb values for each group of mice at the various time points on a specific diet were determined with repeated measures 2-way analysis of variance (ANOVA) with the Bonferroni correction to account for multiple comparisons. Statistical differences between hepatic and splenic tissue iron levels and gene expression levels between groups of mice on the various diets were determined with 1-way ANOVA with the Bonferroni correction. Statistical differences between the serum iron levels from the duodenal loop study were determined by unpaired t test. The level of significance was set to P < 0.05. Statistical analysis were performed with GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA).
RESULTS
Dietary nanoFe3+ is absorbed via a Fpn-dependent pathway
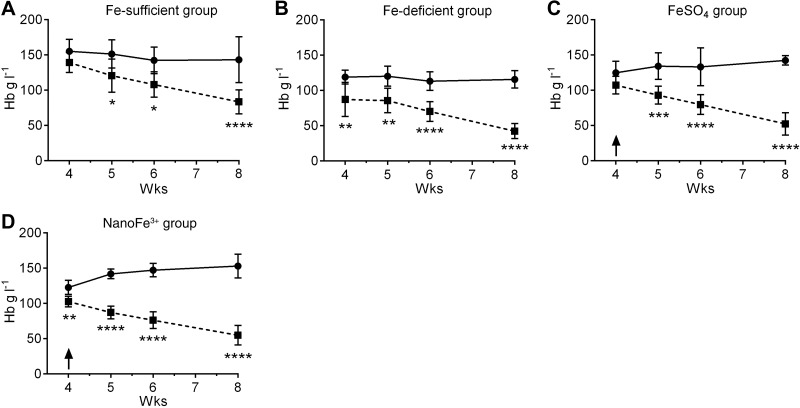
Hb levels in the Fpn-KO mice maintained on the iron-sufficient diet (Fe-sufficient group) were lower than the Hb levels of the WT mice maintained on the same diet, being significant from wk 5 onward (P≤0.03; Fig. 1A). Similarly, Fpn-KO mice maintained on the iron-deficient diet (Fe-deficient group) had lower Hb compared to WT mice on the same diet, and this was statistically significant from wk 4 onward (P<0.003; Fig. 1B). Fpn-KO mice on both iron-supplemented test diets (i.e., FeSO4 and nanoFe3+ groups) also had similar and significantly lower Hb levels throughout the Fe repletion period compared to the WT mice (P≤0.0002 from wk 5 for FeSO4 and P≤0.007 from wk 4 for nanoFe3+; Fig. 1C, D).
Figure 1.
Hb levels in mice during dietary iron repletion. Hb values for WT (solid trace) and Fpn KO (dashed trace) mice in the control Fe-sufficient (i.e., non-iron-depleted) group (A), the Fe-deficient group (B), the FeSO4-supplemented group (C), and the nanoFe3+-supplemented group (D) after iron depletion (study wk 4), 1 wk into the iron repletion period (study wk 5), 2 wk into the iron repletion period (study wk 6), and at the end of the iron repletion period (study wk 8) as per study outline presented in Supplemental Fig. S2. Arrows (C, D) indicate the start of iron repletion with FeSO4- or nanoFe3+-supplemented diets, respectively. Values are means ± sd. Numbers in each group are as follows: Fe-sufficient, n = 6 WT and n = 8 Fpn KO; Fe-deficient, n = 6 WT and n = 6 Fpn KO; FeSO4, n = 4 WT and n = 9 Fpn KO; nanoFe3+, n = 8 WT and n = 6 Fpn KO. *P ≤ 0.01, **P ≤ 0.007, ***P ≤ 0.0002, ****P < 0.0001 vs. Fpn-KO mice.
The body weights of the animals for each dietary group are presented in Supplemental Table S2 alongside the baseline and final Hb values of Fig. 1. There were no significant differences in the body weight of the animals in any diet group at the end of the study. There were no statistically significant differences between final Hb levels of the WT mice supplemented with nanoFe3+ in comparison to WT mice supplemented with FeSO4 or WT mice in the control Fe-sufficient group. However, Hb levels at the end of the study in the nanoFe3+-supplemented WT mice were significantly higher than for WT mice of the Fe-deficient group (P=0.005; Supplemental Table S2). For the Fpn-KO mice, Hb levels at the end of the study in the nanoFe3+ group were similar to the Fe-deficient group and the FeSO4 group, but were lower for these 3 groups than the levels in the Fe-sufficient group (P=0.06 for nanoFe3+, P=0.01 for FeSO4, and P=0.001 for Fe-deficient; Supplemental Table S2).
Throughout, Hb levels of Fpn-KO mice were significantly lower than for WT counterparts (all P≤0.0001 by wk 8; Fig. 1).
Tissue iron levels
The levels of iron in the duodenum, spleen, and liver of animals maintained on diets containing different forms of iron were assessed qualitatively by Perls' Prussian blue staining and quantitatively (only for the spleen and liver) using a colorimetric assay. There was no stainable iron in the small intestinal enterocytes of WT or Fpn-KO mice fed the iron-deficient diet throughout the study (Fig. 2A). Similarly, there was no accumulation of iron in the spleen (Fig. 2A) or the liver in these mice (Supplemental Fig. S4). In the three other groups (i.e., both iron-supplemented groups and the non-iron-depleted control group), no stainable iron was seen in the small intestinal enterocytes of WT mice, but distinct iron accumulation was observed in enterocytes of Fpn-KO mice (Fig. 2B–D). Conversely, iron staining was detected in the spleens of WT mice but not in the spleen of Fpn-KO mice (Fig. 2B–D). No detectable iron staining was observed in the liver of either WT or Fpn-KO animals from any group (Supplemental Fig. S3).
Figure 2.
Tissue Fe distribution. Representative images of Perls' Prussian blue staining of duodenum and spleen of WT and Fpn-KO mice in the Fe-deficient (A), control Fe-sufficient (B), FeSO4-supplemented (C), or nanoFe3+-supplemented (D) groups, defined as per study outline presented in Supplemental Fig. S2. Arrows indicate example locations of iron staining in the tissues. Scale bars = 30 μm.
Quantitation of iron levels in the liver of WT animals showed that both the FeSO4- and the nanoFe3+-supplemented groups were able to increase hepatic Fe stores to levels similar to those observed in the non-iron-depleted control group (Fig. 3). Corresponding increases in spleen iron also occurred in both the FeSO4- and nanoFe3+-supplemented groups, although the levels did not reach that of control mice. In contrast, both liver and spleen iron levels were consistently low in the Fpn-KO mice in all the diet groups and were significantly lower than the levels in the corresponding WT animals (P≤0.006; Fig. 3). Spleen iron levels were an order of magnitude higher than liver iron levels for all diet groups (Fig. 3).
Figure 3.
Liver and spleen nonheme iron levels. Hepatic iron (A) and splenic iron (B) levels of WT (solid bars) and Fpn-KO (open bars) mice of the control Fe-sufficient (i.e., non-iron-depleted) group, Fe-deficient group, FeSO4-supplemented group, and nanoFe3+-supplemented group. Data represent means ± sd. Numbers in each group are as follows: Fe-sufficient, n = 6 WT and n = 8 Fpn KO; Fe-deficient, n = 6 WT and n = 6 Fpn KO; FeSO4, n = 4 WT and n = 9 Fpn KO; nanoFe3+, n = 8 WT and n = 6 Fpn KO. *P ≤ 0.04, **P ≤ 0.006, ***P ≤ 0.0009, ****P < 0.0001.
Tissue expression of Fe-related genes
To investigate how iron derived from nanoFe3+ influenced intestinal and systemic iron homeostasis, we examined the enterocyte mRNA expression of Slc11a2, the apical transporter of ferrous iron in enterocytes (25), and hepatic hepcidin (Hamp1), the master regulator of iron homeostasis (31). In WT mice, Slc11a2 expression in the duodenum was significantly higher (P<0.0001) in the iron-deficient group than in the mice fed the iron-supplemented diets (FeSO4 and nanoFe3+ groups) or the non-iron-depleted controls (Fe-sufficient group) (Fig. 4A). The relative expression of Hamp1 mRNA in the liver was significantly higher in both the WT mice administered the iron-supplemented diets or the non-iron-depleted controls compared to the WT mice on the iron-deficient diet (P≤0.007; Fig. 4B). All Fpn-KO mice had very little expression of either of the iron-regulating genes, irrespective of diet group.
Figure 4.
Expression of Slc11a2 mRNA (A) in isolated enterocytes and Hamp1 (B) in the liver. Data shown for WT (solid bars) and Fpn-KO (open bars) mice in the control Fe-sufficient (i.e., non-iron-depleted) group, Fe-deficient group, FeSO4-supplemented group, and nanoFe3+-supplemented group. Values are means ± sd of the log2-transformed gene expression in relation to the housekeeping gene HPRT. Numbers in each group are as follows: Fe-sufficient, n = 6 WT and n = 8 Fpn KO; Fe-deficient, n = 6 WT and n = 6 Fpn KO; FeSO4, n = 4 WT and n = 9 Fpn KO; nanoFe3+, n = 8 WT and n = 6 Fpn KO. **P ≤ 0.007, ***P ≤ 0.0006, ****P < 0.0001.
Iron transport from nanoFe3+ in isolated duodenal loops in vivo
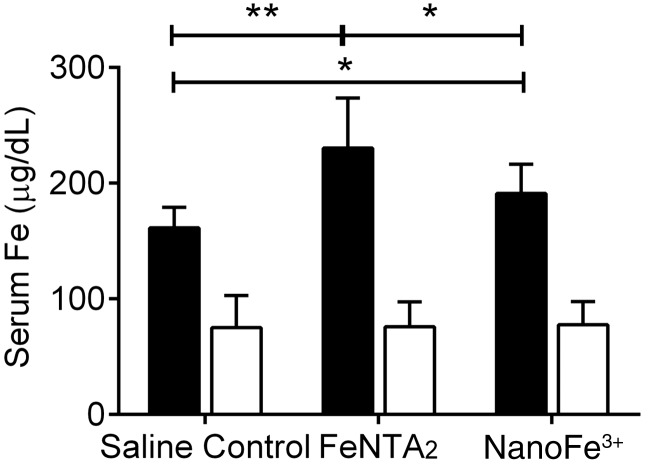
Duodenal loops were used to assess the direct transfer of iron, derived from nanoFe3+ of the lumen, into the blood circulation in WT and Fpn-KO mice. Infusing the duodenal loops with nanoFe3+ provides additional information on the uptake of Fe from the intact nanoparticulate material, that is, bypassing the stomach, unlike with feeding experiments. Serum iron levels were compared to a soluble Fe3+ control (FeNTA2) and a saline control (no iron). All Fpn-KO animals had significantly lower serum iron levels than WT mice in the same diet group (P<0.0001). In WT mice, serum iron levels increased 30 min following infusion with nanoFe3+ (P=0.04) and more so with FeNTA2 (P=0.007) (Fig. 5). In contrast, there was no difference in serum iron levels of Fpn-KO mice 30 min following infusion with either iron preparation in comparison to the saline control (Fig. 5).
Figure 5.
Serum iron levels in Fpn-KO (open bars) and WT (solid bars) mice following 30 min exposure of duodenal loops to iron. Duodenal loops in male mice (8–12 wk old) were infused with either saline (control), 500 μM iron as FeNTA2, or 500 μM iron as nanoFe3+. Data represent means ± sd. Numbers in each group are as follows: saline control, n = 6 WT and n = 7 Fpn KO; FeNTA2, n = 7 WT and n = 7 Fpn KO; nanoFe3+, n = 10 WT and n = 9 Fpn KO. *P ≤ 0.04, **P = 0.007.
DISCUSSION
We have previously shown that nanoFe3+ crosses the apical surface of Caco-2 cells via an endocytic pathway before being dissolved in endosomes or lysosomes inside the cell (17). However, the mechanism by which the iron derived from nanoFe3+ crosses the basolateral membrane is unknown. Since Fpn is the only known mammalian iron export protein (29), we investigated the absorption of nanoFe3+ in intestine-specific Fpn-KO mice and littermate controls. We found that, like other forms of iron in the intestine, nanoFe3+ was ineffective at repleting Hb levels in Fpn-KO mice. Iron was retained in the enterocyte of Fpn-KO animals regardless of whether it was soluble iron or nanoFe3+, consistent with Fpn being the common enterocyte exporter for iron irrespective of its luminal form. The low levels of iron in the liver and spleen of Fpn-KO mice provide further evidence that iron derived from nanoFe3+ cannot bypass the Fpn-mediated efflux mechanism that is used by soluble luminal iron sources.
We further investigated the absorption of nanoFe3+ using intestinal loops in which the iron compound was administered directly into the duodenum of WT and Fpn-KO animals, thereby bypassing the stomach. Although serum iron levels increased significantly with the soluble (FeNTA2) and nanoFe3+ infusions compared to saline, the increase with nanoFe3+ was significantly lower (P=0.03) than with FeNTA2, despite nanoFe3+ being as effective as ferrous sulfate at increasing Hb levels during the dietary intervention study. The difference is not related to the choice of positive controls for the two experiments, as ferrous sulfate is certainly as well absorbed as FeNTA2 (44–46). Instead, the data show that either the passage of nanoFe3+ through the stomach is required for efficient absorption, or nanoFe3+ absorption is slower than that of soluble iron, because the direct uptake of whole nanoparticles requires their endosomal/lysosomal breakdown prior to systemic release of iron (17). Indeed, we suggest that the ca. 30 μg/dl difference in serum Fe levels between the FeNTA2- and nanoFe3+-treated WT animals is mainly due to differences in the absorption kinetics of the two materials. Studies have shown that the rate of absorption of Fe from ferritin, which contains an Fe3+ oxohydroxide core very similar to nanoFe3+ and is taken up by clathrin-dependent endocytosis (47–49), is slower than that of soluble Fe in the rat intestine (50). Moreover, our own observations in human volunteers comparing nanoFe3+ to FeSO4 support this difference in kinetics (unpublished results).
Despite these potential differences in the rate of iron absorption by the intestine, the overall response of the body to nanoFe3+ appears to be almost identical to that of FeSO4. The Hb repletion data in the WT mice demonstrate that iron from nanoFe3+ is as efficient at correcting diet-induced iron deficiency anemia as FeSO4. Gene expression analysis showed that Slc11a2 mRNA levels in enterocytes decreased to similar levels in response to iron repletion by either nanoFe3+ or soluble Fe. Interestingly, the Fpn-KO animals in all the diet groups expressed very low levels of Slc11a2 even though they were iron deficient. This is likely due to the accumulation of iron in Fpn-KO enterocytes, as seen in Fig. 2, which leads to the destabilization of Slc11a2 mRNA via the IRP/IRE system (51). Indeed, similar down-regulation of Slc11a2 levels was observed in sla mice, which develop iron-loaded enterocytes due to a deletion in the gene encoding the ferroxidase hephaestin (52). Liver Hamp1 mRNA expression also responded similarly to iron repletion irrespective of the iron source. WT animals in the iron-supplemented groups showed significantly elevated Hamp1 expression in the liver when compared to iron-deficient animals.
Taken together, our data clearly demonstrate the effectiveness of nanoFe3+ as a dietary supplement, consistent with iron repletion studies in rats (14). The relatively slow release of iron from nanoFe3+, with a consequent reduced rate of absorption, may be an advantage in terms of preventing the generation of nontransferrin bound Fe that can be observed following absorption of therapeutic doses of soluble iron (53–56). Notably, the iron derived from nanoFe3+ does not circumvent systemic iron regulatory mechanisms, as the intestinal efflux of iron following enterocyte uptake of nanoFe3+ is ferroportin mediated in a similar manner to that of soluble iron. This implies that iron derived from nanoFe3+ joins the common enterocyte labile Fe pool, at some point, following the direct brush border uptake of nanoFe3+.
Supplementary Material
Acknowledgments
This work was supported by the UK Medical Research Council (U105960399). M.F.A. is supported by an MRC Ph.D. studentship. G.J.A. is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia. D.M.F. is the recipient of a fellowship from the Australian Liver Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest but note that D.I.A.P., S.F.A.B., N.F., and J.J.P. are inventors on a patent detailing novel Fe3+ polyoxohydroxide structures, as described herein, and that may have further potential as commercial dietary supplements (57). M.F.A., D.M.F., G.J.A., J.J.P., and D.I.A.P. designed the research; S.F.A.B. and N.J.R.F. provided the nanoFe3+ materials; M.F.A., D.M.F., S.J.W., and C.M. conducted the research; M.F.A., D.M.F., and D.I.A.P. analyzed data; M.F.A., J.J.P., and D.I.A.P. had primary responsibility for final content. All authors read, provided input to, and approved the final manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- DMT-1
- divalent metal transporter 1
- FeNTA2
- Fe3+ nitrilotriacetate chelate
- Fpn
- ferroportin-1
- Hb
- hemoglobin
- IDA
- iron deficiency anemia
- KO
- knockout
- nanoFe3+
- nanoparticulate Fe3+ polyoxohydroxide
- NTA
- nitrilotriacetate
- WT
- wild-type
REFERENCES
- 1. World Health Organization (2008) Global Database on Anaemia, World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. World Health Organization (2002) The World Health Report. Reducing Risks, Promoting Healthy Life, World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S., Murray C. J. (2002) Selected major risk factors and global and regional burden of disease. Lancet 360, 1347–1360 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (2008) The Global Burden of Disease: 2004 Update pp. 1–146, World Health Organization, Geneva, Switzerland [Google Scholar]
- 5. Cancelo-Hidalgo M. J., Castelo-Branco C., Palacios S., Haya-Palazuelos J., Ciria-Recasens M., Manasanch J., Pérez-Edo L. (2013) Tolerability of different oral iron supplements: a systematic review. Curr. Med. Res. Opin. 29, 291–303 [DOI] [PubMed] [Google Scholar]
- 6. Peña-Rosas Juan P., De-Regil Luz M., Dowswell T., Viteri Fernando E. (2012) Daily oral iron supplementation during pregnancy. In Cochrane Database of Systematic Reviews, John Wiley & Sons, Ltd., Chichester, UK: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seril D. N., Liao J., Ho K. L., Warsi A., Yang C. S., Yang G. Y. (2002) Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig. Dis. Sci. 47, 1266–1278 [DOI] [PubMed] [Google Scholar]
- 8. Radulescu S., Brookes M. J., Salgueiro P., Ridgway R. A., McGhee E., Anderson K., Ford S. J., Stones D. H., Iqbal T. H., Tselepis C., Sansom O. J. (2012) Luminal iron levels govern intestinal tumorigenesis after apc loss in vivo. Cell Rep. 2, 270–282 [DOI] [PubMed] [Google Scholar]
- 9. Coplin M., Schuette S., Leichtmann G., Lashner B. (1991) Tolerability of iron: a comparison of bis-glycino iron II and ferrous sulfate. Clin. Ther. 13, 606–612 [PubMed] [Google Scholar]
- 10. Dostal A., Chassard C., Hilty F. M., Zimmermann M. B., Jaeggi T., Rossi S., Lacroix C. (2012) Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 142, 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmermann M. B., Chassard C., Rohner F., N′Goran E., K., Nindjin C., Dostal A., Utzinger J., Ghattas H., Lacroix C., Hurrell R. F. (2010) The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am. J. Clin. Nutr. 92, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 12. Werner T., Wagner S. J., Martinez I., Walter J., Chang J. S., Clavel T., Kisling S., Schuemann K., Haller D. (2011) Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut 60, 325–333 [DOI] [PubMed] [Google Scholar]
- 13. Kortman G. A., Boleij A., Swinkels D. W., Tjalsma H. (2012) Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One 7, e29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powell J. J., Bruggraber S. F., Faria N., Poots L. K., Hondow N., Pennycook T. J., Latunde-Dada G. O., Simpson R. J., Brown A. P., Pereira D. I. (2014) A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. [E-pub ahead of print] Nanomedicine 10.1016/j.nano.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan Y. H., Sader K., Powell J. J., Bleloch A., Gass M., Trinick J., Warley A., Li A., Brydson R., Brown A. (2009) 3D morphology of the human hepatic ferritin mineral core: new evidence for a subunit structure revealed by single particle analysis of HAADF-STEM images. J. Struct. Biol. 166, 22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tosha T., Behera R. K., Ng H. L., Bhattasali O., Alber T., Theil E. C. (2012) Ferritin protein nanocage ion channels: gating by N-terminal extensions. J. Biol. Chem. 287, 13016–13025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pereira D. I., Mergler B. I., Faria N., Bruggraber S. F., Aslam M. F., Poots L. K., Prassmayer L., Lonnerdal B., Brown A. P., Powell J. J. (2013) Caco-2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS One 8, e81250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dorey C., Cooper C., Dickson D. P., Gibson J. F., Simpson R. J., Peters T. J. (1993) Iron speciation at physiological pH in media containing ascorbate and oxygen. Br. J. Nutr. 70, 157–169 [DOI] [PubMed] [Google Scholar]
- 19. Simpson R. J., Peters T. J. (1990) Forms of soluble iron in mouse stomach and duodenal lumen: significance for mucosal uptake. Br. J. Nutr. 63, 79–89 [DOI] [PubMed] [Google Scholar]
- 20. Miret S., Simpson R. J., McKie A. T. (2003) Physiology and molecular biology of dietary iron absorption. Annu. Rev. Nutr. 23, 283–301 [DOI] [PubMed] [Google Scholar]
- 21. McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 [DOI] [PubMed] [Google Scholar]
- 22. McKie A. T. (2008) The role of Dcytb in iron metabolism: an update. Biochem. Soc. Trans. 36, 1239–1241 [DOI] [PubMed] [Google Scholar]
- 23. Ohgami R. S., Campagna D. R., McDonald A., Fleming M. D. (2006) The Steap proteins are metalloreductases. Blood 108, 1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gunshin H., Fujiwara Y., Custodio A. O., Direnzo C., Robine S., Andrews N. C. (2005) Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Invest. 115, 1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 [DOI] [PubMed] [Google Scholar]
- 26. Theil E. C. (2004) Iron, ferritin, and nutrition. Annu. Rev. Nutr. 24, 327–343 [DOI] [PubMed] [Google Scholar]
- 27. Donovan A., Brownlie A., Zhou Y., Shepard J., Pratt S. J., Moynihan J., Paw B. H., Drejer A., Barut B., Zapata A., Law T. C., Brugnara C., Kingsley P. D., Palis J., Fleming M. D., Andrews N. C., Zon L. I. (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 [DOI] [PubMed] [Google Scholar]
- 28. Abboud S., Haile D. J. (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 [DOI] [PubMed] [Google Scholar]
- 29. Nemeth E., Ganz T. (2006) Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 26, 323–342 [DOI] [PubMed] [Google Scholar]
- 30. Donovan A., Lima C. A., Pinkus J. L., Pinkus G. S., Zon L. I., Robine S., Andrews N. C. (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 [DOI] [PubMed] [Google Scholar]
- 31. Ganz T. (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788 [DOI] [PubMed] [Google Scholar]
- 32. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 33. Ganz T., Nemeth E. (2012) Hepcidin and iron homeostasis. Biochim. Biophys. Acta 1823, 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drakesmith H., Prentice A. M. (2012) Hepcidin and the iron-infection axis. Science 338, 768–772 [DOI] [PubMed] [Google Scholar]
- 35. Bregman D. B., Morris D., Koch T. A., He A., Goodnough L. T. (2013) Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am. J. Hematol. 88, 97–101 [DOI] [PubMed] [Google Scholar]
- 36. Prentice A. M., Doherty C. P., Abrams S. A., Cox S. E., Atkinson S. H., Verhoef H., Armitage A. E., Drakesmith H. (2012) Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood 119, 1922–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reeves P. G., Nielsen F. H., Fahey G. C., Jr. (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123, 1939–1951 [DOI] [PubMed] [Google Scholar]
- 38. Powell J. J., McNaughton S. A., Jugdaohsingh R., Anderson S. H., Dear J., Khot F., Mowatt L., Gleason K. L., Sykes M., Thompson R. P., Bolton-Smith C., Hodson M. J. (2005) A provisional database for the silicon content of foods in the United Kingdom. Br. J. Nutr. 94, 804–812 [DOI] [PubMed] [Google Scholar]
- 39. Frazer D., Wilkins S., Becker E., Murphy T., Vulpe C., McKie A., Anderson G. (2003) A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut 52, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bunting H. (1949) The histochemical detection of iron in tissues. Biotech. Histochem. 24, 109–115 [DOI] [PubMed] [Google Scholar]
- 41. Rebouche C. J., Wilcox C. L., Widness J. A. (2004) Microanalysis of non-heme iron in animal tissues. J. Biochem. Biophys. Meth. 58, 239–251 [DOI] [PubMed] [Google Scholar]
- 42. Darshan D., Wilkins S. J., Frazer D. M., Anderson G. J. (2011) Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology 141, 300–309 [DOI] [PubMed] [Google Scholar]
- 43. Frazer D. M., Wilkins S. J., Becker E. M., Vulpe C. D., Mckie A. T., Trinder D., Anderson G. J. (2002) Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology 123, 835–844 [DOI] [PubMed] [Google Scholar]
- 44. Barrand M. A., Hider R. C., Callingham B. A. (1990) The importance of reductive mechanisms for intestinal uptake of iron from ferric maltol and ferric nitrilotriacetic acid (NTA). J. Pharm. Pharmacol. 42, 279–282 [DOI] [PubMed] [Google Scholar]
- 45. Zimmermann M. B., Biebinger R., Egli I., Zeder C., Hurrell R. F. (2011) Iron deficiency up-regulates iron absorption from ferrous sulphate but not ferric pyrophosphate and consequently food fortification with ferrous sulphate has relatively greater efficacy in iron-deficient individuals. Br. J. Nutr. 105, 1245–1250 [DOI] [PubMed] [Google Scholar]
- 46. Wollenberg P., Rummel W. (1987) Dependence of intestinal iron absorption on the valency state of iron. Naunyn Schmiedebergs Arch. Pharmacol. 336, 578–582 [DOI] [PubMed] [Google Scholar]
- 47. Antileo E., Garri C., Tapia V., Munoz J. P., Chiong M., Nualart F., Lavandero S., Fernandez J., Nunez M. T. (2013) Endocytic pathway of exogenous iron-loaded ferritin in intestinal epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver. Physiol. 304, G655–G661 [DOI] [PubMed] [Google Scholar]
- 48. Kalgaonkar S., Lonnerdal B. (2009) Receptor-mediated uptake of ferritin-bound iron by human intestinal Caco-2 cells. J. Nutr. Biochem. 20, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. San Martin C. D., Garri C., Pizarro F., Walter T., Theil E. C., Nunez M. T. (2008) Caco-2 intestinal epithelial cells absorb soybean ferritin by mu2 (AP2)-dependent endocytosis. J. Nutr. 138, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Theil E. C., Chen H., Miranda C., Janser H., Elsenhans B., Núñez M. T., Pizarro F., Schümann K. (2012) Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 142, 478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. LaVaute T., Smith S., Cooperman S., Iwai K., Land W., Meyron-Holtz E., Drake S. K., Miller G., Abu-Asab M., Tsokos M. (2001) Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat. Genet. 27, 209–214 [DOI] [PubMed] [Google Scholar]
- 52. Chen H., Su T., Attieh Z. K., Fox T. C., McKie A. T., Anderson G. J., Vulpe C. D. (2003) Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood 102, 1893–1899 [DOI] [PubMed] [Google Scholar]
- 53. Dresow B., Petersen D., Fischer R., Nielsen P. (2008) Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals 21, 273–276 [DOI] [PubMed] [Google Scholar]
- 54. Hurrell R. F. (2011) Safety and efficacy of iron supplements in malaria-endemic areas. Ann. Nutr. Metab. 59, 64–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lomer M. C., Cook W. B., Jan-Mohamed H. J., Hutchinson C., Liu D. Y., Hider R. C., Powell J. J. (2012) Iron requirements based upon iron absorption tests are poorly predicted by haematological indices in patients with inactive inflammatory bowel disease. Br. J. Nutr. 107, 1806–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schumann K., Solomons N. W., Romero-Abal M. E., Orozco M., Weiss G., Marx J. (2012) Oral administration of ferrous sulfate, but not of iron polymaltose or sodium iron ethylenediaminetetraacetic acid (NaFeEDTA), results in a substantial increase of non-transferrin-bound iron in healthy iron-adequate men. Food. Nutr. Bull. 33, 128–136 [DOI] [PubMed] [Google Scholar]
- 57. Powell J., Bruggraber S., Faria N., Pereira D., inventors (2008, August 14) Ligand modified poly oxo-hydroxy metal ion materials, their uses and processes for their preparation. UK patent WO/2008/096130
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.