Abstract
Men with sickle cell disease (SCD) risk developing priapism. Recognizing that SCD is a disease of hypoxia, we investigated the effect of hypoxia on gene expression in corporal smooth muscle (CSM) cells. Rat CSM cells in vitro were treated with CoCl2 or low oxygen tension to mimic hypoxia. Hypoxic conditions increased expression of genes previously associated with priapism in animal models. Variable coding sequence a1 (Vcsa1; the rat opiorphin homologue, sialorphin), hypoxia-inducible factor 1a (Hif-1a), and A2B adenosine receptor (a2br) were increased by 10-, 4-, and 6-fold, respectively, by treatment with CoCl2, whereas low oxygen tension caused increases in expression of 3-, 4-, and 1.5-fold, respectively. Sialorphin-treated CSM cells increased expression of Hif-1a and a2br by 4-fold, and vcsa1-siRNA treatment reduced expression by ∼50%. Using a Hif-1a inhibitor, we demonstrated up-regulation of a2br by sialorphin is dependent on Hif-1a, and knockdown of vcsa1 expression with vcsa1-siRNA demonstrated that hypoxic-up-regulation of Hif-1a is dependent on vcsa1. In CSM from a SCD mouse, there was 15-fold up-regulation of opiorphin at a life stage prior to priapism. We conclude that in CSM, opiorphins are master regulators of the hypoxic response. Opiorphin up-regulation in response to SCD-associated hypoxia activates CSM “relaxant” pathways; excessive activation of these pathways results in priapism.—Fu, S., Tar, M. T., Melman, A., Davies, K. P. Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells.
Keywords: priapism, sickle cell disease, hypoxia inducible factor-1a, A2B-adenosine receptor, sialorphin
Priapism is a condition of persistent penile erection in the absence of sexual excitation, and is particularly prevalent in males with sickle cell disease (SCD). The probability of a man with SCD developing priapism is 29–42% (1). The disorder is a urological emergency requiring prompt and accurate diagnosis and treatment because it is associated with permanent erectile tissue damage and erectile disability. At present, pharmacological treatments are often ineffective, and surgical interventions are the most common treatment option.
The hypothesis that vascular occlusion and ischemia is a mechanism for the development of vascular diseases associated with SCD dates back to 1948 (2). In this scenario, “sludging” of red blood cells in the corpora cavernosa as a result of SCD prevents efflux of blood from corporal tissue, resulting in priapism. In recent years, research has focused on understanding the molecular mechanisms that result in priapism, in the hope of identifying molecular targets useful for the development of pharmacotherapies (3, 4). Many of these studies have considered priapism to result from an imbalance between the “relaxant” and “constrictor” pathways regulating the tone of corporal smooth muscle (CSM), with heightened activation of relaxant pathways leading to excessive erectile tendencies, thereby contributing to the development of priapism (5).
Although nitric oxide (NO) is recognized as the principal mediator of CSM relaxation and thereby penile erection and potentially priapism (6, 7), several other signaling pathways are recognized as playing a potential role in relaxing of smooth muscle tissue. Recently, adenosine (8) and opiorphins (9) signaling pathways have received attention as possible mediators of excessive CSM relaxation that result in priapism.
The adenosine signaling pathways were first shown to potentially play a role in priapism when mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, displayed unexpected priapic activity (10). ADA enzyme therapy successfully corrected the priapic activity both in vivo and in vitro, suggesting that it was dependent on elevated adenosine levels (11). Further genetic and pharmacologic evidence demonstrated that A2B adenosine receptor (A2BR)-mediated cAMP and cGMP induction was required for elevated adenosine-induced prolonged penile erection. In mouse models that mimic the priapic condition associated with human SCD, mouse corporal tissue exhibited elevated adenosine levels and A2BR expression (10).
The opiorphins are a family of pentapeptides shown to be inhibitors of neutral endopeptidase (NEP; refs. 12, 13). They are derived by post-translational processing of their parent proteins (12, 14). The name opiorphin was first applied to the pentapeptide product of the human gene proline-rich lacrimal 1 (proL1; ref. 12). In rats, the opiorphin homologue, called sialorphin, is encoded by the variable coding sequence a1 (vcsa1) gene (14). The vcsa1 gene has tissue-specific expression, being highly expressed in the submandibular gland, lung, prostate, and corporal tissue (15–18). The mouse contains ≥3 genes with homology to vcsa1. Of these, mouse submaxillary gland androgen-regulated protein 3A and 2 (mSmr3a and mSmr2) encode proteins that potentially generate pentapeptides with close homology to the opiorphin family (19). We have demonstrated that opiorphins can directly result in relaxation of CSM tissue (20) and when overexpressed in the corpora of rats by gene transfer can result in priapism (21). In SCD mice, which have a high tendency to develop priapism, there is up-regulation of the mouse opiorphin genes in corporal tissue prior to any detectable indication of priapism (21).
SCD can be viewed as a disease of hypoxia (22). Therefore, we determined whether hypoxia could be a factor in the up-regulation of opiorphins in CSM cells using an in vitro model of hypoxia. In this in vitro model we also noted the up-regulation of hypoxia-inducible factor 1a (Hif-1a) and a2br. Given that both opiorphins and a2br appear to be involved in the development of priapism, we expanded our studies to determine the molecular relationship between opiorphins, Hif-1a, and a2br and their regulation by hypoxia.
MATERIALS AND METHODS
Animals
For isolation of CSM cells, 8-wk-old adult male Sprague-Dawley rats were obtained from Charles River (Wilmington, MA, USA). For studies in SCD mice, 5-wk-old Berk transgenic SCD mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the Albert Einstein Animal Facility till the life stage to be investigated (5 and 12 wk old). Age-matched C57BL/6 animals were used as controls. Numbers of animals for each experiment are reported in the figure captions. All animal studies were approved by the Institute of Animal Studies at Albert Einstein College of Medicine.
Chemicals
Sialorphin (Sigma, St. Louis, MO, USA) was dissolved in H2O at a concentration of 10 mM and stored at −20°C. Hif-1a inhibitor (Millipore, Darmstadt, Germany) was dissolved in dimethyl sulfoxide (DMSO) at concentration of 25 mM and stored at −20°C. The Hif-1a inhibitor is a novel small molecule that reduces Hif-1a protein levels by promoting its proteasomal degradation (23, 24). Pertussis toxin (PTX; Invitrogen, Carlsbad, CA, USA) was dissolved in H2O at a concentration of 0.1 μg/μl and stored at 4°C. CoCl2 was dissolved in H2O at a concentration of 20 mM and made just prior to use.
Cell isolation and culture
Corpus cavernosal tissue was harvested from the rats following euthanasia. After dissection and removal of the urethra, the corpus cavernosal tissues were obtained excluding the glans, cartilaginous portion of the penis, and the overlying ischiocavernous muscle. The corpus cavernosum tissue was washed in phosphate-buffered saline (PBS) several times. The sample was cut into fragments. The tissue fragments were then placed in culture dish and incubated for 10 d. The sprouting cells were subcultured in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 g/ml streptomycin at 37°C and 5% CO2. For all experiments, cultured rat CSM cells were grown to 70–80% confluence. To cause in vitro chemical hypoxia, CoCl2 was added to DMEM to a final concentration of 100 μM as described previously (25, 26). Hypoxic conditions due to low oxygen levels was induced by culture of CSM cells in 1% O2, 5% CO2, and 94% N2 in a 95% humidified atmosphere as described previously (27). Control cells were kept under normoxic conditions (20% O2). In other experiments, sialorphin or the Hif-1a inhibitor was added to the medium at the concentrations and for the time indicated in the figure captions.
Immunoblotting analysis
CSM cells were washed with cold PBS, followed by suspension in lysis buffer (Cell Signaling Technology, Boston, MA, USA) using a standard protocol. The protein concentration was analyzed by a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) with bovine serum albumin standards according to the manufacturer's instructions. Cell lysate was separated by SDS-PAGE and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). Following blocking with PBS-Tween-20 containing 5% nonfat dry milk for 1 h, membranes were incubated overnight at 4°C with primary antibody (anti-Hif-1a antibody, Sigma; or anti-A2BR antibody, Millipore), followed by secondary antibody labeled with horseradish peroxidase–conjugated. GAPDH (Fitzgerald, Acton, MA, USA) was used as loading control. Immunoreactive bands were detected by an enhanced chemiluminescence kit (PerkinElmer, Waltham, MA, USA). The densitometry results were normalized by GAPDH expression and finally normalized by control. The intensities of the resulting bands were quantified using the software ImageJ 1.47q (U.S. National Institutes of Health, Bethesda, MD, USA).
siRNA transfection of rat CSM cells
Vcsa1-siRNA and nontargeting siRNA as control (Thermo Fisher Scientific) were used to knock down expression of the vcsa1 gene in rat CSM cells in vitro, as described previously (28). siRNA transfection was done in 6-well plates using siPORT lipid (Invitrogen) according to the manufacturer's instructions. After 48 h, transfected cells were treated with 100 μM CoCl2 or low oxygen tension for the time indicated in the figures, and then protein and RNA were extracted for analysis.
Transactivation assay
CSM cells were transfected with a luciferase reporter plasmid containing the human Hif-1a promoter (as described in ref. 29) for 6 h. Then transfected cells were trypsinized and divided into 4 wells of a 6-well plate and incubated with 400 nM sialorphin alone, 200 ng/ml PTX alone, or a combination of sialorphin and PTX for 8 h. Cells were lysed and luminescence was developed using a dual luciferase reporter assay kit (Promega, Madison, WI, USA) and measured using a Moonlight FluoStar luminometer (BMG Labtech, Cary, NC, USA). Luciferase activities were normalized based on the amount of protein in the cell lysate. All assays were performed in triplicate and performed 3 times.
Quantitative RT-PCR
The total RNA was extracted from frozen tissue with TRIzol (Invitrogen) according to the manufacturer's instructions. Briefly, ∼50 mg tissue was added to 1 ml of TRIzol reagent and homogenized using a polytron homogenizer (Brinkman, Westbury, NY, USA) for 30 s. The homogenized tissues were incubated for 5 min at room temperature, followed by adding 200 μl of chloroform. After mixing, the aqueous phases were separated by centrifugation (12,000 g for 15 min) at 4°C and then were transferred to a clean tube. The RNA was precipitated from the aqueous phase by addition of isopropyl alcohol and pelleted by centrifugation at 12,000 g for 10 min at 4°C, washed once with 75% ethanol, and again pelleted at 7500 g for 5 min. Then ethanol was aspirated, and the RNA pellet was dissolved in sterile water; 4 μg of total RNA was reverse transcribed to first-strand cDNA primed with Oligo(dT) using the Superscript (Invitrogen) First-Strand Synthesis System for real-time PCR. RNA was denatured for 5 min at 65°C and immediately cooled on ice. Then RNA was combined with reaction buffer and DTT for 2 min at 42°C; Superscript II reverse transcriptase was added, and the reaction mix was incubated for 50 min at 42°C to generate cDNA. The reaction was terminated by heating at 70°C for 15 min. cDNA was amplified using Sybr Green 2xPCR Master Mix (Applied Biosystems, Warrington, UK). Real-time quantitative PCR analysis was performed using the 7300 real-time PCR system (Applied Biosystems). The primers used are shown in Table 1. Expression of the investigated gene was normalized to a housekeeping gene, rpl24 (in rats) or rpl19 (in mice). The PCR reactions for all samples were performed in 96-well plates, with 2 μl cDNA, 100 nM of each primer, and 12.5 μl of Sybr Green in a 25 μl reaction volume. The cycling conditions were as follows: activation of Sybr Green DNA polymerase at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min. Results from real-time PCR were presented as threshold cycles normalized to that of the housekeeping gene rpl24 or rpl19. The expression of transcripts was analyzed by the comparative crossing threshold (Ct) method (also known as the 2−ΔΔCt method).
Table 1.
Primers used in studies
| Species and gene | Forward primer | Reverse primer |
|---|---|---|
| Rat | ||
| vcsa1 | GGGCTACCAAAGATGAAGTC | TGCCACCACCTTCAAAAATA |
| Hif-1a | CTCACCAGACAGAGCAGGAA | AGGCTCCTTGGATGAGCTTT |
| a2br | ATCTTTAGCCTCTTGGCGGT | TGATCCCTCTTGCTCGTGTT |
| rpl24 | TCGAGCTGTGCAGTTTTAGTGG | GCGGACTCACATTTGGCATTA |
| Mouse | ||
| mSmr3a | CTCCTACCGGACCTCCTACCACA | GGCAGCTGGCATTGTAGTTGCTTG |
| mSmr2 | CTCAACCAGACAATATCCCC | GGCAGCTGGTGTTGCATTTG |
| Hif-1a | CTCACCAGACAGAGCAGGAA | GGCTCCTTGGATGAGCTTTG |
| a2br | GTCCTTCCTCCACTGCTCAT | GTCCTTCCTCCACTGCTCAT |
| rpl19 | TCGCCAATGCCAACTCCCGT | GGCCAGGGTGTTTTTCCGGC |
Statistics
Data are shown as means ± sd unless otherwise indicated. Student's t test was performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Significance was attributed to values of P < 0.05.
RESULTS
Rat CSM cells grown in hypoxic conditions show elevated expression of vcsa1, A2BR, and Hif-1a
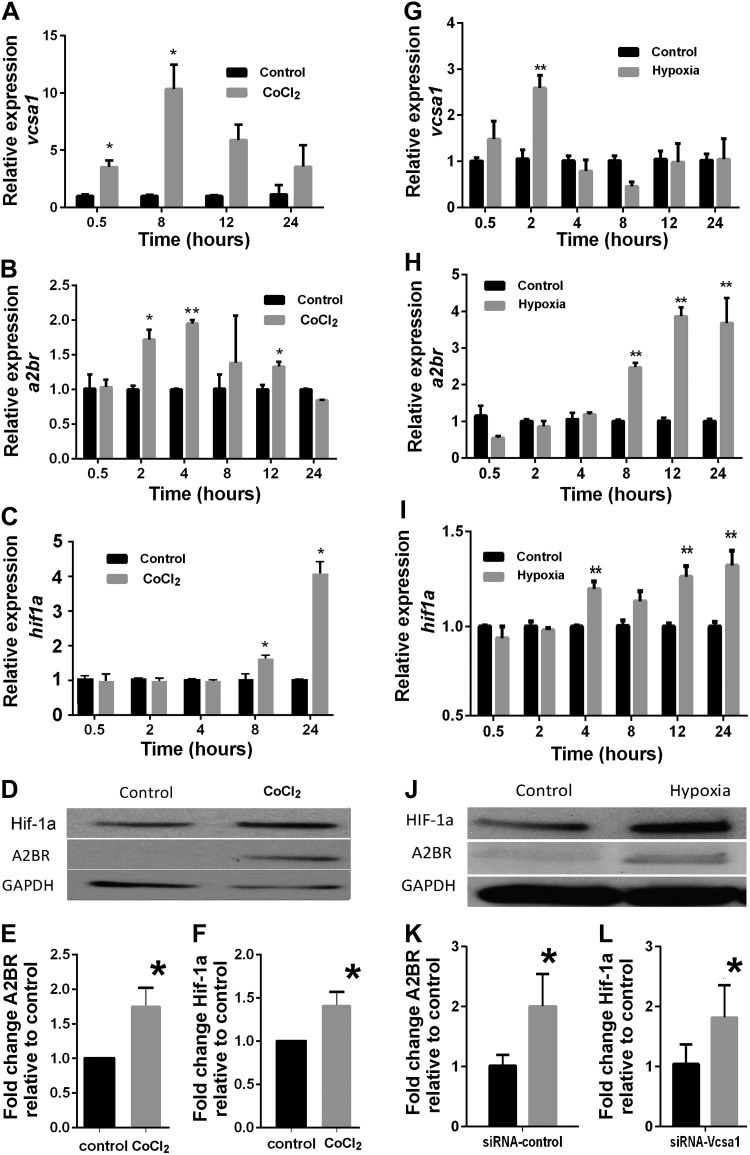
Addition of CoCl2 to culture medium is a commonly used technique in vitro to mimic the hypoxic environment (26, 30, 31). The mechanism involves substitution by cobaltous ions for ferrous ions in heme, causing a conformational change in a heme protein O2 sensor (32). We treated low-passaged rat CSM cells with CoCl2 to simulate a hypoxic environment, and then looked at expression of genes that are thought to be involved in priapism or a response to hypoxia. At various time points after the induction of hypoxia, we determined the levels of gene expression of vcsa1 (the gene encoding the rat opiorphin homologue, sialorphin), a2br, and Hif-1a. As shown in Fig. 1A, there is a rapid elevation in levels of vcsa1, with a ∼4-fold increase in expression after 30 min, increasing to a 10-fold increase after 8 h. There is also an increase in expression of the a2br gene; however, this appears to have a slower onset, with a significant increase in expression being detected after 2 h (Fig. 1B). Hif-1a expression also increased in hypoxic conditions over the time course of the experiments, reaching a peak of ∼4-fold after 24 h (Fig. 1C). Hif-1a is usually considered to be regulated post-transcriptionally through stabilization in hypoxic conditions (33). Therefore, at the 24 h time point, cells were harvested and protein expression of Hif-1a and A2BR was determined (there are no antibodies available suitable for immunoblotting of sialorphin; Fig. 1D). Using densitometry, we demonstrated that hypoxic conditions resulted in significantly increased expression of both A2BR (Fig. 1E) and Hif-1a (Fig. 1F) proteins.
Figure 1.
Vcsa1, A2BR, and Hif-1a expression is up-regulated in rat CSM cells with hypoxia by a chemical mimetic (CoCl2; A–F) or low oxygen tension (G–L). A–C) Gene expression profiles of vcsa1 (A), a2br (B), and Hif-1a (C) at various time points following incubation of rat CSM cells with 100 μM CoCl2. Nonhypoxic environment controls were not incubated with CoCl2. Three separate experiments were performed, with each gene for a particular experiment being determined in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to the nonhypoxic control as means ± sd. *P < 0.05, **P < 0.01 vs. control. D) Representative immunoblot to analyze protein expression of Hif-1a and A2BR in rat CSM cells with or without 100 μM CoCl2 incubation for 24 h. E, F) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a, A2BR, and GAPDH. Expression of Hif-1a and A2BR were normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of A2BR (E) and Hif-1a (F) caused by hypoxia relative to nonhypoxic controls. *P < 0.05 vs. control. G–I) Gene expression profiles of vcsa1 (E), a2br (F), and Hif-1a (G) at various time points following incubation of rat CSM cells in low oxygen tension (1% O2, 5% CO2, and 94% N2) in a 95% humidified atmosphere. Control cells were kept under normoxic conditions (20% O2). Three separate experiments were performed, with each gene for a particular experiment being determined in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to nonhypoxic control as means ± sd. *P < 0.05, **P < 0.01 vs. control. J) Representative immunoblot to analyze protein expression of Hif-1a and A2BR in rat CSM cells incubated for 24 h in low oxygen tension or normoxic conditions. K, L) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a, A2BR, and GAPDH. Expression of Hif-1a and A2BR was normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of A2BR (K) and Hif-1a (L) caused by hypoxia relative to nonhypoxic controls. *P < 0.05 vs. control.
Although CoCl2 treatment of cells remains a commonly employed chemical mimetic of hypoxia, its use has been associated with redox effects and other nonhypoxic influences. It has been suggested that some of the effects of CoCl2 may be mediated by signaling pathways not necessarily shared by the “true” hypoxic response and may cause, therefore, different and oxygen-independent biological effects (34–36). Several articles have demonstrated that there are differences in gene expression and metabolism between cells treated with CoCl2 and hypoxia (37, 38). Therefore, we repeated the experiments using low oxygen tension as a hypoxia mimetic. As shown in Fig. 1G, as with CoCl2 treatment, there is a rapid elevation in levels of vcsa1, reaching a ∼3-fold increase in expression after 2 h. This is followed by a slower increase in the expression of the a2br gene, with an increase in expression of ∼4-fold at 12 h (Fig. 1H). Significant increases in Hif-1a expression were observed at 4 h, earlier in the low oxygen tension model of hypoxia than CoCl2 (Fig. 1I). Changes in protein expression of Hif-1a and A2BR were similar between the CoCl2 and low oxygen tension methods of mimicking hypoxia. Using Western blot densitometry (Fig. 1J), we demonstrated that low oxygen tension hypoxia resulted in significantly increased (∼2-fold) expression of both A2BR (Fig. 1K) and Hif-1a (Fig. 1L) proteins.
Although the change in gene expression in CSM cells is qualitatively similar with both types of hypoxia mimetics (i.e., there is elevation of vcsa1, a2br, and Hif-1a), there is earlier significant up-regulation of vcsa1 and Hif-1a gene expression with low oxygen tension. The sequence in which these genes become up-regulated with low oxygen tension is vcsa1, Hif-1a, and a2br.
Sialorphin regulates expression of Hif-1a and A2BR in a dose- and time-dependent manner
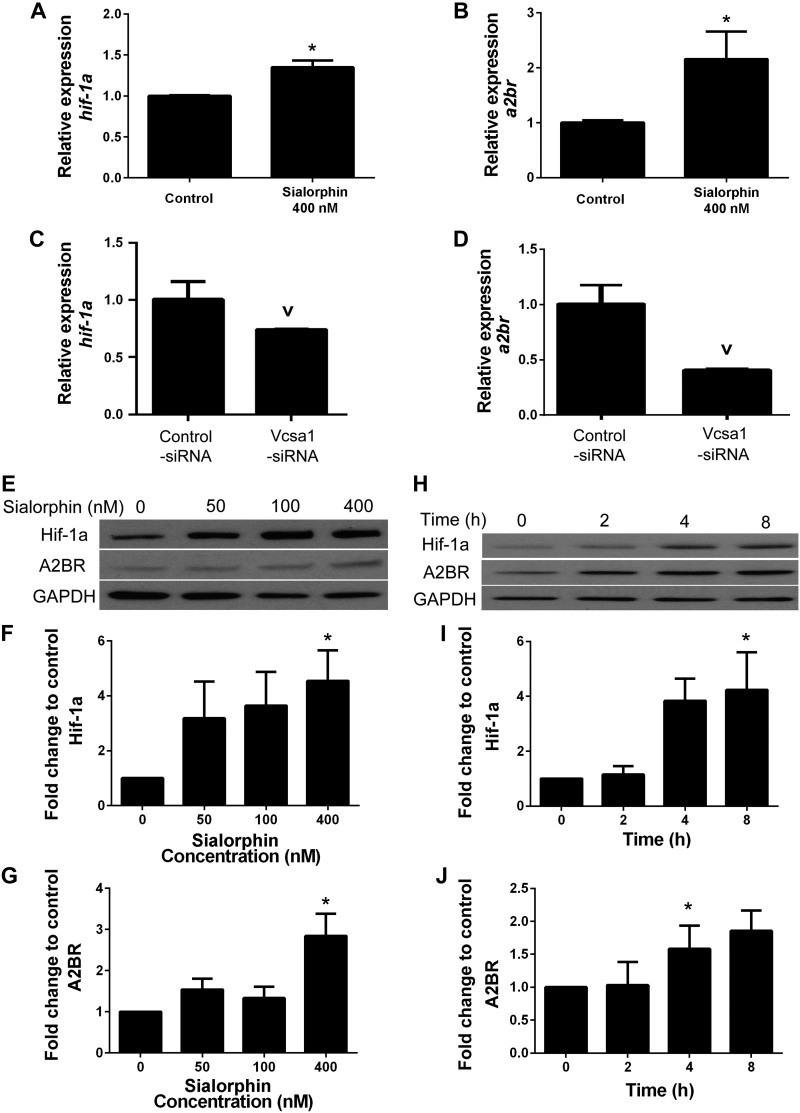
We noted that in the in vitro hypoxia model, the increase in vcsa1 expression coincided with or preceded that of a2br and Hif-1a. Therefore, we investigated whether sialorphin may itself regulate expression of these genes. CSM cells were incubated in the presence of 400 nM sialorphin, and the gene expression of Hif-1a and a2br (Fig. 2A, B) was determined after 8 h. There was a significant increase in the level of transcription of both Hif-1a and a2br (Fig. 2A, B, respectively). To further demonstrate the dependence of Hif-1a and a2br expression on sialorphin, we performed gene-knockdown experiments using siRNA against vcsa1 (vcsa1-siRNA) in the CSM cells, which have been reported to have high levels of endogenous vcsa1 expression compared to other cell types (15–18). Treating CSM cells with vcsa1-siRNA caused a significant decrease of ∼50% Hif-1a and a2br expression (Fig. 2C, D). We then performed dose-dependent (Fig. 2E) and time-dependent (Fig. 2H) studies on the effect of sialorphin on expression of Hif-1a and A2BR proteins. There was a dose-dependent increase in Hif-1a expression (∼4-fold with 400 nm sialorphin after an 8 h incubation; Fig. 2F). Incubation of cells with 400 nM sialorphin also caused a time-dependent increase in Hif-1a (Fig. 2I). Similarly, incubation of CSM with sialorphin caused a dose-dependent (Fig. 2G) and time-dependent (Fig. 2J) increase in A2BR expression. We determined that sialorphin does not change the expression if Hif-2 (data not shown).
Figure 2.
Sialorphin regulates expression of HIF-1a and A2BR in a dose- and time-dependent manner. A, B) Gene expression of Hif-1a (A) and a2br (B) in rat CSM cells with or without (control) 400 nM sialorphin for 8 h. Three separate experiments were performed, with each gene for a particular experiment being determined in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to untreated control. Bars represent means ± sd. *P < 0.05 significantly increased fold-change in gene expression compared to untreated control. C, D) Gene expression of Hif-1a (C) and a2br (D) in rat CSM cells treated with vcsa1-siRNA or nonspecific control siRNA for 30 h. Three separate experiments were performed, with each gene for a particular experiment being determined in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to the untreated control. Bars represent means ± sd. vP < 0.05 vs. control. E) Immunoblot to analyze protein expression of Hif-1a and A2BR in rat CSM after 8 h incubation with increasing concentrations of sialorphin. F, G) Densitometry analysis of ratio of Hif-1a (F) and A2BR (G) to GAPDH protein expression with increasing sialorphin concentration. Densitometric analysis of 3 separate experiments to determine expression of Hif-1a, A2BR, and GAPDH. Expression of Hif-1a and A2BR were normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of Hif-1a (F) and A2BR (G) caused by increasing sialorphin concentration relative to untreated controls. *P < 0.05 vs. control. H) Immunoblot to analyze time-dependent protein expression of Hif-1a and A2BR in rat corporal cells with 400 nM sialorphin. I, J) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a, A2BR, and GAPDH. Expression of Hif-1a and A2BR was normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of Hif-1a (I) and A2BR (J) at different time points compared to protein expression at baseline (T=0). *P < 0.05 vs. baseline.
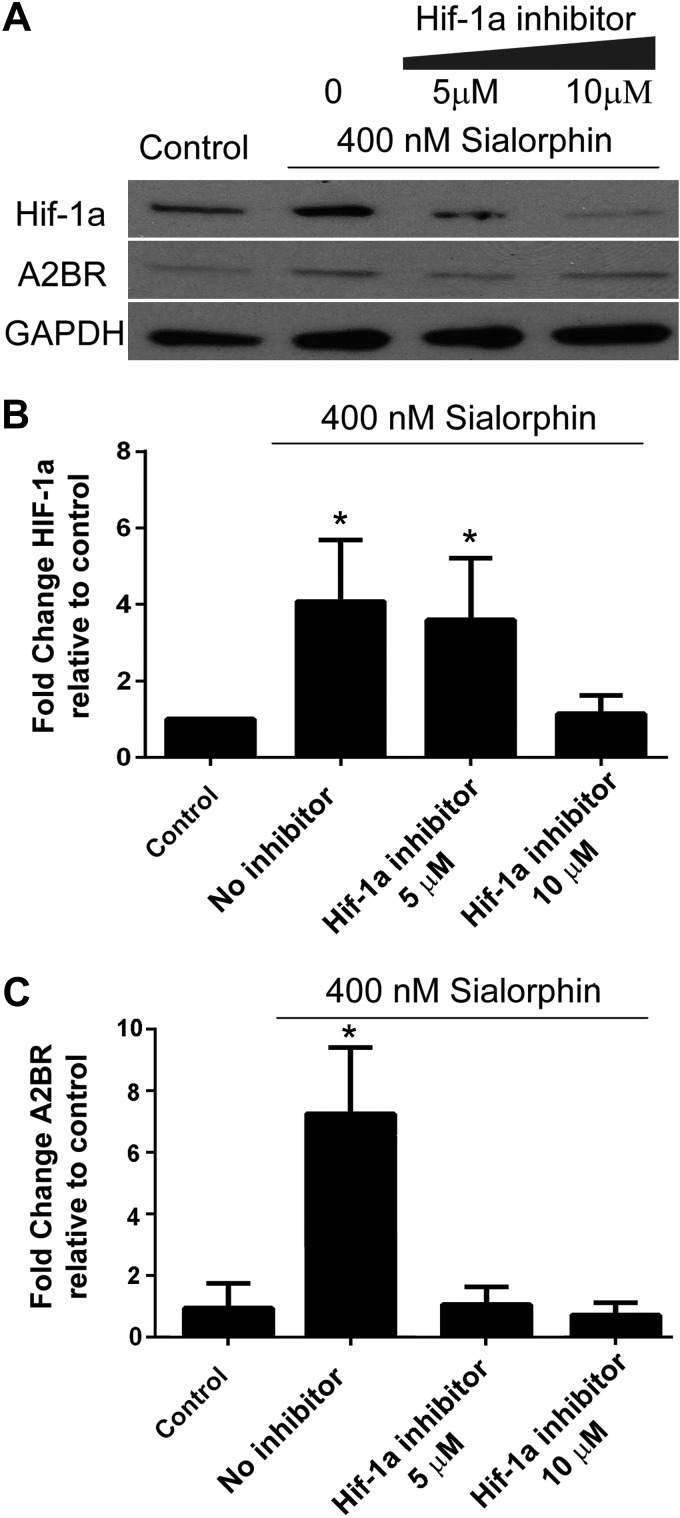
Sialorphin activation of A2BR is dependent on activation of Hif-1a
The promoter region for a2br has a Hif-1a response element (39), and therefore a2br activation could be through sialorphin-mediated up-regulation of Hif-1a; alternatively, sialorphin could independently activate A2BR and Hif-1a expression. To discern these two possibilities, the sialorphin activation of A2BR was investigated in the presence of increasing concentrations of a Hif-1a inhibitor (Fig. 3A). Increasing concentrations of the Hif-1a inhibitor in the presence of sialorphin caused a decrease in the expression of Hif-1a (Fig. 3B), which corresponded to a decrease in the expression of A2BR (Fig. 3C). These data demonstrate that in CSM cells, the up-regulation of A2BR by sialorphin is mediated through Hif-1a.
Figure 3.
Activation of A2BR by sialorphin is dependent on Hif-1a activation. A) Representative immunoblot to determine protein expression of Hif-1a, A2BR, and GAPDH in rat CSM cells with 400 nM sialorphin alone, or in combination with Hif-1a inhibitor for 8 h. B, C) Densitometric analysis of 3 separate experiments to determine expression of A2BR and GAPDH from 6 separate experiments to determine expression of Hif-1a. Expression of Hif-1a and A2BR were normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of Hif-1a (B) and A2BR (C) caused by increasing Hif-1a inhibitor concentration relative to untreated controls (CSM cells without sialorphin or Hif-1a inhibitor treatment). *P < 0.05 vs. controls.
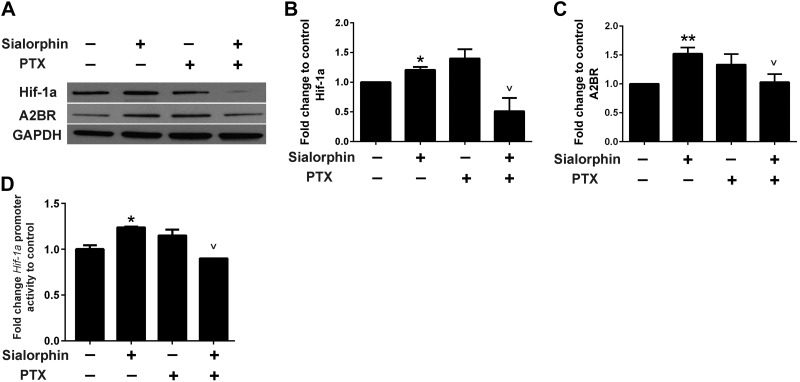
Sialorphin activates Hif-1a expression though a G-protein coupled receptor (GPCR) mechanism
As an NEP inhibitor, sialorphin can potentially affect peptide-dependent signaling mediated through GPCRs. We have also previously shown that sialorphin can modulate the transcription of several types of GPCR (28). Therefore, using PTX, which inhibits the activity of GPCRs by preventing the αi/o subunits of the heterotrimeric G proteins from associating with GPCR, we determined whether sialorphin activation of Hif-1a was dependent on GPCR activation (Fig. 4A). PTX itself did not show any significant effect on the expression of either Hif-1a or A2BR, though there was a tendency for it to increase expression of both these proteins. However, PTX prevents sialorphin activation of Hif-1and A2BR (Fig. 4B, C, respectively), suggesting that activation of these proteins is mediated through GPCR. We also investigated the ability of sialorphin to directly activate the Hif-1a promoter, by using a Hif-1a promoter driving expression of a luciferase reporter gene (Fig. 4D). Using this reporter construct, we demonstrated that sialorphin can activate the Hif-1a promoter, confirming the results in Fig. 2A that sialorphin can regulate Hif-1a transcription. However, PTX prevented this activation, confirming the involvement in GPCR in sialorphin activation of the Hif-1a promoter.
Figure 4.
Sialorphin activates Hif-1a though a GPCR pathway. A) Representative blot showing protein expression of Hif-1a and A2BR in rat corporal cells treated with sialorphin alone, PTX, or a combination of sialorphin and PTX. B, C) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a, A2BR, and GAPDH. Expression of Hif-1a and A2BR were normalized to the housekeeping gene GAPDH. Data represent mean ± sd fold change in expression of Hif-1a (B) and A2BR (C) normalized to untreated controls are. *P < 0.05 vs controls; vP < 0.05 vs. sialorphin alone. D) Hif-1a luciferase reporter assay. Confluent CSM cells were transiently transfected with a reporter construct in which the Hif-1a promoter drives expression of luciferase. After 24 h, transfected cells were treated with 400 nM sialorphin alone, 200 ng/ml PTX alone, or a combination of sialorphin and PTX for 8 h. Cells were lysed, and luciferase activity was normalized to protein. Results are expressed as fold change in luciferase activity compared to activity in the untreated control. Data are expressed as means ± sd. *P < 0.05 vs. controls; vP < 0.05 vs. sialorphin alone.
Hypoxic up-regulation of Hif-1a is dependent on sialorphin
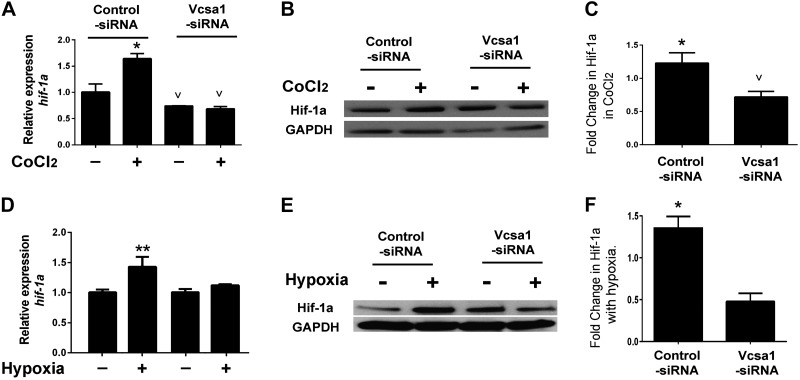
Given the observation that sialorphin regulates expression of Hif-1a in CSM, cells, we investigated whether the up-regulation of Hif-1a by hypoxia was mediated through sialorphin (Fig. 5). We repeated the treatment of cultured rat CSM cells with CoCl2; however, one group of cells was treated with vcsa1-siRNA for 48 h prior to hypoxic conditions. Controls were treated with control siRNA. As shown in Fig. 5A, control cells treated with CoCl2 exhibited elevated transcription of Hif-1a, whereas cells treated with vcsa1-siRNA hypoxia no longer elevated Hif-1a transcription. A similar effect was seen at the protein level (Fig. 5B, C); treating CSM cells with vcsa1-siRNA prevented the up-regulation of Hif-1a protein by CoCl2.
Figure 5.
Up-regulation of Hif-1a expression by hypoxia is dependent on sialorphin. CSM cells were pretreated for 48 h with control-siRNA or vcsa1-siRNA, and cells were then exposed for 8 h to CoCl2 (A–C) or low oxygen tension (D–E) to mimic hypoxia. A) Gene expression of Hif-1a and rpl24 was determined in the presence or absence of CoCl2, in CSM cells pretreated with control-siRNA or vcsa1-siRNA. Three separate experiments were performed, with each gene being measured in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to nonhypoxic CSM cells treated with control-siRNA. Data are expressed as means ± sd. Note that in the vcsa1-siRNA treated cells, there was no significant difference between cells maintained under hypoxic and nonhypoxic conditions. *P < 0.05 for increase vs. controls; vP < 0.05 for decrease vs. controls. B) Representative immunoblot for the expression of Hif-1a and GAPDH. C) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a and GAPDH. Expression of Hif-1a was normalized to the housekeeping gene, GAPDH. Data represent mean ± sd fold change in expression of Hif-1a, compared between cells in hypoxic conditions with nonhypoxic cells for each treatment condition. *P < 0.05 for increase vs. controls; vP < 0.05 for decrease vs. controls. D) Gene expression of Hif-1a and rpl24 was determined in CSM cells grown in low oxygen tension (1% O2, 5% CO2, and 94% N2) in a 95% humidified atmosphere or under normoxic conditions (20% O2) pretreated with control-siRNA or vcsa1-siRNA. Three separate experiments were performed, with each gene being measured in triplicate. Data were normalized to the housekeeping gene, rpl24, and expressed relative to nonhypoxic CSM cells treated with control-siRNA. Data are expressed as means ± sd. Note that in the vcsa1-siRNA treated cells, there was no significant difference between cells maintained under hypoxic and nonhypoxic conditions. *P < 0.05 vs. control. E) Representative immunoblot for the expression of Hif-1a and GAPDH. F) Densitometric analysis of 3 separate experiments to determine expression of Hif-1a and GAPDH. Expression of Hif-1a was normalized to the housekeeping gene, GAPDH. Data represent mean ± sd fold change in expression of Hif-1a, compared between cells in hypoxic conditions with nonhypoxic cells for each treatment condition. *P < 0.05 vs. controls.
We repeated these experiments using low oxygen tension as the mimetic of hypoxia. As shown in Fig. 5D, control cells incubated in low oxygen tension exhibited elevated transcription of Hif-1a, whereas cells previously treated with vcsa1-siRNA hypoxia no longer elevated Hif-1a transcription. A similar effect was seen at the protein level (Fig. 5E, F); treating CSM cells with vcsa1-siRNA prevented the up-regulation of Hif-1a protein in conditions of low oxygen tension.
In SCD mice, there is up-regulation of genes encoding the mouse opiorphin homologues, A2BR and Hif-1a
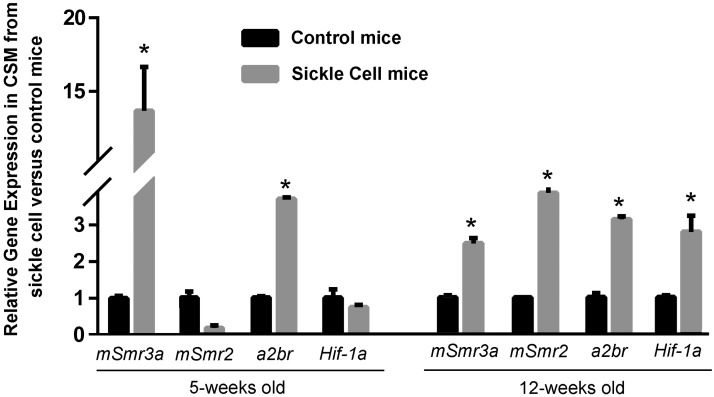
Although the clinical condition of priapism is associated with ischemia/hypoxia (40), the nature of SCD likely results in low oxygen tension in corporal tissue even prior to the onset of priapism (22). We therefore looked at the expression of genes identified as regulated by hypoxia in the in vitro model described above (Fig. 1) to determine whether their expression was also changed in SCD mice at life stages that are prepriapic and priapic to a control. As shown in Fig. 6, we compared gene expression of the mouse opiorphin homologues (mSmr3a and mSmr2), a2br, and Hif-1a in the corpora of SCD mice to controls at a life stage prior to the exhibition of any physiological evidence of priapism. There was up-regulation of mSmr3a and a2br in the corporal tissue of a 5-wk-old SCD animal compared to a control, and in a 12-wk-old animal, there was up-regulation of mSmr3a, mSmr2, a2br, and Hif-1a. These are life stages of the mice prior to any physiological evidence of priapism and therefore the changes in gene expression are unlikely due to priapism itself, but rather due to the hypoxic conditions resulting from SCD.
Figure 6.
Up-regulation of genes encoding the mouse opiorphin homologues A2BR and HIF-1a in corporal tissue of SCD mice prior to physiological evidence of priapism. Expression profiles of mSmr3a, mSmr2, a2br, and Hif-1a in corporal tissue in control and SCD mice at the age of 5 and 12 wk, normalized to the housekeeping gene rpl19. Bars represent mean change in gene expression in SCD mice compared to controls; 5 animals/age group. *P < 0.05 vs. control.
DISCUSSION
We demonstrate that in rat CSM cells, there is transcriptional regulation of Hif-1a and a2br by the rat opiorphin homologue, sialorphin. We show that the up-regulation of a2br by sialorphin is dependent on Hif-1a activation and is mediated through GPCR activity. Under hypoxic conditions, mimicked by both CoCl2 treatment and low oxygen tension, there is up-regulation of vcsa1 (the gene encoding sialorphin), Hif-1a, and a2br. The up-regulation of Hif-1a transcription in CSM cells by hypoxic conditions is mediated by vcsa1. When we looked at gene expression in the corporal tissue of SCD mice, we observed that the mouse opiorphin homologue mSmr3a was highly up-regulated at an age (5 wk) at which we have previously demonstrated there is no physiological evidence of priapism (9). These observations lead us to propose the following hypothesis for the development of priapism as a result of SCD, depicted in Fig. 7. SCD, which is considered a disease of hypoxia (22) causes increased expression of opiorphin in corporal tissue. Opiorphin then acts as a master regulator of compensatory smooth muscle relaxant pathways aimed at increasing blood flow, and thereby oxygen supply, to corporal tissue. There are examples in other systems, such as the microcirculation of the retina or vascular system, where low oxygen tension activates compensatory mechanisms to increase blood flow (41, 42). However, the molecular response mechanisms to hypoxia may be unique in corporal tissue because of the higher levels of opiorphins expressed in this tissue. Previously, we have demonstrated that opiorphins can activate genes involved in smooth muscle relaxation (such as the ornithine decarboxylase pathway and NOS; ref. 9), and our work presented here demonstrates that they also can regulate expression of Hif-1a and a2br. It is the excessive activation of these relaxant pathways that results in priapism. In many aspects, this hypothesis connects the 60-yr-old hypothesis that ischemia associated with SCD is a mechanism for the development of vascular diseases (2) to the more recent studies in which activation of relaxant pathways in corporal tissue are considered a primary mechanism for the development of priapism (5).
Figure 7.
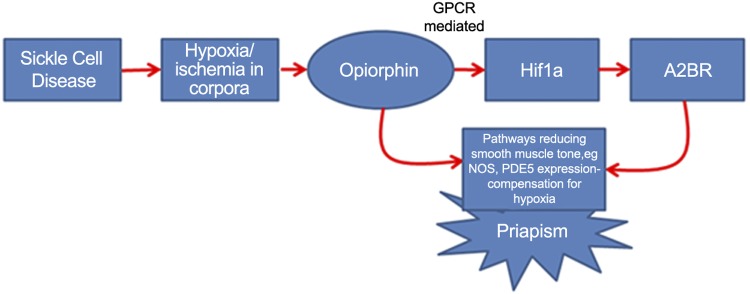
Hypothesis generated from our study on the role of opiorphins in the development of priapism as a result of SCD. SCD causes hypoxia in corporal tissue, which up-regulates opiorphin expression. Increased opiorphin expression activates pathways involved in smooth muscle relaxation. Excessive up-regulation of these pathways results in excessive blood flow into the penis, causing priapism.
The transcriptional regulation of Hif-1a by opiorphin in CSM cells is a novel finding. Hif-1a is generally considered as a key regulator responsible for the induction of a broad range of cellular and systemic responses to hypoxia, and >100 Hif-1a-regulated genes have been identified (43). Although it is generally considered that Hif-1a is regulated at the post-translational level (44), nonhypoxic stimuli, such as lipopolysaccharides, thrombin, and angiotensin II (Ang II), have been proven to enhance Hif-1a transcription (44). The biochemical mechanism by which opiorphins regulate Hif-1a remains to be determined, however, our preliminary data presented in Fig. 4 suggests that one potential mechanism involves activation of GPCRs. The opiorphins are potent endogenous NEP inhibitors (45), which potentially exert their effect through modulating the activity of signaling peptides bound at the GPCR. Given that the major biochemical activity of opiorphins is inhibition of NEP, in Fig. 4 we determined whether this biochemical activity of sialorphin might be a potential mechanism by which opiorphins regulate Hif-1a expression. PTX prevents the G proteins from interacting with GPCR, and we used this as a tool to demonstrate that opiorphin activation of Hif-1a expression is mediated through GPCR. Interestingly, previous studies have shown that when the expression of vcsa1 was knocked down in rat CSM cells, microarray analysis revealed modulated expression of GPCRs as an ontological group (28). Among these receptors, the Ang II receptor was among the greatest fold-change in gene expression. It has been demonstrated that stimulation of vascular smooth muscle cells with Ang II strongly increases Hif-1a gene expression (46) a potential mechanism for the activation of Hif-1a by opiorphins may be through modulation of Ang II receptor activity, stabilizing the activity of Ang II bound to its receptor.
Our results demonstrate that sialorphin activation of a2br expression in CSM cells is mediated through Hif-1a, confirming previous reports that the promoter region for a2br has a Hif-1a response element (39). Recently, activation of adenosine signaling pathways has been identified as being associated with the development of priapism (8, 10). A priapic phenotype was identified in ADA-knockout (ada−/−), mice and high levels of adenosine caused prolonged CSM relaxation in vitro. In SCD mouse models, corporal tissue exhibited elevated A2BR expression and adenosine levels (10). Our results would suggest that in SCD mice, the up-regulation of a2br in the corporal tissue is mediated through hypoxic up-regulation of Hif-1a by sialorphin.
The mechanism described here, where hypoxic up-regulation of sialorphin results in transcriptional activation of Hif-1a and A2BR, may be restricted to CSM tissue, which under normal physiological conditions expresses high levels of vcsa1 relative to other tissues (15–18). However, we have previously demonstrated that changes in expression of vcsa1 in rat corporal tissue are reflected by circulating levels of sialorphin in the blood (47). We have demonstrated that gene transfer of vectors into rat corporal tissue can increase sialorphin levels in the blood of diabetic rats, and the increased levels of sialorphin in blood are associated with reduced systemic blood pressure. Therefore, it is possible that up-regulation of vcsa1 expression in corporal tissue caused by hypoxia associated with SCD may affect circulating levels of opiorphins, resulting in systemic effects on biochemistry and physiology. Definitive proof of the involvement of opiorphins in the development of priapism awaits the development of mouse opiorphin gene-knockout/knockdown models. However, the development of these models will likely be complicated by the presence of multiple homologues of the vcsa1 gene in mice with overlapping biochemical functions.
Our results suggesting that opiorphin expression is elevated in CSM cells in response to hypoxia, and then acts a master regulator of pathways leading to activation of smooth muscle cell relaxant pathways, has several potential clinical translational applications. Since SCD results in systemic hypoxia, the hypoxic activation of opiorphin expression, and the downstream pathways that opiorphins regulate, may serve as potential targets for preventing and treating priapism in patients with SCD. One approach might be to use siRNA technology to lower opiorphin expression in corporal tissue of patients with SCD. Clinical trials have been conducted using intracorporeal gene transfer of vectors expressing potassium channels to treat erectile dysfunction (48, 49), and we demonstrate here the use of vcsa1-siRNA to modulate the hypoxic response in CSM cells. Potentially, these technologies could be combined to generate vectors that reduce opiorphin expression in corporal tissue. An alternative approach would be to use knowledge concerning the factors regulating opiorphin expression. For example, opiorphin expression is regulated by testosterone (50), and, potentially, treatments lowering testosterone levels, and thereby opiorphin levels, could prevent priapism. Indeed, there is some evidence that ketoconazole and prednisone, which reduce circulating testosterone levels, can help prevent recurrent ischemic priapism (51), and, potentially, some of their physiological activity could be mediated through decreased opiorphin expression. In addition, as described above, opiorphin can be detected in the bloodstream and saliva of patients (52), and in rats, levels of vcsa1 expression in corporal tissue are reflected by sialorphin levels in the blood (47). Therefore, opiorphin levels in the blood are potentially a useful biomarker for SCD-related priapic crisis. At present, there is no method for the identification of patients at risk of developing priapism; assay of opiorphins levels in the blood of patients with SCD may be a predictive assay for a priapic crisis and could be a basis to initiate preventive strategies.
Acknowledgments
This work was supported by U.S. National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK087872 to K.P.D.
Footnotes
- A2BR
- A2B adenosine receptor
- ADA
- adenosine deaminase
- Ang II
- angiotensin II
- CSM
- corporal smooth muscle
- GPCR
- G-protein coupled receptor
- Hif-1a
- hypoxia-inducible factor 1a
- NEP
- neutral endopeptidase
- NO
- nitric oxide
- PBS
- phosphate-buffered saline
- ProL1
- proline-rich lacrimal 1
- mSmr2
- mouse submaxillary gland androgen-regulated protein 2
- mSmr3a
- mouse submaxillary gland androgen-regulated protein 3A
- PTX
- pertussis toxin
- SCD
- sickle cell disease
- Vcsa1
- variable coding sequence a1
REFERENCES
- 1. Emond A. M., Holman R., Hayes R. J., Serjeant G. R. (1980) Priapism and impotence in homozygous sickle cell disease. Arch. Intern. Med. 140, 1434–1437 [PubMed] [Google Scholar]
- 2. Kimmelsteil P. (1948) Vascular occlusion and ischemic infarction in sickle cell disease. Am. J. Med. Sci. 216, 11–19 [PubMed] [Google Scholar]
- 3. Yuan J., Desouza R., Westney O. L., Wang R. (2008) Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J. Androl. 10, 88–101 [DOI] [PubMed] [Google Scholar]
- 4. Kato G. J., Hebbel R. P., Steinberg M. H., Gladwin M. T. (2009) Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 84, 618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bivalacqua T. J., Musicki B., Kutlu O., Burnett A. L. (2012) New insights into the pathophysiology of sickle cell disease-associated priapism. J. Sex. Med. 9, 79–87 [DOI] [PubMed] [Google Scholar]
- 6. Burnett A. L., Lowenstein C. J., Bredt D. S., Chang T. S., Snyder S. H. (1992) Nitric oxide: a physiologic mediator of penile erection. Science 257, 401–403 [DOI] [PubMed] [Google Scholar]
- 7. Lagoda G., Sezen S. F., Hurt K. J., Cabrini M. R., Mohanty D. K., Burnett A. L. (2014) Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 28, 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai Y., Zhang Y., Phatarpekar P., Mi T., Zhang H., Blackburn M. R., Xia Y. (2009) Adenosine signaling, priapism and novel therapies. J. Sex. Med. 6(Suppl. 3), 292–301 [DOI] [PubMed] [Google Scholar]
- 9. Kanika N. D., Tar M., Tong Y., Kuppam D. S., Melman A., Davies K. P. (2009) The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am. J. Physiol. Cell Physiol. 297, C916–C927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mi T., Abbasi S., Zhang H., Uray K., Chunn J. L., Xia L. W., Molina J. G., Weisbrodt N. W., Kellems R. E., Blackburn M. R., Xia Y. (2008) Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J. Clin. Invest. 118, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen J., Jiang X., Dai Y., Zhang Y., Tang Y., Sun H., Mi T., Kellems R. E., Blackburn M. R., Xia Y. (2010) Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J. Sex. Med. 9, 3011–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wisner A., Dufour E., Messaoudi M., Nejdi A., Marcel A., Ungeheuer M. N., Rougeot C. (2006) Human opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. U. S. A. 103, 17979–17984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rougeot C., Messaoudi M., Hermitte V., Rigault A. G., Blisnick T., Dugave C., Desor D., Rougeon F. (2003) Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc. Natl. Acad. Sci. U. S. A. 100, 8549–8554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rougeot C., Rosinski-Chupin I., Njamkepo E., Rougeon F. (1994) Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur. J. Biochem. 219, 765–773 [DOI] [PubMed] [Google Scholar]
- 15. Mathison R. D., Davison J. S., St Laurent C. D., Befus A. D. (2012) Autonomic regulation of anti-inflammatory activities from salivary glands. Chem. Immunol. Allergy 98, 176–195 [DOI] [PubMed] [Google Scholar]
- 16. Rosinski-Chupin I., Rougeot C., Courty Y., Rougeon F. (1993) Localization of mRNAs of two androgen-dependent proteins, SMR1 and SMR2, by in situ hybridization reveals sexual differences in acinar cells of rat submandibular gland. J. Histochem. Cytochem. 41, 1645–1649 [DOI] [PubMed] [Google Scholar]
- 17. Rougeot C., Rosinski-Chupin I., Mathison R., Rougeon F. (2000) Rodent submandibular gland peptide hormones and other biologically active peptides. Peptides 21, 443–455 [DOI] [PubMed] [Google Scholar]
- 18. Davies K. P., Tong Y., Tar M., Lowe D., Melman A. (2006) Vcsa1 (SMR1) as a molecular marker for treatment of erectile dysfunction. J. Sex. Med. 3, 414 [Google Scholar]
- 19. Tronik-Le Roux D., Senorale-Pose M., Rougeon F. (1994) Three novel SMR1-related cDNAs characterized in the submaxillary gland of mice show extensive evolutionary divergence in the protein coding region. Gene 142, 175–182 [DOI] [PubMed] [Google Scholar]
- 20. Davies K. P., Tar M., Rougeot C., Melman A. (2007) Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 99, 431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanika N. D., Tar M., Tong Y., Kuppam D. S., Melman A., Davies K. P. (2009) The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am. J. Physiol. Cell Physiol. 297, C916–C927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun K., Xia Y. (2013) New insights into sickle cell disease: a disease of hypoxia. Curr. Opin. Hematol. 20, 215–221 [DOI] [PubMed] [Google Scholar]
- 23. Lee K., Kang J. E., Park S. K., Jin Y., Chung K. S., Kim H. M., Lee K., Kang M. R., Lee M. K., Song K. B., Yang E. G., Lee J. J., Won M. (2010) LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 80, 982–989 [DOI] [PubMed] [Google Scholar]
- 24. Lee K., Lee J. H., Boovanahalli S. K., Jin Y., Lee M., Jin X., Kim J. H., Hong Y. S., Lee J. J. (2007) (Aryloxyacetylamino)benzoic acid analogues: a new class of hypoxia-inducible factor-1 inhibitors. J. Med. Chem. 50, 1675–1684 [DOI] [PubMed] [Google Scholar]
- 25. Yang Y. F., Chen Z., Hu S. L., Hu J., Li B., Li J. T., Wei L. J., Qian Z. M., Lin J. K., Feng H., Zhu G. (2011) Interleukin-1 receptor associated kinases-1/4 inhibition protects against acute hypoxia/ischemia-induced neuronal injury in vivo and in vitro. Neuroscience 196, 25–34 [DOI] [PubMed] [Google Scholar]
- 26. Goldberg M. A., Schneider T. J. (1994) Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J. Biol. Chem. 269, 4355–4359 [PubMed] [Google Scholar]
- 27. Roemeling-van Rhijn M., Mensah F. K., Korevaar S. S., Leijs M. J., van Osch G. J., Ijzermans J. N., Betjes M. G., Baan C. C., Weimar W., Hoogduijn M. J. (2013) Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front. Immunol. 4, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tong Y., Tiplitsky S. I., Tar M., Melman A., Davies K. P. (2008) Transcription of G-protein coupled receptors in corporeal smooth muscle is regulated by the endogenous neutral endopeptidase inhibitor sialorphin. J. Urol. 180, 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sengupta T., Abraham G., Xu Y., Clurman B. E., Minella A. C. (2011) Hypoxia-inducible factor 1 is activated by dysregulated cyclin E during mammary epithelial morphogenesis. Mol. Cell. Biol. 31, 3885–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldberg M. A., Glass G. A., Cunningham J. M., Bunn H. F. (1987) The regulated expression of erythropoietin by two human hepatoma cell lines. Proc. Natl. Acad. Sci. U. S. A. 84, 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y., Du K. M., Xue Z. H., Yan H., Li D., Liu W., Chen Z., Zhao Q., Tong J. H., Zhu Y. S., Chen G. Q. (2003) Cobalt chloride and low oxygen tension trigger differentiation of acute myeloid leukemic cells: possible mediation of hypoxia-inducible factor-1alpha. Leukemia 17, 2065–2073 [DOI] [PubMed] [Google Scholar]
- 32. Goldberg M. A., Dunning S. P., Bunn H. F. (1988) Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242, 1412–1415 [DOI] [PubMed] [Google Scholar]
- 33. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 34. Chachami G., Simos G., Hatziefthimiou A., Bonanou S., Molyvdas P. A., Paraskeva E. (2004) Cobalt induces hypoxia-inducible factor-1alpha expression in airway smooth muscle cells by a reactive oxygen species- and PI3K-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 31, 544–551 [DOI] [PubMed] [Google Scholar]
- 35. Triantafyllou A., Liakos P., Tsakalof A., Georgatsou E., Simos G., Bonanou S. (2006) Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radical Res. 40, 847–856 [DOI] [PubMed] [Google Scholar]
- 36. Triantafyllou A., Mylonis I., Simos G., Bonanou S., Tsakalof A. (2008) Flavonoids induce HIF-1alpha but impair its nuclear accumulation and activity. Free Radic. Biol. Med. 44, 657–670 [DOI] [PubMed] [Google Scholar]
- 37. Han Y. H., Xia L., Song L. P., Zheng Y., Chen W. L., Zhang L., Huang Y., Chen G. Q., Wang L. S. (2006) Comparative proteomic analysis of hypoxia-treated and untreated human leukemic U937 cells. Proteomics 6, 3262–3274 [DOI] [PubMed] [Google Scholar]
- 38. Befani C., Mylonis I., Gkotinakou I. M., Georgoulias P., Hu C. J., Simos G., Liakos P. (2013) Cobalt stimulates HIF-1-dependent but inhibits HIF-2-dependent gene expression in liver cancer cells. Int. J. Biochem. Cell Biol. 45, 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kong T., Westerman K. A., Faigle M., Eltzschig H. K., Colgan S. P. (2006) HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 20, 2242–2250 [DOI] [PubMed] [Google Scholar]
- 40. Melman A., Serels S. (2000) Priapism. Int. J. Impot. Res. 12(Suppl. 4), S133–S139 [DOI] [PubMed] [Google Scholar]
- 41. Nagaoka T., Sakamoto T., Mori F., Sato E., Yoshida A. (2002) The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest. Ophthalmol. Vis. Sci. 43, 3037–3044 [PubMed] [Google Scholar]
- 42. Segal S. (1992) Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. News Physiol. Sci. 7, 152–156 [Google Scholar]
- 43. Maxwell P. H. (2005) Hypoxia-inducible factor as a physiological regulator. Exp. Physiol. 90, 791–797 [DOI] [PubMed] [Google Scholar]
- 44. Huang L. E., Gu J., Schau M., Bunn H. F. (1998) Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U. S. A. 95, 7987–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Javelot H., Messaoudi M., Garnier S., Rougeot C. (2010) Human opiorphin is a naturally occurring antidepressant acting selectively on enkephalin-dependent delta-opioid pathways. J. Physiol. Pharmacol. 61, 355–362 [PubMed] [Google Scholar]
- 46. Page E. L., Robitaille G. A., Pouyssegur J., Richard D. E. (2002) Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J. Biol. Chem. 277, 48403–48409 [DOI] [PubMed] [Google Scholar]
- 47. Calenda G., Tong Y., Kanika N. D., Tar M. T., Suadicani S. O., Zhang X., Melman A., Rougeot C., Davies K. P. (2011) Reversal of diabetic vasculopathy in a rat model of type 1 diabetes by opiorphin-related peptides. Am. J. Physiol. Heart Circ. Physiol. 301, H1353–H1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melman A., Davies K. P. (2009) Gene therapy in the management of erectile dysfunction (ED): past, present, and future. ScientificWorldJournal 9, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melman A., Bar-Chama N., McCullough A., Davies K., Christ G. (2006) hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum. Gene Ther. 17, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 50. Chua R. G., Calenda G., Zhang X., Siragusa J., Tong Y., Tar M., Aydin M., DiSanto M. E., Melman A., Davies K. P. (2009) Testosterone regulates erectile function and Vcsa1 expression in the corpora of rats. Mol. Cell. Endocrinol. 303, 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abern M. R., Levine L. A. (2009) Ketoconazole and prednisone to prevent recurrent ischemic priapism. J. Urol. 182, 1401–1406 [DOI] [PubMed] [Google Scholar]
- 52. Dufour E., Villard-Saussine S., Mellon V., Leandri R., Jouannet P., Ungeheuer M. N., Rougeot C. (2013) Opiorphin secretion pattern in healthy volunteers: gender difference and organ specificity. Biochem. Anal. Biochem. 2, 136 [Google Scholar]