Abstract
Fibromuscular dysplasia (FMD) is a rare, nonatherosclerotic arterial disease for which the molecular basis is unknown. We comprehensively studied 47 subjects with FMD, including physical examination, spine magnetic resonance imaging, bone densitometry, and brain magnetic resonance angiography. Inflammatory biomarkers in plasma and transforming growth factor β (TGF-β) cytokines in patient-derived dermal fibroblasts were measured by ELISA. Arterial pathology other than medial fibrodysplasia with multifocal stenosis included cerebral aneurysm, found in 12.8% of subjects. Extra-arterial pathology included low bone density (P<0.001); early onset degenerative spine disease (95.7%); increased incidence of Chiari I malformation (6.4%) and dural ectasia (42.6%); and physical examination findings of a mild connective tissue dysplasia (95.7%). Screening for mutations causing known genetically mediated arteriopathies was unrevealing. We found elevated plasma TGF-β1 (P=0.009), TGF-β2 (P=0.004) and additional inflammatory markers, and increased TGF-β1 (P=0.0009) and TGF-β2 (P=0.0001) secretion in dermal fibroblast cell lines from subjects with FMD compared to age- and gender-matched controls. Detailed phenotyping of patients with FMD allowed us to demonstrate that FMD is a systemic disease with alterations in common with the spectrum of genetic syndromes that involve altered TGF-β signaling and offers TGF-β as a marker of FMD.—Ganesh, S. K., Morissette, R., Xu, Z., Schoenhoff, F., Griswold, B. F., Yang, J., Tong, L., Yang, M.-L., Hunker, K., Sloper, L., Kuo, S., Raza, R., Milewicz, D. M., Francomano, C. A., Dietz, H. C., Van Eyk, J., McDonnell, N. B. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features.
Keywords: biomarker, FMD, TGF-β, pathway aneurysm, human genetics
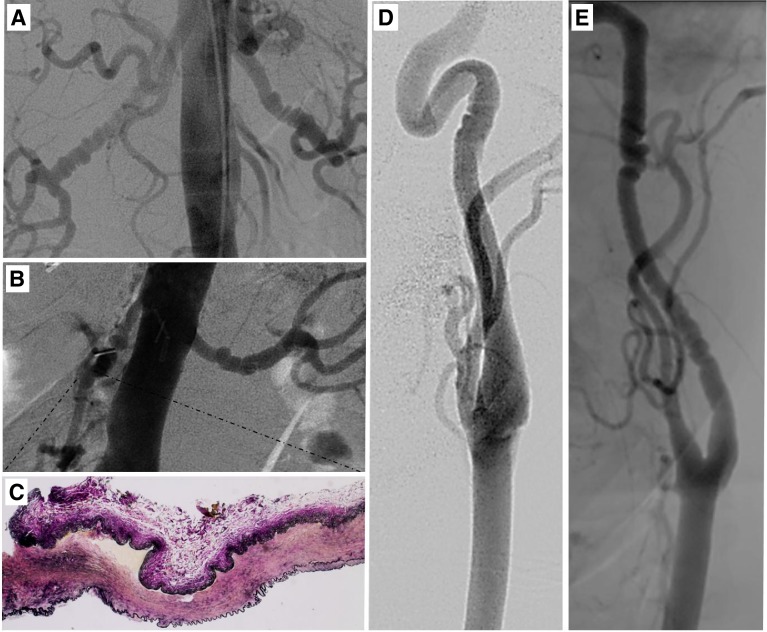
Fibromuscular dysplasia (FMD) is a rare, nonatherosclerotic vascular disease, originally described in 1938 (1), characterized by arterial dysplasia and obstruction to arterial blood flow by neointimal lesions rich with cells with a smooth muscle phenotype (2). It most commonly affects the renal arteries (presenting as hypertension) and internal carotid arteries (presenting as stroke or transient ischemic attack) but has been described in virtually all arterial beds. Hyperplasia of the intimal, medial, or adventitial layers of the arterial wall leads to distinct FMD forms (3). Medial fibroplasia FMD accounts for ∼85% of cases, (3, 4) and is diagnosed on imaging due to a characteristic “string of beads” appearance of alternating arterial stenosis and aneurysmal dilation (Fig. 1).
Figure 1.
FMD angiographic phenotype and histopathology. A, B, D, E) Each subject in the study had a clinical FMD diagnosis on the basis of invasive or noninvasive angiography. Representative images from 4 subjects are shown with classic FMD changes consistent with medial fibroplasia. A, B) Bilateral renal disease is shown in two different subjects. C) Histopathology shows fibrous dysplasia of the excised segment of the renal artery from the subject in panel B, with elastin stained black, at ×4 view. D, E) Carotid artery FMD is shown, with multifocal stenosis seen in both images, but one focus of disease in panel D and two regions of disease in panel E.
Virtually no new information on FMD pertaining to mechanisms of disease has been published in the last 4 decades (5). Currently there is no medical therapy aimed at preventing progression or treating the underlying vascular pathology. Furthermore, there are no biomarkers or defined clinical features to identify who is at risk or who has the disorder. The current hypothesis of the pathophysiology of FMD is that mechanical factors or local trauma incite aberrant vascular remodeling in genetically predisposed individuals. Histopathologic studies show that medial fibroplasia-type FMD demonstrates lesions comprised of smooth muscle cell hyperplasia in the narrowed portions of arteries, haphazard fibroblast organization, and accumulation of compact fibrous collagen (6, 7). A paucity of inflammatory cells has been noted in FMD lesions, although whether the arterial remodeling is a sequelae of long-term inflammation or excess cytokine expression as seen in other fibrotic diseases is unknown (6, 8). Both sporadic and familial cases have been reported. Limited pedigree studies show an autosomal dominant inheritance pattern with incomplete penetrance and variable expression (9). Lesions with similar radiological appearance have been noted in genetically mediated syndromes; however, no causative genes for FMD have been identified to date (10–13).
We undertook a deep phenotyping study of 47 individuals with FMD to evaluate relevant genetic, clinical, and anatomic features of this vascular disease.
MATERIALS AND METHODS
Study subjects
Individuals with a primary clinical diagnosis of FMD made at an outside clinical center and who contacted the U.S. National Institutes of Health, National Institute on Aging (NIH/NIA) study center were enrolled and included in the current analysis on confirmation of the presence of medial fibroplasia-type FMD as demonstrated by arterial beading or multifocal stenosis (14, 15) on an outside angiographic imaging study (conventional angiogram, computerized tomography, or magnetic resonance angiography) done as part of each subject's routine clinical care prior to enrollment in the study. The study protocol was approved by the MedStar Health and NIA (protocol 2003-086) Institutional Review Board. Each subject provided informed consent. Study participants were enrolled between 2005 and 2012 and underwent a comprehensive history and physical examination, full spine MRI, and a bone densitometry scan. Skin biopsies were performed on patients with FMD and submitted to the Coriell Repository (Camden, NJ, USA; http://ccr.coriell.org) to derive individual dermal fibroblast cell lines. Deidentified apparently healthy dermal fibroblasts were obtained from the Coriell repository (http://ccr.coriell.org). Age- and gender-matched healthy controls for plasma analysis and bone densitometry were obtained from the Baltimore Longitudinal Study of Aging (BLSA), a community-based longitudinal study with >50 yr of follow-up (16). Control subjects for all analyses were matched to FMD case subjects for age (within 5 yr) and gender. Dermal fibroblast derivation methods and cell culture passage number were equivalent in the FMD case subject and control samples, and fibroblasts were analyzed at the same passage number. Plasma samples from individuals with Marfan syndrome (MFS) were obtained through the same NIH protocol (2003-086) and analyzed as an additional comparison group.
Clinical examination
A physical examination was performed by a clinical geneticist (N.B.M.) and included auscultation for arterial bruits, skin examination, and objective joint hypermobility measures using the Beighton scale with the following maneuvers: passive dorsiflexion of the little fingers beyond 90°, 1 point for each hand; passive apposition of the thumbs to the flexor aspect of the forearm, 1 point for each hand; hyperextension of the elbows beyond 10°, 1 point for each elbow; hyperextension of the knees beyond 10°, 1 point for each knee; and forward flexion of the trunk with knees fully extended so the palms of the hand rest flat on the floor, 1 point. Other clinical features of hereditary connective tissue disorders were assessed utilizing established clinical criteria for cardinal features of each disorder. Medical and family history was noted for each subject.
Spine MRI
MRI studies were completed on an Intera Philips 1.5 T magnet with software version 11.1.4.4 (Philips, Amsterdam, The Netherlands). All images were read by a single clinical radiologist (R.R.) in a blinded procedure. In the spine MRI interpretation, we utilized the narrow, but widely accepted, definition of Chiari I malformation as tonsillar herniation of >5 mm below the foramen magnum (17). Tonsillar ectopia was defined as tonsillar descent of 0 to 4 mm below the foramen magnum.
Bone densitometry scanning
Dual-energy X-ray absorptiometry (DEXA) studies on FMD case subjects and BLSA controls were performed on a GE Lunar Prodigy Advance scanner (GE Healthcare, Piscataway, NJ, USA) at the same location for all individuals. Population-specific z scores were calculated similarly for FMD case subjects and healthy controls.
Magnetic resonance angiography (MRA)
Vascular anatomy was assessed in each subject able to undergo a study MRA on enrollment at the NIA clinical center with gadolinium contrast (i.e., no renal dysfunction or other contraindications) from the brain through the pelvis by MRA images of the entire arterial tree. MR studies were completed on an Intera Philips 1.5 T magnet with software version 11.1.4.4. All images were read by the same clinical radiologist experienced in arterial disease determination, particularly artifacts that may appear as beading (R.R.), and were also reviewed by the principal investigator of clinical protocol 2003-086 (N.B.M.). Characteristics defined in each artery included the presence of stenosis, defined as ∼20% or greater luminal loss compared to the proximal reference diameter in arteries sufficiently large to visualize changes due to the special resolution of MRA (approximate artery diameter typically ≥5 mm); aneurysm, defined as 50% increase from the reference diameter immediately proximal or distal to the involved segment, and smaller changes were called ectatic; dissection, as evidenced by the presence of an intimal flap; beading, defined as sequential stenosis with or without aneurysmal dilation between stenotic segments; and arterial tortuosity, coded when it appeared in arteries normally expected to follow a straight course in otherwise healthy individuals. Other vascular abnormalities, including anatomic variants and atherosclerotic changes, were noted. Hypoplastic or absent segments or arteries were categorized as arteries not well visualized by MRA. Previously stented or bypassed arteries were coded as not visualized.
Fibroblast cell culture
Dermal fibroblasts were cultured from a 4 mm punch skin biopsy (18). Fibroblasts between passages 4 and 10 from case subjects and age- and sex-matched controls were cultured to confluence in high-glucose Dulbecco's modified Eagle's medium, 10.0% fetal bovine serum, penicillin, and streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in 5.0% CO2. FMD fibroblasts appeared morphologically similar to control fibroblasts. For Western blot experiments, untreated cells (for pERK1/2 and p-p38) were grown to confluence before lysis. Treated cells were grown to ∼80% confluence and then serum starved for 18 h. Cells were then treated for 1 h with either 10 ng/ml transforming growth factor β1 (TGF-β1) or TGF-β2 (for pSmad2) or 50 ng/ml BMP-4 (for pSmad1/5/8) (R&D Systems, Minneapolis, MN, USA) before lysis.
Enzyme-linked immunosorbent assays (ELISAs) for protein marker measurement
Background TGF-β found in fetal bovine serum was eliminated by a series of washes and the addition of serum-free medium for secretion experiments. Total TGF-β1 and TGF-β2 concentrations in secreted medium from skin fibroblasts and total TGF-β2 in platelet-poor EDTA-plasma were measured by ELISA with the human TGF-β1 and TGF-β2 Quantikine ELISA kits (R&D Systems); samples were acid activated according to the manufacturer's instructions. Total TGF-β1, MCP-1, TNF-α, CRP, SAA, ICAM-1, VCAM-1, and IL-8 in human plasma were measured using a ruthenium-based commercially available electrochemiluminescence platform to measure systemic inflammation, according to the manufacturer's instructions (Meso Scale Discovery, Gaithersburg, MD, USA). Secreted IL-8 was measured using a human CXCL8/IL-8 Quantikine ELISA kit (R&D Systems). Samples used for TGF-β1 were acid activated prior to assaying. All samples were assayed in duplicate. The percentage coefficient of variance was below 20% and above the lower level of quantification for each analyte. Secretion data were normalized to protein concentration. Secretion of total TGF-β1 and TGF-β2 from human skin fibroblasts was measured in a subset of subjects with FMD and healthy subjects who provided dermal biopsy samples and whose cell lines provided adequate in vitro cell growth. The plasma TGF-β2 was measured at the same time as the secreted TGF-β2 in the same subset of patients so the results could be directly compared.
Cell cycle analysis
Cell cycle analysis was performed on dermal fibroblasts at ∼80% confluence using methods for propidium iodide (PI) and FACS analysis (19). Cells were grown in culture to 90–95% confluence, harvested, washed, and suspended in 1× PBS, fixed in ice-cold 70% ethanol, pelleted, and suspended in 500 μl PI stain (50 μg/ml PI and 200 μg/ml RNase A) for 60 min in the dark. Samples were assayed by flow cytometry using FACSCalibur (BD Biosciences, San Jose, CA, USA), and the raw data were analyzed using the Modfit LT program (BD Biosciences). Bromodeoxyuridine (BrdU) assays were performed in parallel as a second method to assess cellular proliferation. Dermal fibroblasts were seeded in a 96-well plate (5000 cells/well). After 18 h, BrdU was added to cells in triplicate according to the BrdU Cell Proliferation Assay instructions (EMD Millipore, Billerica, MA, USA). The BrdU incorporated for 6 h, and then the plate was processed according to the manufacturer's instructions. Values were collected on a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Triplicate values were averaged and then subtracted from values from cells not treated with BrdU.
Western blot analysis
Protein expression was analyzed by SDS-PAGE electrophoresis and Western blot. Fibroblasts were lysed with RIPA buffer (Pierce, Rockford, IL, USA) containing protease and phosphatase inhibitors (cocktail sets I–III; Calbiochem, Gibbstown, NJ, USA). Protein (30 μg, as determined by a BCA protein assay of whole-cell protein extracts and using BSA as a standard; Pierce) was loaded onto a 4–20% Novex Tris-Glycine precast gel (Invitrogen). Proteins were then electrotransferred onto a PVDF membrane using Invitrogen's iBlot dry blotting system, and immunoblotting was done using the appropriate human antibodies. Specifically, rabbit polyclonal anti-phospho-Smad1/5/8 (1:500; Cell Signaling Technology, Danvers, MA, USA), anti-Smad1 (1:500, Cell Signaling Technology), anti-phospho-Smad2 (1:500; Millipore, Billerica, MA, USA), anti-phospho-Erk1/2 (1:1000; Cell Signaling Technology), anti-p38 MAPK (1:500; Cell Signaling Technology), anti-β-tubulin (1:2000; Cell Signaling Technology) or rabbit monoclonal anti-Smad2 (1:500; Cell Signaling Technology), anti-Erk1/2 (1:1000; Cell Signaling Technology), anti-phospho-p38 MAPK (1:500; Cell Signaling Technology), and anti-β-actin (1:1000; Cell Signaling Technology) were used overnight at 4°C, followed by 1 h incubation with a secondary donkey anti-rabbit IgG ECL-HRP linked antibody (1:5000; GE Healthcare, Piscataway, NJ, USA). Immunoreactive products were visualized by chemiluminescence using the ECL Plus kit (GE Healthcare). Quantification of immunoblots was performed using ImageJ software (NIH, Bethesda, MD, USA).
Genetic mutation analysis
DNA fragments covering coding regions and flanking sequences of genes of interest were amplified and then sequenced using the Big Dye Terminator V3.1 on the ABI Genetic Analyzer 3130xl (Applied Biosystems, Foster City, CA, USA). Alignment was performed with Sequencher 4.10.1 (Gene Codes Corp., Ann Arbor, MI, USA). Genes sequenced are shown in Supplemental Tables S1 and S2, respectively.
Statistical analysis
Analyses were carried out using SAS 9.3 statistical software (SAS Institute, Cary, NC, USA) and Microsoft Excel 2007 (Microsoft, Redmond, WA, USA); t tests were used to compare DEXA measurements between case and control subjects who were matched at a 1:3 ratio by age (±5 yr) and gender. Group comparisons for biomarkers were made with the independent, 2-tailed sample t test, with statistical significance defined as P ≤ 0.05, and represented as means ± sem. Based on the MRA data, an arterial disease severity score was constructed by counting the number of arterial beds with beading, aneurysm, stenosis, dissection or tortuosity in carotid, vertebral, cerebral, mesenteric, iliac, and upper extremity arteries, and the aorta. A second score was constructed counting beading only in each arterial bed. Multivariable regression was performed on the resulting score. Variable selection was applied by a stepwise regression method using the adjusted R2 as the criterion to determine which variables to include as predictors in the model. In our initial model, severity score was the dependent variable, and TGF-β results, exogenous hormone usage, MRA findings, age, and gender were included as independent variables. After variable selection, the final model had the maximum adjusted R2, which can be seen as a fraction index of the variance explained by the model.
RESULTS
FMD patient population
In our study of 47 FMD case subjects, every subject with FMD had received an outside angiographic diagnosis of FMD in the clinical setting, and each subject showing classic string-of-beads-type changes consistent with medial fibrodysplasia, as confirmed by the study team, was included in the cohort (Fig. 1). As part of their clinical care prior to study enrollment, approximately half (51.1%) of the study subjects had invasive treatment (percutaneous or surgical) for the lesions in the presenting arterial bed (Table 1).
Table 1.
Demographics, medical history, symptoms, and physical examination
| Parameter | Range | Mean ± sd | N | % |
|---|---|---|---|---|
| Demographics | ||||
| Gender, female | 43 | 91.5 | ||
| Ethnicity, European ancestry | 46 | 97.9 | ||
| Medical history | ||||
| Hypertension | 31 | 66.0 | ||
| Stroke or transient ischemic attack | 23 | 48.9 | ||
| Percutaneous coronary intervention for FMD | 24 | 51.1 | ||
| Surgery for FMD | 10 | 21.3 | ||
| Renal failure | 1 | 2.1 | ||
| Menopause | 31 | 66.0 | ||
| Hysterectomy | 13 | 27.7 | ||
| Venous thrombosis | 0 | 0 | ||
| Hyperlipidemia | 21 | 44.7 | ||
| Myocardial infarction | 3 | 6.4 | ||
| Osteoporosis | 4 | 8.5 | ||
| Age at study evaluation | 19–67 | 51.0 ± 9.0 | ||
| Age at FMD presentation | 16–65 | 44.5 ± 12.8 | ||
| Age of hypertension diagnosis | 16–56 | 36.5 ± 13.3 | ||
| Age at stroke or transient ischemic attack | 32–58 | 47.2 ± 8.1 | ||
| Age at percutaneous coronary intervention for FMD | 16–58 | 45.7 ± 11.2 | ||
| Age at surgery for FMD | 16–56 | 40.9 ± 13.6 | ||
| Age at menopause | 28–54 | 45.2 ± 7.8 | ||
| Myopia | 28 | 59.6 | ||
| Retinal detachment | 9 | 19.1 | ||
| Raynaud's phenomenon | 11 | 23.4 | ||
| Livedo reticularis | 14 | 29.8 | ||
| Symptom and activity history | ||||
| Competitive sports | 4 | 8.5 | ||
| Runner, competitive or noncompetitive | 5 | 10.6 | ||
| Miles running per week > 15 | 3 | 6.4 | ||
| Days running per week > 4 | 4 | 8.5 | ||
| Swooshing sound in ears | 17 | 36.2 | ||
| Headache | 25 | 53.2 | ||
| Claudication | 1 | 2.1 | ||
| Neck pain | 32 | 68.1 | ||
| Back pain | 32 | 68.1 | ||
| Abdominal pain | 12 | 25.5 | ||
| Flank pain | 1 | 2.1 | ||
| Dizziness | 13 | 27.7 | ||
| Tinnitus | 12 | 25.5 | ||
| Neurologic deficit | 14 | 29.8 | ||
| Joint pain | 32 | 68.1 | ||
| Medication history | ||||
| Angiotensin receptor blocker, at study evaluation | 10 | 21.3 | ||
| Angiotensin receptor blocker, ever | 11 | 23.4 | ||
| Oral contraceptive pills, at study evaluation | 2 | 4.3 | ||
| Oral contraceptive pills, current or former use | 18 | 38.3 | ||
| Hormone replacement therapy, at study evaluation | 13 | 27.7 | ||
| Hormone replacement therapy, current or former use | 16 | 34.0 | ||
| Any history of exogenous estrogen use | 29 | 61.7 | ||
| Anxiolytic or antidepressant, at study evaluation | 19 | 40.4 | ||
| Narcotic use, at study evaluation | 6 | 12.8 | ||
| Tobacco and alcohol use | ||||
| Current or former smoker | 18 | 38.3 | ||
| Alcohol consumption > 7 drinks/wk | 7 | 14.9 | ||
| Physical examination | ||||
| Height (cm) | 149.4–181.0 | 165.9 ± 6.8 | ||
| Weight (kg) | 48.4–100.4 | 70.2 ± 12.1 | ||
| Body surface area | 1.5–2.1 | 1.8 ± 0.1 | ||
| Body mass index | 19.5–35.7 | 25.5 ± 3.6 | ||
| Systolic blood pressure | 96–159 | 129.0 ± 14.7 | ||
| Diastolic blood pressure | 54–90 | 70.2 ± 9.7 | ||
| Pulse | 46–91 | 65.6 ± 10.1 | ||
| Beighton score > 5 | 27 | 57.4 |
Family history
Self-reported vascular diagnoses in first- and second-degree family members were tallied for each FMD case subject (Table 2). None of our FMD case subjects were related to one another. In our study there were no subjects with known connective tissue diseases, and there were no family members with Ehlers-Danlos syndrome (EDS), which has been reported in families of FMD patients elsewhere (20, 21). Aneurysm was reported in 15 (31.9%) families, and specifically abdominal aortic aneurysm was reported in 8 families (17.0%). Arterial dissection was uncommon and reported in only 1 family member. Stroke was relatively common and seen in 28 (59.6%) families.
Table 2.
FMD case subjects' self-reported family history of vascular and genetic diseases
| Disease or condition | N | % |
|---|---|---|
| Abdominal aortic aneurysm | 8 | 17.0 |
| Any aneurysm | 15 | 31.9 |
| Arterial dissection | 1 | 2.1 |
| Hypertension | 22 | 46.8 |
| Ehlers-Danlos syndrome | 0 | 0 |
| FMD diagnosed by a physician | 1 | 2.1 |
| Scoliosis diagnosed by a physician | 12 | 25.5 |
| Stroke | 28 | 59.6 |
| Sudden death | 8 | 17.0 |
N. number of subjects with a positive family history for the listed diagnoses.
Genetic analysis
Based on established clinical criteria for genetic testing for various gene mutations, subjects meeting the corresponding criteria underwent molecular genetic screening for mutations in COL3A1, FBN1, PLOD1, TGFBR1, TGFBR2, TGFB2, SMAD3, ACTA2, and COL5A1. The selection criteria are outlined in Supplemental Table S1. No pathogenic mutations were identified.
Clinical features
Of the 47 subjects, 43 were female (91.5%) with a median age at FMD presentation of 50 yr (mean 44.5 yr) and median age at study enrollment of 53 yr (mean 51.0 yr) (Table 1); 48.9% had a history of stroke or transient ischemic attack (TIA), 53.2% had chronic headache, and 66.0% had a history of hypertension. History of exogenous estrogen use of any kind was noted in 61.7%; similar to prior reports, parity rates and pregnancy complications were not elevated above population estimates (6). Current or former smoking history was present in 38.3%.
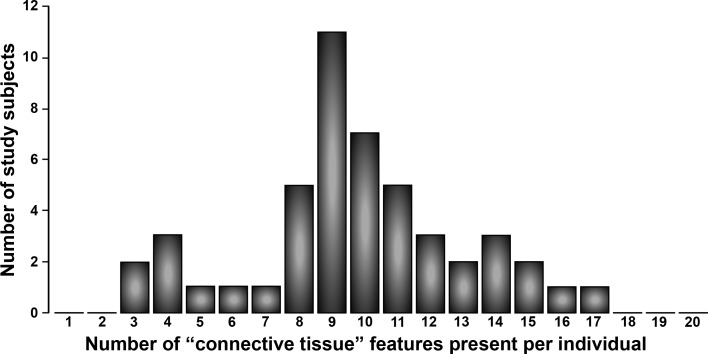
On MRI of the spine, despite the relatively young age of our cohort, 95.7% showed degenerative disc disease, and 74.5% showed degenerative facet changes at any location (Table 3 and refs. 22, 23). Spinal stenosis was noted in 66.0% of subjects. Surprisingly, a high incidence of Chiari I malformation (6.4%) and tonsillar ectopia (14.9%) was seen on cervical spine MRI, and 42.6% of the subjects had evidence of dural ectasia. Scoliosis was severe enough to be noted by MRI in 29.8% of subjects (Table 3), which was lower than the estimate of 78.7% by clinical examination in the setting of weight bearing in a patient with joint laxity (Table 1 and refs. 1, 2). On DEXA scans, we found a loss of bone density on examination of the femoral neck (P<0.001), with adjustment for age, gender, and BMI. We did not analyze spinal bone density due to extensive degenerative disease seen on MRI in our subjects. On physical examination, FMD subjects demonstrated significant musculoskeletal features. By clinical examination, a Beighton score ≥ 5 was found on examination in 57.4% of our subjects (Table 1), which is a rare finding in this age group in the Caucasian population (24, 25). Hip laxity, as evidenced by the ability to place the foot over the head in a seated position, was seen in 83.0% of our subjects. Additional features specifically interrogated, which have been associated with other forms of hereditary disorders of connective tissue, included small joint hyperextension, (n=38, 80.9%), pes planus (n=34, 72.3%), hammertoes (n=9, 19.1%), hypertelorism (n=10, 21.3%), any abnormality of uvula (n=7, 14.9%), dolichocephaly (n=5, 10.6%), malar hypoplasia (n=17, 36.2%), palate abnormality (high palate or palate torus; n=41, 87.2%), blue-tinged sclera (n=30, 63.8%), pectus deformity of any kind (n=19, 40.4%), downsloping ribs (n=12, 25.5%), velvety skin (n=25, 53.2%), stretchy skin (n=27, 57.4%), abnormal scars (n=27, 57.4%), piezogenic papules (n=30, 63.8%), scoliosis on examination, which includes functional scoliosis in the setting of excessive joint laxity in some of the subjects (n=37, 78.7%), arachnodactyly (n=7, 14.9%), ability to touch tongue to nose (n=11, 23.4%), and ability to touch foot to forehead (n=39, 83.0%). Of the 20 connective tissue features interrogated, 45 of 47 FMD case subjects (95.7%) had ≥4 features present on clinical examination (Fig. 2).
Table 3.
Spine magnetic resonance imaging features
| Feature | N | % |
|---|---|---|
| Degenerative disc disease at any location in the spine | 45 | 95.7 |
| Degenerative disc disease noted in cervical spine | 39 | 83.0 |
| Degenerative disc disease noted in thoracic spine | 21 | 44.7 |
| Degenerative disc disease noted in lumbar spine | 39 | 83.0 |
| Facet changes noted at any location | 35 | 74.5 |
| Facet changes at cervical spine | 6 | 12.8 |
| Facet changes at thoracic spine | 5 | 10.6 |
| Facet changes at lumbar spine | 34 | 72.3 |
| Dural ectasia | 20 | 42.6 |
| Chiari I malformation | 3 | 6.4 |
| Tonsillar ectopia | 7 | 14.9 |
| Scoliosis on magnetic resonance imaging | 14 | 29.8 |
| Spinal stenosis at any location in the spine | 31 | 66.0 |
| Spinal stenosis at cervical spine | 22 | 46.8 |
| Spinal stenosis at thoracic spine | 5 | 10.6 |
| Spinal stenosis at lumbar spine | 17 | 36.2 |
Figure 2.
Distribution of the number of connective tissue dysplasia features per subject with FMD. For each FMD case subject, the number of connective tissue features is shown, tallying the number of the 20 features summarized in Results: Clinical features.
The study MRA was performed in 43 subjects able to undergo the procedure. Multifocal stenosis was observed in 26 subjects, and the lack of observed beading in the primary arterial territory in the remaining subjects is consistent with the clinical history of surgery, often with resection of the diseased arterial segment and end-to-end graft implantation, and/or percutaneous intervention with stent implantation, precluding artery visualization. Intracerebral aneurysm was seen in 6 of 47 subjects. In the multivariable regression, plasma TGF-β2 levels were significantly predictive of the arterial severity score of systemic burden of arterial disease (Table 4).
Table 4.
MRA characteristics and arterial disease severity scores
| Characteristic, in any vessel | N | % |
|---|---|---|
| Aneurysm | 30 | 63.8 |
| Dissection | 2 | 4.3 |
| Beading | 26 | 55.3 |
| Stenosis | 30 | 63.8 |
| Tortuosity | 6 | 12.8 |
| Other vascular abnormality | 20 | 42.6 |
| Not visualized by MRA | 14 | 29.8 |
| Severity score | Score 1: beading, dissection, aneurysm, or stenosis on MRA |
Score 2: beading only |
||
|---|---|---|---|---|
| N | % | N | % | |
| 0 | 4 | 8.5 | 20 | 42.6 |
| 1 | 17 | 36.2 | 13 | 27.7 |
| 2 | 13 | 27.7 | 11 | 23.4 |
| 3 | 7 | 14.9 | 1 | 2.1 |
| 4 | 2 | 4.3 | 0 | 0 |
| 5 | 3 | 6.4 | 1 | 2.1 |
| 6 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| Sum | 47 | 47 | ||
| ≥2 territories | 53.2 | 27.7 | ||
TGF-β2 plasma: score 1, P = 0.003; score 2, P = 0.004.
TGF-β pathway biomarker assays
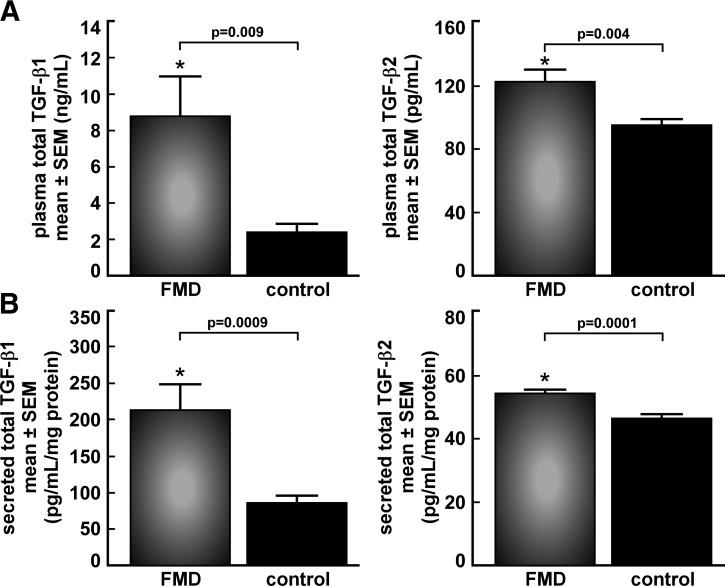
We analyzed plasma samples from the first 38 FMD case subjects enrolled and 74 age-, sex-, and BMI-matched apparently healthy control subjects, matched 1:2 when possible. Case control identities were blinded to the researcher performing the assays. Circulating TGF-β1 levels were higher in subjects with FMD compared to control subjects (8.8±2.2 vs. 2.5±0.4 ng/ml, P=0.009). Similarly, plasma samples from a smaller cohort of 15 FMD case subjects and 16 age-, sex-, and BMI-matched apparently healthy control subjects showed increased circulating total TGF-β2 in FMD case subjects (124.2±7.8 vs. 95.8±3.6 pg/ml, P=0.004; Fig. 3A).
Figure 3.
Circulating and secreted TGF-β in FMD. A) Elevated levels of total TGF-β1 and -β2 in platelet-poor EDTA-plasma from FMD case subjects vs. age- and sex-matched healthy controls. Samples were acid-activated prior to assaying by ELISA. B) Secreted total TGF-β1 and -β2 from cultured fibroblasts from FMD case subjects is elevated vs. healthy controls ex vivo. Samples were acid activated prior to assaying by ELISA, and secretion data are normalized to protein concentration.
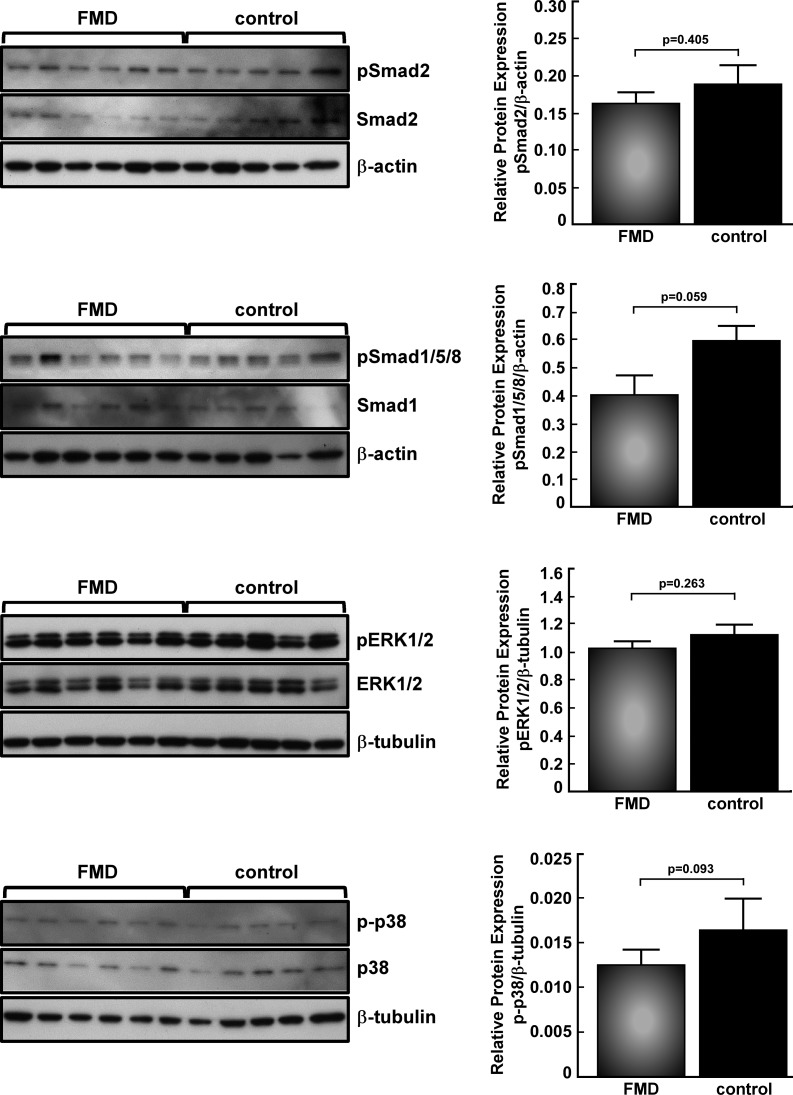
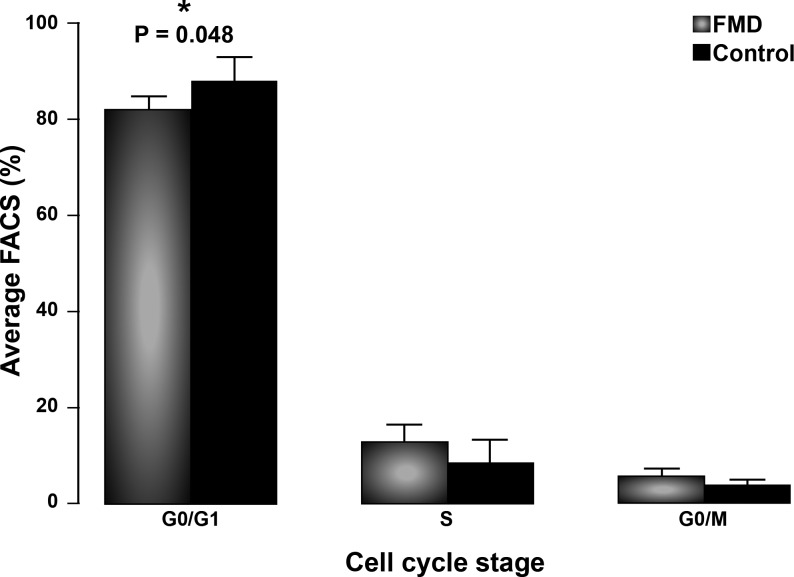
Secretion of total TGF-β1 and TGF-β2 from human skin fibroblasts was measured in a subset of the 25 subjects who provided dermal biopsy samples, including 16 FMD case subjects and 14 age- and sex-matched apparently healthy control subjects, whose cell lines provided adequate in vitro cell growth. Significant elevation of secreted TGF-β1 (215.2±32.1 vs. 73.9±7.5 pg/ml/mg protein, P=0.0009) and TGF-β2 (54.6±1.2 vs. 46.8±1.2 pg/ml/mg protein, P=0.0001) was seen in FMD case subjects vs. controls (Fig. 3B). There was a positive correlation between plasma and secreted levels of TGF-β2 (r=0.55). Intracellular downstream TGF-β pathway biomarkers (pSmad2, pSmad1/5/8, pERK1/2, and p-p38 MAPK) were analyzed by immunoblot and showed no significant differences, even when stimulated with TGF-β1, TGF-β2, or BMP-4 (Fig. 4, TGF-β2 results same as TGF-β1, data not shown). FACS analysis for cell cycle suggested excess cellular proliferation in FMD case subject fibroblasts in vitro (Fig. 5), although assessment of cellular proliferation in the same cell lines by BrdU assay showed no statistically significant difference (P=0.36). Protein expression of TGF-β receptors I and II, collagens I, III, and V, fibronectin, and decorin was not different between patients with FMD and control subjects (data not shown).
Figure 4.
Western blot analysis of TGF-β pathway biomarkers. Whole-cell lysates from FMD case subject (n=18) and control fibroblasts (n=14) were probed for canonical pathway markers pSmad2 and pSmad1/5/8, treated with TGF-β1 or BMP-4, respectively, or untreated noncanonical markers pERK1/2 and p-p38 MAPK. No significant differences in biomarker levels were seen between FMD and control fibroblasts. Representative blots are shown; however, quantitation graphs include all samples above. Data were normalized to the loading control.
Figure 5.
FACS analysis of cell cycle profiles of dermal fibroblasts from FMD case samples with corresponding TGF-β data. FACS analysis shows a shift in the cell cycle profiles of dermal fibroblasts from subjects with FMD compared to healthy controls, with a lower proportion of cells in quiescence (G0/G1 phase).
Inflammatory biomarkers in circulating blood
Plasma levels of MCP-1, TNF-α, CRP, SAA, ICAM-1, and VCAM-1 were all significantly elevated in FMD case subjects relative to age- and sex-matched apparently healthy control subjects, while IL-8 was significantly decreased in FMD case subjects (Table 5). IL-8 secreted from fibroblasts was not different compared to controls (data not shown). When comparing FMD samples to the MFS group, we found alterations in TGF-β1, TGF- β 2, MCP-1, SAA, ICAM-1, VCAM-1, and IL-8 that were in similar direction and magnitude. In addition, TGF-β1 levels in FMD were similar to those found in an independent analysis of circulating TGF-β1 in MFS (26). However, TNF-α and CRP, which were elevated in subjects with FMD, were not significantly different in individuals with MFS as compared to the healthy control subjects, indicating a distinct pattern of protein markers in FMD (Table 5).
Table 5.
Circulating biomarker comparison between FMD and MFS
| Marker | FMD, n = 38 |
MFS, n = 20 |
Control, n = 74 | ||
|---|---|---|---|---|---|
| Value | P | Value | P | ||
| TGF-β1 (ng/ml) | 8.8 ± 2.2 | 0.009 | 9.2 ± 1.4 | 0.0001 | 2.5 ± 0.4 |
| TNF-α (pg/ml) | 5.76 ± 0.25 | <0.0001 | 5.04 ± 0.30 | NS | 4.33 ± 0.18 |
| CRP (ng/ml) | 2958 ± 701 | 0.027 | 2700 ± 747 | NS | 1264 ± 171 |
| MCP-1 (pg/ml) | 256 ± 11 | 0.001 | 270 ± 15 | 0.0004 | 211 ± 0.7 |
| SAA (ng/ml) | 2698 ± 588 | 0.026 | 3142 ± 825 | 0.037 | 1281 ± 104 |
| ICAM-1 (ng/ml) | 231 ± 11 | <0.0001 | 265 ± 27 | 0.005 | 179 ± 6 |
| VCAM-1 (ng/ml) | 454 ± 37 | 0.009 | 542 ± 40 | 0.0001 | 347 ± 12 |
| IL-8 (pg/ml) | 1.86 ± 0.13 | <0.0001 | 1.98 ± 0.21 | <0.0001 | 3.46 ± 0.18 |
Values are presented as means ± sem. P ≤ 0.05 considered significant; NS, not significant.
DISCUSSION
Recent data have suggested that FMD represents a systemic vasculopathy, with the finding of a clinical manifestation of the disease in multiple arterial beds in at least one-third of patients (20). However, systematic evaluation for clinical and musculoskeletal features has not previously been performed. Our results indicate that FMD is a systemic disease with clinical features that extend beyond arterial pathology to include low bone density, joint laxity, and degenerative disease in the spine. The major clinical manifestations of FMD in our study are related to the vascular pathology and pain often related to early onset arthritis and degenerative joint disease in the spine. Based on the constellation of findings, consistent with a mild and subclinical connective tissue dysplasia, we hypothesized that TGF-β expression alterations may drive these findings in patients with FMD. We tested this hypothesis through assays of circulating plasma and dermal fibroblasts, and we demonstrated that levels of TGF-β isoforms and other inflammatory cytokines are altered.
The demographics and clinical histories of our subjects closely align with a U.S. registry of 447 patients with FMD (20) despite differences in study methodologies. We also are aware of 12 subjects enrolled in our cohort who are also enrolled in the same U.S. registry. Similar features included percentage of female sex (91.5 vs. 91.0%), mean age at presentation (44 vs. 47 yr), prevalence of hypertension (66.0 vs. 72.0% at study enrollment), headache (53.2 vs. 60.0%), current or former history of smoking (38.3 vs. 37.2%), and a history of exogenous hormone use (61.7 vs. 69.6%). However, in our study, 21.3% of our subjects had undergone surgical treatment for FMD with histopathologic confirmation, in contrast to 3.3% in the U.S. registry. A higher proportion of our cohort had experienced a cerebrovascular event (48.9%) as compared to the U.S. registry, which noted TIA in 8.7% and stroke in 6.9% of its participants. This may reflect an ascertainment bias for patients who are more severely affected in being motivated to undertake the travel requirements for participation at the NIH. Our cohort did not include any individuals with asymptomatic disease discovered incidentally, whereas the U.S. registry included 5.6% with no symptoms or signs. In our study, surveillance MRA imaging of the entire arterial tree showed that 53.2% of our study subjects had vascular disease (aneurysm, beading, stenosis, or dissection) involving ≥2 arterial beds. Multiple vascular bed involvement was noted in 26.0–35.3% of patients with FMD in the U.S. registry who had imaging performed on additional arterial territories as clinically necessitated. Several caveats to the interpretation of our study MRA findings include that motion artifact from breathing is generally problematic for abdominal studies, previously treated arterial beds in cases of prior surgery or stent implantation could not be assessed, and we were unable to study every subject in the cohort because of contraindications to MR and/or gadolinium contrast administration. These caveats may have led to underestimating the severity and burden of systemic disease. Brain MR imaging is less susceptible to motion artifact, and we found that 12.8% of subjects had an intracerebral aneurysm. In the U.S. registry, 9 of 76 (11.8%) individuals with imaging data had a cerebral artery aneurysm, and 16 of 76 (21.1%) had carotid artery aneurysm, including both extracranial and intracranial internal carotid artery. Overall, the close alignment of demographic variables in our study as compared to the U.S. registry indicates that our cohort is a representative sample of U.S. patients with FMD, but likely with more severe clinical disease.
While subjects with FMD had findings often associated with connective tissue dysplasias, including joint laxity, scoliosis, craniofacial features, and pes planus, the findings ranged in severity, with most exhibiting mild and subclinical findings on physical examination. Unlike individuals with hypermobile EDS, FMD case subjects in our study had not come to medical attention due to recurrent dislocations, refractory joint pain, or multiple joint surgeries. Tissue fragility, hollow organ rupture, and lack of healing from surgical scars seen in the vascular form of EDS were not observed. Scoliosis and pes planus, when seen in the FMD case subjects, were much less severe than is typically seen in MFS. Although the severity of the musculoskeletal findings was mild, the pattern of these features in our FMD case subjects may be clinically relevant. Features that were similar to patients with SMAD3 mutations included degenerative or facet changes on spine MRI and spinal stenosis, and neck or back pain in our cohort was common. In addition, extravascular features associated with Loeys-Dietz syndrome and MFS, including dural ectasia, tonsillar ectopia, and Chiari 1 malformation, were observed (27, 28). In the clinical setting, the diagnosis of genetic syndromes often requires the consideration of multiple clinical features simultaneously, which may present heterogeneously in individual subjects, thus necessitating the use of scores or multivariable criteria to define a syndrome (28). In our study, while there was heterogeneity in the number of connective tissue features observed in individuals with FMD, the vast majority (95.7%) showed ≥4 of the features that have been associated with other genetically mediated connective tissue disorders with vascular abnormalities. The pattern of these features most closely resembles that of other connective tissue dysplasias with vascular phenotypes, such as Loeys-Dietz syndrome, MFS, or ACTA2 mutation syndromes. In terms of vascular pathology, the vascular lesion observed in medial fibroplasia-type FMD appears to be closest to disorders caused by ACTA2 and NF1 mutations, where aneurysms and stenotic lesions can be found simultaneously, rather than primary mutations in TGF-β pathway genes that typically cause aortic aneurysms and arterial dissections. To ensure that our cohort did not contain individuals with pathogenic mutations in these genes, we screened individuals for specific gene disorders based on the established clinical criteria for genetic testing. We used sequencing to evaluate the target genes in case previously unreported mutations in the same genes caused a milder phenotype overlapping with FMD in our study. The yield of these analyses was low, with no pathogenic mutations identified, consistent with prior reports (21).
Environmental factors contributing to the development of FMD have been proposed and include smoking and mechanical risk factors (6). Hypercholesterolemia was not more prevalent in our patients compared to population estimates (29). Given the preponderance of disease in women and prior data suggesting that exogenous estrogen use is higher in the FMD patient population, hormonal factors appear to play a role (30, 31). Our data showed similar trends as prior reports, with many subjects reporting a history of exogenous hormone use. Hormonal milieu also affects joint laxity and may play a role in the musculoskeletal features of FMD (25, 32). However, loss of bone density and arthritis are not associated with exogenous estrogen use.
Our protein marker analyses showed evidence of systemic inflammation in subjects with FMD compared to age- and gender-matched healthy controls with elevation of circulating plasma TGF-β1, TGF-β2, MCP-1, TNF-α, CRP, SAA, ICAM-1, and VCAM-1 (20, 33, 34). TGF-β1 and TGF-β2 are members of the TGF superfamily of cytokines that regulate a variety of cellular functions in the arterial wall that lead to cellular proliferation, differentiation, modulation of extracellular matrix production, and ultimately, arterial remodeling (35). The finding of TGF-β elevation in FMD is intriguing, given the similarity of the FMD phenotype to the spectrum of disorders with altered TGF-β signaling, particularly the extravascular manifestations we identified (36–38). MCP-1 mediates vascular inflammatory cell adhesion and contributes to neointimal hyperplasia in the setting of arterial injury (39). The finding of lower circulating IL-8 is surprising, but not unprecedented. IL-8 is known to play an important role in endothelial cell survival, proliferation, and angiogenesis (40, 41). In addition, TGF-β1 inhibits IL-8 secretion during active inflammation in the vascular endothelium, (42) consistent with our findings in patients with FMD. Furthermore, it was recently shown that patients with vascular EDS similarly have reduced IL-8 and elevated circulating TGF-β1 (43). Though the exact mechanism behind reduced IL-8 is unknown, a potential role in aneurysmal disorders is intriguing and warrants further study. Elevated CRP, SAA, and TNF-α are seen in acquired autoimmune disorders, although our FMD patients did not present with usual rheumatologic symptoms and signs. These findings suggest a state of systemic inflammation in FMD, although vascular lesions have not characteristically shown active cellular inflammation (6). For comparison purposes, we evaluated the same markers in a group of individuals with MFS, a known disorder of TGF-β signaling, and we found a distinct pattern of protein markers in FMD as compared to MFS. TGF-β1 was elevated in both groups, but individuals with MFS did not have significant differences in TNF-α or CRP levels as compared to the healthy controls as was seen in subjects with FMD, an intriguing difference between these disorders.
Although the underlying molecular defects are unknown, our protein marker findings in FMD plasma and fibroblast secretion suggest a disturbance of homeostasis in the TGF-β axis (44) and other pathways involved in inflammatory signaling. Since access to vascular tissue through clinical care is limited, we utilized dermal fibroblasts to study key pathological features as has been frequently done in the study of other hereditary disorders of connective tissue with vascular features (45–47). It is interesting and informative that the dermal fibroblast lines obtained from a large number of FMD case subjects in our study recapitulated the phenotype of increased TGF-β1 and TGF-β2 expression ex vivo despite removal from their hormonal and other potentially interacting milieu. This suggests that an intrinsic genetic abnormality may contribute to altered TGF-β expression. Since expression of downstream TGF-β biomarkers did not show clear activation patterns, the exact mechanism invoked by excess extracellular TGF-β in FMD is unclear at this time and suggests possible locus or allelic heterogeneity, requiring further study. It is also possible that increased TGF-β expression is not a primary defect leading to FMD. Rather, there may be altered downstream activation or signaling, leading to possible compensatory pathway activation resulting in elevated TGF-β. The finding that TGF-β2 levels are associated with burden of arterial disease suggests that TGF-β2 may drive the extent of vascular disease, but this hypothesis needs to be formally tested, ideally in vascular tissues and in vivo given the context dependency of TGF-β effects. Fibroblasts obtained in this study have been donated to the Coriell repository and are available to the scientific community. Taken together, the results of our study show a pattern of clinical manifestations consistent with TGF-β alterations as have been observed in other hereditary vascular disorders, with specific vascular manifestations that are unique to FMD.
These findings have potential clinical implications. Our data suggest that an evaluation of degenerative spine arthritis and bone density may be helpful in identifying the cause and possible therapies for musculoskeletal symptoms. The yield of genetic testing for currently known connective tissue dysplasias in the FMD population is low in the absence of features that would otherwise indicate specific genetic testing, consistent with another published study (21). Given the rapid evolution of our understanding of vascular genetic diseases, formal genetic evaluation of patients with connective tissue features may be helpful to rule out other potentially overlapping disorders where FMD can be seen, such as vascular or type IV EDS. Finally, anecdotal evidence supports the rationale for therapy for FMD with pharmacologic agents that are known to decrease vascular TGF-β expression; (48) however, it is premature to make this treatment recommendation without a clinical trial. Further studies to identify the underlying molecular defect in FMD will be needed to clarify these issues.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the U.S. National Institutes of Health (NIH), National Institute on Aging. FACS analysis studies were supported in part by grant R00HL089413 at the University of Michigan. The Johns Hopkins Institutional Clinical and Translational Science Award (CTSA) grant 1U54RR023561-01A1 and the American Recovery and Reinvestment Act (ARRA) Novel Biomarkers in Aortic Aneurysms and Acute Aortic Dissection grant 5RC1HL100021-02 were used for plasma biomarker studies. S.K.G. was supported by grants from the National Heart, Lung, and Blood Institute/NIH (P30HL101290) and the Doris Duke Charitable Foundation. S.K.G. and N.B.M. are unpaid medical advisors for the nonprofit Fibromuscular Dysplasia Society of America (FMDSA).
No FMDSA funds were used in this study.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BLSA
- Baltimore Longitudinal Study of Aging
- BrdU
- bromodeoxyuridine
- DEXA
- dual-energy X-ray absorptiometry
- EDS
- Ehlers-Danlos syndrome
- ELISA
- enzyme-linked immunosorbent assay
- FMD
- fibromuscular dysplasia
- MFS
- Marfan syndrome
- MRA
- magnetic resonance angiography
- PI
- propidium iodide
- TGF-β
- transforming growth factor β
- TIA
- transient ischemic attack
REFERENCES
- 1. Leadbetter W. F., Burkland C. F. (1938) Hypertension in unilateral renal disease. J. Urol. 39, 611–626 [Google Scholar]
- 2. Slovut D. P., Olin J. W. (2004) Fibromuscular dysplasia. N. Engl. J. Med. 350, 1862–1871 [DOI] [PubMed] [Google Scholar]
- 3. Harrison E. G., Jr., McCormack L. J. (1971) Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin. Proc. 46, 161–167 [PubMed] [Google Scholar]
- 4. Stanley J. C., Fry W. J. (1975) Renovascular hypertension secondary to arterial fibrodysplasia in adults: criteria for operation and results of surgical therapy. Arch. Surg. 110, 922–928 [DOI] [PubMed] [Google Scholar]
- 5. Olin J. W., Sealove B. A. (2011) Diagnosis, management, and future developments of fibromuscular dysplasia. J. Vasc. Surg. 53, 826–836, e821 [DOI] [PubMed] [Google Scholar]
- 6. Stanley J. C., Gewertz B. L., Bove E. L., Sottiurai V., Fry W. J. (1975) Arterial fibrodysplasia: histopathologic character and current etiologic concepts. Arch. Surg. 110, 561–566 [DOI] [PubMed] [Google Scholar]
- 7. Stanley J. C., Whitehouse W. M., Jr., (1984) Occlusive and aneurysmal disease of the renal arterial circulation. Dis. Mon. 30, 1–62 [DOI] [PubMed] [Google Scholar]
- 8. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gladstien K., Rushton A. R., Kidd K. K. (1980) Penetrance estimates and recurrence risks for fibromuscular dysplasia. Clin. Genet. 17, 115–116 [DOI] [PubMed] [Google Scholar]
- 10. Schievink W. I., Limburg M. (1989) Angiographic abnormalities mimicking fibromuscular dysplasia in a patient with Ehlers-Danlos syndrome, type IV. Neurosurgery 25, 482–483 [DOI] [PubMed] [Google Scholar]
- 11. Milewicz D. M., Kwartler C. S., Papke C. L., Regalado E. S., Cao J., Reid A. J. (2010) Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet. Med. 12, 196–203 [DOI] [PubMed] [Google Scholar]
- 12. Lancman M., Mesropian H., Serra P., Granillo R. (1991) Turner's syndrome, fibromuscular dysplasia, and stroke. Stroke 22, 269–271 [DOI] [PubMed] [Google Scholar]
- 13. Schievink W. I., Bjornsson J., Piepgras D. G. (1994) Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan's syndrome and bilateral carotid artery dissections. Stroke 25, 2492–2496 [DOI] [PubMed] [Google Scholar]
- 14. Olin J. W. (2012) Is fibromuscular dysplasia a single disease? Circulation 126, 2925–2927 [DOI] [PubMed] [Google Scholar]
- 15. Savard S., Steichen O., Azarine A., Azizi M., Jeunemaitre X., Plouin P. F. (2012) Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation 126, 3062–3069 [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T., Scheet P., Giusti B., Bandinelli S., Piras M. G., Usala G., Lai S., Mulas A., Corsi A. M., Vestrini A., Sofi F., Gori A. M., Abbate R., Guralnik J., Singleton A., Abecasis G. R., Schlessinger D., Uda M., Ferrucci L. (2009) Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am. J. Hum. Genet. 84, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milhorat T. H., Bolognese P. A., Nishikawa M., McDonnell N. B., Francomano C. A. (2007) Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and Chiari malformation type I in patients with hereditary disorders of connective tissue. J. Neurosurg. Spine 7, 601–609 [DOI] [PubMed] [Google Scholar]
- 18. Normand J., Karasek M. A. (1995) A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In Vitro Cell. Dev. Biol. Anim. 31, 447–455 [DOI] [PubMed] [Google Scholar]
- 19. Tanner F. C., Yang Z. Y., Duckers E., Gordon D., Nabel G. J., Nabel E. G. (1998) Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ. Res. 82, 396–403 [DOI] [PubMed] [Google Scholar]
- 20. Olin J. W., Froehlich J., Gu X., Bacharach J. M., Eagle K., Gray B. H., Jaff M. R., Kim E. S., Mace P., Matsumoto A. H., McBane R. D., Kline-Rogers E., White C. J., Gornik H. L. (2012) The United States registry for fibromuscular dysplasia: results in the first 447 patients. Circulation 125, 3182–3190 [DOI] [PubMed] [Google Scholar]
- 21. Poloskey S. L., Kim E. S. H., Sanghani R., Al-Quthami A. H., Arscott P., Moran R., Rigelsky C. M., Gornik H. L. (2012) Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc. Med. 17, 371–378 [DOI] [PubMed] [Google Scholar]
- 22. Boden S. D., Davis D. O., Dina T. S., Patronas N. J., Wiesel S. W. (1990) Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J. Bone Joint Surg. Am. 72, 403–408 [PubMed] [Google Scholar]
- 23. Boden S. D., McCowin P. R., Davis D. O., Dina T. S., Mark A. S., Wiesel S. (1990) Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J. Bone Joint Surg. Am. 72, 1178–1184 [PubMed] [Google Scholar]
- 24. Dolan A. L., Hart D. J., Doyle D. V., Grahame R., Spector T. D. (2003) The relationship of joint hypermobility, bone mineral density, and osteoarthritis in the general population: the Chingford Study. J. Rheumatol. 30, 799–803 [PubMed] [Google Scholar]
- 25. Hakim A. J., Cherkas L. F., Grahame R., Spector T. D., MacGregor A. J. (2004) The genetic epidemiology of joint hypermobility: a population study of female twins. Arthritis Rheum. 50, 2640–2644 [DOI] [PubMed] [Google Scholar]
- 26. Matt P., Schoenhoff F., Habashi J., Holm T., Van Erp C., Loch D., Carlson O. D., Griswold B. F., Fu Q., De Backer J., Loeys B., Huso D. L., McDonnell N. B., Van Eyk J. E., Dietz H. C. (2009) Circulating transforming growth factor-beta in Marfan syndrome. Circulation 120, 526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodrigues V. J., Elsayed S., Loeys B. L., Dietz H. C., Yousem D. M. (2009) Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. Am. J. Neuroradiol. 30, 1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rose P. S., Levy H. P., Ahn N. U., Sponseller P. D., Magyari T., Davis J., Francomano C. A. (2000) A comparison of the Berlin and Ghent nosologies and the influence of dural ectasia in the diagnosis of Marfan syndrome. Genet. Med. 2, 278–282 [DOI] [PubMed] [Google Scholar]
- 29. Arnett D. K., Jacobs D. R., Jr., Luepker R. V., Blackburn H., Armstrong C., Claas S. A. (2005) Twenty-year trends in serum cholesterol, hypercholesterolemia, and cholesterol medication use: the Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation 112, 3884–3891 [DOI] [PubMed] [Google Scholar]
- 30. D'Anglejan Chatillon J., Ribeiro V., Mas J. L., Bousser M. G., Laplane D. (1990) [Dissection of the extracranial internal carotid artery. 62 cases.] Presse Méd. 19, 661–667 [PubMed] [Google Scholar]
- 31. Mas J. L., Bousser M. G., Hasboun D., Laplane D. (1987) Extracranial vertebral artery dissections: a review of 13 cases. Stroke 18, 1037–1047 [DOI] [PubMed] [Google Scholar]
- 32. Shultz S. J., Gansneder B. M., Sander T. C., Kirk S. E., Perrin D. H. (2006) Absolute serum hormone levels predict the magnitude of change in anterior knee laxity across the menstrual cycle. J. Orthop. Res. 24, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gabay C., Kushner I. (1999) Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 [DOI] [PubMed] [Google Scholar]
- 34. Kisilevsky R., Manley P. N. (2012) Acute-phase serum amyloid A: perspectives on its physiological and pathological roles. Amyloid 19, 5–14 [DOI] [PubMed] [Google Scholar]
- 35. Nabel E. G., Shum L., Pompili V. J., Yang Z. Y., San H., Shu H. B., Liptay S., Gold L., Gordon D., Derynck R. (1993) Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc. Natl. Acad. Sci. U.S.A. 90, 10759–10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Erkula G., Sponseller P. D., Paulsen L. C., Oswald G. L., Loeys B. L., Dietz H. C. (2010) Musculoskeletal findings of Loeys-Dietz syndrome. J. Bone Joint Surg. Am. 92, 1876–1883 [DOI] [PubMed] [Google Scholar]
- 37. Loeys B. L., Chen J., Neptune E. R., Judge D. P., Podowski M., Holm T., Meyers J., Leitch C. C., Katsanis N., Sharifi N., Xu F. L., Myers L. A., Spevak P. J., Cameron D. E., De Backer J., Hellemans J., Chen Y., Davis E. C., Webb C. L., Kress W., Coucke P., Rifkin D. B., De Paepe A. M., Dietz H. C. (2005) A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 37, 275–281 [DOI] [PubMed] [Google Scholar]
- 38. Wrana J. L., Attisano L., Wieser R., Ventura F., Massague J. (1994) Mechanism of activation of the TGF-beta receptor. Nature 370, 341–347 [DOI] [PubMed] [Google Scholar]
- 39. Ling S., Nheu L., Komesaroff P. A. (2012) Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini. Rev. Med. Chem. 12, 175–183 [DOI] [PubMed] [Google Scholar]
- 40. Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258, 1798–1801 [DOI] [PubMed] [Google Scholar]
- 41. Li A., Dubey S., Varney M. L., Dave B. J., Singh R. K. (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 170, 3369–3376 [DOI] [PubMed] [Google Scholar]
- 42. Smith W. B., Noack L., Khew-Goodall Y., Isenmann S., Vadas M. A., Gamble J. R. (1996) Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J. Immunol. 157, 360–368 [PubMed] [Google Scholar]
- 43. Morissette R., Schoenhoff F., Xu Z., Shilane D. A., Griswold B. F., Chen W., Yang J., Zhu J., Fert-Bober J., Sloper L., Lehman J., Commins N., Van Eyk J. E., McDonnell N. B. (2014) Transforming growth factor-beta and inflammation in vascular (type IV) Ehlers-Danlos syndrome. Circ. Cardiovasc. Genet. 7, 80–88 [DOI] [PubMed] [Google Scholar]
- 44. Akhurst R. J. (2012) The paradoxical TGF-beta vasculopathies. Nat. Genet. 44, 838–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doyle A. J., Doyle J. J., Bessling S. L., Maragh S., Lindsay M. E., Schepers D., Gillis E., Mortier G., Homfray T., Sauls K., Norris R. A., Huso N. D., Leahy D., Mohr D. W., Caulfield M. J., Scott A. F., Destree A., Hennekam R. C., Arn P. H., Curry C. J., Van Laer L., McCallion A. S., Loeys B. L., Dietz H. C. (2012) Mutations in the TGF-beta repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat. Genet. 44, 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. St Hilaire C., Ziegler S. G., Markello T. C., Brusco A., Groden C., Gill F., Carlson-Donohoe H., Lederman R. J., Chen M. Y., Yang D., Siegenthaler M. P., Arduino C., Mancini C., Freudenthal B., Stanescu H. C., Zdebik A. A., Chaganti R. K., Nussbaum R. L., Kleta R., Gahl W. A., Boehm M. (2011) NT5E mutations and arterial calcifications. N. Engl. J. Med. 364, 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aoyama T., Francke U., Gasner C., Furthmayr H. (1995) Fibrillin abnormalities and prognosis in Marfan syndrome and related disorders. Am. J. Med. Genet. 58, 169–176 [DOI] [PubMed] [Google Scholar]
- 48. Tanemoto M., Takase K., Yamada T., Satoh A., Abe T., Ito S. (2007) Dilation of renal artery stenosis after administration of losartan. Hypertens. Res. 30, 999–1002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.