Abstract
Eosinophilic esophagitis (EoE) is characterized with eosinophils and mast cells predominated allergic inflammation in the esophagus and present with esophageal dysfunctions such as dysphagia, food impaction, and heartburn. However, the underlying mechanism of esophageal dysfunctions is unclear. This study aims to determine whether neurons in the vagal sensory ganglia are modulated in a guinea pig model of EoE. Animals were actively sensitized by ovalbumin (OVA) and then challenged with aerosol OVA inhalation for 2 wk. This results in a mild esophagitis with increases in mast cells and eosinophils in the esophageal wall. Vagal nodose and jugular neurons were disassociated, and their responses to acid, capsaicin, and transient receptor potential vanilloid type 1 (TRPV1) antagonist AMG-9810 were studied by calcium imaging and whole cell patch-clamp recording. Compared with naïve animals, antigen challenge significantly increased acid responsiveness in both nodose and jugular neurons. Their responses to capsaicin were also increased after antigen challenge. AMG-9810, at a concentration that blocked capsaicin-evoked calcium influx, abolished the increase in acid-induced activation in both nodose and jugular neurons. Vagotomy strongly attenuated those increased responses of nodose and jugular neurons to both acid and capsaicin induced by antigen challenge. These data for the first time demonstrated that prolonged antigen challenge significantly increases acid responsiveness in vagal nodose and jugular ganglia neurons. This sensitization effect is mediated largely through TRPV1 and initiated at sensory nerve endings in the peripheral tissues. Allergen-induced enhancement of responsiveness to noxious stimulation by acid in sensory nerve may contribute to the development of esophageal dysfunctions such as heartburn in EoE.
Keywords: antigen, transient receptor potential vanilloid type 1
eosinophilic esophagitis (EoE) is an inflammatory disorder characterized with elevations of eosinophils and mast cells in the esophagus. Patients with EoE often have concurrent responses to food and airborne allergens and present with the symptoms related to esophageal dysfunctions, such as dysphagia, food impaction, and heartburn (1). The mechanisms underlying esophageal dysfunctions in EoE are poorly understood, but many of the symptoms of this disorder are consistent with a dysregulation of the neuronal control of the esophagus. Here we address the hypothesis that increased infiltrations of eosinophils and mast cells in the tissue lead to an increase in vagal sensory neuronal responsiveness to acid.
Recently, we have developed a guinea pig model of EoE by repetitive antigen inhalation in antigen-sensitized animals. Our study demonstrated that prolonged antigen challenge not only increases infiltrations of eosinophils and mast cells in the esophagus but also leads to an increase in epithelial permeability. The increased epithelial permeability enhanced the ability of luminal acid to stimulate action potential discharge in nociceptive afferent terminals underneath (15, 19).
In addition to acute effects on nerve excitability, and to the effect of permeability, inflammation may change acid sensitivity by altering gene expression and ion channel production in the distant vagal sensory ganglia through a process referred to as neuroplasticity. Inflammation-induced tissue damage starts the tissue repair process, which involves production and release of growth factors such as neurotrophins at the injury site. Nerve growth factors bind to high-affinity receptors at the nerve terminal and are then transported back to the cell body where they can regulate gene expression (10, 13). Our previous studies have showed that allergic inflammation induced by antigen aerosol challenge in guinea pigs induced the production of glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) in airway tissues, which led to the induction of transient receptor potential vanilloid type 1 (TRPV1) in vagal afferent neurons (8, 9). TRPV1 is a ligand-gated, nonselective cationic channel expressed mainly in small-sized peripheral sensory neurons. It can be activated by the pungent compound capsaicin, heat, and acid (2). A recent study showed a chemically challenged esophagus using acid and capsaicin enhanced the activity of thoracic spinal neurons. These results suggest that acid activation via TRPV1 containing spinal visceral afferents from the esophagus may explain the heartburn (11). Our previous studies demonstrated that TRPV1 was expressed in both nodose and jugular small-sized neurons and that the TRPV1 agonist capsaicin activated both nodose and jugular C fibers (16). Because TRPV1 plays an important role in sensing tissue pH in the noxious range (2), an increase in its synthesis in vagal afferent neurons may also contribute to an increase in acid responsiveness in inflamed esophagus.
In the present study, we use the allergen-induced EoE in guinea pigs to address the hypothesis that the eosinophilic inflammation is associated with increases in acid responsiveness in vagal afferent neurons, and this is mediated mainly through a TRPV1-dependent mechanism. Our data are consistent with the hypothesis that allergic inflammation leads to signals at the nerve terminals that are transmitted to cell bodies in the vagal sensory ganglia leading to increases in neuronal acid responsiveness, largely secondary to increases in TRPV1 activity.
METHODS
Male Hartley guinea pigs (150–200 g) were purchased from Hilltop Laboratory Animals (Scottsdale, PA). All experiments were approved by the Johns Hopkins University Animal Care and Use Committee. N represents the number of animals in the study, and n represents the number of neurons in the study.
Allergen sensitization, challenge, and vagotomy.
Antigen sensitization and aerosol challenge were performed according to our previous studies (14, 15). Briefly, adult male guinea pigs were given three intraperitoneal injections of ovalbumin (OVA, 10 mg/kg in normal saline) every 48 h as the active sensitization. Three weeks after the last injection, these guinea pigs were challenged by an aerosol of OVA (0.1% in normal saline) for 30 s each time, 7 times/wk for 2 wk. During antigen challenge, guinea pigs were placed in a plastic chamber (Thermo Fisher Scientific, Hampton, NH) and breathed spontaneously and continuously in the stream of air-aerosol mixture. Once animals developed an asthma-like response, they were released from the chamber and allowed to breathe fresh air.
To determine the involvement of vagal afferent nerve endings in OVA inhalation-induced hypersensitivity of vagal sensory neurons to acid, unilateral vagotomies were performed on seven guinea pigs before OVA inhalation. Briefly, the guinea pig was kept under anesthesia with ketamine (50 mg/ml in PBS) and xylazine (2.5 mg/ml in PBS), a cervical midline incision was made, the left side of the vagus nerve was exposed, and a 2-mm-long vagal trunk (later determined at about 50 mm from the gastric-esophageal junction) was transected. Three days later, these guinea pigs were challenged by OVA inhalation for 2 wk. The weight of OVA-challenged guinea pigs reached to 400–500 g, and naïve animals of the same size were used as controls.
Dissociation of nodose and jugular neurons.
Nodose and jugular ganglia from guinea pigs were collected in an ice-cold, oxygenated Krebs bicarbonate solution (118 mM NaCl, 1.0 mM NaH2PO4, 25.0 mM NaHCO3, 5.4 mM KCl, 1.9 mM CaCl2, 1.2 mM MgSO4, and 11.1 mM dextrose, pH 7.4, and gassed with 95% O2 and 5% CO2) and then incubated in digest cocktail (2 mg/ml collagenase and 2 mg/ml dispase in Ca2+- and/or Mg2+-free HBSS buffer) at 37°C for 2 h. During the incubation, neurons were dissociated by mild trituration with a different size of fire-polished Pasteur pipettes (large/medium/small in order) every 30 min. After that, the neurons were washed with L15 media containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and then harvested by centrifuged at 200 g for 5 min. After being resuspended in fresh L15 media, these neurons were transferred to square 8-mm glass cover slips (Warner Instrument) precoated with poly-d-lysine (0.1 mg/ml) and laminin (5 μg/ml) and allowed to adhere to the cover slips for 2 h in an incubator at 37°C. After that, all of the media were removed from the cover slips, and the cells were washed with fresh media for one time, cultured in fresh L15 media overnight in the incubator at 37°C, and used within 24 h.
Calcium imaging.
Calcium ions generate intracellular signals that control key functions in all types of neurons. TRPV1 is a nonspecific cationic channel. At the cell soma, it is well understood that, upon activation, calcium enters the cell via TRPV1 (2). Imaging calcium in neurons is an important way to study neuron response to certain specific stimuli, including TRPV1 agonist capsaicin and proton. Calcium-imaging studies were performed as described previously (7). Briefly, cultured vagal sensory neurons were loaded with 2 mM fura 2-AM dissolved in normal extracellular solution (ECS: 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.4 with NaOH) supplemented with 0.05% Pluronic F-127 in a dark environment at 37°C for 45 min. After being rinsed three times with ECS, these neurons were allowed to deesterify for at least 30 min before use. For imaging, the cover slips were placed in a P-3 model chamber (VMR Instruments) and perfused with ECS for 5∼10 min before recording. [Ca2+]i changes were measured by fura 2 excitation at 340 and 380 nm using a Zeiss Upright Scope equipped with PTI-RatioMaster. Ratios were calculated after background subtraction by setting regions of interest. Chemicals were applied with a custom-built perfuse system. At the end of every experiment, a 50 mM KCl buffer (95 mM NaCl, 50 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.4 with NaOH) was applied to distinguish excitable cells. Only KCl-responsive cells were considered to be excitable cells and used for analysis.
Patch-clamp recording.
Whole cell patch-clamp experiments were performed according to our previously described methods (7, 18). Briefly, the pipette solution was used, containing (in mM): 140 KCl, 1 MgCl2, 5 MgATP, 5 EGTA, and 10 HEPES (pH 7.2 with KOH). Patch pipettes were pulled from borosilicate glass capillaries using a P-97 Flaming-Brown micropipette puller (Sutter Instrument, Novato, CA) with resistance of 2∼5 MΩ when filled with pipette solution. Whole cell patch-clamp was performed using an Axopatch 200B patch-clamp amplifier and Axograph software (Axon Instruments, Foster City, CA). Currents were typically digitized at 100 kHz. Macroscopic records were filtered at 5 kHz. Cells were patched with a holding potential of −60 mV.
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate labeling of esophageal nodose and jugular neurons.
In a separate study, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) retrograde labeling of nodose and jugular neurons from the esophagus was performed in four guinea pigs (2 received OVA inhalation, 2 as naïve control) according to our previously described method (18). Dil (1 μl DiI solution, 1% diluted in 50% dimethyl sulfoxide in saline) was injected in the wall of the esophagus at 50–60 mm above the gastric-esophageal junction (the injection site was confirmed by the time to dissect the ganglia). Each esophagus received two to three injections. Postoperatively, animals were carefully monitored, and, if necessary, treated for pain until totally recovery (15, 18). After 2 wk OVA challenge, both nodose and jugular ganglia (2 of each from each animal) were collected and processed for whole cell patch-clamp recordings as we previously described (7).
Chemicals.
All chemicals used in the experiments were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Collagenase/dispase was purchased from Roche Applied Science (Indianapolis, IN). AMG-9810 was obtained from Tocris (Bristol, UK). Fetal bovine serum, Hanks' balanced salt solution (HBSS), and Pluronic F-127 were purchased from Life Technologies (Grand Island, NY). The stock solution of capsaicin (10 mM) was prepared in ethanol; those of collagenase/dispase (2 mg/ml) and laminin (5 μg/ml) were in sterile Ca2+/Mg2+-free HBSS. AMG-9810 were diluted in dimethyl sulfoxide, fura 2-AM (2 M) was prepared in acetone, and poly-l-lysine (1 mg/ml) was diluted in sterile water. All of the stock solutions were separated into small aliquots and stored in −20°C, and working solutions were prepared 1∼2 days before use. Acid solution was made by adding hydrochloric acid (1 N; Fisher Scientific) to normal ECS and adjusting pH to 6.4, 5.4, or 4.4.
Data analysis.
In calcium-imaging studies, neurons were defined as “responders” to a given compound if the mean response was greater than the mean baseline plus two times the standard deviation using unpaired t-test. Patch-clamp data were analyzed with Sigmaplot 11.0 (SPSS). Dose-response curves for the antagonist concentration dependence of current inhibition were fitted with a modified Hill equation
where IC50 is the half-maximum inhibition concentration, Inorm is the normalized current by the currents elicited by pH 4.4 ECS buffer with and without the AMG-9810, and [T] is the concentration of AMG-9810. All data are presented as means ± SE. Statistical comparisons were made with unpaired Student's t-test and Wilcoxon rank-sum test, and differences were considered significant at P < 0.05.
RESULTS
OVA inhalation increases acid responsiveness of vagal sensory neurons.
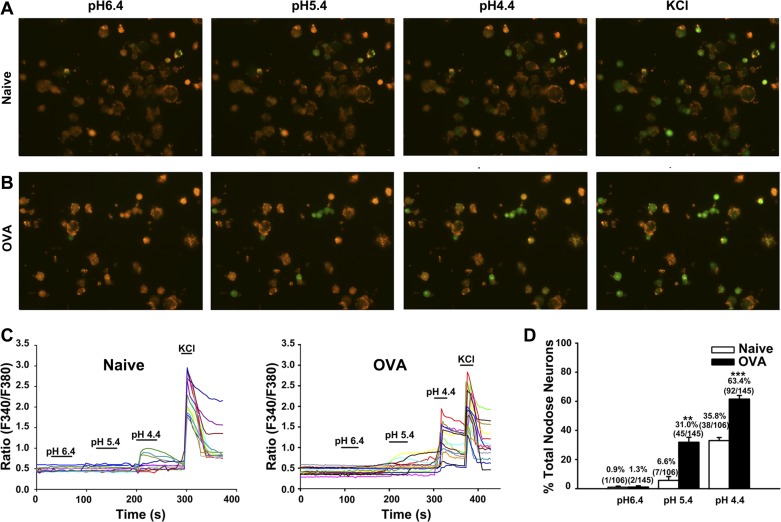
Our previous studies have demonstrated that OVA inhalation in vivo in sensitized guinea pig induces mast cell and eosinophil infiltration in the esophagus (14). However, whether increased eosinophils and mast cells subsequently sensitize esophageal afferents in the proximity and enhance their responses to acid is still unclear. To determine the possible responsive change in the vagal afferent neurons to acid, calcium-imaging assays were performed in the nodose and jugular neurons from naïve animals as control group (naïve, N = 6) and an OVA inhalation group (OVA, N = 4). Perfusion of acid at pH 6.4 activated only a small percentage of nodose neurons in both groups. A much larger percentage of vagal sensory neurons isolated from the OVA group responded to acid at pH 5.4 compared with the control group (P = 0.002); 31.0% (45 of 145) of all nodose neurons from the OVA inhalation group were found to be excited by acid at pH of 5.4, whereas 6.6% (7 of 106) of the nodose neurons from the control group elicited calcium response by the same treatment (Fig. 1, A–C). Similarly, perfusion with acid at pH 4.4 activated 63.4% (92 of 145) of nodose neurons from OVA inhalation animals compared with 35.8% (38 of 106) of nodose neurons from the control group (Fig. 1D). These results suggest that OVA inhalation increases acid responsiveness at pH 5.4 (P = 0.002 for naïve vs. OVA inhalation) and pH 4.4 (P < 0.001 for naïve vs. OVA inhalation) in nodose neurons.
Fig. 1.
Ovalbumin (OVA) inhalation increases acid responsiveness in nodose neurons. A and B: fluorescence images of nodose neurons activated with acid (pH 6.4, 5.4, and 4.4) and KCl (50 mM) from naïve and OVA inhalation (OVA) guinea pigs. C: representative traces of nodose neurons from naïve (left) and OVA inhalation (right) guinea pigs that were activated by acid (pH 6.4, 5.4, and 4.4) and KCl (50 mM). D: summary of percentages of acid-responsive nodose neurons in all KCl-responsive nodose neurons from naïve (N = 6) and OVA inhalation (N = 4) guinea pigs. **P < 0.01 and ***P < 0.001 are the levels of significance for naïve vs. OVA inhalation (2-tailed unpaired t-test).
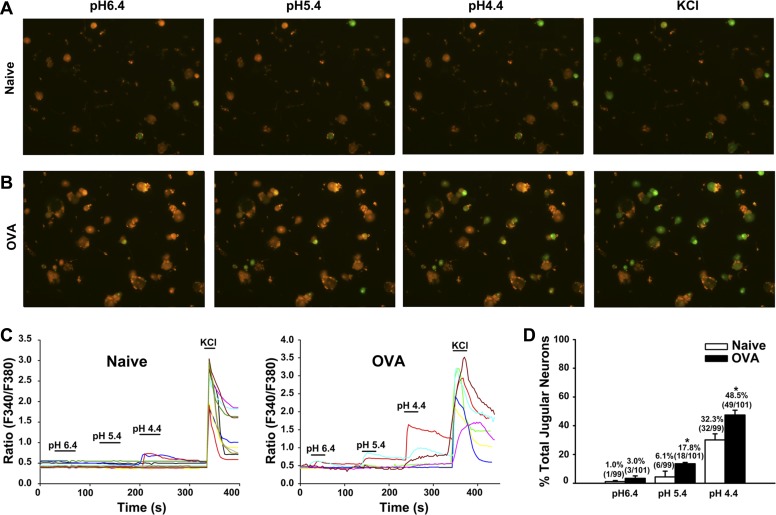
OVA treatment also increased the acid responsiveness of jugular neurons, especially at pH 5.4 (P = 0.04 for naïve vs. OVA inhalation) and pH 4.4 (P = 0.024 for naïve vs. OVA inhalation) (Fig. 2, A–C). The percentages of pH 5.4-responsive jugular neurons increased from 6.1% (6/99) in control to 17.8% (18/101) in OVA inhalation animals. Furthermore, the percentages of pH 4.4-responsive jugular neurons were increased from 32.3% (32/99) in control to 48.5% (49/101) in OVA groups (Fig. 2D).
Fig. 2.
OVA inhalation increases acid responsiveness in jugular neurons. A and B: fluorescence images of jugular neurons activated with acid (pH 6.4, 5.4, and 4.4) and KCl (50 mM) from naïve guinea pigs and OVA inhalation guinea pigs. C: representative traces from jugular neurons from naïve (left) and OVA inhalation (right) guinea pigs that were activated by acid (pH 6.4, 5.4, and 4.4) and KCl (50 mM). D: summary of percentages of acid-responsive jugular neurons in KCl-responsive jugular neurons in naïve (N = 6) and OVA inhalation (N = 4) guinea pigs. *P < 0.05 for naïve vs. OVA inhalation.
OVA inhalation also increases capsaicin responsiveness in vagal sensory neurons.
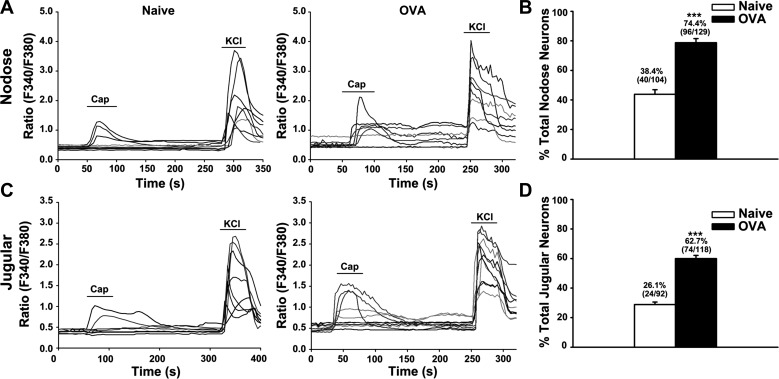
In this part of the study, we set out to test the hypothesis that antigen inhalation-induced allergic inflammation sensitized TRPV1 in vagal sensory neurons and increased their responses to the TRPV1 agonist capsaicin. Perfusion with capsaicin (100 nM) elicited calcium responses in both nodose and jugular neurons from naïve guinea pigs (N = 6 animals). OVA treatment (N = 4 animals) significantly increased the capsaicin responses of nodose and jugular neurons (P < 0.001). The percentage of capsaicin-responsive nodose neurons was increased from 38.4% (40/104) in naïve animals to 74.4% (96/129) in animals challenged with OVA (Fig. 3, A and B). Similarly, about 26.1% (24/92) of the jugular neurons could be excited by 100 nM capsaicin in the control group compared with 62.7% (74/118) of jugular neurons that responded to capsaicin after OVA inhalation (Fig. 3, C and D).
Fig. 3.
OVA inhalation enhanced capsaicin responses in nodose and jugular neurons. A: representative traces of nodose neurons from naïve (left) and OVA inhalation (right) guinea pigs. The calcium response elicited by capsaicin (100 nM) was significantly enhanced in the OVA inhalation group. B: summary of percentages of capsaicin-responsive nodose neurons related to KCl-responsive nodose neurons. C: representative traces of jugular neurons from naïve (left) and OVA inhalation (right) guinea pigs. The calcium response elicited by capsaicin (100 nM) was enhanced in the OVA inhalation group. D: summary of percentages of capsaicin-responsive jugular neurons in KCl-responsive jugular neurons in naïve (N = 6) and OVA inhalation (N = 4) guinea pigs. ***P < 0.001 for naïve vs. OVA.
TRPV1 antagonist inhibits acid-elicited responses in vagal sensory neurons.
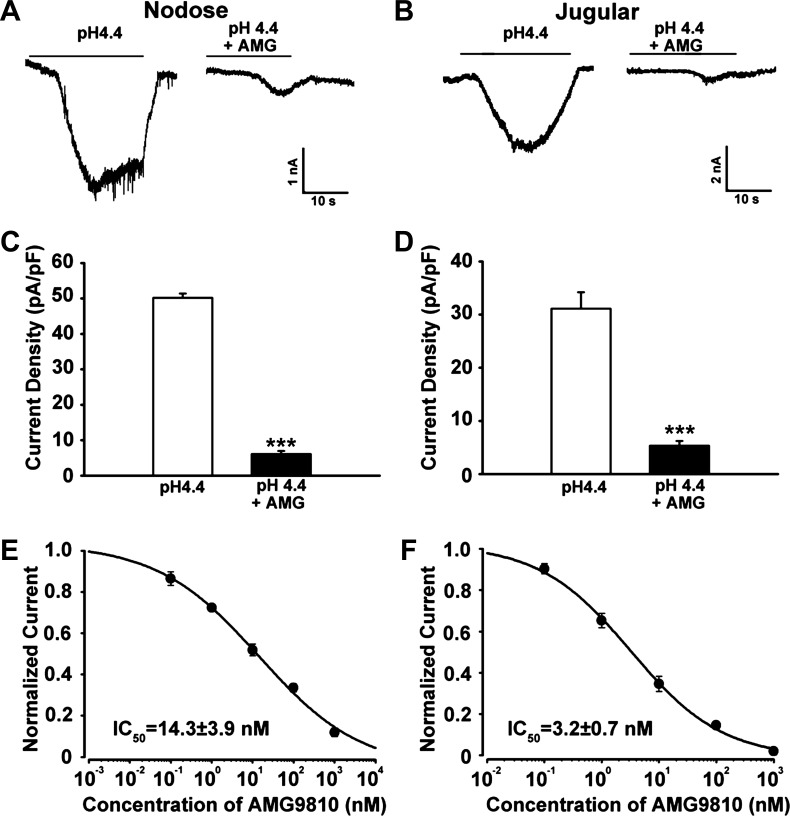
Because capsaicin is a well-known TRPV1 agonist with an EC50 of 0.7 μM, we predicted that increased acid responsiveness might be mediated by TRPV1. In this study, we selected the TRPV1 antagonist AMG-9810 to investigate the possible role of TRPV1 in OVA inhalation-induced acid hypersensitivity. Patch-clamp experiments were performed to ensure the contribution of TRPV1 channel in acid-induced activation in vagal sensory neurons from naïve guinea pigs (N = 5). As shown in Fig. 4, A and B, acid evoked an inward current in nodose and jugular neurons that is largely inhibited by the TRPV1 antagonist AMG-9810 (P < 0.001). AMG-9810 inhibited the acid-induced currents in nodose neurons with an IC50 of 14.3 ± 1.9 nM (Fig. 5E) and 3.1 ± 0.7 nM in jugular neurons (Fig. 5F), both of which were comparable with the inhibition effect on TRPV1-transfected CHO cells as reported (4).
Fig. 4.
Transient receptor potential vanilloid type 1 (TRPV1) antagonist AMG-9810 inhibited the acid-evoked currents in nodose and jugular neurons in a dose-dependent manner. A–D: AMG-9810 significantly inhibited acid (pH 4.4)-evoked current in both nodose and jugular neurons (***P < 0.001). E and F: dose-dependence curve of the inhibition effects of AMG-9810 on acid-induced currents in nodose and jugular neurons from naïve guinea pigs. ***P < 0.001 for pH 4.4 vs. pH 4.4 + AMG-9810.
Fig. 5.
TRPV1 antagonist AMG-9810 reverses the acid-induced calcium response in nodose and jugular neurons from both naïve and OVA inhalation guinea pigs. A and B: representative traces of nodose and jugular neurons from naïve (N = 4) guinea pigs. The repeatable calcium response elicited by acid (pH 4.4) was inhibited by AMG-9810 (AMG 1 μM + acid pH 4.4). C and D: representative traces of nodose and jugular neurons from OVA inhalation (N = 3) guinea pigs. The repeatable calcium response elicited by acid (pH 4.4) was inhibited by AMG-9810 (AMG 1 μM + acid pH 4.4). E and F: summary of calcium-imaging experiments as shown in A–D. ***P < 0.001 for pH 4.4 vs. pH 4.4 + AMG-9810.
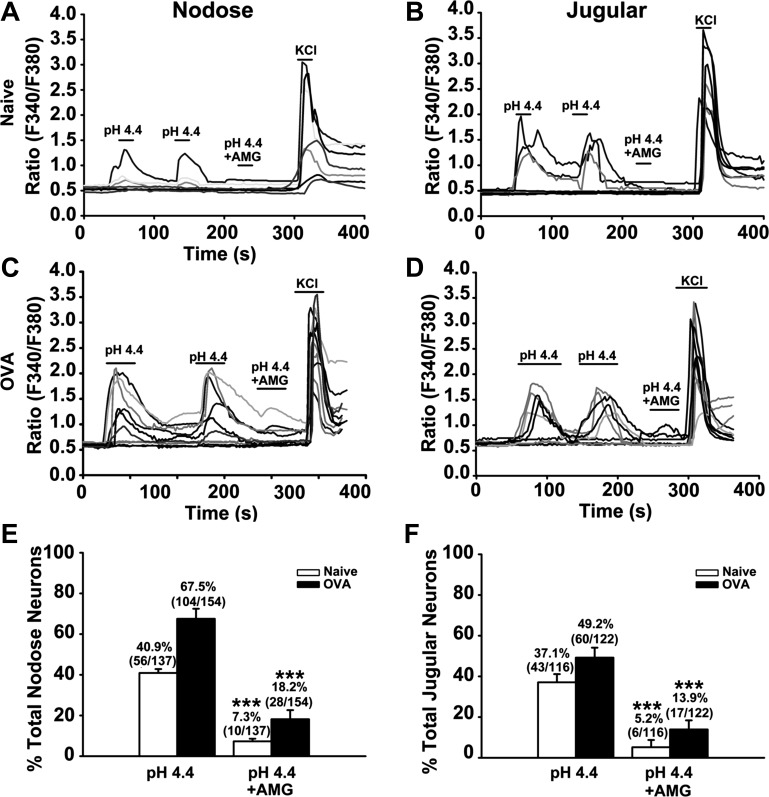
Consistent with the above result, about 40.9% (56/137) of all nodose neurons from naïve guinea pigs (N = 5) can be activated by acid (pH = 4.4). However, in the presence of 1 μM AMG-9810, only 7.3% (10/137) of these neurons were excited by acid (pH = 4.4) (Fig. 5, A and E). The same phenomenon was also observed in the jugular neurons. About 37.1% (43/116) of all jugular neurons were sensitive to acid (pH = 4.4). However, in the presence of 1 μM AMG-9810, only 5.2% (6/116) of them were activated by acid (pH = 4.4) (Fig. 5, B and F).
In OVA-challenged animals (N = 3), the acid-evoked calcium responses were also inhibited by AMG-9810 (P < 0.001 for pH 4.4 vs. pH 4.4 + AMG-9810 in both nodose and jugular neurons). Among all nodose neurons, 67.5% (104/154) were activated by acid (pH = 4.4). However, in the presence of 1 μM AMG-9810, only 18.2% (28/154) were activated by acid (pH = 4.4) (Fig. 5, C and E). The inhibition effect was also observed in jugular neurons. Only 13.9% (17/122) of all jugular neurons were activated by acid (pH = 4.4) in the presence of AMG-9810 compared with 49.2% (60/122) activated by acid (Fig. 5, D and F). To exclude the possibility that the inhibition of acid response was secondary to the desensitization to the first instillation of acid, a second acid perfusion was performed. As shown in Fig. 5, A∼D, the second acid perfusion induced the same percentage of calcium response as the first perfusion.
Increased acid responsiveness of vagal sensory neurons depends on allergic inflammation-induced sensitization of sensory nerve endings in the peripheral tissues.
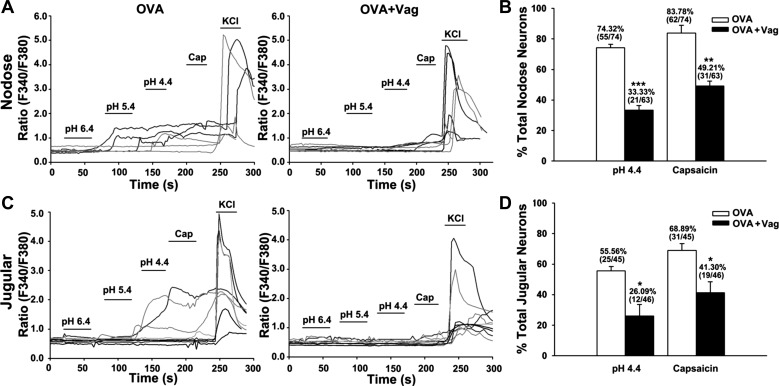
OVA challenge-induced increases in the responses to acid and capsaicin have been evaluated at the cell bodies situated in the nodose and jugular ganglia, a long distance from their nerve endings in visceral tissues. To determine if this sensitization effect depended on an intact vagus nerve in inflamed peripheral tissues, we repeated the OVA inhalation protocol in animals in which the vagal nerve was unilaterally cut. Consistent with the above results, OVA inhalation enhanced the responses to acid and capsaicin in nodose and jugular neurons, but only on the side with an intact vagal nerve and not on the side in which the vagal nerve trunk was transected. In nodose neurons, vagotomy significantly attenuated OVA inhalation-induced responsive rates for acid from 74.32% (intact vagus) to 33.33% (P < 0.001) and for capsaicin from 83.78% to 49.21% (P = 0.005). Similarly, vagotomy attenuated OVA inhalation-induced responsive rates for acid from 55.56% (intact vagus) to 26.09% (P = 0.022) and for capsaicin from 68.89% to 41.30% (P = 0.032) in jugular neurons (Fig. 6). This result suggested that allergic inflammation-induced increases of acid responsiveness in vagal sensory neurons depends on the nerve-immune interactions in peripheral inflamed tissues. To exclude the possibility that vagatomy itself changes the neuron responses to acid and capsaicin, control experiments were performed in naïve guinea pigs, and the results revealed that the responses of nodose and jugular neurons to capsaicin and acid were not significantly different between the intact side and the transected side (animal number = 3, cell number >100, P > 0.5, data not shown).
Fig. 6.
Vagotomy attenuated OVA inhalation-induced enhancements of acid and capsaicin responses in nodose and jugular neurons. A and C: representative traces of acid (pH 4.4)- and capsaicin (100 nM)-responsive nodose and jugular neurons in the control side (with intact vagus, labeled as OVA) and vagotomy side (labeled as OVA + vag) from OVA inhalation guinea pigs. B and D: summary of calcium-imaging experiments. Compared with the control side (with intact vagus), both acid- and capsaicin-responsive nodose and jugular neurons were significantly decreased in the vagotomy side after OVA inhalation. *P < 0.05, **P < 0.01, and ***P < 0.001 for OVA vs. OVA + vagotomy.
Increased acid-induced inward current in esophageal-specific nodose and jugular neurons.
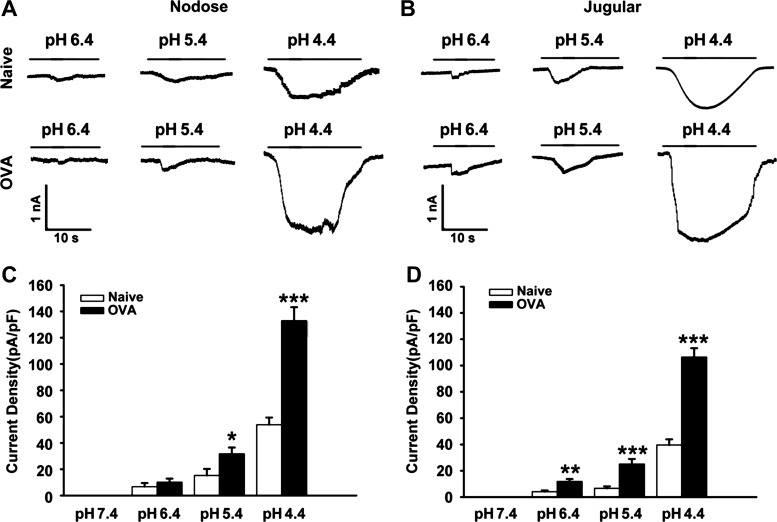
To identify the possible change of sensory neurons innervating the esophagus, DiI retrogradely labeled esophageal nodose and jugular neurons were dissociated for patch-clamp experiments. Acidic buffer, ranging from pH 6.4 to 4.4, evoked inward current in sensory neurons from both naïve animals and OVA inhalation animals in a dose-dependent manner. As shown in Fig. 7, acid (pH 6.4) evoked very small currents in nodose neurons (6.8 ± 2.7 pA/pF, n = 6) and jugular neurons (4.1 ± 1.0 pA/pF, n = 7) from the naïve animal. When pH was decreased to 5.4 and 4.4, bigger currents were elicited, respectively, with the current densities from 15.2 ± 5.0 pA/pF (pH 5.4, n = 6) to 53.9 ± 5.5 pA/pF (pH 4.4, n = 6) in nodose neurons and from 6.6 ± 1.5pA/pF (pH 5.4, n = 7) to 39.6 ± 4.3 pA/pF (pH 4.4, n = 7) in jugular neurons.
Fig. 7.
OVA inhalation increased acid-evoked inward current in Dil-labeled esophageal nodose and jugular neurons. A and B: representative traces of acid-evoked currents in nodose and jugular neurons from naïve and OVA-challenged (OVA) guinea pigs (N = 2 in each group). C and D: acid-elicited currents in nodose and jugular neurons from naïve (both n = 6) and OVA-challenged (both n = 7) guinea pigs. *P < 0.05, **P < 0.01, and ***P < 0.001 for naïve and OVA.
In the OVA inhalation group, these inward currents by acid at the same pH were increased compared with the naïve group. Mild acid (pH 6.4) activated bigger currents in nodose neurons (10.2 ± 2.7 pA/pF, n = 6) and jugular neurons (11.8 ± 1.9 pA/pF, n = 7) from OVA inhalation animals. Furthermore, such increases were more significant at lower pH, such as pH 5.4 (P < 0.001 for naïve vs. OVA inhalation in nodose neurons and P = 0.003 in jugular neurons) and pH 4.4 (P < 0.001 in both nodose neurons and jugular neurons). The inward currents triggered at pH 5.4 were much bigger in nodose neurons (31.7 ± 4.9 pA/pF, n = 6) and jugular neurons (25.1 ± 3.8 pA/pF, n = 7), and the most significant increases were observed at pH 4.4 with the current densities of 132.9 ± 10.3 pA/pF (n = 6) in nodose and 106.4 ± 6.7 pA/pF (n = 7) in jugular neurons. Increased current densities in esophageal vagal nodose and jugular neurons evoked by acid after OVA challenge indicated that the excitabilities of esophageal vagal sensory neurons to acid are increased after antigen challenge.
DISCUSSION
Patients with allergic disorders often present with symptoms that are secondary to the alterations in the nervous system in affected tissues or organs (13). In patients with EoE, the typical symptoms of dysphagia, food impaction, and heartburn are considered to relate to sensory/motor dysfunctions in the esophagus, but the underlying mechanism is still unclear. Increased infiltration and activation of eosinophils and mast cells in the esophagus could release mediators and growth factors in the tissue, which may subsequently sensitize sensory nerves in the proximity and increase their responses to noxious stimuli. Gastric acid refluxed in the esophagus with a lower pH is a noxious stimulation that can activate nociceptive afferent nerve and induce a burning sensation, heartburn. This is a typical symptom not only presented in refluxed disorders but also in EoE. Whether allergic inflammation sensitizes esophageal afferents and potentiates their responses to acid has yet to be determined. Using our established guinea pig EoE model that is typified by increases in tissue eosinophils and mast cells (15, 19), the acid responsiveness in vagal sensory neurons was substantively enhanced. The data support the hypothesis that this largely involves an increase in TRPV1 function, secondary to neurotrophic factor regulation arising from the nerve terminals within esophageal, and potentially other visceral tissue.
Our previous studies have demonstrated that guinea pig esophageal vagal afferents have distinctive subtypes of nociceptive C fibers with their neuron cell bodies in either nodose or jugular ganglia. They are able to discriminate noxious and innocuous mechanical (distension) stimuli and can be activated by noxious chemical stimuli such as capsaicin and lower pH acid (16, 19). Mast cell activation-released mediators such as histamine, tryptase, and PGD2 can acutely enhance their electrical excitability (13, 18). The present study extended these findings from afferent nerve endings to sensory neuron cell bodies situated in the vagal ganglion where EoE was associated with a decreased threshold to acid stimulation and an increased percentage of neurons responding to acid. This indicates that, in addition to neuroexcitability changes as shown in our previous studies, EoE is associated with phenotypic changes in these sensory neurons that likely involve transcriptional regulation of certain acid-responsive ion channels.
There are basically three general mechanisms by which the inhaled OVA could influence these neuronal cell bodies in the vagal sensory ganglia (13). First, the OVA may have reached the circulation and stimulated mast cells within the ganglia. There are mast cells within vagal sensory ganglia, and these mast cells are sensitized by the active sensitization procedure such that they would be activated should OVA reach the ganglia (12). Second, the OVA-induced inflammation in the esophagus (and respiratory tract) may lead to cytokines or mediators that reach the systemic circulation in concentrations sufficient to affect the cell bodies in the vagal ganglia. Third, the inflammation in the visceral tissue may lead to the formation of neurotrophic factors that interact with the nerve endings and are then transported back to the cell bodies (6). Of these three mechanisms, only the latter would require an intact vagus nerve. This is because, upon binding to high-affinity receptors at the terminals, neurotrophic factors are transported axonally back to the cell body where they can modify gene transcription. Our finding that the increase in acid sensitivity of vagal sensory neurons required an intact vagus nerve provides supportive evidence of a neurotrophic mechanism. The neurons in the adult guinea pig nodose ganglion express primarily neurotrophic tyrosine kinase receptor type 2 (TrkB), the receptor for BDNF and neurotrophin-4, whereas jugular neurons express largely tyrosine kinase receptor type 1 (TrkA), the receptor for NGF. The neurons in both ganglia express the GDNF receptor GFRa1 and RET (8). Which, if any of these, factors participate in the increased acid sensitivity is open for speculation, but agonists for TrkA, TrkB, and GFRa1 are associated with allergen challenge (13).
The results indicate that the ionic mechanism for the increased acid responsiveness in vagal sensory neurons involves TRPV1. TRPV1 is the capsaicin receptor, but it can also be stimulated by heat and protons. TRPV1 function can be regulated by inflammatory mediators and at the transcriptional level by neurotrophins (13). Our previous studies have demonstrated that repeated OVA inhalation leads to a phenotypic switch in the type of neurons that express TRPV1 in the nodose ganglion (6, 9). In healthy animals, TRPV1 expression is largely limited to C-fiber neurons. Our previous studies showed that, after OVA inhalation, not only do the small-diameter C-fiber-type neurons express TRPV1 but also the larger A fiber neurons (9, 17). Allergen-induced induction of TRPV1 can be mimicked by GDNF and BDNF (9). The present study provided functional evidence that prolonged OVA inhalation significantly increased capsaicin-responsive neurons in both nodose and jugular neurons, suggesting upregulated TRPV1 functions in these neurons. When the influence of TRPV1 was blocked with the TRPV1 antagonist AMG-9810, the response to acid was substantially reduced in both healthy animals, and the upregulated response in OVA-treated animals was largely normalized. This is consistent with the result that TRPV1 involves OVA inhalation-induced increase in acid sensitivity of vagal sensory neurons.
The EoE model involves the inhalation of OVA in actively sensitized guinea pigs. With respect to gastrointestinal tissues, the eosinophilic inflammation is limited to the esophagus and is not observed in the intestine or colon (15). The OVA inhalation will, however, also result in an eosinophilic inflammation of the respiratory tract. In the calcium-imaging studies, the neurons that reveal an increase in sensitivity to acid therefore likely involve both respiratory and esophageal sensory neurons. To prove that the acid sensitivity of esophageal neurons in particular are increased in this model, we evaluated the sensitivity of esophageal-specific neurons using retrograde tracing techniques. When acid-induced inward currents were quantified in esophageal-specific neurons, we noted a striking increase in responsiveness in those neurons isolated from the OVA-challenged animals. As with the calcium assay, most of this inward current was blocked when TRPV1 was antagonized. It should be pointed out that acid can stimulate vagal afferent neurons via many ionic channels other than TRPV1 (5). Ionic channels of the acid-sensing ion channel (ASIC) family, for example, are expressed in these neurons. Some ASIC channels are rapidly inactivating and require rapid acid exposure to investigate. Our experimental design may have therefore reduced the apparent influence of these channels.
In summary, the present study demonstrated for the first time in a guinea pig EoE model that prolonged antigen challenge increases acid responsiveness in cell bodies of vagal afferent jugular and nodose neurons, including those innervating the esophagus. This sensitization effect is mediated largely through TRPV1 and initiated from nerve-immune interactions at sensory nerve endings in the peripheral tissues. This adds to our knowledge of the development of esophageal dysfunctions, such as heartburn and dysphagia, in EoE.
GRANTS
This study is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-087991 and the Blaustein Pain Research Fund (S. Yu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.H., P.J.P., B.J.U., and S.Y. conception and design of research; Y.H., Z.L., and X.Y. performed experiments; Y.H., X.Y., and S.Y. analyzed data; Y.H., Z.L., X.Y., and S.Y. interpreted results of experiments; Y.H. and S.Y. prepared figures; Y.H. and S.Y. drafted manuscript; Y.H., P.J.P., B.J.U., and S.Y. approved final version of manuscript; P.J.P., B.J.U., and S.Y. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Xinzhong Dong (Department of Neuroscience, Johns Hopkins University) for providing support on the calcium-imaging study.
REFERENCES
- 1. Abonia JP, Rothenberg ME. Eosinophilic esophagitis: rapidly advancing insights. Annu Rev Med 63: 421–434, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Chuaychoo B, Hunter DD, Myers AC, Kollarik M, Undem BJ. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 116: 325–331, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313: 474–484, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 201: 63–75, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 161: 1985–1990, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Lee MG, Macglashan DW, Jr, Undem BJ. Role of chloride channels in bradykinin- induced guinea pig airway vagal C-fibre activation. J Physiol 566: 205–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol 300: L790–L798, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low- threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302: L941–L948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–38, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Qin C, Farber JP, Foreman RD. Intraesophageal chemicals enhance responsiveness of upper thoracic spinal neurons to mechanical stimulation of esophagus in rats. Am J Physiol Gastrointest Liver Physiol 294: G708–G716, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Undem BJ, Hubbard W, Weinreich D. Immunologically induced neuromodulation of guinea pig nodose ganglion neurons. J Auton Nerv Syst 44: 35–44, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol 13: 1847–1852, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long- lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 293: G850–G856, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine- mediated pathway. Life Sci 82: 324–330, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563: 831–842, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang S, Grabauskas G, Wu X, Joo MK, Heldsinger A, Song I, Owyang C, Yu S. Role of prostaglandin D2 in mast cell activation-induced sensitization of esophageal vagal afferents. Am J Physiol Gastrointest Liver Physiol 304: G908–G916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S, Liu Z, Heldsinger A, Owyang C, Yu S. Intraluminal acid activates esophageal nodose C fibers after mast cell activation. Am J Physiol Gastrointest Liver Physiol 306: G200–G207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]